Abstract
Leptin receptor (LEPR) signaling controls appetite and energy expenditure. Somatotrope-specific deletion of the LEPRb signaling isoform causes GH deficiency and obesity. The present study selectively ablated Lepr exon 1 in somatotropes, which removes the signal peptide, causing the loss of all isoforms of LEPR. Excision of Lepr exon 1 was restricted to the pituitary, and mutant somatotropes failed to respond to leptin. Young (2–3 mo) males showed a severe 84% reduction in serum GH levels and more than 60% reduction in immunolabeled GH cells compared with 41%–42% reductions in GH and GH cells in mutant females. Mutant males (35 d) and females (45 d) weighed less than controls and males had lower lean body mass. Image analysis of adipose tissue by magnetic resonance imaging showed that young males had a 2-fold increase in abdominal fat mass and increased adipose tissue density. Young females had only an overall increase in adipose tissue. Both males and females showed lower energy expenditure and higher respiratory quotient, indicating preferential carbohydrate burning. Young mutant males slept less and were more restless during the dark phase, whereas the opposite was true of females. The effects of a Cre-bearing sire on his non-Cre-recombinase bearing progeny are seen by increased respiratory quotient and reduced litter sizes. These studies elucidate clear sex differences in the extent to which somatotropes are dependent on all isoforms of LEPR. These results, which were not seen with the ablation of Lepr exon 17, highlight the severe consequences of ablation of LEPR in male somatotropes.
Leptin is an anorexigenic hormone most commonly known for its role in food intake and regulation of appetite. Its message is conveyed by leptin receptors (LEPRs) in the hypothalamus (1). LEPRs are found in most cell and tissue types throughout the brain and body. However, the anterior pituitary cells are of particular interest when looking at the endocrine effects related to obesity and metabolic diseases. Animals with leptin or Lepr gene mutations show reduced somatotrope and gonadotrope functions, infertility, hyperphagia, obesity, and reduced energy expenditure (2, 3).
The association between somatotropes and leptin is interesting because GH is needed for lipolysis and production of muscle. Adult onset GH deficiency (GHD) presents with broad changes in body composition due to increased fat mass and decreased muscle mass, metabolic disorders, and poor quality of life (4).
Mice have 5 LEPR isoforms but only the long form (LEPRb) binds and activates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (5, 6). Most somatotropes express LEPRs (7–9) and leptin has been shown to stimulate GH secretion (10–13). We recently created a somatotrope-specific LEPR-null mouse model by deleting exon 17 of the Lepr gene (14), its removal leads to loss of signaling (5). These deletion mutant mice were GH deficient, and males and females had adult onset obesity.
The next series of studies selectively deleted exon 1 of LEPR in somatotropes. As described by the developers of this transgenic mouse (15), because exon 1 contains the signal peptide sequence targeting the mRNA to the rough endoplasmic reticulum, its deletion removes all isoforms (5). We hypothesized that this ablation might cause a more severe phenotype and further clarify the role of leptin in the regulation of somatotrope functions. We visually observed an increased abdominal obesity in prepubertal mice and therefore elected to study young mice. This report presents the validation of this new deletion mutant line in 2- to 3-month-old mice, the first in a series of studies on this model. We report the results of magnetic resonance imaging (MRI) studies that show early accumulation of abdominal fat, even in light of weight loss. We also report distinct sex differences in the impact of the total loss of somatotrope LEPR on serum GH, activity, and sleep.
Materials and Methods
Production and validation of somatotrope LEPR exon 1 deletion mutants
C57/B6 mice bearing homozygous floxed exon 1 Lepr alleles were obtained from Dr Jeffrey Friedman (15). These mice (Leprexon 1 fl/fl) were then crossed with heterozygous mice expressing Cre-recombinase (CreGH+/−) behind the GH promoter in order to target the deletion to somatotropes (16). In the first cross with 1 Cre-bearing parent, all offspring were heterozygous for floxed leptin, and 50% carried 1 allele of CreGH. Heterozygous floxed Lepr exon 1 mice bearing CreGH were then backcrossed to homozygous Leprexon 1 fl/fl mice. The resulting offspring carried 2 floxed Lepr alleles and 25% of the progeny were deletion mutants carrying 1 allele of CreGH and Leprexon 1 fl/fl. Weanlings were tail snipped at 21 days and genotyped using 3 sets of exon 1 primers: (CZO81) 5′-TCT AGC CCT CCA GCA CTG GAC-3′, (CZ121) 5′-GCA ATT CAT ATC AAA ACG CC-3′, and (CZ083) 5′-GTC ACC TAG GTT AAT GTA TTC-3′ and Cre primers as previously described (14, 16). PCR conditions for exon 1 primers began with step 1: 94°C for 5 minutes; step 2: 30 cycles of 94°C for 1 minute, 62°C for 1 minute, and 68°C for 2 minutes; and step 3: 72°C for 10 minutes followed by a 4°C hold.
Male and female controls and deletion mutants (4 per group) were collected and 16 organs (pituitary, hypothalamus, cerebrum, cerebellum, fat, muscle, spleen, kidney, adrenal, stomach, pancreas, liver, heart, lung, ovaries/testes, uterus/uterine tubes) were harvested for genotyping to validate the specificity of the knockout. This was done to verify that Cre-recombinase activity was isolated in the pituitary. In addition, we collected fat samples to assay leptin levels and a subset of samples were fixed in paraformaldehyde, and embedded in paraffin for measurements of fat cells.
