Abstract
Morphological assessments are used to select embryos with the highest implantation potential, however it is still very limited. The development of new technologies, such as Raman spectroscopy have improved quantitative and qualitative analysis, and consequently led to a better characterization of embryos and improvements on the prediction of their potential. Therefore, we propose a method based on the conventional in vitro culture system of bovine embryos, and the subsequent analysis of the culture media drops by Raman spectroscopy. Our results obtained by PCA analysis clearly showed a separation of the spectral profiles from culture media drops with and without embryos.
OCIS codes: (170.0170) Medical optics and biotechnology, (170.5660) Raman spectroscopy, (170.1420) Biology
1. Introduction
The main goal of in vitro production (IVP) of bovine embryos is increase pregnancy rates from IVP embryos transferred to the recipients, resulting in several economical and social benefits to cattle producers [1]. To reach this goal, researchers seek for improvements in the embryo selection criteria, aiming to transfer the best embryos to recipients, with high development potential and pregnancy success [2, 3].
Several methods have been proposed for the evaluation of embryo quality, such as assessment of eclosion rates, survival to cryopreservation, cell count of the inner cell mass and trophoectoderm, evaluation of the number of apoptotic cells, and embryo biopsy for chromosomal abnormalities analysis. However, many of these techniques are not feasible for practical application in IVP, since they can compromise the embryo viability and prevent its transfer to recipients [1]. Thus, bovine embryos selection for embryo transfer (ET) is based on morphology and embryonic stage of development, but these criteria are not good predictors of embryo survival [4–6].
Therefore, new methods has been sought - preferably non-invasive ones - to provide more accurate information that can be correlated to the quality and development potential of bovine IVP embryos [7, 8]. Among non-invasive techniques, the evaluation of embryo culture medium is a new approach that has been gaining attention recently, since it is the direct microenvironment of the IVP embryo [9, 10]. Studies suggest that the detection of chemical composition changes in the culture medium can be correlated with the embryo quality and development potential [3, 11–13].
Several technologies have been used to evaluate culture media, such as Nuclear Magnetic Resonance (NMR) [14], Mass Spectrometry (MS) [15], Fourier Transform Infrared Spectroscopy (FT-IR) [1], Near-infrared Spectroscopy (NIR) [16], Raman Spectroscopy [10], among others.
The Raman spectroscopy technique has special interest due to their high sensitivity to detect tiny biochemical and molecular variations in tissues or biological samples [17]. The characteristic spectra profiles generated by this technique can be applied as a non destructive analytical method. Other advantages are related to the small sample volume required (only some µL) and the minimal previous preparation needed [10, 18]. Therefore, Raman spectroscopy can be considered as a very promising technique for the evaluation of embryo culture media.
Using this technique, some authors have demonstrated the presence of different spectra profiles of IVP culture media for embryos with good development and implantation potential in comparison with ones that resulted in implantation failure after transfer [10, 16, 18]. It’s important to mention that the evaluation of embryo culture media by Raman spectroscopy has been performed in humans, but the same is not true for other species, such as bovine. Therefore, the objective of this study was the development and standardization of a method based on Raman micro-spectroscopy for the evaluation of the culture media of IVP bovine embryos.
2. Materials and methods
All procedures and protocols were performed in accordance with the Ethical Principles in Animal Research set forth by the Brazilian College of Animal Experimentation, with approval from “Ethic Committee in the use of animal” of Universidade Federal do ABC (Protocol No. 008/2014).
2.1 Sample preparation
Embryos were produced following standard protocols for in vitro maturation (IVM) and fertilization (IVF) [19]. During the in vitro culture (IVC) the zygotes were transferred individually into microdroplets of 20μl of culture medium and the individual cultures were placed in an incubator at 38.5°C and 5% CO2 in air and high humidity for seven days.
For evaluation by Raman spectroscopy three standard culture media for the IVC of the embryos were used: (i) Potassium Simplex Optimization Medium (KSOM) (MR-106-D Millipore) supplemented with 10% of fetal bovine serum (FBS), gentamicin and non-essential amino acids; (ii) Synthetic Oviduct Fluid (SOF) supplemented with 5% FBS, essential and non-essential amino acids (SOFaa); (iii) SOFaa collected after seven days of embryo in vitro culture (SOFaaE). All samples remained in an incubator at 38.5°C and 5% CO2 in air and high humidity for seven days before being collected and stored at −80°C until analysis.
