Abstract
Prenatal testosterone (T)-treated ewes display a constellation of reproductive defects that closely mirror those seen in PCOS women, including altered hormonal feedback control of GnRH. Kisspeptin/neurokinin B/dynorphin (KNDy) neurons of the arcuate nucleus (ARC) play a key role in steroid feedback control of GnRH secretion, and prenatal T treatment in sheep causes an imbalance of KNDy peptide expression within the ARC. In the present study, we tested the hypothesis that prenatal T exposure, in addition to altering KNDy peptides, leads to changes in the morphology and synaptic inputs of this population, kisspeptin cells of the preoptic area (POA), and GnRH cells. Prenatal T treatment significantly increased the size of KNDy cell somas, whereas POA kisspeptin, GnRH, agouti-related peptide, and proopiomelanocortin neurons were each unchanged in size. Prenatal T treatment also significantly reduced the total number of synaptic inputs onto KNDy neurons and POA kisspeptin neurons; for KNDy neurons, the decrease was partly due to a decrease in KNDy-KNDy synapses, whereas KNDy inputs to POA kisspeptin cells were unaltered. Finally, prenatal T reduced the total number of inputs to GnRH cells in both the POA and medial basal hypothalamus, and this change was in part due to a decreased number of inputs from KNDy neurons. The hypertrophy of KNDy cells in prenatal T sheep resembles that seen in ARC kisspeptin cells of postmenopausal women, and together with changes in their synaptic inputs and projections to GnRH neurons, may contribute to defects in steroidal control of GnRH observed in this animal model.
Polycystic ovarian syndrome (PCOS) is a common reproductive endocrine disorder, characterized by hyperandrogenism, polycystic ovaries and anovulatory infertility (1–5). Reproductive defects associated with PCOS include disturbances at the hypothalamic-pituitary-gonadal axis, with decreased sensitivity to steroid feedback control of GnRH secretion (6–10) and abnormally rapid LH pulsatility (11, 12). Although the symptoms of PCOS manifest during adolescence, the neuroendocrine defects of PCOS may originate in prenatal life. Prenatal testosterone (T)-treated female sheep (treated from d 30 to 90 of the 150-d gestation period) show a constellation of symptoms (7, 13–18) almost identical to that of women with PCOS, including deterioration of estrous cycles (14, 16, 18), development of multifollicular ovaries (15, 17), and ultimately, infertility (14, 18). Associated with these abnormalities is an alteration in gonadotropin secretion characterized as a hypersecretion of LH but not FSH, similar to that in PCOS women (19). Studies using the sheep as an animal model have shown that prenatal (T) treatment interferes with 17β-estradiol (E2) negative and positive feedback (7, 20–23). For example, ewes exposed to excess T in utero during days 30–90 show a delayed and attenuated or absent LH surge (7). Altered sensitivity to steroid feedback and reproductive abnormalities have also been shown in women with PCOS (10, 24) and in other animal models for this disease, including female rhesus monkeys (25), rats (26), and mice (24) exposed to prenatal T or dihydrotestosterone.
The reproductive outcomes of prenatal (T) treatment are well described in the ewe, but the neural mechanisms responsible for these detrimental effects are just beginning to be explored. Recent work has focused on the role of a subset of neurons in the arcuate nucleus (ARC) of the sheep that coexpress the neuropeptides kisspeptin/neurokinin B (NKB)/dynorphin (KNDy) (27–30). These cells play a critical role in steroid feedback regulation of GnRH secretion, and represent a final common pathway of multiple hormonal and environmental signals regulating GnRH (28, 31). Because of their reciprocal connections with each other, and the presence of synchronous activity in this portion of the ARC corresponding to GnRH/LH pulses, the KNDy cells have been hypothesized to comprise the “pulse generator” driving pulsatile secretion of GnRH and LH (32, 33). In addition, in the sheep, there is evidence that KNDy cells may also be involved in the generation of the preovulatory GnRH/LH surge (34). A very high percentage of KNDy neurons colocalize estrogen receptor (ER)α, progesterone receptors (PRs), and androgen receptors (27, 28, 35, 36), consistent with the view that these neurons are key targets for the actions of sex steroids in the adult brain. These neurons are also targets for sex steroid action in the developing brain. Specifically, prenatal T-treated ewes show a reduction in the neuropeptide content of dynorphin and NKB in KNDy cells, whereas kisspeptin content in this population remains similar to control females (27). Because dynorphin in KNDy cells is thought to mediate the inhibitory influence of progesterone on GnRH/LH pulses (32, 37, 38), the decrease in this inhibitory peptide (dynorphin) with no change in stimulatory peptide (kisspeptin) has been hypothesized to underlie the reduced responsiveness to progesterone negative feedback seen in this model and women with PCOS (8, 14, 38).