Validation of loss of LEPR in somatotropes
Male and female deletion mutants and littermate controls were taken at 3–4 months of age and the pituitaries dispersed and plated, as previously described (14). Cells were fixed after 24 hours in culture and immunolabeled for GH with an avidin-peroxidase protocol as previously described by our lab (17). The selective loss of LEPRs in somatotropes was detected by immunolabeling for LEPR and also by demonstrating lack of production of phosphorylated STAT3 (pSTAT3) in response to 3 hours of 10nM leptin exposure. With respect to the LEPR immunolabeling, the developers of Lepr exon 1 flox/flox line have stated that it is likely that no isoforms of LEPR are produced, because exon 1 contains the signal peptide (15). To detect a loss in LEPR, we used a commercial antibody (catalog ab5593; Abcam) that recognizes the extracellular domain of all LEPR isoforms (1:10 000 dilution), which we validated in studies of LEPR expression in somatotropes (14) and gonadotropes (18). However, the loss in immunolabeled GH made it impossible to determine whether the reduction was specifically in somatotropes. Therefore, we introduced a Cre-recombinase reporter into our mutant and control mouse populations to facilitate identification of somatotropes (19). This line bears the floxed tdTomato transgene linked to enhanced green fluorescent protein (eGFP) (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/) (Jackson Laboratories). All cells fluoresce red; however, in the presence of Cre-recombinase, the tdTomato is deleted leaving the membrane promoter to drive the expression of eGFP. We validated the GH content of eGFP cells by in situ hybridization in control and mutant mice and showed that most eGFP-expressing cells in mutants are GH cells (19).
Animals
Deletion mutants bearing CreGH and Leprexon 1 fl/fl were bred to the N4/N5 generations as previously described. Mice were housed 5 per cage and maintained at 14-hour light, 10-hour dark cycle until the age of 2.5–3 months. The CreGH was passed by the males to avoid problems during pregnancy from GHD female mutants. This study focused on young, preobese animals to detect metabolic changes that may contribute to the adult-onset obesity. The animals were fed Teklad 8640 or Teklad 22/5 rodent diet (Teklad), which is 22% protein, 5% fat, and 4.5% fiber. Although breeding, the mice were fed Teklad Global 19% protein diet 2019, which is 19% protein, 9% fat, and 5% fiber. The Animal Care and Use Committee approved all animal use and protocols (AUP number 3339). At the time of collection, mice were anesthetized with isoflurane and then decapitated when fully unconscious.
Comprehensive Laboratory Animal Monitoring System (CLAMS)
The O2 and CO2 levels, activity levels, food intake, and sleep were calculated using Oxymax CLAMS (Columbus Instruments) for a period of 72 hours, as described in recent studies (20). Each CLAMS run consisted of 8 male or female deletion mutants and littermate controls. The averages include data from 12 mice/group. However, the data will not include feeding data for 2 reasons. First, the young mice were small enough to get under the barrier and dig out their food from the dish, thus invalidating the weighing mechanism. Second, during this period of use of the CLAMS, we experienced occasional equipment failure with the sensors on the balances that weighed the food. Each animal's lean body mass was calculated using Kleiber's law, which is body mass (M) to ¾ power equals lean body mass (21, 22). Activity was measured by infrared beam breaks that are affixed to the sides of cages. Sleep was indirectly determined by activity measurements where a sleep epoch is defined as a 60-second bout without activity.
Magnetic resonance imaging
Subsets of animals that had been studied in the CLAMS unit were also taken for MRI (Bruker PharmaScan 7T MRI). They were anesthetized with an isoflurane/O2 combination unique to each mouse that kept their respiration at 60–80 breaths per minute during imaging. Images were taken from the cervical spine region to the sacral region. T2 coronal images without fat suppression were analyzed using ImageJ software (National Institutes of Health). Statistical analysis used ANOVA and Student's t test. Area was calculated by counting the number of pixels imaging adipose tissue in a defined region, comparing either abdominal or whole body. Adipose density was determined by optical density.
Multiplex enzyme immunoassays and immunocytochemistry
Trunk blood was collected between 9 and 10 am and stored at 20°C for serum analysis of pituitary hormones, cytokines, and adipokines using Luminex LX200 (Luminex Corp) xPONENT 3.1 with the Millipore MAP Multiplex kits (Millipore Corp), as previously described (20). Pituitaries were collected and dispersed as previously described (14, 20). Cells were plated 12 000 per coverslip in 24-well plates and fixed as described in previous studies (14, 20).
Statistical analysis
At least 5 animals per group were tested. Unless otherwise described in results, statistical tests were done with PRISM software and ANOVA followed by Bonferroni's post hoc test or Student's t test. P < .05 was considered significant. Power analyses have been described in recent reports (14, 20).
Results
Validation of excision of Lepr in pituitaries by organ genotyping and responses to leptin
Organ genotyping demonstrated that the excision of Lepr exon 1 was only in the pituitaries of Cre-bearing animals (Figure 1). As described previously for this CreGH line (14, 20), there was no evidence of Cre-recombinase activity in ovaries or testes of Cre-bearing animals, nor was there evidence in any of the 16 organs tested. We also detected the excised alleles as early as post natal day (PND) 1 by genotyping pituitaries from 1-day-old pups (data not shown).
Figure 1.

Organ genotyping to detect extrapituitary Cre-recombinase activity. Cre-recombinase activity was selective only for the pituitary in both males and females. The top gels in males and females show the detection of Cre-recombinase (166 bp) in pituitaries of mice 5–8, whereas mice 1–4 are controls. The second gels detect floxed, deleted, and wild-type (wt) Lepr. A 400-bp band marks the deleted Lepr exon 1 and is found only in the pituitary of the Cre-bearing animals (mice 5–8). The other band found in all mice marks the 2 floxed alleles of Lepr (250 bp), which are found in the nonsomatotropes. TCR-Δ, T-cell receptor-Δ; housekeeping gene; Cre, Cre-recombinase; Lepr ΔΔ, excised Lepr exon 1.
To determine whether LEPR proteins were reduced in somatotropes, we used antisera to the extracellular domain of LEPR with control populations that also expressed eGFP in cells bearing Cre-GH. Our counts showed that 57 ± 0.06% of all pituitary cells were labeled for LEPR, which fits with previous studies of this antibody (14, 20). The overall percentages of LEPR cells in deletion mutants lacking LEPR in somatotropes was reduced to 27 ± 0.02% (P = .0005). Mutants and control populations had similar numbers of eGFP cells (53 ± 0.05% controls; 56 ± 0.1% mutants), which were identified as somatotropes (20). However, only 2.6 ± 0.01% of mutant eGFP-labeled cells expressed LEPR compared with 63 ± 0.05%% of eGFP labeled cells in controls (P < .0001). The magnitude of the reduction is similar to that reported for the somatotrope-LEPR exon 17 deletion mutants (14).