2.2 Raman spectroscopy
The equipment used on this study was the Triple T64000 Raman Spectrometer (Horiba Jobin-Yvon S.A.S., France) with microanalysis option and CCD detector 1024x256 – OPEN-3LD/R with quantum response of approximately 40%. The excitation laser was a 532 nm (Verdi G5, Coherent Inc. USA) focused on a spot with 5 mW power. Spectra were acquired from four biological replicates. Each replicate was composed by five embryos at blastocyst stage. One spectrum was collected per droplet.
2.3 Spectra acquisition
During the standardization of the method parameters such as droplet volume, presence or not of mineral oil, kind of substrate, objective magnification, degree of confocability, and acquisition time/number of accumulations were tested. Figure 1 shows a schematic diagram of the experiment using Petri dish as substrate and microdrops culture media covered with mineral oil (Fig. 1(A)). That set was placed in the upright micro-Raman microscope. The laser was focused through the mineral oil layer (Fig. 1(B)).
Fig. 1.
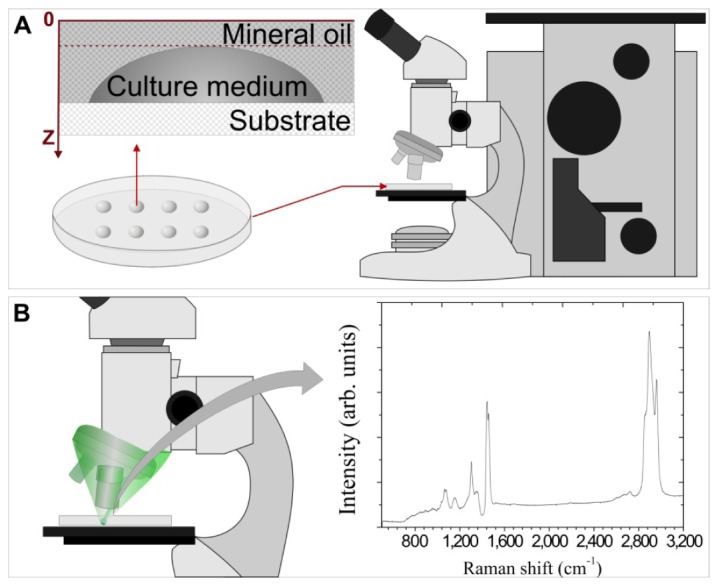
Schematic diagram of the proposed experimental model. A) culture medium droplets covered with mineral oil on Petri dish in the micro-Raman system; B) incidence of the laser beam on the sample with the spectra acquisition.
The characterization of the culture medium droplets was performed using the optimized parameters found. It was used a confocal analysis with the spectra being acquired from the mineral oil surface and at every 2 µm interval in the z-axis (Fig. 1(A)) using the microscope micrometer.
2.4 Spectra analysis
Experimental data were plotted using the Origin 8.0 software (OriginLab, Northampton, MA). Spectra were acquired by Labscap 5.0 software and pre-processed by removing spikes. The baseline correction and normalization by area of the spectra was performed by Fityk 0.9.8 software [20]. Spectra and loading plot were analyzed by Principal Component Analysis (PCA), subroutines of the software Minitab 16 (Minitab Inc.). Vector normalization was made for the analysis done by PCA, and PCs were obtained from a correlation matrix.
3. Results and discussion
3.1 Standardization of spectra parameters
The choice of the substrate was the first step of the standardization procedure. It was tested Petri dish (polystyrene), commercial aluminum foil, CaF2 window and glass slides. A chemically stable substrate that does not interfere in the spectra acquisition of the samples, such as quartz [16, 18] and gold [9, 10] is the usual option of choice for Raman experiments in culture media. Nevertheless, the selected substrate for this experiment was the Petri dish. Others substrates generated contamination with spurious Raman bands. Moreover, Petri dish is a widely employed culture cell consumable which enables evaluation of the proposed methodology in a closer IVP lab scenario.
As for the sample preparation, volumes of 10 and 20 µL of culture medium, with and without a cover of mineral oil on the polystyrene Petri dish, were tested. The droplet with 10 µL of culture medium covered with mineral oil on the Petri dish resulted in the best configuration for spectra acquisition and applicability of the proposed method. The use of a smallest volume of culture medium is essential because of the limited availability of material derived from embryo culture. The choice to scan the samples covered with mineral oil was made because it is also the method used for in vitro culture [19, 21] increasing, therefore, the commercial application of this method. Furthermore, the tests carried out without the use of mineral oil covering the culture medium droplets, showed that the incidence of the laser beam, even with low power, caused sample evaporation during the spectra acquisition. This reduction of the sample volume could generate erroneous data, especially in a quantitative analysis, and also prevent the reproducibility of the results [17].
