Abstract
Since the presence of brown adipose tissue (BAT) was confirmed in adult humans, BAT has become a therapeutic target for obesity and insulin resistance. We examined whether human subcutaneous white adipose tissue (sWAT) can adopt a BAT-like phenotype using a clinical model of prolonged and severe adrenergic stress. sWAT samples were collected from severely burned and healthy individuals. A subset of burn victims were prospectively followed during their acute hospitalization. Browning of sWAT was determined by the presence of multilocular adipocytes, uncoupling protein 1 (UCP1), and increased mitochondrial density and respiratory capacity. Multilocular UCP1-positive adipocytes were found in sWAT samples from burn patients. UCP1 mRNA, mitochondrial density and leak respiratory capacity in sWAT increased after burn trauma. Our data demonstrate that human sWAT can transform from an energy storing to energy dissipating tissue, which opens new research avenues in our quest to prevent and treat obesity and its metabolic complications.
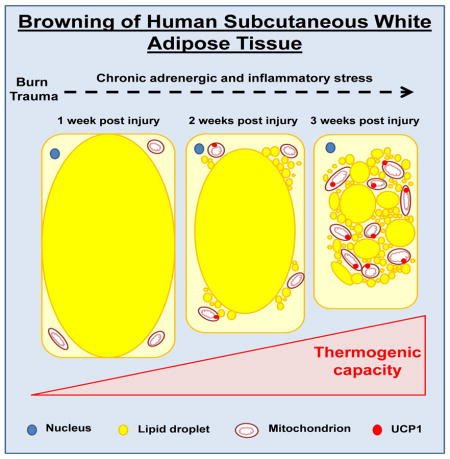
Graphical Abstract
INTRODUCTION
In the context of the global obesity epidemic, impacting energy balance in humans without altering either energy consumption or physical activity would be of significant clinical importance. The recent discovery of functional brown adipose tissue (BAT) in adult humans (Cypess AM, 2009; Nedergaard et al., 2007; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009) has renewed scientific interest in adipose tissue and its role in energy expenditure. While there is still debate as to whether this tissue is genuine brown adipose tissue (Shinoda et al., 2015; Sidossis and Kajimura, 2015; Wu et al., 2012; Wu et al., 2013), it is indisputable that it contains numerous mitochondria expressing uncoupling protein 1 (UCP1). When activated UCP1 ‘short circuits’ the electron-transport chain, which can increase energy expenditure, glucose and fatty acid oxidation, and improve insulin sensitivity (Chondronikola et al., 2014; Lee et al., 2014; van Marken Lichtenbelt et al., 2009; Yoneshiro et al., 2013). The ability of UCP1-positive adipocytes to uncouple oxidative phosphorylation holds much promise for the treatment of the metabolic complications associated with obesity and sedentary lifestyles in humans. Thus, strategies which activate BAT in humans may have significant health implications.
While activation of the supraclavicular BAT has clinically significant effects, in humans BAT is found in small volumes, where older and obese individuals often have negligible amounts of BAT (Yoneshiro et al., 2013). Indeed, individuals with small BAT depots do not have measureable metabolic responses to cold exposure (Chondronikola et al., 2014). Intriguing, and potentially more physiologically significant for humans, are data from cell e.g., (Petrovic et al., 2010) and rodent e.g., (Young et al., 1984) studies which suggest that white adipocytes can adopt a BAT-like (beige or brite) phenotype. Indeed, it has recently been shown that human pre-adipocytes can be differentiated into beige adipocytes in vitro (Gustafson et al., 2015; Lee et al., 2014). In addition, it has previously been shown that reduced insulin sensitivity is associated with reduced expression of transcripts involved in BAT adipogenesis within subcutaneous white adipose tissue (sWAT) in humans (Yang et al., 2003). Further, Frontini and colleagues reported that ~50% of omental adipocytes from patients with pheochromocytoma (which causes chronically elevated catecholamine levels), contained some multi-locular adipocytes, which expressed UCP1 (Frontini et al., 2013). However, despite a wealth of data from cells and rodents, and the cross sectional study by Frontini et al. in omental adipocytes, direct evidence of browning of human sWAT is currently lacking.
The adrenergic stress response to prolonged cold exposure induces browning of WAT in rodents (Cousin et al., 1992). However, in humans, ten days of cold exposure, which resulted in activation of pre-existing BAT depots and modest increases in catecholamine levels, did not result in the browning of sWAT (van der Lans et al., 2013). These results suggest that if a white-to-beige adipocyte trans-differentiation is possible within human sWAT, greater and/or more prolonged adrenergic stress is likely required.
Burn trauma represents a unique model of severe and prolonged adrenergic stress, as burn injury results in prolonged elevations in circulating norepinephrine levels, which persist for several weeks post injury (Wilmore et al., 1974). Moreover, expression of UCP1 mRNA acutely increases in the sWAT of burned rodents (Zhang et al., 2008). Finally, we have recently demonstrated that burn trauma induces thermogenically functional mitochondrial within the sWAT of mice (Porter et al., 2015), suggesting that burn injury is an ideal locale to study sWAT browning in humans.
The aim of this study was to determine if browning of sWAT is possible in humans. Using both a cross-sectional design in a large group of burn patients, and a prospective design in a subset of patients from whom serial samples were obtained after injury, we report morphological, molecular, and functional evidence of browning of human sWAT.