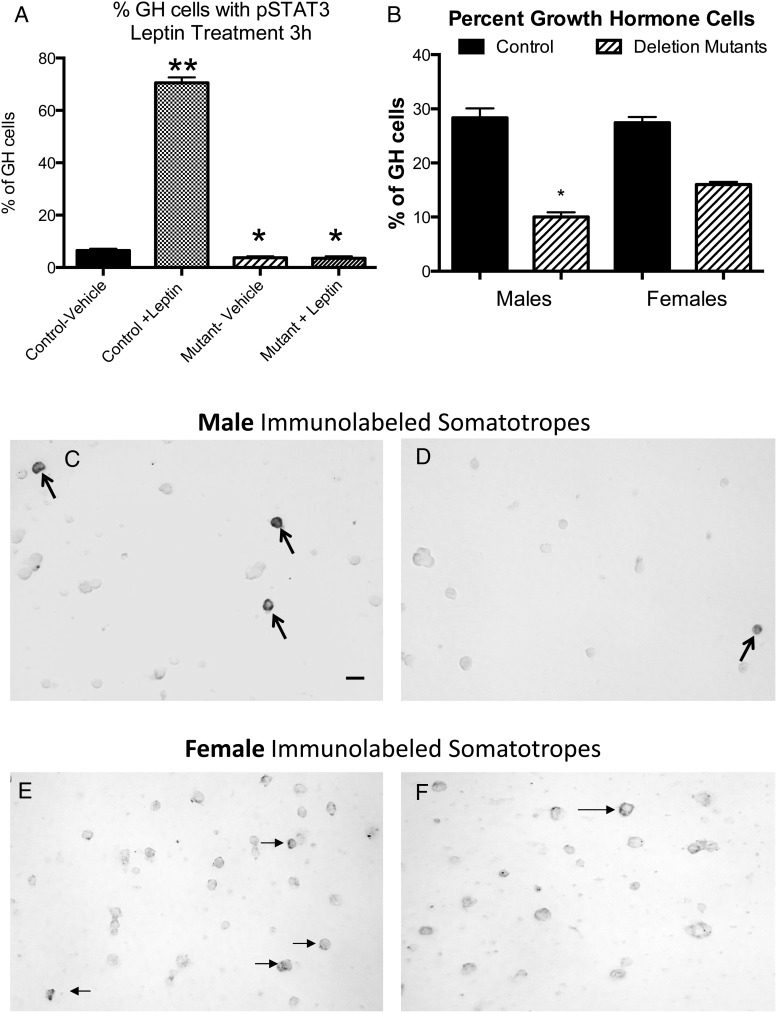
To determine whether mutant somatotropes were responsive to leptin, we analyzed somatotropes from control mice for pSTAT3 immunoreactivity and found that 70.5 ± 2% of somatotropes showed pSTAT3 labeling after leptin stimulation, compared with 6.6 ± 0.67% in vehicle-treated control populations (Figure 2A). In contrast, only 3.7 ± 0.3% of Lepr exon 1-null somatotropes showed pSTAT3 labeling in vehicle-treated population, and there was no response to 10nM leptin. Cells from mutants had only 3.5 ± 0.7% somatotropes with pSTAT3 labeling.
Figure 2.
In vitro tests of response of mutants and controls to leptin in overnight cultures from control and mutant mice. A, pSTAT immunolabeling of female somatotropes after leptin stimulation showed 70.5 ± 2% labeling, compared with 6.6 ± 0.67% in vehicle-treated control populations. Deletion mutants had 3.7 ± 0.3% GH cells with pSTAT3 labeling after vehicle treatment, and there was no response to 10nM leptin. Single asterisk, significantly lower than control vehicle; double asterisk, significantly higher that control vehicle. B, Immunolabeling of GH cells showed a 65% decrease in males, 28 ± 1.7% to 10 ± 0.9% of pituitary cells, and a 42% decrease in females, from 27 ± 1% to 16 ± 0.5%. Immunolabeling of GH cells of male and female controls (C and E) and deletion mutants (D and F). Scale bar, 10 nm. Values shown are mean ± SEM. Student's t tests were used to determine differences between groups.
Sex differences in GH production
Counts of immunolabeled somatotropes also showed significant reductions in cells with GH proteins in mutants (Figure 2B). However, the young male pituitaries had a more severe reduction with a 65% loss in immunolabeled GH cells from 28 ± 1.7% to 10 ± 0.9% of pituitary cells. In comparison, young females had a 42% loss in immunolabeled somatotropes from 27 ± 1% to 16 ± 0.5% of pituitary cells. Both values are significantly different from controls and the values from male and female mutants are significantly different from one another (ANOVA, Bonferroni). Figure 2 also illustrates the loss in immunolabeled GH cells comparing populations from control (Figure 2, C and E) and deletion mutant (Figure 2, D and F) males and females.
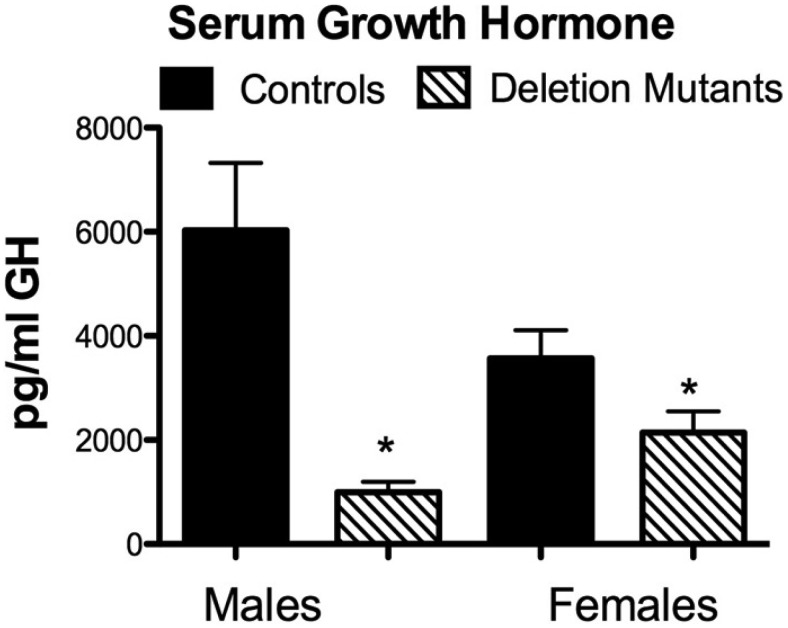
The reduction in immunolabeled GH cells also affected serum GH, with a more pronounced GHD in males than females (Figure 3). In males, a simple average of serum GH levels from 12–14 mice showed a clear significant difference in serum GH levels with an 84% reduction (P = .002). In contrast, the 12–14 females showed only a 41% reduction in GH levels (P = .046).