In addition to alterations in the expression of KNDy peptides, there is also evidence suggesting that KNDy cells in the adult female brain may be subject to plasticity in their morphology and synaptic connections. KNDy neurons in postmenopausal women undergo hypertrophy with an increase in somal size (39–42), a change thought to reflect a compensatory action in response to decreased E2 during menopause (39). Thus, we explored the possibility that prenatal T treatment in the sheep, in addition to altering the balance of KNDy peptides, leads to long-term changes in the somal size of KNDy neurons. In addition, the ARC has long been known to be a site of neuroplasticity in the adult female brain, with changes observed in synaptic inputs to ARC neurons across the estrous cycle (43). KNDy cells project directly to GnRH cell bodies in the sheep (44) and the total number of synaptic inputs to GnRH cells are decreased in prenatal T-treated ewes (45). Thus, we also examined the possibility that prenatal T alters the synaptic connections of KNDy neurons with each other (KNDy-KNDy connections) (31), as well as with GnRH cells. Because NKB and dynorphin are decreased in prenatal T animals, we were unable to use colocalization of these peptides with kisspeptin to identify KNDy efferents. As an alternative marker, we used colocalization of kisspeptin and the vesicular glutamate transporter-2 (vGlut2) to identify KNDy terminals, because in the sheep, a vast majority of dual-labeled (ie, kisspeptin and dynorphin) KNDy fibers (>92%) colocalize vGlut2, whereas single-labeled kisspeptin fibers (originating from preoptic area [POA] kisspeptin neurons) do not contain this marker (44). Finally, to unambiguously confirm that varicosities in close contact to KNDy neurons were indeed presynaptic terminals, we also used the synaptic vesicle marker, synaptophysin (Syn), as part of our multiple-label immunostaining protocol.
Materials and Methods
Animals
To generate prenatal T-treated ewes, pregnant Suffolk ewes (mothers) were administered twice weekly im injections of T propionate (100 mg/injection, catalog item T1875; Sigma-Aldrich) suspended in cottonseed oil (catalog item C7767; Sigma-Aldrich) from 30 to 90 days of pregnancy (term, 147 d). This dose of T propionate administered results in levels of T in the female fetus comparable with those seen in fetal males. Controls were not treated with vehicle, because in previous studies, we found no effect of vehicle treatment on reproductive attributes (46). Lambs were born in March and April. After weaning, they were maintained outdoors under natural photoperiods with a daily maintenance feeding and free access to water at the Sheep Research Facility of the University of Michigan until the age of 2 years. Four prenatal T-treated ewes and 5 control ewes were used in this experiment. Brains were collected during the breeding season when females were 2 years of age. Because prenatal-T-treated ewes no longer have regular reproductive cycles at this age (6, 7, 14, 16), we normalized the internal hormonal milieu using a well-established model for the ovine follicular phase (47). Three-four weeks before tissue collection, animals were ovariectomized and treated sequentially for 11–12 days with 2 controlled internal drug release progesterone implants (controlled internal drug release) (InterAG) and a 1-cm-long SILASTIC capsule (inner diameter, 3.35 mm and outer diameter, 4.65 mm; Dow Corning Corp) filled with E2 (Sigma-Aldrich). Controlled internal drug releases were then removed and an additional 4 3-cm-long E2 implants were inserted sc to simulate ovarian steroid levels during the late follicular phase of the cycle. Eighteen hours after the E2 implants, animals were killed.
Tissue collection and preparation
At the time of tissue collection animals received 2 iv injections of heparin (25 000 U given 10 min apart; catalog item 402588B; Abraxiz Pharmaceutical Products) and then deeply anesthetized with sodium pentobarbital (2–3 g, iv, catalog item P3761; Sigma-Aldrich) and were immediately decapitated. The heads were perfused via both internal carotids with 6-L 4% paraformaldehyde in 0.1M phosphate buffer (PB) (pH 7.3) containing 0.1% sodium nitrate and 10-U/mL heparin. The brains were removed after perfusion, and a block of tissue containing the POA and hypothalamus was dissected out. Tissues were placed in 4% paraformaldehyde in 0.1M PB overnight for postfixation at 4°C and then transferred into 30% sucrose in 0.1M PB for cryoprotection until the filtration was completed. Frozen coronal sections (45 μm) were cut using a freezing microtome (Microm HM400R) and stored at −20°C in a cryopreservative solution until being processed for immunodetection of kisspeptin, GnRH, vGlut2, or Syn (marker of all synaptic terminals), or histochemically stained with DAPI (nuclear marker). Within each experiment, tissue sections from all experimental groups were processed simultaneously as described below.