RESULTS AND DISCUSSION
Patients
We enrolled 42 severely burned children (9.3±4.8 years old, 31 males and 11 females) with burns encompassing 54±18% of their total body surface area (TBSA), including 37±22% of their TBSA with full thickness (3rd degree) burns. Six severely burned adults (39±19 years old, 4 males and 2 females) were also recruited. On average, adult burn victims had burns covering 69±19% of their TBSA, including full-thickness burn wounds covering 64±22 of their TBSA. Ten metabolically healthy children (9.5±6.2 years, 6 males and 4 females) and 9 healthy adults (42.3±15.7 years, all male) were recruited as healthy controls.
sWAT samples from burned patients were stratified into two groups depending on the day post burn on which they were collected. The early burn group included samples collected on or before post burn day seven (4±1 days post burn), whereas the late burn group were studied after post burn day seven but prior to their discharge from the intensive care unit (15±6 days post burn). The early and late burn groups did not differ significantly in any characteristic other than the day on which sWAT samples were collected. A subgroup of pediatric (n=18) and the six adult burn patients were followed prospectively after burn injury with serial sWAT biopsies performed during their hospital stay. Whole body resting energy expenditure (REE) and sWAT metabolic measurements were made in this patient group at approximately one week (6±3 days) and three weeks (20±6 days) post burn.
Morphological and immunohistochemical evidence of browning in human sWAT
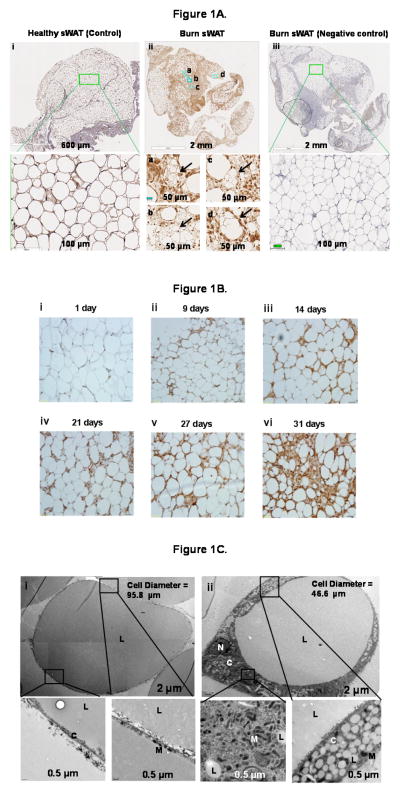
Brown or beige adipocytes can be distinguished from white adipocytes by their smaller size, multilocular appearance, UCP1 expression, and numerous mitochondria. We show morphological alterations in sWAT collected from severely burned children, particularly those in the late burn group (Fig. 1). Compared to healthy sWAT (Fig. 1A panel i), many of the white adipocytes from burn victims stained for UCP1 and contained multilocular cells (Fig. 1A panel ii). Furthermore, in patients biopsied sequentially after burn, we show a gradual decline in cell size and an increase in UCP1 staining (Fig. B panels i–vi), suggesting progressive browning of sWAT in response to burn trauma. This is in line with our findings in rodents, showing a gradual change in sWAT morphology after burn injury (Porter et al., 2015). Finally, transmission electron microscopy confirmed the presence of adipocytes with an abundance of small (0.5–1μm) lipid droplets and numerous electron-dense, rod-shaped mitochondria in the sWAT from burn patients (Fig. 1C panel ii), which was in contrast to sWAT from healthy subjects (Fig. 1C panel i). Collectively, these morphological data provide evidence of the presence of BAT-like or beige adipocytes in the sWAT of humans following burn injury.
Figure 1.
Figure 1A. Morphological and immunohistochemical evidence of browning.
Micrographs of paraffin sections of subcutaneous tissue from burned and healthy children (Figure 1A). All images show immunohistochemical staining for uncoupling protein 1 (UCP1) protein. (Panel i) Subcutaneous white adipose tissue (sWAT) from a healthy child obtained at the time of surgery. No UCP1 staining was evident. The adipocytes contain one large lipid droplet and there is very little tissue between the adipocytes. (Panel ii) Representative appearance of sWAT from a burned patient (at 45 days post burn; 14 yr old male). The largest observed adipocytes are smaller than those in the control tissue. Multiple fat droplets are observable in some adipocytes, and some cells between the large adipocytes contain multiple small fat droplets and are positive for UCP1. (Panel iii) Negative control from a burn patient.
Figure 1B. Prospective evidence of UCP1 induction in human WAT.
(Panels i–vi) Morphological and immunohistochemical evidence of browning over time in a representative patient. One day after burn, the adipocytes appear similar to the control samples. Small blood vessels appear to stain positively for UCP-1. At 9–21 days after burn, a peripheral rim of UCP-1-positive cytoplasm containing many tiny fat droplets is present at the periphery of a large fat droplet. A few small cells staining positive for UCP1 are observed between the adipocytes. At 27–31 days after burn, large adipocytes are separated by an interstitium that contains multiple elongated cells positive for UCP1, some containing small lipid droplets. In addition, scattered large adipocytes contain multiple fat droplets rather than one large fat droplet per cell (multilocular and paucilocular cells).
Figure 1C. Ultrastructural evaluation of white adipocytes from control and burn patients.