Figure 3.
Assays of serum GH in control and mutant mice. Serum GH is reduced in male (P = .002) and female (P = .046) deletion mutants. Values shown are mean ± SEM; n = 12 for all groups. Student's t tests were used to determine differences between groups.
Deletion mutants weighed less and males had decreased lean body mass
The deletion mutants were studied at a relatively young age to detect factors that might lead to obesity. However, Figure 4 shows that 75-day-old male deletion mutants in which exon 1 of Lepr was deleted in somatotropes actually weighed less (P < .0150) and had lower lean body mass (P < .0302) than control littermates (Figure 4A). Female mutants (75 d old) also weighed less (P < .0486) but did not show any differences in lean body mass. The weight change was seen as early as 35 days of age in males and 45 days of age in females.
Figure 4.
Mice (age 2.5–3 mo) were weighed (B) and lean body mass (A) was calculated using Kleiber's law. Mutants weight less, but only males had lower lean body mass (P < .0302). Values shown are mean ± SEM; n = 12 for all groups. Student's t tests were used to determine differences between groups.
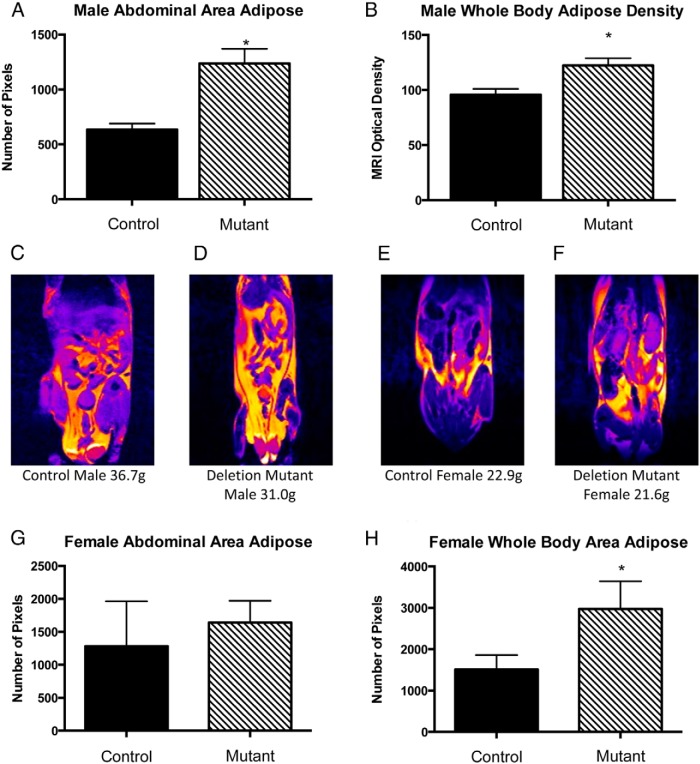
In spite of their decreased weight, MRI analyses indicated that somatotrope Lepr-null deletion mutants had increased adiposity. Young male deletion mutants had increased abdominal fat area (P < .001) (Figure 5A) and increased whole-body adipose density (P < .03) (Figure 5B) as analyzed using pixel optical density in ImageJ. These changes correlated well with the more extreme GHD seen in the males (Figures 2 and 3). Young female mutants also showed an increase in adipose tissue distributed throughout the body with size measured by number of pixels (P < .04) (Figure 5D) but no differences in abdominal adipose tissue distribution (Figure 5C). Analysis of adipocyte size showed no differences between controls and mutants of either sex.
Figure 5.
Small animal MRI was used to image fat depots throughout the trunk of 2- and 5-month-old mice. A, ImageJ analyses of T2 MRI images revealed mutant males had more adipose in the abdominal areas as compared with littermate controls (P ≤ .001). B, OD measured density of adipose tissue. Mutant males had a greater adipose tissue density than controls (P ≤ .03). C–F, Images were color coded to show areas of fat (yellow) and the greater fat deposition in the mutants (D and F) compared with controls (C and E). G, Graph showing that mutant females did not have significantly greater abdominal adipose distribution (P ≤ .754). H, Females did have a significant increase in whole-body adipose distribution. Values shown are mean ± SEM, n = 12. Student's t tests were used to determine differences between groups.
Respiratory quotient (RQ) and energy expenditure is reduced in young mutants
Indirect calorimetry revealed that both deletion mutant males (Figure 6, A–C) and females (Figure 6, E–G) had higher RQ values during the dark phase than control littermates. However, control littermates also had a higher RQ than expected from analyses of previous lines of mice (20) examined with the same equipment. To determine whether there might be a contribution from the floxed Lepr exon 1 transgene, we tested founders bearing only floxed Lepr exon 1 alleles that had never been exposed to Cre-recombinase bearing mice. These founders exhibited RQ values more like those of controls studied in previous lines, including lines bearing only Cre-GH (20). The values from founders that had never been exposed to a parent bearing Cre-GH were lower than both control littermate and deletion mutants (Figure 6, A and E). In males the higher RQs are correlated with increased production of CO2 (VCO2) (Figure 6C) and decreased consumption of O2 (VO2) (Figure 6B). The increased RQ in male and female deletion mutants also correlated with decreased energy expenditure (Figure 6, D and H).
Figure 6.
CLAMS of deletion mutant mice, littermate controls, and control mice bearing 2 alleles of floxed leptin exon 1. A and E, Deletion mutant males and females had a higher RQ (RQ = VCO2/VO2) than littermate controls. In addition, founder controls had lower RQ values than littermate controls. C and G, Detection of CO2 output indicates that increased RQ is caused by increased CO2 levels in males. D and H, Detection of energy expenditure shows that deletion mutants have lower energy expenditure than littermates and founder controls. Single asterisk indicates significant difference comparing littermate control and deletion mutant (P ≤ .05). Two asterisks indicate significant difference comparing founder controls and deletion mutants and littermate controls (P ≤ .0001). Θ, between controls and founders; Φ, significant difference between deletion mutants and founders. Values shown are mean ± SEM, n = 12. Student's t tests were used to determine differences between groups.