Immunohistochemistry. General methods
Sections were processed free floating with gentle agitation and all steps were performed at room temperature. Antibodies were dissolved in incubation solution consisting of 0.1M PBS, 0.4% Triton X-100 (catalog item BP151-500; Sigma-Aldrich) containing 4% normal goat serum (catalog item 005-000-121; Jackson ImmunoResearch) (for antibodies, please see Table 1). Tissue sections were washed with 0.1M PBS (pH 7.35) between steps. Before incubation with first primary antibody sections were incubated with 1% hydrogen peroxide (10 min, H2O2; catalog item H325; Fisher Scientific) and incubation solution (1 h) to prevent nonspecific background labeling. All primary antibodies have previously been validated for use in ovine tissues (44). Negative controls were performed by omission of primary antibody, which eliminated all labeling corresponding to that antigen.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| vGlut2 | AA 510–582 | VGLUT-2 | Synaptic Systems, catalog 135402 | Rabbit, polyclonal | 1:5000 |
| Syn | N/A | Monoclonal anti-Syn | Sigma; catalog S-5768 | Mouse, monoclonal | 1:200 |
| Kisspeptin | YNWNSFGLRY (AA 43–52) | Kisspeptin antibody | Gift from Alain Caraty; catalog 564 | Rabbit, polyclonal | 1:300 000 |
| GnRH | N/A | GnRH (LHRH) | Covance; catalog SMI-41R | Mouse, monoclonal | 1:400 |
| POMC | AA 27–52 | POMC precursor (POMC) | Phoenix Pharmaceuticals; catalog H-029-30 | Rabbit, polyclonal | 1:4000 |
| AgRP | 82–131-NH2 | Agouti-related protein (AgRP) | Antibodies Australia; catalog GPAAGRP.1 | Guinea pig, polyclonal | 1:750 |
Triple-label detection of kisspeptin, vGlut2, and Syn
In order to investigate synaptic inputs on Kiss cells in POA and ARC 1 series of every sixth section containing POA or MBH from each animal were processed for kisspeptin, vGlut2, Syn, and DAPI (a nuclear marker) using a previously described immunostaining protocol (28, 31). Because the antibodies against kisspeptin and vGlut2 were both raised in rabbits, the kisspeptin antisera was used at a high dilution (1:300 000) and amplified using tyramide signal amplification this procedure has been previously validated to prevent cross-reactivity of second antibodies under these circumstances (29). First, tissue sections were incubated with rabbit anti-kisspeptin (1:300 000 dilution, 17 h; kp10, lot 564; gift from Dr Alain Caraty, Nouzilly, France), biotinylated goat antirabbit IgG (1:500, 1 h, catalog item BA-9200; Vector Laboratories), avidin, and biotinylated horseradish peroxidase complex (Avidin-Biotin Complex) (1:500, 1 h, catalog item PK-6100; Vector Laboratories), biotinylated tyramine (1:250, catalog NEL700A; diluted PerkinElmer Life Sciences), adding 1 μL of 3% H2O2/mL of total volume (10 min), and Alexa Fluor 488 streptavidin (1:100, 30 min, green, S-32354; Invitrogen/Molecular Probes). Tissue was protected from light from this step forward. Next, sections were coincubated with rabbit anti-vGlut2 (1:5000, 17 h, lot 135402; Synaptic Systems) and mouse anti-Syn (1:200, 17 h, S-5768; Sigma). After overnight incubation, sections were washed and incubated with goat anti-rabbit Alexa Fluor 555 (1:100, 30 min, red, catalog A-21428; Invitrogen/Molecular Probes), then donkey anti-mouse Cy5 (1:100, 30 min, blue, 715-175-151, lot 75139; Jackson ImmunoResearch). Finally, sections triple-labeled for kisspeptin, vGlut2, and Syn were incubated in DAPI (10 mg/mL, 10 min, catalog D-3571; Invitrogen) to visualize the nuclear area of the cells. Tissue sections were mounted onto slides, air dried, coverslipped with Gelvatol mounting medium, and stored at 4°C until analyzed.
Triple-label detection of GnRH, kisspeptin, and vGlut2
In order to examine synaptic inputs onto GnRH cells in POA and MBH, 1 series of every sixth section containing POA or MBH from each animal were processed for GnRH, kisspeptin, and vGlut2 (28, 31). As previously described, sections were coincubated with rabbit antikisspeptin and mouse anti-GnRH (1:400, 17 h, catalog SMI-41R, lot 3; Covance). Next, sections were washed and incubated with goat anti-mouse Alexa Fluor 488 (1:100, 30 min, green, lot 41139A; Molecular Probes). Tissue was protected from light from this step forward, and sections were incubated with biotinylated goat anti-rabbit IgG (1:500, 1 h, catalog item BA-9200; Vector Laboratories), followed by Avidin-Biotin Complex reagent, biotinylated tyramine (as previously described), and Cy5 streptavidin (1:100, 30 min; blue, lot 55606; Jackson ImmunoResearch). Sections were incubated with the primary antibody rabbit anti-vGlut2 (17 h), then with goat anti-rabbit Alexa Fluor 555 (1:100, 30 min, red, catalog A-21428; Invitrogen/Molecular Probes). Sections were mounted onto slides, coverslipped with Gelvatol mounting medium, covered, and stored at 4°C until analyzed.
Image capture and analysis. Synaptic inputs
Confocal Z-stacks comprised of 1.0-μm optical sections through immunostained sections were captured at ×60 magnification using an LSM 510 META/ConfoCor2 Confocal Microscope. Sections through the ARC and POA (inputs to kisspeptin cells, 3 and 6 sections for each region, respectively) and the MBH and POA (inputs to GnRH cells, 6 and 3 sections for each region, respectively) in each animal were analyzed. A total of 15–20 kisspeptin-positive cells per region (ARC and POA) were analyzed in each animal; similarly, 10–15 GnRH positive cells per region (MBH and POA) were analyzed in each animal.