Composite electron micrograph of a typical adipocyte (diameter 95.8 μm) from a healthy child undergoing elective surgery (Panel i). The nucleus is out of the plane of the section (Bar = 1 μm). As shown at higher magnification below, only a very thin rim of cytoplasm with few mitochondria (M) separates the lipid droplet (L) from extracellular collagen fibers (C). Representative adipocyte (diameter 46.6 μm) from a child 21 days after burn (Bar = 2 μm) (Panel ii). Surrounding a single large lipid droplet is a rim of cytoplasm (Cy) that contains many small (0.5 – 1 μm) lipid droplets (L). The adipocyte nucleus (N) and many small and medium size electron-dense mitochondria (M) are also present in the cytoplasmic rim (Cy).
Molecular evidence of browning in sWAT
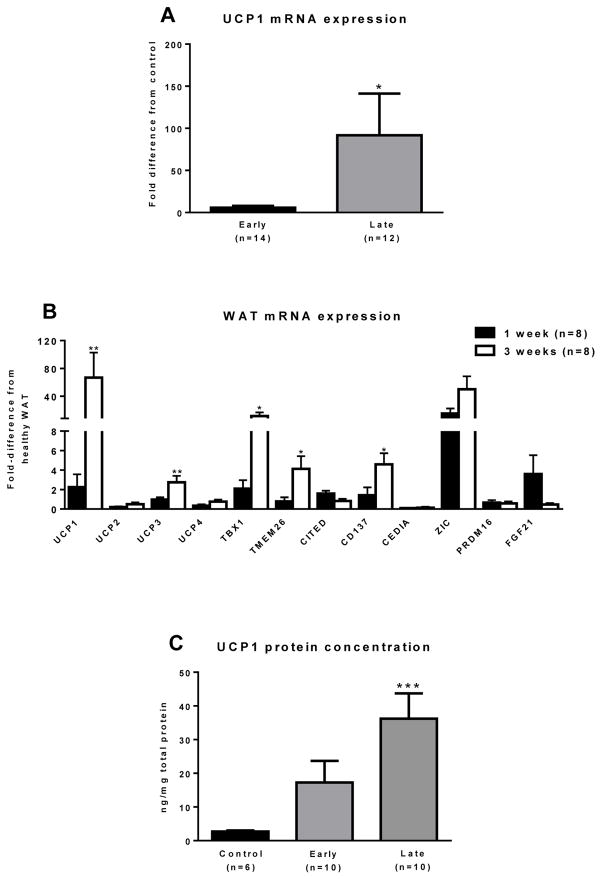
In agreement with our immunohistochemistry data, UCP1 mRNA was ~ 80-fold higher (P<0.05) in the late burn group relative to the healthy control and ~15-fold higher relative to the early burn group (Fig. 2A), suggesting UCP1 transcription in sWAT in response to burn injury. These human data are in agreement with those from rodent models of burns, where UCP1 expression is induced in BAT and sWAT depots following burn trauma (Yo et al., 2012; Zhang et al., 2008). While data presented in Fig 2A were from cohorts of burn victims, we saw similar induction of UCP1 in a sub-group of burn victims followed prospectively after injury (P<0.01, Fig. 2B), providing the novel evidence of UCP1 induction in human sWAT in vivo.
Figure 2. Molecular evidence of browning in sWAT.
(A) UCP1 mRNA expression was determined in sWAT from 9 healthy controls, 14 burned children in the early burn group and 12 burned children in the late burn group. Controls had minimal UCP1 expression. UCP1 expression was ~15-fold greater in the late group vs. the early group and ~80-fold greater in the late burn group vs. the healthy control group (*, P<0.05 vs. healthy control group). (B) UCP1 and its homologs UCP2-4 were determined in sWAT. There was a ~ 75-fold increase in sWAT UCP1 following burn trauma (P<0.01). Similarly, UCP3 was also significantly increased in sWAT following burn (P<0.01). UCP2 and UCP4 were not significantly altered by burn. The beige adipocyte markers TBX1, TMEM and CD137 were all significantly elevated in the sWAT of burn victims at the late time point. The brown adipocyte marker Zic1 was greater in sWAT of burn victims vs. controls, in particularly, Zic1 mRNA expression was an ~ 50-fold greater in burn patients sWAT at the late time point vs. controls (P<0.01). All data are presented as fold-change from healthy sWAT (*, P<0.05 and **, P<0.01 vs. healthy sWAT, respectively). (C) UCP1 protein concentration, as determined by ELISA, was significantly higher in the sWAT samples from the late burn group vs. the healthy control group (***, P<0.001 vs. healthy control group).
In addition to UCP1, other UCP homologs and transcripts considered markers of beige or brown adipocytes were determined in sWAT samples from patients followed prospectively post-burn. The expression of UCP3 was ~ 3-fold greater in the sWAT of the late burn group relative to the early burn group (Fig 2B, P<0.01). TBX1 (~20-fold), TMEM26 (~4-fold) and CD137 (~4-fold) were significantly greater in the sWAT of the late burn group when compared to the early burn group (Fig 2B, P<0.05). The brown fat marker ZIC1 was significantly greater in sWAT of the early and late burn groups when compared to healthy controls, ~30-fold and ~40-fold respectively (p<0.05), relative to healthy sWAT (Fig. 2B). Collectively, these data provide evidence that sWAT gene expression is altered in response severe and prolonged burn-induced adrenergic stress, towards a more thermogenic genotype
In addition to our RNA and immunohistochemistry data, we also quantified UCP1 protein concentrations by ELISA. UCP1 protein concentrations in sWAT did not differ significantly between the healthy control group and the early burn group (Fig. 2C) but were significantly higher in the late burn group when compared to healthy controls (2.7±0.3 vs. 36.2±7.5 ng/mg; P<0.001) (Fig. 2C), again suggesting a time dependent increase in sWAT UCP1 content after burn injury.