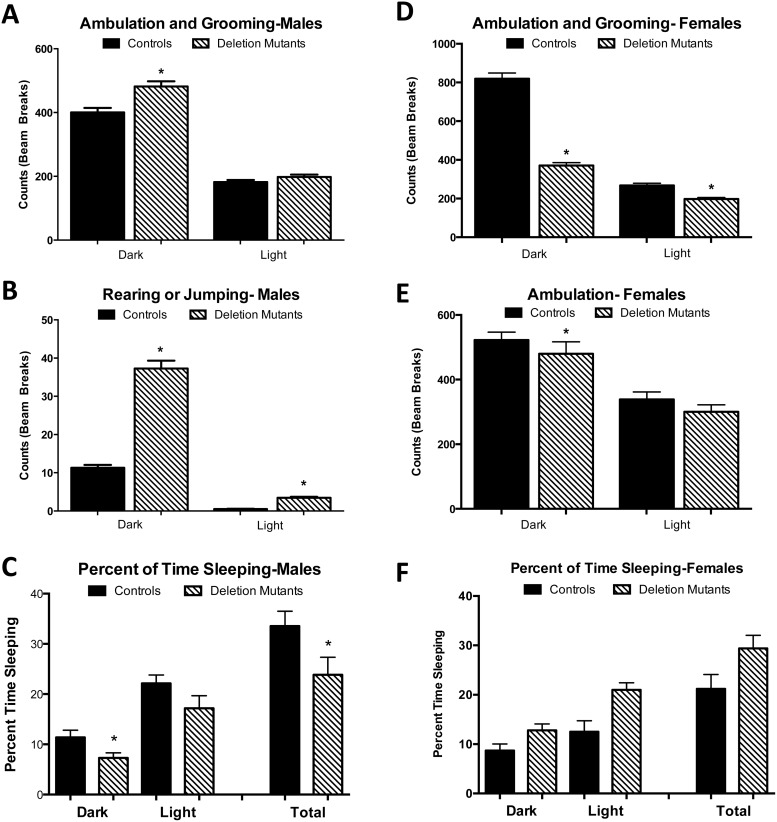
Sex differences in activity and sleep
Activity measurements showed that the loss of Lepr exon 1 in somatotropes impacted males and females differently. Although both groups of young females were much more active than males overall (Figure 7, A, B, D, and E), deletion mutant females were less active than their control littermates (Figure 7, D and E), showing fewer beam breaks by ambulating or grooming behaviors in the dark and light phases. In contrast, young mutant males were more active than controls, showing significant increases in z-axis activity (raising up) during both dark and light phases and increased grooming and ambulation during the dark phase (Figure 7, A and B). Sleep analysis showed that mutant males slept less than controls correlating with their increased restlessness (Figure 7C). In contrast, mutant females slept more than controls, which correlated well with their lower activity (Figure 7F). It should be noted that indirect calorimetry calculation of energy expenditure does not account for changes in activity and thus does not correlate with activity measurements, which are dependent on beam breaks (23). For example, a single beam break can be done with varying levels of energy expenditure (running vs walking); however, the computer does not differentiate calories burned.
Figure 7.
CLAMS detected activity levels by beam breaks in the x- and z-axes and calculated periods of sleep (lack of activity). A and B, Deletion mutant males were more active than controls and slept less than controls (C). D–F, Females were more active than males throughout light and darks phases. However, deletion mutant females were less active and slept more than littermate controls. All P values were P ≤ .02 or better. Values shown are mean ± SEM, n = 12. Student's t tests were used to determine differences between groups.
Adipokines, cytokines, pituitary hormones
Serum cytokines (TNF-α and IL-6) were assayed to detect any indications of inflammatory disease in these young, preobese mice. Data on serum cytokines can be found in Supplemental Table 1. There were no significant changes in IL-6 (females controls 9.188 ± 0.9423 pg/mL; mutants 10.98 ± 1.007 pg/mL; males controls 16.98 ± 3.063 pg/mL; mutants 19.79 ± 6.674 pg/mL) or TNF-α (females controls 5.952 ± 0.507 pg/mL; mutants 6.062 ± 0.3496 pg/mL; males controls 8.173 ± 0.259 pg/mL; mutants 11.34 ± 3.393 pg/mL). These were within normal ranges for young mice. There were no differences in glucose regulatory hormones (insulin and glucagon) and C-peptide or in the next adipokines (adiponectin, leptin, gastric inhibitory peptide, resistin, peptide tyrosine tyrosine, and plasminogen activator inhibitor-1). Deletion mutant males had a borderline change in the anorexigenic hormone PYY, which was not significant (P < .054). There were no significant changes in serum triglycerides or IGF-1. These data can be found in Supplemental Table 2.
All anterior pituitary hormone products were assayed to look for any changes caused by the total deletion of somatotrope LEPR. Pituitary hormones data can be found in Supplemental Table 3. There were no differences in prolactin, TSH, LH, FSH, and ACTH between young adult mutants and controls.
Discussion
With the use of Cre-Lox technology to remove exon 1 of the LEPR in somatotropes, we successfully created a somatotrope-selective LEPR-null mouse model. The location of the loxP around exon 1 causes deletion of a region that contains the signal peptide, thus preventing translocation of the protein into the rough endoplasmic reticulum (5). As was reported by Cohen et al (15), this will result in complete loss of any isoforms. This contrasts with our previous model, in which only the JAK binding site of LEPRb was deleted (14). As in the previous study (14), we verified specificity of the deletion by genotyping 16 organs and showed that there was no extrapituitary excision. We also showed that mutant GH cells were unresponsive to leptin in vitro as detected by pSTAT3 immunolabeling and that fluorescent mutant somatotropes exhibited a severe loss in LEPR labeling. The immunolabeling for LEPR must be interpreted with caution, however, because the antisera will bind to unknown proteins in a Western blotting.