The number of triple-labeled (Kiss/vGlut2/Syn), double-labeled (vGlut2/Syn; Kiss/Syn), and single-labeled Syn-positive inputs were counted for each kisspeptin cell body in the ARC and POA, and numbers were compared between control (OVX+Estrogen) and prenatal (T)-treated females (OVX+Estrogen). A synaptic input was defined as an immunolabeled terminal that was 1) positive for Syn, 2) and in direct apposition to a cell body without any intervening pixels. Orthogonal views were used to confirm contacts in all planes of section and no overlap of the synaptic contact with kisspeptin immunoreactivity in the soma (eg, see Figure 3 below). Markers were placed (eg, see Figure 4 below) to ensure inputs were not counted twice and the numbers of triple-, dual-, or single-labeled inputs were recorded. The somal perimeter of each cell was measured for each optical section of the cell where the nucleus was seen and averaged per number of sections. The average somal membrane was calculated for each cell. The number of inputs per KNDy cell soma was normalized per 10 μm of somal perimeter to control for any difference in somal perimeter between the 2 groups. Similarly, the number of double-labeled (kiss/vGlut2) and single-labeled (kiss and vGlut2) inputs was counted for each GnRH soma in the MBH and POA and compared between control and prenatal (T)-treated females. The number of inputs was calculated per 10 μm of GnRH somal perimeter. All analyses were performed by investigators who were blinded to the animal number and experimental group.
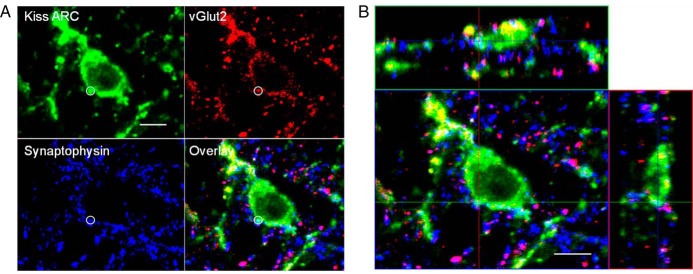
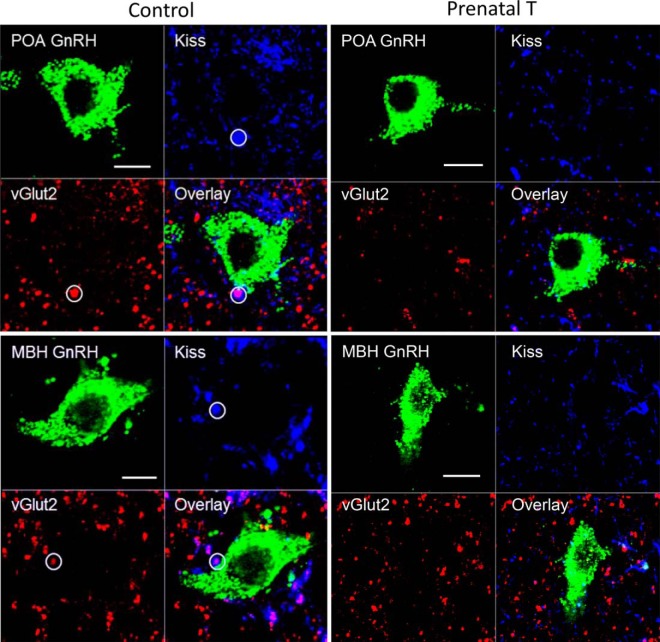
Figure 3.
A, Confocal 1-μm-thick optical section through an ARC kisspeptin cell from a prenatal T ewe, showing a triple-labeled kisspeptin/vGlut2/Syn-positive input (white circle). B, Orthogonal views from the same Z-stack, showing the direct contact between this terminal and the ARC kisspeptin neuron in all 3 planes. Scale bar, 10 μm.
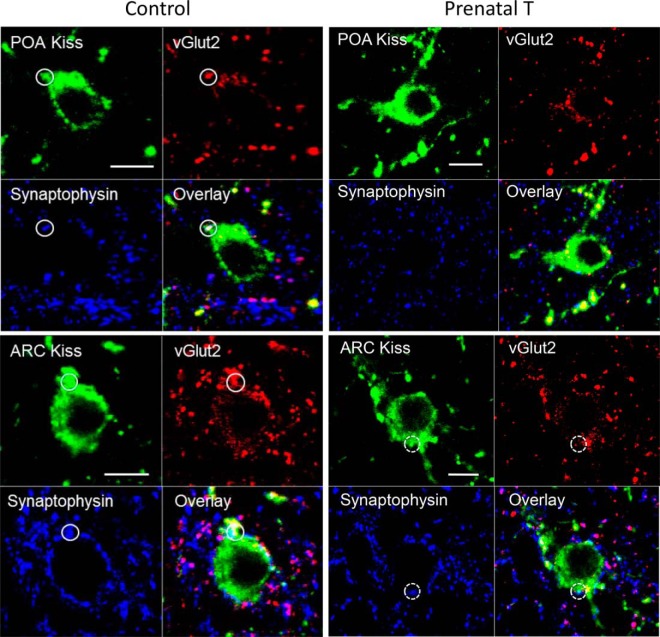
Figure 4.
Confocal 1-μm-thick optical sections from POA (top panels) and ARC kisspeptin (bottom panels) neurons from control (left) and prenatal T (right) animals, showing examples of inputs (circles with solid perimeter) which are triple-labeled for kisspeptin (green), vGlut2 (red), and Syn (blue), and the overlay of the 3 channels. In addition, a double-labeled kisspeptin/Syn input onto an ARC kisspeptin cell from a prenatal T ewe is shown (dotted white circle). Scale bar, 10 μm.