Regarding the use of objective lenses for spectral acquisition, tests were conducted using 10, 50, and 100X magnification objectives. At long working distance (10.6 µm), the plan achromatic 50X objective (NA = 0.50) showed better results, with best signal-to-noise ratio. The confocability of the system was also tested, ranging the confocal aperture in 1, 3, 5 and 6.5 µm. The best results were observed with the 6.5 µm confocal aperture which presented spectra with best signal-to-noise ratio and higher bands intensity.
Moreover tests were conducted varying the spectral range to analyze the samples: 500 to 1800 cm−1, 1800 to 4000 cm−1, 500 to 3200 cm−1 and 500 to 4000 cm−1. The spectra were acquired on the 500 - 3200 cm−1 spectral window. It is also a region that contains bands with biological relevance (see Table 1), such as proteins, amino acids, lipids, among other bands [9, 10, 16–18]. Finally, tests were also made by varying acquisition number x time of exposure: 1 x 10 s, 2 x 30 s, 3 x 20 s. The best acquisition number x time of exposure was 3 x 20 s since it produced bands with higher signal intensity.
Table 1. Raman bands assignments of the culture medium with embryos (SOFaaE).
| Experimental (cm−1) | Literature (cm−1) | Assigment |
|---|---|---|
| 845 | 844 [24] | Proteins (v (C-C)) |
| 891 | 865-942 [25] | Threonine (δ(CO2H)) |
| 952 | 955 [26] | v (PO4) |
| 1001 | 1001 [26] | Phenylalanine (Symmetric CC aromatic ring breathing) |
| 1062 | 1063–1295 [25] | Ɣt (CH2) (Weak, couples with adjacent CH2 groups) |
| 1076 | 1082 [17] | Lipids (C─C or C─O stretching mode) |
| 1152 | 1156 [27] | β-carotene (C═C stretch) |
| 1300 | 1301 [27] | Amide III (δ (N─H)-30%, α-helix,ν (C─N)-40% & δ(CH3)) |
| 1346 | 1342 [17] | Collagen (CH3CH2 wagging mode) |
| 1442 | 1428–1471[27]/1442 [28] | Lipid, protein (δ (CN) bending, δ(CH3))/ β-carotene |
| 1455 | 1451 [29] | Lipids, proteins- Kerantin (CH2 bending) |
| 2719 | 2727 [27] | T, A, G of DNA in bend overtone(CH3 in phase deformation) |
| 2892 | 2891 [27] | Poly methylene chain (v (CH2, FR)) |
| 2957 | 2960 [30] | υ(C−Η) |
Different vibration types: δ: deformation vibration; ρ: rocking vibration; Ɣt: twisting vibration; v: stretching vibration; δ: bending vibration; FR: Fermi resonance
3.2 Confocal analysis of the droplet
The opening of the confocal aperture was also optimized. The best value was 6.5 µm. In order to identify the oil-culture interface spectra were acquired for several z-values (Fig. 2). It was possible to identify three distinct regions. The first consists of the spectra collected in the oil surface (0 µm) to 14 µm z-axis displacement, which it is noted that the spectra show fairly constant relative intensity of the bands. The second region occurs between 16 and 18 µm z-axis, comprises the interface between the mineral oil and the culture medium, evidenced by the slight increase in the intensity of the bands. The third region is from 20 µm of z axis, shows the spectra collected from the culture medium itself, evidenced by a gradual increase in the intensity of the bands.
Fig. 2.
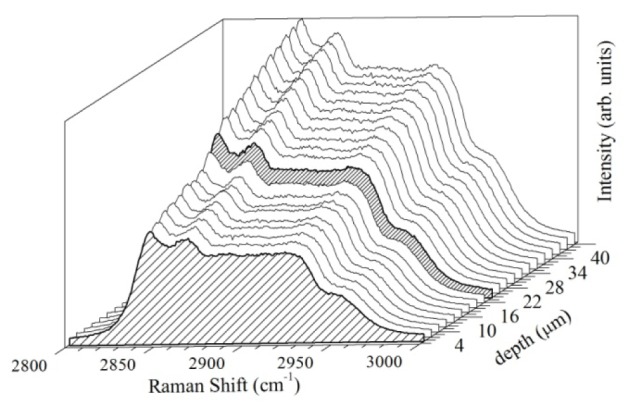
Oil-culture interface identification in the droplet. The two highlighted regions corresponding to the spectrum of the surface of the mineral oil (0 µm in the z-axis) and the interface between oil and culture medium (18 µm z-axis) are shown.