Functional evidence of browning in sWAT
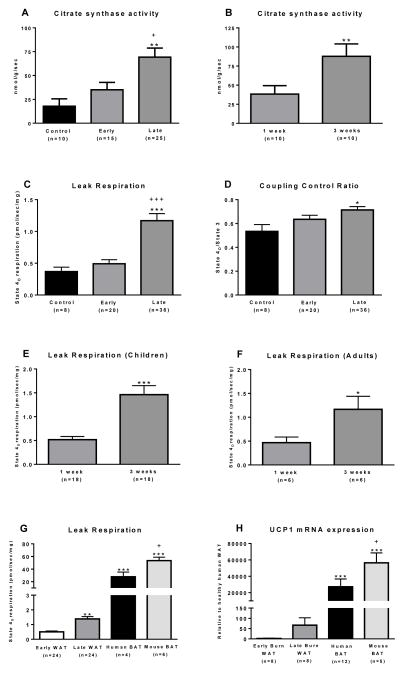
To determine the functional significance of the alterations in sWAT morphology and UCP1 expression, we determined mitochondrial enzyme activity and respiration rate. We observed a significant increase in maximal citrate synthase (CS) activity (a proxy of mitochondrial density) in a time dependent manner following burn injury (Fig. 3A; P<0.01). CS activity was significantly greater in the late burn group (69±8 nmol/g/sec) than the healthy control (18±7 nmol/g/sec; P<0.01) or early burn group (33±7 nmol/g/sec; P<0.05). CS activity was also determined in sWAT samples of prospectively followed patients (Fig. 3B). We found that CS activity significantly increased by 130% (38±11 vs. 89±16 nmol/g/sec) in the two weeks that patients were followed (P<0.01), which is agreement with our recent data showing greater CS activity in sWAT of burned mice (Porter et al., 2015). In addition to our morphological data (Fig 1C), these data provide further evidence that mitochondrial biogenesis occurs in sWAT following burn injury, similar to that which occurs in murine sWAT in response to chronic cold exposure (Shabalina et al., 2013). Since mitochondrial density is low in sWAT, an increase in mitochondrial density is a necessary component of browning of human sWAT.
Figure 3. Functional evidence of browning in sWAT.
(A) The maximal activity of citrate synthase was measured in WAT as a proxy of mitochondrial density. Sufficient tissue was available for this analysis from n=10 controls, n=15 patients in the early burn group and n=25 patients in the late burn group. Adipose tissue citrate synthase activity was greater in burn patients than healthy controls (**, P<0.01) and the early burn cohort (+, P<0.05). (B) Maximal sWAT citrate synthase activity from burned children who were biopsied sequentially post burn. Citrate synthase activity significantly increased in these burned children over the two-week study period (**, P<0.01), suggesting that the abundance of mitochondrial protein increased over this time interval. (C) Oligomycin insensitive leak respiration (i.e., state 4O respiration) was determined in digitonin-permeabilized WAT samples following the titration of substrates, ADP and oligomycin. In cohorts of healthy children (control, n=8) and severely burned children study approximately 1 week (early, n=20) and 3 weeks (late, n=36), leak mitochondrial respiration is significantly greater in the late burn group relative to control (***, P<0.001) and the early burn group (+++, P<0.001). (D) The oligomycin coupling control ratio, calculated by dividing state 4O respiration by state 3 respiration, was significantly greater in the late burn group compare to control (*, P<0.05). A greater oligomycin coupling control ratio means reduced oligomycin sensitivity and thus greater capacity for leak respiration per mitochondrion. (E) In a cohort of 18 burned children followed prospectively, we saw a ~3-fold increase in sWAT leak respiration following burn injury (***, P<0.001), indicating induction of altered sWAT respiratory capacity within burn victims. (F) A significant increase in sWAT leak respiration following burn injury was also seen in six adults followed prospectively after burn (*, P<0.05), demonstrating that this phenomenon occurs in both pediatric and adult burn victims. (G) Leak respiration is plotted for sWAT from 24 burn victims (n=18 children and n=6 adults) with a measurement early (≤ 7 days) and late (>7 days) after burn; and BAT from both humans (n=4) and mice (n=6). Human sWAT respiration is the same as that reported in Figure 3E and 3F, and is presented here for comparison with genuine BAT. Human BAT samples were collected by a PET-CT guided percutaneous needle biopsy from the supraclavicular region. Mouse BAT was harvested from the intrascapular BAT depot. Leak respiration was ~ 20-fold (***, P<0.001) and 30-fold (***, P<0.001) greater in human and murine BAT when compared to sWAT from burn victims (i.e. the early burn time point). Thus, while we show a ~3-fold increase in leak respiration in sWAT following burn trauma (**, P<0.01), sWAT tissue remains very much distinct from genuine BAT in terms of respiratory and thus thermogenic capacity (+, P<0.05 vs. late burn group). (H) This is further reflected in the UCP1 signatures of sWAT and BAT. UCP1 mRNA expression was ~ 20,000-fold greater in human BAT (***, P<0.001) and ~ 50,000-fold greater in murine BAT (***, P<0.001) when compared to sWAT from of burn victims in the early time-point post burn, and ~ 400-fold greater in human BAT and ~ 1,000-fold greater in murine BAT (+, P<0.05) when compared to sWAT from of burn victims in the late time-point post burn. These comparisons put the ~ 50 to 100-fold increase in WAT UCP1 signal seen following burn trauma into context.