Our previous model in which the JAK binding site was deleted showed no signs of obesity in males until 3–4 months of age and no weight loss until late in life (>10 mo of age) (14). We detected metabolic disturbances at 3–4 months (20). In contrast, in the present study, we observed truncal fat deposition in the young mutant mice from this line as early as 15 days of age. Therefore, we chose to examine the metabolic profile of somatotrope Lepr exon 1-null mice as young as 2–3 months of age. The objective was to determine whether the metabolic dysfunction after the complete deletion of the somatotrope LEPR would be greater than that seen in the previous model. Our results to be discussed below clearly indicate that with this model, truncal obesity occurs earlier in both sexes and there are sex differences in the severity of the GHD.
GHD is more severe in young mutant males than in young mutant females
GHD during childhood in both males and females is characterized by short stature, slow or compromised bone growth, and atypical body composition (24). Adult-onset GHD is associated with changes in body composition, along with cardiovascular and metabolic complications, osteopenia or osteoporosis, and a reduced quality of life (4, 25, 26). In addition, there is substantial data showing sexual dimorphism in GH secretion, which is not apparent until just before puberty (27). For example, the age-associated decline in 24 hours GH secretion seen during pubertal development is 2-fold greater in men that women (4, 24, 27). GH pulse amplitude and frequency also varies between sexes (27–31).
In the present studies, GHD was seen in both the young males and females. This was evident both in the decreased numbers of immunolabeled somatotropes and the decreased serum GH levels. However, the males had a much more severe phenotype with a more than 60% loss in GH cells and an 84% reduction in serum GH whereas females showed only 41%–42% reductions in somatotropes and serum GH. This sex difference was not seen in the previous model of somatotrope Lepr exon 17 deletion, suggesting that all isoforms of the somatotrope LEPR may be vital for GH maintenance, especially in males (17). There may be LEPR isoforms available to the somatotrope Lepr exon 17-null mice that support some aspect of somatotrope function. At this point, little is known about the function of the nonsignaling LEPR isoforms, although LEPRe is considered to be an important transport protein (5). Also, the previous model was studied later in the adult period (4–10 mo). It is possible that the sex difference will not be as extreme in older mice as the deletion of Lepr exon 17 in somatotropes did not show significant increases in fat mass and obesity in females until they were 6 months of age (14).
Decreased lean body mass and adipose distribution
In humans, GHD is associated with a decrease in lean body mass and muscle composition, which can be restored with GH therapy (32–34). In our young deletion mutants, MRI imaging and analysis showed that lean body mass is reduced in deletion mutants as young as 2–3 months. Male and female deletion mutants also weigh less than their littermate controls. This is in spite of their increased fat load detected by MRI. These findings contrast with data from the previous model (14), which showed normal weight increases from weaning until 4 months of age in males and 6 months of age in females. Thereafter, both sexes showed weight gain, which was associated with an increased fat load.
In humans, energy metabolism and storage is related to sex-specific functions that reflect requirements in females for reproductive needs, whereas males may represent a default energy state (35). Females have greater adiposity as defined by percent body fat (36), less visceral white adipose, and more sc adipose tissue distributed in the abdominal and gluteofemoral areas (37, 38). Body fat distribution is a better predictor of metabolic health than body mass index, because the location and type of fat are more precise than the general body mass index measurement (35, 37, 38). Subcutaneous white adipose tissue is favorable, whereas more visceral white adipose is undesirable, because it is linked to altered lipid and glucose homeostasis (39).
In the present study, young male deletion mutants had greater visceral adipose distribution and adipose density than control littermates, which indicates a greater sensitivity to the reduction in serum GH in this depot. Young mutant females had more adipose throughout the body correlating with differences in whole storage. These sex differences may indicate compartmentalization of free fatty acid (FFA) metabolism. FFA flux is similar in men and women under basal postabsorptive conditions, whereas upper body white adipose is more lipolytically active than lower body (40, 41). However, nonoxidative FFA clearance through reesterification is higher in women than men, suggesting that women tend to store, whereas men tend to oxidize circulating FFAs. The evidence in our mutants suggests that the severe reduction in serum GH in young mutant males has compromised normal lipolysis in visceral fat whereas the less severe reduction in GH in females affected lipolysis more generally. The mutation did not affect adipocyte size, however, and triglyceride levels were normal in the young mutant animals.
Studies of older mutant male mice will likely detect increased insulin resistance as a result of the build-up of visceral fat and exposure of the liver to elevated FFA levels. Indeed, the older mice (5–6 mo) in which LEPR exon 17 was deleted in somatotropes had a significant increase in the adipokine resistin, which increases insulin resistance (20).
GHD effects on RQ and reduced activity levels
Adult patients with GHD are insulin-resistant, have reduced LBM, and impaired physical performance (42). The greatest indication of metabolic dysfunction in our deletion mutants was observed in the calculation of RQ (RQ = VCO2/VO2). Indirect calorimetry demonstrated that deletion mutant males and females had higher RQs. The oxidation of fat requires more O2 than the oxidation of carbohydrates; therefore, a high RQ indicates that animals are oxidizing carbohydrates rather than fat (43). This in addition to low energy expenditure would cause increased adipose accumulation.
The RQs observed in control littermates raised concern, because they appeared higher than those of previous control groups monitored by the same equipment (20). Therefore, a second control group was chosen for analysis, with founder mice that bear floxed Lepr exon 1 but have had no exposure to mice bearing Cre-GH. These founders were of normal weight and showed RQ values similar to those of our control groups bearing Cre-GH only or floxed LEPR exon 17 (20). Thus, the higher RQ did not appear to come from the floxed Lepr exon 1 transgene alleles.
These findings for the metabolic activity of the littermate control group suggest possible influences from the Cre-GH bearing males, which may affect the Cre-negative littermate controls. We had chosen to pass the Cre-recombinase through the male parent to avoid possible complications from Cre-GH bearing females during pregnancy, which might result from their low GH levels. However, it is possible that the severe GHD in the males compromised spermatogenesis and sperm health so as to affect the metabolic health of future generations, even those that did not bear Cre-GH.