It was determined z = 20 µm the suitable depth for culture medium data acquisition. It is important to notice that the distinct droplet regions presented almost the same set of Raman bands. Only an overall intensity variation was observed. It is well-reported that some culture medium molecules, as lipids, could be absorbed by the mineral oil [9, 21, 22] which justify this finding.
It was described a method for culture media analysis based on the deposition and drying of the droplet on a solid substrate [17]. The pre-concentration of the sample constituents on the substrate allows their capture with a high intensity signal in the Raman spectrum. However, the droplet drying technique results in a coffee ring effect, in which proteins precipitate and accumulate in the peripheral region of the ring, providing a Raman spectrum with high intensity signal. The central region, though, contains a large precipitation of salts, mainly NaCl and KCl, with low intensity signal. Thus, the method proposed in this study, with no drying up of the droplet, has the advantage of faithfully representing the composition of the culture medium.
3.3 Sample analysis
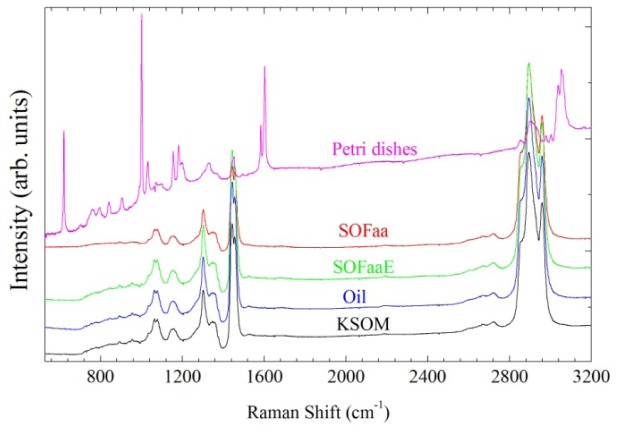
Raman spectra were acquired from the substrate (Petri dish), mineral oil and the culture media with and without embryos. Figure 3 shows the spectra of the culture media without embryos (KSOM and SOFaa), with embryos (SOfaaE), Petri dish and mineral oil. It was evident that the substrate showed a very distinct and characteristic spectrum in comparison with the culture media spectra, indicating that it didn’t interfere in the culture media analysis. Some of the mineral oil bands coincided with those from the culture media, however they had distinct intensities. The presence of coincident bands between these samples could be caused by the moving of culture medium molecules to the oil during the embryo culture [21–23]. It is also important to note that mineral oil is a product derived from crude oil, and therefore, has a chemical composition variable in quality [21], which can result in various bands in the Raman spectra.
Fig. 3.
Average Raman spectra of the following groups: culture media without embryos (KSOM and SOFaa), with embryos (SOFaaE), mineral oil and Petri dish in the 500-3200 cm−1 region.
The analysis of embryo culture medium (SOFaaE) showed vibrational modes of important biomolecules, especially large classes of proteins, lipids and nucleic acids, as expected [9, 10, 17]. Figure 4 shows the Raman spectra of SOFaaE with the corresponding bands, which are identified in Table 1.
Fig. 4.