In line with our CS activity data, oligomycin insensitive leak respiration was significantly higher in sWAT from the late burn cohort compared to the healthy control cohort (1.09±0.10 vs. 0.37±0.06 pmol/sec/mg) and the early burn group (1.09±0.14 vs. 0.49±0.06 pmol/sec/mg) (Fig. 3C; P<0.001), indicating greater mitochondrial respiratory capacity in sWAT after burn injury. Further, the coupling control ratio for oligomycin was significantly greater in the late burn cohort compared to healthy control cohort (P<0.05; Fig. 3D), suggesting that each mitochondrion has a greater intrinsic capacity for leak respiration in the late burn group. These data are similar to our recent findings in burned mice, where sWAT respiratory capacity increase after injury, in addition to a shift in mitochondrial coupling control toward a more thermogenic phenotype (Porter et al., 2015). In pediatric patients followed prospectively after injury, leak respiration increased post injury (0.52±0.07 vs. 1.46±0.19 pmol/sec/mg) (Fig. 3E; P<0.001). This suggests that increased mitochondrial respiratory capacity accompanies the increase in mitochondrial density and UCP1 expression seen in sWAT after burn injury. Similarly, we also noted increased sWAT leak respiration in a group of adult burn victims followed prospectively (0.47±0.12 vs. 1.17±0.27 pmol/sec/mg) (Fig. 3F; P<0.05), suggesting that this response is not exclusive to pediatric patients.
Collectively, we show that greater sWAT oxidative capacity accompanies the morphological and transcriptional alterations seen in sWAT of burn victims. Importantly, this indicates that these morphological and genomic adaptations bring about a meaningful change in sWAT physiological function. Moreover, in the patients studied prospectively, we demonstrate an increase in sWAT oxidative capacity, suggesting a dynamic process of browning. Specifically, increased mitochondrial enzyme activity and respiratory capacity in sWAT of burned children indicates greater capacity for thermogenesis. While we recognize that we did not measure UCP1-specific respiration in sWAT by titration of the UCP1 inhibitor GDP, the increase in leak respiration in sWAT following burn trauma suggests greater sWAT mitochondria respiratory capacity. Further, our results suggest that this is not an effect limited to children.
While our data offers strong evidence of altered sWAT thermogenic capacity in response to chronic adrenergic stress in humans, comparison to human and murine BAT respiration data, puts these findings into context (Fig. 3G and 3H). While leak respiration significantly increases in the sWAT of burn victims, it is still ~20-fold lower than that of supraclavicular BAT of humans and ~30-fold the intrascapular BAT of mice (Fig. 3G). These findings indicate that sWAT of burn victims, while significantly different to that of healthy human sWAT remains distinct from supraclavicular BAT. Indeed, this is confirmed when contrasting the UCP1 mRNA expression in sWAT of burn victims compared to that of human and murine BAT (Fig. 3H). While we see a significant increase in UCP1 transcription within sWAT after burn, its expression remains a fraction of that seen in human and murine BAT (Fig. 3H).
Nevertheless, the large difference in thermogenic capacity between BAT and sWAT does not necessarily diminish the potential functional/clinical significance of sWAT browning. In contrast to BAT, of which humans have no more that a few hundred grams of, most individuals have tens of kilograms of sWAT. If all sWAT in the body was capable of developing a thermogenic capacity akin to that of BAT, when activated, sWAT UCP1 could increase REE by tens of thousands of calories. Increasing energy needs by 10-fold or more has devastating consequences, and in the long term seems incompatible with life (Luft et al., 1962). Thus, the inability of sWAT to develop a phenotype identical to that of WAT is likely protective in terms of preventing an unquenchable metabolic rate.
Physiological significance of browning of human sWAT
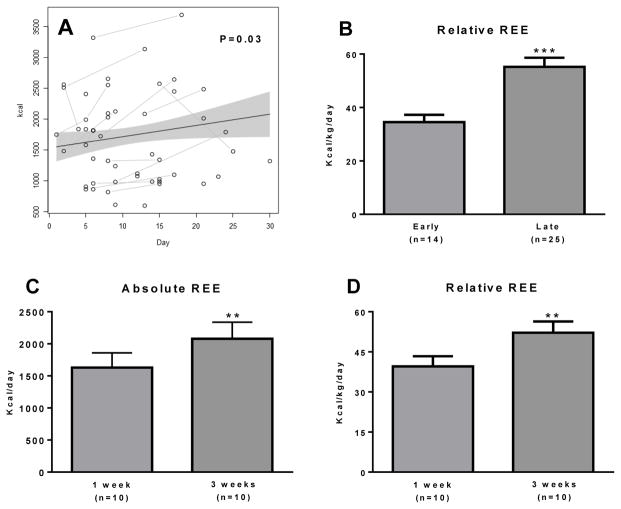
Burn victims become hypermetabolic after injury. REE was positively correlated with days post injury (Fig. 4A; P=0.03) when an analysis of covariance was used to correct for body mass. It was critical to perform an analysis of covariance on REE data since children ranging from 2 to 17 years were studied. Indeed, absolute REE was strongly correlated with age (Supplemental Figure 1A) and body mass (Supplemental Figure 1B) in our patient cohort, but negatively correlated with age when normalized to body mass (Supplemental Figure 1C). Thus, there was no correlation between absolute REE and days post burn (Supplemental Figure 1D), but a significant correlation between REE normalized to body mass and days post burn (Supplemental Figure 1E).