Overall reproductive health may be affected as well, because generations after N3 produced significantly fewer pups as well as fewer males than females, suggesting that the GHD of the mutant male may have caused fragility in the Y-bearing sperm. Ongoing studies of this phenomenon find no correlation between the reduction in male pups with expression of Cre-recombinase. The loss in males appears to be equally distributed between control and mutant males, which also points to influential factors from the Cre-bearing male parent. The previous model showed no obvious reproductive defects (14). The data may highlight the importance of normal serum GH levels for optimal male reproduction. Further tests of the mutant male or female reproductive competence are definitely needed before conclusions can be drawn.
Analyses of activity, cytokines, and adipokines
Older female deletion mutant mice lacking Lepr exon 17 in somatotropes had significant changes in glucagon, insulin, and C-peptide, suggesting increased insulin sensitivity as a result of GHD (20). There were no differences found in the younger mice lacking Lepr exon 1 in somatotropes in these hormones or in any of the adipokines tested. It is possible that changes may eventually be observed with increased age and buildup of fat depots. A full discussion of the impact of the adipokines can be found in our previous paper (20).
Because obesity can be classified as a low grade inflammatory response, proinflammatory cytokines were measured, including TNF-α, a proinflammatory cytokine secreted by macrophages and adipocytes, and IL-6, which acts as both a proinflammatory cytokine and antiinflammatory myokine. As described previously for mice lacking Lepr exon 17 in somatotropes, these factors were not elevated in these young deletion mutants. This may indicate that the degree of visceral adiposity had not advanced to promote the transition to inflammatory cells in the adipose tissue of these young mice.
With respect to activity, GHD in adult humans is associated with decreased physical performance (42), and this is demonstrated in both sleep and activity parameters. The older mutant males lacking Lepr exon 17 in somatotropes did show reduced sleep and this was correlated with increased restlessness, especially during the dark phase (20). Their response was similar to that of the young deletion mutant males lacking Lepr exon 1 in somatotropes in the present study.
Older deletion mutant females lacking Lepr exon 17 in somatotropes had increased activity, but no evidence of sleep disturbance (20). In contrast, in the present study, young deletion mutant females had decreased activity and increased sleep, which indicates a sluggishness that is characteristic of GHD.
Finally, feeding data was unavailable for these animals due to inconsistencies with the young mice digging out food and equipment malfunctions. However, unlike the Lepr exon 17 deletion mutants (20), there was trend that suggested that young deletion mutants ate less than control littermates.
Summary and conclusions
These studies report the impact of deletion of all isoforms of LEPR in somatotropes. After an initial study of very young (prepubertal) mutants showed unusual abdominal obesity, we elected to focus on the analysis of metabolic activity in the 2–3 month old group. We report severe changes in young male deletion mutants lacking Lepr exon 1 in somatotropes that include an 84% reduction in serum GH, a 65% reduction in somatotropes, loss of lean body mass and weight and significant increases in abdominal adiposity detected by MRI as early as 2 months of age. Indirect calorimetry data indicate preferential burning of carbohydrates. The non-Cre-bearing offspring from these male deletion mutants appear to show metabolic changes that indicate reduced fat burning in spite of their normal serum GH levels, suggesting an as yet unexplained influence from the Cre-GH bearing sire. Collectively, these changes in mutant males are more severe than those seen in mutant mice in which Lepr exon 17 was deleted in somatotropes (20). Thus, this may reflect a greater dependency on all isoforms of the LEPR to promote somatotrope function in males. There may also be an impact on reproductive competence, which requires further analyses. We also report that the young female deletion mutants lacking Lepr exon 1 are not as severely GHD; however, they do show an overall increase in adiposity along with a decrease in weight, neither of which were shown when Lepr exon 17 was ablated in somatotropes. These data point to a vital role for leptin in the optimization of somatotrope lipolytic function, especially in the young male.
Acknowledgments
We thank the University of Arkansas for Medical Sciences Center for Translational Neuroscience for allowing us to use the CLAMS metabolic cages and the Luminex Analyzer, Dr Mohsin Syed and Dr Noor Akhter for their expertise in dissections and immunolabeling, Streamson Chua for his interpretation of metabolic data, and A.F. Parlow and the Hormone Distribution Program for the antisera to rat GH. This article is submitted in partial fulfillment of the PhD degree in the Department of Neurobiology and Developmental Sciences.
This work was supported by National Institutes of Health (NIH) Grant 1R01HD059056 and NIH P30 NS 047546 (Cores), NIH National Institute of General Medical Sciences Institutional Development Award IDeA Program Awards NIH P20 GM103425 and NIH P30 GM110702, a Sturgis Charitable Trust award to support diabetes research and NIH R03 HD082793.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CLAMS
- Comprehensive Laboratory Animal Monitoring System
- eGFP
- enhanced green fluorescent protein
- FFA
- free fatty acid
- GHD
- GH deficiency
- JAK
- Janus kinase
- LEPR
- leptin receptor
- MRI
- magnetic resonance imaging
- pSTAT3
- phosphorylated STAT3
- RQ
- respiratory quotient
- STAT
- signal transducer and activator of transcription
- VCO2
- production of CO2
- VO2
- consumption of O2.