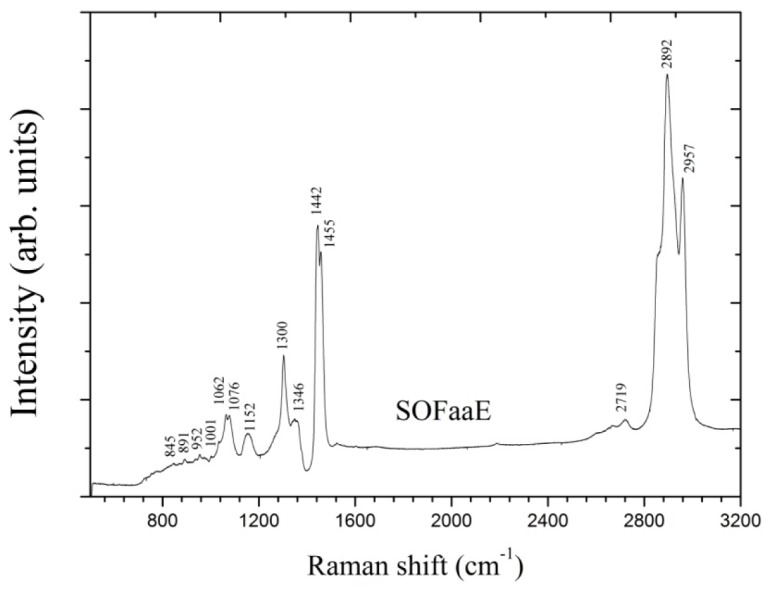
Raman spectra of culture medium with embryos (SOFaaE) with their corresponding vibrational bands in the 500 to 3200 cm−1 region.
The technique sensitivity was evaluated from the PCA analysis of the culture media spectra without embryos (KSOM, SOFaa) and with embryos (SOFaaE). Since the spectra scan was set on a broad region, the PCA analysis was separated in two ranges, one from 500 to 1800 cm−1 (fingerprint) and the other from 1800 to 3200 cm−1 (high wavenumber). The percentage of variance for each PC was PC1: 98.4%; PC2: 1.5%; PC3: 0.1%; PC4 < 0.1% for fingerprint region. Similar values were found for high wavenumber region. Due to the negligible contribution from PCs greater than 4 they were not considered in the analysis. Figure 5 presents the dispersion curves for PC1, PC2, and PC3. It was possible to see a clear separation between the spectroscopic profiles of the three groups analyzed in both ranges evaluated. This separation shows the sensitivity of the technique to generate specific and characteristic spectroscopic profiles of the culture media with and without embryos.
Fig. 5.
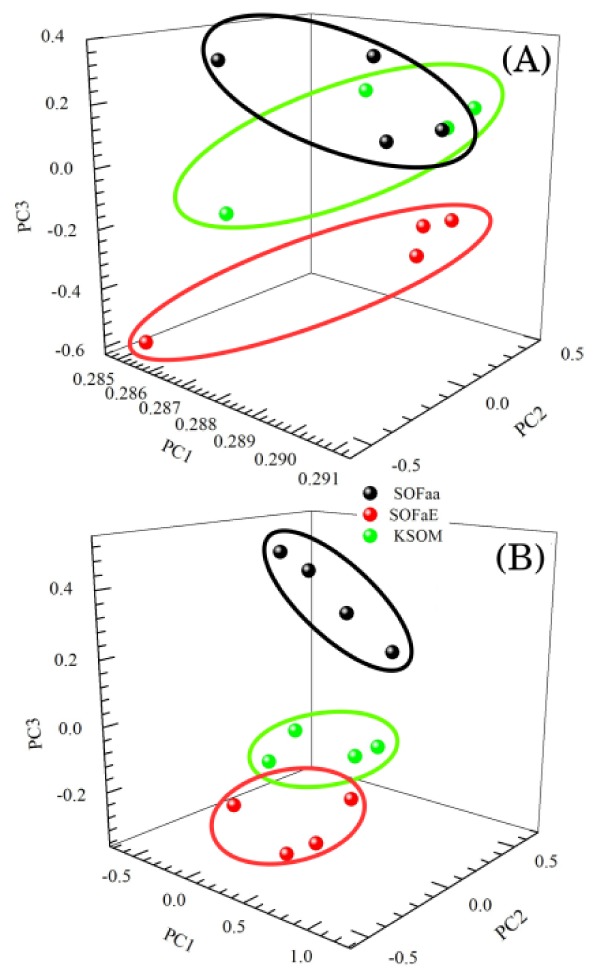
Principal Component Analysis (PCA) of the Raman spectra of the culture media without embryos (KSOM and SOFaa) and with embryos (SOFaaE) in the Raman bands from 500 to 1800 cm−1 (A) and from 1800 to 3200 cm−1 (B).
Loading plot analysis from PCs responsible for the separation of the groups are shown in Fig. 6. Bands that contribute in a higher proportion to the separation of the groups were identified: 1442 and 1455 cm−1 (lipids and proteins bands) in 500 to 1800 cm−1 region, and 2892 cm−1 (poly methylene chain band) in 1800 to 3200 cm−1 region.