Image capture and analysis. Somal size analysis
To determine whether prenatal T treatment induced morphological changes in the KNDy and/or GnRH cells, the somal and nuclear areas of kisspeptin cells in the ARC (KNDy) and POA, and GnRH cells (POA and MBH) were measured. Using sections from the same animals (control, n = 5; prenatal T, n = 4) as for the synaptic input analysis, the somal and nuclear (DAPI) areas of approximately 15–20 kisspeptin cells/animal in the ARC and POA, and 10 GnRH positive-labeled cells/animal in the POA and MBH, were measured and averaged from 3–4 optical sections per cell using the Zeiss Image Browser (version 4.2.0.121). In addition, as control neuronal populations in the ARC, we also analyzed the somal areas of neurons that contain agouti-related peptide (AgRP) and proopiomelanocortin (POMC) cells. Dual label immunofluorescence was performed on tissue sections from the middle ARC for POMC and AgRP. Briefly, to visualize POMC, tissue sections were incubated in polyclonal rabbit anti-POMC serum (1:4000; Phoenix Pharmaceuticals) for 17 hours. Tissue sections were then processed with a biotinylated tyramide signal amplification protocol with Cy5 donkey anti-rabbit (1:100; Jackson ImmunoResearch) for 30 minutes to visualize POMC. Next, AgRP was visualized using a polyclonal Guinea pig anti-AgRP serum (1:750, incubated for 17 h; Antibodies Australia) and then fluorophore-conjugated secondary antibodies Alexa Fluor 488 goat anti-Guinea pig (1:100; Jackson ImmunoResearch) for 30 minutes. Sections were mounted on gelatinized slides, dried and coverslipped with gelvatol. Z-stacks from 15–20 AgRP and POMC cells per animal were captured, somal areas measured and averaged from 3–4 optical sections per cell. All confocal Z-stacks of immunostained sections were captured at ×60 magnification using a LSM 510 META/ConfoCor2 Confocal Microscope or a LSM 510 META Confocal Microscope (laser diode 405nm to visualize the DAPI and Cy5 staining).
Statistical analysis
Values of somal and nuclear areas, and the density of specific synaptic inputs and total number of inputs, were averaged for each animal. These means were then used for statistical comparisons between control and prenatal T-treated ewes by t tests. P < .05 was considered statistically significant for all analyses.
Results
Changes in somal area of ARC kisspeptin cells
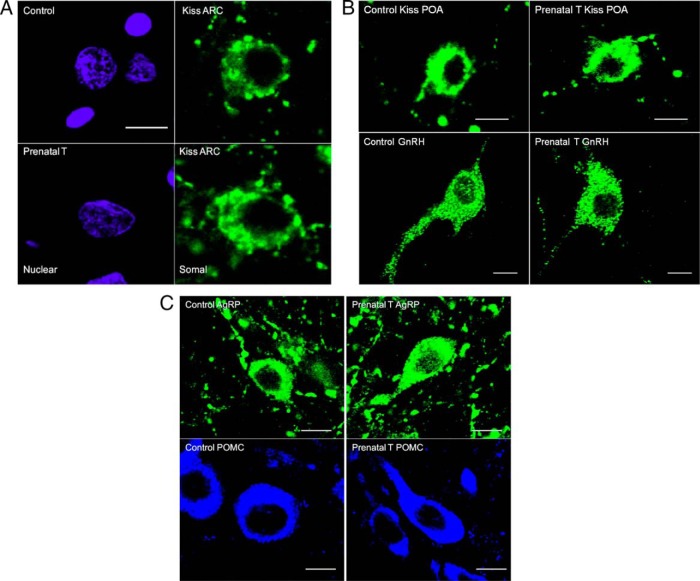
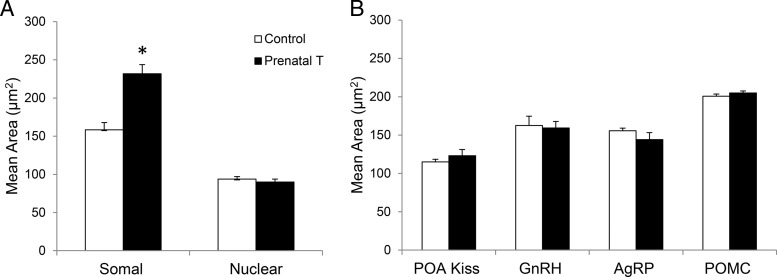
Representative images of ARC kisspeptin, POA kisspeptin, GnRH, AgRP, and POMC neurons used for somal and nuclear (ARC kisspeptin) size analyses are shown in Figure 1. Prenatal T treatment significantly increased the somal perimeter of ARC kisspeptin neurons compared with control females (prenatal T: 51.23 ± 0.90 μm, control: 43.46 ± 0.64 μm; P = .009) and area (Figure 2) but had no effect on the area of their nuclei (Figures 1A and 2A). By contrast, prenatal T treatment had no effect on somal area of POA kisspeptin cells, GnRH cells from the POA and MBH, or AgRP or POMC cells of the ARC (Figures 1, B and C, and 2B).