References
- 1. Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140(10):4551–4557. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd RV, Jin L, Tsumanuma I, et al. Leptin and leptin receptor in anterior pituitary function. Pituitary. 2001;4(1–2):33–47. [DOI] [PubMed] [Google Scholar]
- 3. Mann DR, Plant TM. Leptin and pubertal development. Semin Reprod Med. 2002;20(2):93–102. [DOI] [PubMed] [Google Scholar]
- 4. Glynn N, Agha A. Diagnosing growth hormone deficiency in adults. Int J Endocrinol. 2012;2012:972617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chua SC, Jr, Koutras IK, Han L, et al. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45(2):264–270. [DOI] [PubMed] [Google Scholar]
- 6. Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J Biol Chem. 1998;273(40):26194–26201. [DOI] [PubMed] [Google Scholar]
- 7. Sone M, Nagata H, Takekoshi S, Osamura RY. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell Tissue Res. 2001;305(3):351–356. [DOI] [PubMed] [Google Scholar]
- 8. Akhter N, Johnson BW, Crane C, et al. Anterior pituitary leptin expression changes in different reproductive states: in vitro stimulation by gonadotropin-releasing hormone. J Histochem Cytochem. 2007;55:151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDuffie IA, Akhter N, Childs GV. Regulation of leptin mRNA and protein expression in pituitary somatotropes. J Histochem Cytochem. 2004;52(2):263–273. [DOI] [PubMed] [Google Scholar]
- 10. Chen C, Roh SG, Nie GY, et al. The in vitro effect of leptin on growth hormone secretion from primary cultured ovine somatotrophs. Endocrine. 2001;14(1):73–78. [DOI] [PubMed] [Google Scholar]
- 11. Saleri R, Giustina A, Tamanini C, et al. Leptin stimulates growth hormone secretion via a direct pituitary effect combined with a decreased somatostatin tone in a median eminence-pituitary perifusion study. Neuroendocrinology. 2004;79(4):221–228. [DOI] [PubMed] [Google Scholar]
- 12. Luque RM, Huang ZH, Shah B, Mazzone T, Kineman RD. Effects of leptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am J Physiol Endocrinol Metab. 2007;292(3):E891–E899. [DOI] [PubMed] [Google Scholar]
- 13. Baratta M, Saleri R, Mainardi GL, Valle D, Giustina A, Tamanini C. Leptin regulates GH gene expression and secretion and nitric oxide production in pig pituitary cells. Endocrinology. 2002;143(2):551–557. [DOI] [PubMed] [Google Scholar]
- 14. Childs GV, Akhter N, Haney A, et al. The somatotrope as a metabolic sensor: deletion of leptin receptors causes obesity. Endocrinology. 2011;152(1):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108(8):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luque RM, Amargo G, Ishii S, et al. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148(5):1946–1953. [DOI] [PubMed] [Google Scholar]
- 17. Childs GV, Unabia G, Miller BT. Cytochemical detection of gonadotropin-releasing hormone-binding sites on rat pituitary cells with luteinizing hormone, follicle-stimulating hormone, and growth hormone antigens during diestrous up-regulation. Endocrinology. 1994;134(4):1943–1951. [DOI] [PubMed] [Google Scholar]
- 18. Akhter N, Crane C, Childs GV. Pituitary leptin-a paracrine regulator of gonadotropes: a review. Open Neuroendocrinol J. 2011;4:25–42. [Google Scholar]
- 19. Childs GV, Allensworth-James ML, Odle AK, Haney AC, MacNicol AM. Validation of Cre-reporter strategies to address challenges in the purification of GH-deficient somatotropes. Program of the 97th Annual Meeting of The Endocrine Society, San Diego, CA, 2015 (Abstract THR-452). [Google Scholar]
- 20. Akhter N, Odle AK, Allensworth-James ML, et al. Ablation of leptin signaling to somatotropes: changes in metabolic factors that cause obesity. Endocrinology. 2012;153(10):4705–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–351. [Google Scholar]
- 22. Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27:511–541. [DOI] [PubMed] [Google Scholar]
- 23. Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes. 2011;60(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19(6):717–797. [DOI] [PubMed] [Google Scholar]
- 25. Doga M, Bonadonna S, Gola M, Mazziotti G, Giustina A. Growth hormone deficiency in the adult. Pituitary. 2006;9(4):305–311. [DOI] [PubMed] [Google Scholar]
- 26. Hoffman DM, O'Sullivan AJ, Baxter RC, Ho KK. Diagnosis of growth-hormone deficiency in adults. Lancet. 1994;343(8905):1064–1068. [DOI] [PubMed] [Google Scholar]
- 27. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27(2):101–140. [DOI] [PubMed] [Google Scholar]
- 28. Veldhuis JD, Iranmanesh A, Ho KK, Waters MJ, Johnson ML, Lizarralde G. Dual defects in pulsatile growth hormone secretion and clearance subserve the hyposomatotropism of obesity in man. J Clin Endocrinol Metab. 1991;72(1):51–59. [DOI] [PubMed] [Google Scholar]
- 29. Xu J, Bekaert AJ, Dupont J, et al. Exploring endocrine GH pattern in mice using rank plot analysis and random blood samples. J Endocrinol. 2011;208(2):119–129. [DOI] [PubMed] [Google Scholar]
- 30. Veldhuis JD, Patrie J, Wideman L, Patterson M, Weltman JY, Weltman A. Contrasting negative-feedback control of endogenously driven and exercise-stimulated pulsatile growth hormone secretion in women and men. J Clin Endocrinol Metab. 2004;89(2):840–846. [DOI] [PubMed] [Google Scholar]
- 31. Wideman L, Weltman JY, Patrie JT, et al. Synergy of L-arginine and growth hormone (GH)-releasing peptide-2 on GH release: influence of gender. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1455–R1466. [DOI] [PubMed] [Google Scholar]
- 32. Götherström G, Elbornsson M, Stibrant-Sunnerhagen K, Bengtsson BA, Johannsson G, Svensson J. Muscle strength in elderly adults with GH deficiency after 10 years of GH replacement. Eur J Endocrinol. 2010;163(2):207–215. [DOI] [PubMed] [Google Scholar]
- 33. Elbornsson M, Götherström G, Bosæus I, Bengtsson BÅ, Johannsson G, Svensson J. Fifteen years of GH replacement improves body composition and cardiovascular risk factors. Eur J Endocrinol. 2013;168(5):745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carroll PV, Christ ER, Bengtsson BA, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab. 1998;83(2):382–395. [DOI] [PubMed] [Google Scholar]
- 35. Varlamov O, Bethea CL, Roberts CT., Jr Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne). 2014;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackson AS, Stanforth PR, Gagnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes Relat Metab Disord. 2002. June;26(6):789–796. [DOI] [PubMed] [Google Scholar]
- 37. Fuente-Martín E, Argente-Arizón P, Ros P, Argente J, Chowen JA. Sex differences in adipose tissue: it is not only a question of quantity and distribution. Adipocyte. 2013;2(3):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues – the biology of pear shape. Biol Sex Differ. 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48(2):301–308. [DOI] [PubMed] [Google Scholar]
- 40. Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest. 1995;96(5):2297–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88(2):609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Møller N, ***Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. [DOI] [PubMed] [Google Scholar]
- 43. Lusk G. The Elements of the Science of Nutrition. Philadelphia, PA: W.B. Saunders; 1909. [Google Scholar]