Fig. 6.
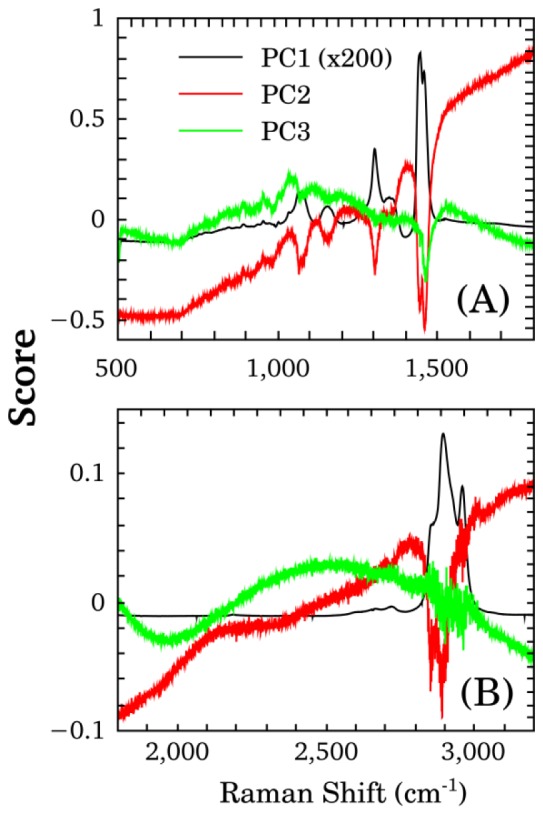
Loading plot analysis of the Raman spectra of the culture media without embryos (KSOM and SOFaa) and with embryos (SOFaaE) in the Raman bands from 500 to 1800 cm−1 (A) and from 1800 to 3200 cm−1 (B).
Considering the results of the method proposed in this study (summarized in Fig. 1), it’s possible to state that it is efficient for the evaluation of culture media used in IVP bovine embryos. This technique can be used as a fast and accurate diagnostic method that allow us to characterize the bovine embryo culture media in order to find global standards and characteristic profiles. The use of this profiles combined with embryo morphology data can allow a better evaluation of embryo quality, which can lead to an improvement in embryo selection and an increase in pregnancy rates.
4. Conclusion
This study proposes, for the first time, a method for the evaluation of culture media of in vitro produced bovine embryos based on Raman spectroscopy. The proposed method has potential for commercial application and a series of advantages such as: the equipment is relatively easy to use and handle and it is not expensive; no high cost analysis and results can be obtained immediately; use of small sample volume with no need of previous preparation or processing of the samples; and it is a non-invasive and non-destructive technique. Therefore, this technology has potential as a diagnostic application for global characterization of spectroscopic profiles of culture media used in IVP bovine embryos, which can be correlated with viability in order to improve embryo selection and consequently the pregnancy rates.
Acknowledgments
The authors would like to thank the Brazilian agencies FAPESP, CAPES, and CNPq for financial support; Multiusers Central Facilities of UFABC for the experimental support.
References and links
- 1.Muñoz M., Uuyar A., Correia E., Diez C., Fernandez-gonzales A., Caamaño J. N., Martinez-bello D., Trigal B., Humblot P., Ponsart C., Guyader-joly C., Carrocera S., Martin D., Marquant Le Guienne B., Seli E., Gomez E., “Metabolomic Prediction of Pregnancy Viability in Superovulated Cattle Embryos and Recipients with Fourier Transform Infrared Spectroscopy,” J. Dairy Sci. 97(9), 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes A. S., Greve T., Callesen H., “Quantification of embryo quality by respirometry,” Theriogenology 67(1), 21–31 (2007). 10.1016/j.theriogenology.2006.09.026 [DOI] [PubMed] [Google Scholar]
- 3.Seli E., Botros L., Sakkas D., Burns D. H., “Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization,” Fertil. Steril. 90(6), 2183–2189 (2008). 10.1016/j.fertnstert.2008.07.1739 [DOI] [PubMed] [Google Scholar]
- 4.Brison D. R., Houghton F. D., Falconer D., Roberts S. A., Hawkhead J., Humpherson P. G., Lieberman B. A., Leese H. J., “Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover,” Hum. Reprod. 19(10), 2319–2324 (2004). 10.1093/humrep/deh409 [DOI] [PubMed] [Google Scholar]
- 5.Vergouw C. G., Botros L. L., Roos P., Lens J. W., Schats R., Hompes P. G. A., Burns D. H., Lambalk C. B., “Metabolomic profiling by near-infrared spectroscopy as a tool to assess embryo viability: a novel, non-invasive method for embryo selection,” Hum. Reprod. 23(7), 1499–1504 (2008). 10.1093/humrep/den111 [DOI] [PubMed] [Google Scholar]