Figure 4. Physiological significance of browning of human sWAT.
(A) Absolute REE as a function of days post-injury. Due to the age range of children studied and thus large variation in patient body mass, REE was analyzed by ANCOVA to correct for body mass. REE was significantly related to days post burn (P=0.036). (B) REE was significantly greater in the late burn group vs. the early burn group (***, P<0.001; unpaired t test). (C) In patients followed prospectively after injury, absolute REE significantly increased over the two-week study period (**, P<0.01). (D) Relative REE (i.e. absolute REE, presented in Figure 4C, normalized to body mass) also significantly increased in these burned children over the two-week study period (**, P<0.01).
REE (per kg body mass) was significantly higher in the late burn group compared to the early burn group (37±2 vs. 54±3 kcal/kg/day, early vs. late group, respectively; Fig. 4B; P<0.001). In agreement with our cross-sectional data, absolute REE increased with time post injury in the group of severely burned children (1631±229 vs. 2079±260 kcal/kg/day) (Fig. 4C; P<0.01). Further, this held true when REE was expressed relative to body mass (40±4 vs. 52±4 kcal/kg/day) (Fig. 4D; P<0.01). Importantly, these data demonstrate that browning of sWAT occurs in parallel with an increase in whole body REE. We do not suggest that browning of sWAT is wholly responsible for the hypermetabolic state after burns, since ATP turnover and thermogenesis increase in response to burns in a number of tissues (Carter et al., 2011; Porter et al., 2014; Yu et al., 1999), however, our data does demonstrate an association between increased thermogenic capacity in sWAT and increased whole body REE.
Patients with burns have a severely compromised skin barrier, which means they cannot efficiently conserve body heat. Thermogenesis must be increased to maintain body temperature within a physiological range. This increase in heat production is presumably accomplished via stimulation of mitochondrial thermogenesis in various tissues such as muscle (Porter et al., 2014) and brown adipocytes (Carter et al., 2011). Pre-existing BAT depots are most likely recruited within the first few days of burn injury, as suggested by rodent studies (Carter et al., 2011). At the same time, browning of white adipocytes appears to occur as a long-term (days to weeks) adaptive response. Indeed, in agreement with others, who were unable to show browning of sWAT in humans expose to mild cold for 10 days (van der Lans et al., 2013), we detected no significant browning in sWAT samples in the first week after burn injury. Significant browning of sWAT was only apparent in the late burn group, from whom tissue samples were collected between 2–5 weeks post injury. These data indicate that chronic adrenergic stress over several weeks is required for browning of sWAT to occur in humans.
Mitochondrial respiration uncoupled from ATP production in UCP1 positive mitochondria increases metabolic rate by converting energy stored in glucose and fatty acids to heat. Our data suggest a 2- to 3-fold increase in sWAT thermogenic capacity following burn trauma. Since sWAT is thought to account for ~4.5% of REE in healthy individuals (Gallagher et al., 1998), we speculate that sWAT may account for up to 13.5% of REE in burn survivors. In the current patient cohort, total REE increased from ~1400 to ~1950 kcal/day, which represents a 550 kcal/day (40%) increase. In the early bun group, 4.5% of 1400kcal is ~60kcal; in the late burn 13.5% of 1950kcal equals 263kcal. In the context of obesity, and assuming that a similar degree of browning of sWAT could be achieved in unburned individuals, REE and substrate utilization could be significantly altered.
Factors contributing to the browning of human sWAT
Patients with full-thickness burns involving more than 30% of their TBSA endure a prolonged pathophysiological stress response. Circulating levels of epinephrine and norepinephrine increase immediately post injury and remain elevated for weeks to months (Jeschke et al., 2008). In the current study, at approximately one week post-injury, 24hr urinary epinephrine and norepinephrine excretion in burn patients were 10-fold (150±129 vs. <15 μg/day) and 6-fold (93±58 vs. <15 μg/day) greater, respectively, than our previously published values for healthy unburned individuals. Urinary epinephrine excretion remained elevated at ~ 3 weeks post burn (28±9 μg/day), as did urinary norepinephrine excretion (96±48 μg/day). Our current data are in agreement with our previously reported values for burn victims (Jeschke et al., 2008), where marked and prolonged adrenergic stress is accompanied with hypermetabolism. Since animal studies indicate that adrenergic stimulation mediates the browning of WAT e.g., (Cousin et al., 1992), we speculate that the so-called catecholamine surge associated with large burns likely contribute to browning of sWAT, making burn injury a unique pathophysiology in which to study the mechanisms responsible for sWAT browning in humans. Indeed, this large ~ 10-fold increase in circulating norepinephrine, which persisted for several weeks post-burn is significantly greater than the transient increases (~1.5-fold) in plasma norepinephrine levels seen in humans exposed to mild chronic cold exposure (Chondronikola et al., 2014; van der Lans et al., 2013).