Figure 1.
A, Confocal images showing examples of ARC kisspeptin cells from control and prenatal T animals that were used for somal and nuclear size analyses. Cells were labeled by immunofluorescence for kisspeptin (green, right panels) to measure somal perimeter and counterstained for DAPI (purple, left panels) for assessment of nuclear perimeter. Scale bar, 10 μm. B, Confocal images showing examples of POA kisspeptin (top panels) and GnRH neurons (bottom panels) from control and prenatal T animals. Scale bars, 10 μm. C, Confocal images showing examples of AgRP (top panels) and POMC neurons (bottom panels) in the ARC from control and prenatal T animals. Scale bars, 10 μm. Kisspeptin, GnRH, and AgRP were labeled using Alexa Fluor 488 as the flourophore (green), whereas POMC was labeled with Cy5 (blue); see Materials and Methods for more details.
Figure 2.
Effects of prenatal T treatment on (A) mean somal and nuclear area (±SEM) of kisspeptin neurons in the ARC and (B) mean somal area (±SEM) of POA kisspeptin neurons, GnRH, AgRP, and POMC neurons. *, P < .05.
Inputs to kisspeptin cells in the POA and ARC
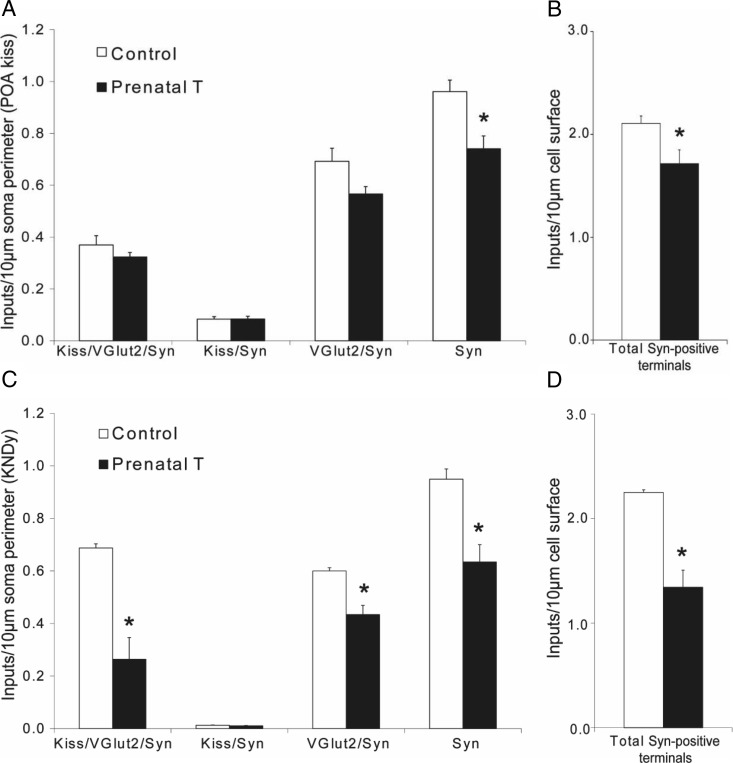
The number of triple-labeled (Kiss/vGlut2/Syn), double-labeled (Kiss/Syn and vGlut2/Syn), and single-labeled (Syn) inputs onto kisspeptin cells in both the POA and ARC was analyzed (for examples, see Figures 3 and 4). Inputs were identified as Syn-positive terminals that were in direct contact with kisspeptin cell bodies (no intervening unlabeled pixels) (Figure 3A) as viewed in 1-μm optical sections through all 3 planes of the contact (Figure 3B).
In the POA, there was no significant difference in the number of Kiss/vGlut2/Syn inputs or Kiss/Syn inputs per 10-μm somal perimeter of kisspeptin cells between prenatal T and control females (Figure 5A). However, there was a trend towards decreased density of vGlut2/Syn inputs (P = .068) and a significant decrease in the density of Syn-only inputs (P = .019) in prenatal T sheep. As a consequence, when the total number of Syn-positive inputs was analyzed (Figure 5B), POA kisspeptin cells in prenatal T animals were found to receive significantly fewer inputs per unit of cell surface than in control ewes (P = .026). This change was paralleled by a significant decrease in the total number of Syn-positive inputs per cell in prenatal T animals compared with controls (Table 2).
Figure 5.
Prenatal T alters the density of synaptic inputs onto kisspeptin cells in the POA (A and B) and ARC (C and D). Graphs at left (A and C) show the number (mean ± SEM) of triple-labeled (Kiss/vGlut2/Syn), double-labeled (Kiss/Syn and vGlut2/Syn), and single-labeled (Syn) terminals per 10-μm somal perimeter in control (n = 5) and prenatal T-treated (n = 4) adult female ewes. Graphs at right (B and D) show the total number (mean ± SEM) of Syn-positive terminals per 10-μm somal perimeter in the same ewes. *, P < .05.
Table 2.