- 6.Pudakalakatti S. M., Uppangala S., D’Souza F., Kalthur G., Kumar P., Adiga S. K., Atreya H. S., “NMR studies of preimplantation embryo metabolism in human assisted reproductive techniques: a new biomarker for assessment of embryo implantation potential,” NMR Biomed. 26(1), 20–27 (2013). 10.1002/nbm.2814 [DOI] [PubMed] [Google Scholar]
- 7.Herrero J., Meseguer M., “Selection of high potential embryos using time-lapse imaging: the era of morphokinetics,” Fertil. Steril. 99(4), 1030–1034 (2013). 10.1016/j.fertnstert.2013.01.089 [DOI] [PubMed] [Google Scholar]
- 8.Gardner D. K., Lane M., Stevens J., Schoolcraft W. B., “Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential,” Fertil. Steril. 76(6), 1175–1180 (2001). 10.1016/S0015-0282(01)02888-6 [DOI] [PubMed] [Google Scholar]
- 9.Shen A. G., Peng J., Zhao Q. H., Su L., Wang X. H., Hu J. M., Yang Q., “Accurate and noninvasive embryos screening during in vitro fertilization (IVF) assisted by Raman analysis of embryos culture medium,” Laser Phys. Lett. 9(4), 322–328 (2012). 10.1002/lapl.201110134 [DOI] [Google Scholar]
- 10.Zhao Q., Yin T., Peng J., Zou Y., Yang J., Shen A., Hu J., “Noninvasive Metabolomic Profiling of Human Embryo Culture Media Using a Simple Spectroscopy Adjunct to Morphology for Embryo Assessment in in Vitro Fertilization (IVF),” Int. J. Mol. Sci. 14(4), 6556–6570 (2013). 10.3390/ijms14046556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner D. K., Leese H. J., “Assessment of embryo viability prior to transfer by the noninvasive measurement of glucose uptake,” J. Exp. Zool. 242(1), 103–105 (1987). 10.1002/jez.1402420115 [DOI] [PubMed] [Google Scholar]
- 12.Hardarson T., Ahlström A., Rogberg L., Botros L., Hillensjö T., Westlander G., Sakkas D., Wikland M., “Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial,” Hum. Reprod. 27(1), 89–96 (2012). 10.1093/humrep/der373 [DOI] [PubMed] [Google Scholar]
- 13.Lee Y. S., Thouas G. A., Gardner D. K., “Developmental kinetics of cleavage stage mouse embryos are related to their subsequent carbohydrate and amino acid utilization at the blastocyst stage,” Hum. Reprod. 30(3), 543–552 (2015). 10.1093/humrep/deu334 [DOI] [PubMed] [Google Scholar]
- 14.Wallace M., Cottell E., Cullinane J., McAuliffe F. M., Wingfield M., Brennan L., “(1)H NMR based metabolic profiling of day 2 spent embryo media correlates with implantation potential,” Syst. Biol. Reprod. Med. 60(1), 58–63 (2014). 10.3109/19396368.2013.854426 [DOI] [PubMed] [Google Scholar]
- 15.Cortezzi S. S., Cabral E. C., Trevisan M. G., Ferreira C. R., Setti A. S., Braga D. P., Figueira R. C., Iaconelli A., Jr, Eberlin M. N., Borges E., Jr., “Prediction of embryo implantation potential by mass spectrometry fingerprinting of the culture medium,” Reproduction 145(5), 453–462 (2013). 10.1530/REP-12-0168 [DOI] [PubMed] [Google Scholar]
- 16.Seli E., Sakkas D., Scott R., Kwok S. C., Rosendahl S. M., Burns D. H., “Noninvasive metabolomic profiling of embryo culture media using Raman and near-infrared spectroscopy correlates with reproductive potential of embryos in women undergoing in vitro fertilization,” Fertil. Steril. 88(5), 1350–1357 (2007). 10.1016/j.fertnstert.2007.07.1390 [DOI] [PubMed] [Google Scholar]
- 17.Huang Z., Sun Y., Wang J., Du S., Li Y., Lin J., Feng S., Lei J., Lin H., Chen R., Zeng H., “Rapid and nondestructive method for evaluation of embryo culture media using drop coating deposition Raman spectroscopy,” J. Biomed. Opt. 18(12), 127003 (2013). 10.1117/1.JBO.18.12.127003 [DOI] [PubMed] [Google Scholar]