In addition to adrenergic stress, burn patients had elevated plasma levels of the inflammatory cytokine interleukin-6 (IL-6) at ~1 (128±27 pg/ml) and 3 (86±19 pg/ml) weeks post burn compared to our historical data from healthy individuals (<5 pg/ml) (Jeschke et al., 2008). Similarly, the inflammatory cytokine interleukin-8 (IL-8) was massively elevated above normal values (<5 pg/ml) at ~1 (445±133 pg/ml) and 3 (391±79 pg/ml) weeks post burn. Also, granulocyte colony-stimulating factor (G-CSF) was profoundly greater in the plasma of burn victims at ~1 (1142±299 pg/ml) and 3 (1109±650 pg/ml) weeks post burn compared to our historical data from healthy individuals (<5 pg/ml). Finally, monocyte chemotactic protein 1(MCP-1) was markedly greater in plasma of burn patients) at ~1 (1328±327 pg/ml) and 3 (1538±456 pg/ml) weeks post burn compared to our historical data from healthy individuals (<200 pg/ml) (Jeschke et al., 2008). Thus, along with elevated catecholamine levels, inflammatory stress may also contribute to the browning of sWAT in humans.
Summary
Given the proven difficulty in achieving reduced energy intake or increased energy expenditure via exercise, the ability to alter whole body energy balance and substrate utilization without such lifestyle changes could have significant implications for human health. A better understanding of the biochemical pathways responsible for browning of sWAT might lay the foundation for the development of pharmacologic interventions to prevent or attenuate obesity and its metabolic complications.
EXPERIMENTAL PROCEEDURES
Patients and sample collection
Written patient consent, assent and parental consent were obtained. The Institutional Review Board and the Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston approved the studies. Eligible burn patients had burn lesions covering at least 30% of their total body surface area (TBSA). Both healthy controls and the burned patients were free of pre-existing metabolic disease. For details on sample collection, see the Supplemental Appendix.
Resting energy expenditure
Resting energy expenditure (REE) was measured in burn patients by calculating respiratory gas exchange (oxygen consumption and carbon dioxide production) using a ventilated hood system (Sensor Medics, Yorba Linda, CA), according to equations described previously (Weir, 1949). All REE measurements were acquired at the normal ambient temperature of the patient rooms in our acute burns intensive care unit (29±2°C).
Histology, Immunohistochemistry Analysis, and Transmission Electron Microscopy
sWAT samples were dehydrated, embedded in paraffin and cut into 5 μm sections. Sections were stained with hematoxylin and eosin for histology (see the Supplementary Appendix for a detailed description). For electron microscopy, fresh adipose tissue was fixed in 2.5% glutaraldehyde in sodium cacodylate buffer (Electron Microscopy Sciences, Hatfield, PA), and post fixed in 1% osmium tetroxide and embedded in an Epon-Araldite mixture. The 2 μm sections were stained with toluidine blue. Ultrathin (80 nm) sections were obtained with an Ultracut E ultramicrotome (Leica Microsystems, Buffalo Grove, IL) and post stained with 3% uranyl acetate, followed by 0.4% lead citrate. The ultrathin sections were examined with a JEOL 100CX transmission electron microscope and images were recorded digitally.
Analysis of UCP1 protein content
UCP1-protein levels in sWAT were measured by a human mitochondrial uncoupling protein ELISA kit (MyBioSource Inc., San Diego, CA). Approximately 100 mg of frozen sWAT was used to prepare homogenates by freeze thawing. After centrifugation of homogenates, the supernatant was removed and assayed immediately according to the manufacturer’s protocol. Light absorbance was measured at 450 nm (reference 540 nm) using a Biotek Eon™ spectrophotometer. A skeletal muscle sample was run in parallel to sWAT samples control for non-specific binding of the ELISA antibody. The data was normalized to total protein as measured by the Bradford protein assay kit (BioRad, Hercules, CA) and expressed as ng of UCP1 per mg of total protein.
Citrate synthase activity measurements
The activity of citrate synthase was measured in sWAT tissue lysates as a proxy of mitochondrial density (see the Supplementary Appendix).
Gene expression
Approximately 100 mg of sWAT was used for extraction of RNA using a pure link RNA isolation mini kit (Life Technologies, Carlsbad, CA). cDNA was synthesized and amplified using a high capacity RNA-cDNA kit and TaqManpre Amp master mix kit, respectively (Life Technologies, Carlsbad, CA). Quantitative real-time PCR analyses were performed on an ABI PRISM 7900HT using standard TaqMan master-mix reagents and gene-specific primer assays (Life Technologies, Carlsbad, CA). GAPDH was used as the housekeeping gene to normalize the expression of the target gene. sWAT from healthy children and adults was used as a control to calculate the delta CT values and the fold change.
Statistical analysis
All values are presented as group means ± standard error with the exception of patient characteristics, which are presented as group means ± standard deviation. An ANCOVA was used to determine the effect of days post burn of REE, correcting for body mass as a covariate. The model blocked on subject to compensate for repeated measures. Paired data from consecutive biopsies from the same patients were compared using a Paired t-test or Wilcoxon test where appropriate. Statistical analysis was performed in GraphPad Prism version 6 (GraphPad Software, Inc., La Jolla, CA), with the exception of the analysis of co-variance performed on REE data, which was performed using R statistical software (R Foundation for Statistical Computing, Vienna, Austria). A 95% level of confidence was assumed.