Total Number of Synaptic Inputs/Cell in Control and Prenatal T Ewes
| Cell Type | Control Ewes (Mean ± SEM) | Prenatal T Ewes (Mean ± SEM) |
|---|---|---|
| Total inputs per kisspeptin cell (POA) | 37 ± 2.1 | 29 ± 1.0a |
| Total inputs per kisspeptin cell (ARC) | 80 ± 3.6 | 66 ± 3.0b |
| Total inputs per GnRH cell (POA) | 22 ± 0.4 | 17 ± 0.2b |
| Total inputs per GnRH cell (MBH) | 20 ± 0.9 | 15.0 ± 1.4a |
P < .05, control vs prenatal T.
P < .01, control vs prenatal T.
ARC kisspeptin cells in prenatal T animals received significantly fewer Kiss/vGlut2/Syn, vGlut2/Syn and Syn-only inputs per 10-μm somal perimeter than in control ewes (Figure 5C). Moreover, when the total number of Syn-positive inputs was analyzed, ARC kisspeptin cells in prenatal T-treated ewes received approximately 40% fewer contacts per unit somal surface than control ewes (Figure 5D). Similarly, when the total numbers of Syn-positive inputs per cell were analyzed, ARC kisspeptin cells in prenatal T animals received significantly fewer inputs than in control ewes (Table 2). In both ARC and POA kisspeptin cells, very few dual-labeled Kiss/Syn inputs were seen (Figure 5, A and C), and there was no difference between prenatal T and control ewes in this type of input to either kisspeptin cell population.
Inputs to GnRH cells
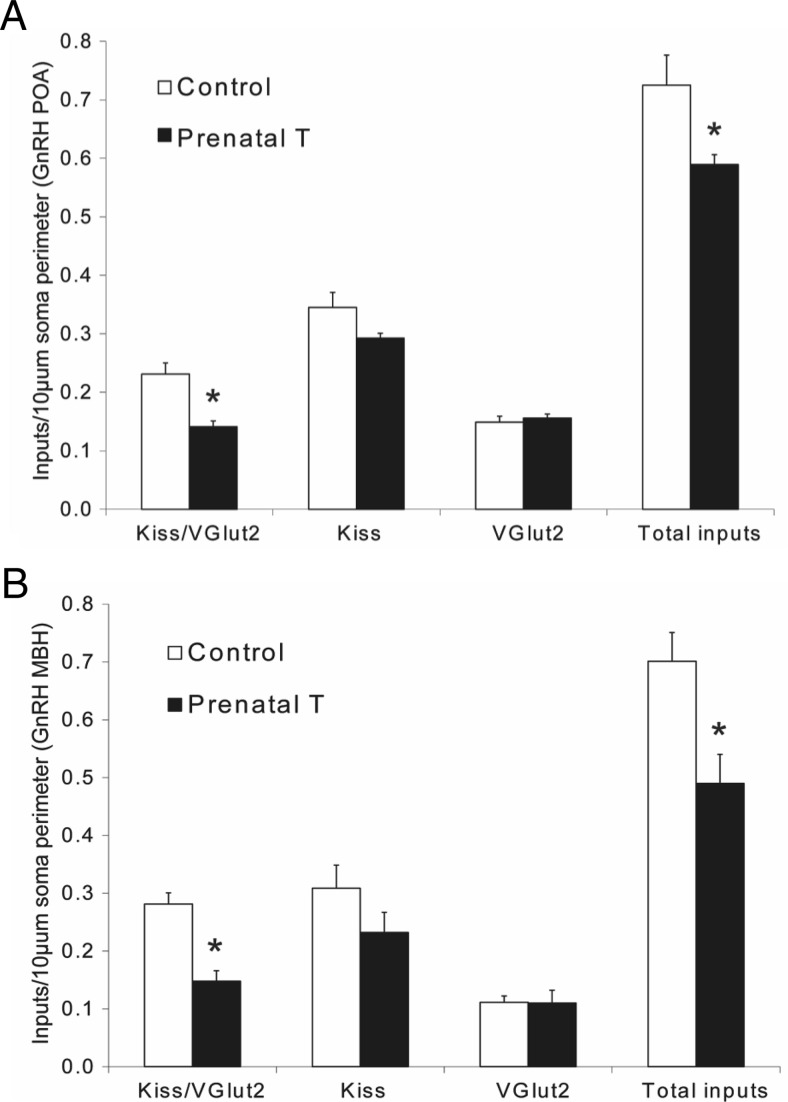
GnRH neurons in both the POA and MBH were observed to receive a mix of single-labeled Kiss and vGlut2 inputs, as well as dual-labeled Kiss/vGlut2 inputs (Figure 6). Prenatal T animals showed a significant decrease in the number of Kiss/vGlut2 inputs per 10-μm somal cell surface in the POA (soma-control: 0.23 ± 0.02, prenatal T: 0.14 ± 0.01; P = .003) (Figure 7A) and the MBH (control: 0.28 ± 0.02, prenatal T: 0.15 ± 0.02; P = .0009) (Figure 7B). In addition, prenatal T treatment reduced the total number of identified inputs (Kiss/vGlut2 dual, kisspeptin single, and vGlut2 single) onto GnRH cells in both the POA (control: 0.73 ± 0.05, prenatal T: 0.59 ± 0.05; P < .036) and MBH (control: 0.70 ± 0.05, prenatal T: 0.49 ± 0.05; P = .017). This was paralleled by significant decreases in the total number of identified inputs per cell for GnRH neurons in either the POA or MBH (Table 2). No significant differences between prenatal T and control ewes were found in the number of kisspeptin or vGlut2 single-labeled inputs onto GnRH neurons in either area.