- 18.Scott R., Seli E., Miller K., Sakkas D., Scott K., Burns D. H., “Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study,” Fertil. Steril. 90(1), 77–83 (2008). 10.1016/j.fertnstert.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 19.Soares C. A., Annes K., Dreyer T. R., Magrini T., Sonoda M. T., da Silva Martinho H., Nichi M., d’Ávila Assumpção M. E., Milazzotto M. P., “Photobiological effect of low-level laser irradiation in bovine embryo production system,” J. Biomed. Opt. 19(3), 035006 (2014). 10.1117/1.JBO.19.3.035006 [DOI] [PubMed] [Google Scholar]
- 20.Wojdyr M., “Fityk: a general-purpose peak fitting program,” J. Appl. Cryst. 43(5), 1126–1128 (2010). 10.1107/S0021889810030499 [DOI] [Google Scholar]
- 21.Martinez C. A., Nohalez A., Cuello C., Vazquez J. M., Roca J., Martinez E. A., Gil M. A., “The use of mineral oil during in vitro maturation, fertilization, and embryo culture does not impair the developmental competence of pig oocytes,” Theriogenology 83(4), 693–702 (2015). 10.1016/j.theriogenology.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 22.Shimada M., Kawano N., Terada T., “Delay of nuclear maturation and reduction in developmental competence of pig oocytes after mineral oil overlay of in vitro maturation media,” Reproduction 124(4), 557–564 (2002). 10.1530/rep.0.1240557 [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves P. B. D., Oliveira M. A. L., Mezzalira A., Montagner M. M., Visitin J. A., Costa L. F. S., Biotécnicas aplicadas à reprodução animal (Roca, 2008). [Google Scholar]
- 24.Bergholt M. S., Zheng W., Lin K., Ho K. Y., Teh M., Yeoh K. G., Yan So J. B., Huang Z., “In vivo diagnosis of gastric cancer using Raman endoscopy and ant colony optimization techniques,” Int. J. Cancer 128(11), 2673–2680 (2011). 10.1002/ijc.25618 [DOI] [PubMed] [Google Scholar]
- 25.Barth A., Zscherp C., “What vibrations tell us about proteins,” Q. Rev. Biophys. 35(4), 369–430 (2002). 10.1017/S0033583502003815 [DOI] [PubMed] [Google Scholar]
- 26.Cinta Pinzaru S., Falamas A., Dehelean C. A., “Molecular conformation changes along the malignancy revealed by optical nanosensors,” J. Cell. Mol. Med. 17(2), 277–286 (2013). 10.1111/jcmm.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y., Liu C. H., Sun Y., Pu Y., Boydston-White S., Liu Y., Alfano R. R., “Human brain cancer studied by resonance Raman spectroscopy,” J. Biomed. Opt. 17(11), 116021 (2012). 10.1117/1.JBO.17.11.116021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samek O., Jonáš A., Pilát Z., Zemánek P., Nedbal L., Tříska J., Kotas P., Trtílek M., “Raman Microspectroscopy of Individual Algal Cells: Sensing Unsaturation of Storage Lipids in vivo,” Sensors (Basel) 10(9), 8635–8651 (2010). 10.3390/s100908635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue L., Sun P., Ou D., Chen P., Chen M., Yan B., “Diagnosis of pathological minor salivary glands in primary Sjogren’s syndrome by using Raman spectroscopy,” Lasers Med. Sci. 29(2), 723–728 (2014). 10.1007/s10103-013-1398-y [DOI] [PubMed] [Google Scholar]
- 30.Moshaverinia A., Ansari S., Movasaghi Z., Billington R. W., Darr J. A., Rehman I. U., “Modification of conventional glass-ionomer cements with N-vinylpyrrolidone containing polyacids, nano-hydroxy and fluoroapatite to improve mechanical properties,” Dent. Mater. 24(10), 1381–1390 (2008). 10.1016/j.dental.2008.03.008 [DOI] [PubMed] [Google Scholar]