Supplementary Material
Chronic stress causes the browning of subcutaneous white adipose tissue (sWAT) in rodents. Whether this phenomenon occurs in humans is unknown. Using burn trauma as a unique human model of prolonged stress, we show that human sWAT does indeed undergo browning.
Human sWAT exhibits the plasticity to undergo browning.
Prolonged stress (>2 weeks) alters sWAT morphology and function.
sWAT adopts a more thermogenic phenotype after prolonged stress.
Browning of sWAT was associated with increased whole body metabolic rate.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P50-GM60338, R01-GM05668, T32 GM008256, H133P110012, and the Clinical and Translational Science Award UL1TR000071), grants from the Shriners Hospitals for Children (85310, 85300, 85130, 84090, 84080, 84060, 71001, 71008, 79141, and 71006), the John Sealy Memorial Endowment Fund for Biomedical Research (66992), ADA (1-14-TS-35) and the Sealy Center on Aging at UTMB. CP was supported in part by a National Institute of Disability and Rehabilitation Research Training Grant (H133P110012). MC is supported by the Onassis Foundation.
The authors thank the study participants, the technical staff at Shriners Hospitals for Children – Galveston, Mr. Clark Andersen for statistical analyses, and Drs. Susan M. Carlton, Michael B. Sherman, Ding Zhixia and the staff of the Sealy Center for Structural Biology and Molecular Biophysics at UTMB.
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carter E, Bonab A, Hamrahi V, Pitman J, Winter D, Macintosh L, Cyr E, Paul K, Yerxa J, Jung W, et al. Effects of burn injury, cold stress and cutaneous wound injury on the morphology and energy metabolism of murine brown adipose tissue (BAT) in vivo. Life Sci. 2011;89:78–85. doi: 10.1016/j.lfs.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäk S, Liddell ME, Saraf M, Labbe S, Hurren S, et al. Brown adipose tissue activation improves glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- Cypess AMLS, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, Guerrieri M, Cinti S. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochimica et biophysica acta. 2013;1831:950–959. doi: 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer F, Heymsfield S. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:249–258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Hammarstedt A, Hedjazifar S, Hoffmann J, Svensson P, Grimsby J, Rondinone C, Smith U. BMP4 and BMP antagonists regulate human white and beige adipogenesis. Diabetes. 2015 doi: 10.2337/db14-1127. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Werner C, Kebebew E, Celi F. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond) 2014;38:170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft R, Ikkos D, Palmieri G, Ernster L, Aafeluis B. A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study. J Clin Invest. 1962;41:1776–1804. doi: 10.1172/JCI104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Petrovic N, Walden T, Shabalina I, Timmons J, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C, Herndon D, Bhattarai N, Ogunbileje J, Szczesny B, Szabo C, Toliver-Kinsky T, Sidossis L. Severe Burn Injury Induces Thermogenically Functional Mitochondria in Murine White Adipose Tissue. Shock. 2015 doi: 10.1097/SHK.0000000000000410. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C, Herndon D, Borscheim E, Chao T, Reidy P, Borack M, Rasmussen B, Chondonikola M, Saraf M, Sidossis L. Uncoupled skeletal muscle mitochondria contribute to hypermetabolism in severely burned adults. Am J Physiol Endocrinol Metab. 2014;307:462–467. doi: 10.1152/ajpendo.00206.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina I, Petrovic N, de Jong J, Kalinovich A, Cannon B, Nedergaard J. UCP1 in Brite/Beige Adipose Tissue Mitochondria Is Functionally Thermogenic. Cell Rep. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Luijten I, Hasegawa Y, Hong H, Sonne S, Kim M, Xue R, Chondronikola M, Cypess A, Tseng Y, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lans A, Hoeks J, Brans B, Vijgen G, Visser M, Vosselman M, Hansen J, Jörgensen J, Wu J, Mottaghy F, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W, Vanhommerig J, Smulders N, Drossaerts J, Kemerink G, Bouvy N, Schrauwen P, Teule G. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen K, Lidell M, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto N, Enerbäck S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D, Long J, Mason AJ, Skreen R, Pruitt BJ. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–669. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Boström P, Sparks L, Ye L, Choi J, Giang A, Khandekar M, Virtanen K, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman B. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Enerbäck S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res. 2003;11:1182–1191. doi: 10.1038/oby.2003.163. [DOI] [PubMed] [Google Scholar]
- Yo K, Yu Y, Zhao G, Bonab A, Aikawa N, Tompkins R, Fischman A. Brown Adipose Tissue and Its Modulation by a Mitochondria-targeted Peptide in Rat Burn Injury Induced Hypermetabolism. Am J Physiol Endocrinol Metab. 2012;304:331–341. doi: 10.1152/ajpendo.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Arch J, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–168. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ma B, Fischman A, Tompkins R, Carter E. Increased uncoupling protein 1 mRNA expression in mice brown adipose tissue after burn injury. J Burn Care Res. 2008;29:358–362. doi: 10.1097/BCR.0b013e318166739c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.