Figure 6.
Confocal 1-μm-thick optical sections through GnRH neurons in the POA (top panels) and MBH (bottom panels) from control (right) and prenatal T (right) ewes, showing examples of inputs in control ewes (white circles) that were double-labeled for kisspeptin (blue) and vGlut2 (red), along with the overlay of the 3 channels. Scale bar, 10 μm.
Figure 7.
Prenatal T alters the density of kisspeptin and vGlut2-positive inputs onto GnRH neurons in the POA (A) and MBH (B). Graphs show the number (mean ± SEM) of double-labeled (Kiss/vGlut2), single-labeled (Kiss and vGlut2), and total number of these 3 types of inputs per 10-μm somal perimeter in control (n = 5) and prenatal T-treated (n = 4) adult female ewes. *, P < .05.
Discussion
Our results reveal that prenatal T treatment in female sheep leads to 2 types of morphological changes in ARC kisspeptin (KNDy) cells: 1) an increase in the size of KNDy cell bodies, and 2) decreases in the density of KNDy-KNDy synaptic contacts, of KNDy inputs to GnRH neurons, and of nonidentified inputs onto KNDy as well as POA kisspeptin cells (Figure 8). Importantly, the increase in somal size was specific to ARC kisspeptin cells, and not seen for other neuronal subpopulations of the ARC (AgRP and POMC), nor for other kisspeptin cells (POA) or GnRH neurons in the same brains. ARC and POA kisspeptin neurons in prenatal T-treated ewes both exhibited a reduced total number of synaptic inputs per cell (Table 2); in ARC kisspeptin cells, this decrease reflected decreased reciprocal inputs from ARC kisspeptin cells, as well as other (eg, single-labeled vGlut2) inputs. Finally, prenatal T treatment also altered the density of inputs from ARC kisspeptin cells to GnRH neurons, contributing to a reduction in the total number of identified inputs per cell onto GnRH neurons in both the POA and MBH (Table 2).
Figure 8.
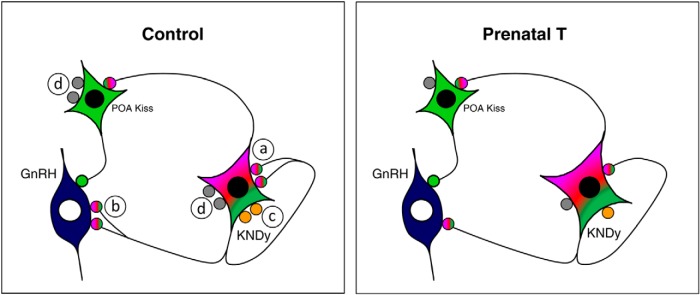
Schematic drawing summarizing the differences observed in this study between control and prenatal T ewes in 1) somal size of KNDy neurons, and 2) in the number of KNDy-KNDy inputs (a), KNDy inputs onto GnRH cells (b), vGlut2 inputs onto KNDy cells (c), and nonidentified inputs (gray circles) onto both KNDy and POA kisspeptin cells (d).
It should be noted that these conclusions are based on the assumption that vGlut2 expression in KNDy neurons is not inhibited by prenatal T treatment. If this occurred there would have to be a corresponding increase in terminals that contained only kisspeptin in the T-treated females. As no such increase was seen (Figures 5 and 7), vGlut2 appears to be a valid marker for KNDy neurons in this study.
Although the changes in synaptic input reported here are based on light microscopic confocal observations, we have previously shown that close associations between axon terminals and cell bodies/dendrites in the sheep hypothalamus, as observed in 1-μm-thick confocal sections, are confirmed as bona fide synaptic inputs when that material is viewed under the electron microscope (46). The use of Syn as a marker in this study lends additional support in confirming the identity of our confocal contacts as synaptic. However, a limitation to the set of results reporting changes in synaptic inputs to KNDy cells is that they were restricted to observations at the level of cell somas and not dendrites; this is because immunodetectable kisspeptin only filled the proximal dendrites of KNDy cells in our material. Thus, it is conceivable that the effects of prenatal T treatment on axo-dendritic inputs to KNDy cells differ from that reported here for axo-somatic inputs. However, we would note that synaptic inputs at the level of cell bodies are likely to be of greater consequence in influencing membrane excitability. Nonetheless, it would be worthwhile to replicate this portion of the results incorporating approaches to enable visualization of the complete KNDy dendritic arbor, for example, transfection of KNDy cells by viral vectors incorporating KNDy-specific promoters to drive reported genes (eg, enhanced green fluorescent protein) (44), or the use of diolistics to label KNDy cells in tissue slices with carbocyanine dyes and produce in them a “Golgi-like” appearance (48).
What is the functional impact of changes in synaptic inputs to kisspeptin and GnRH cells?
Because KNDy-KNDy reciprocal connections have been hypothesized to underlie the coordinated multiunit activity recorded from the ARC that closely parallels LH pulses (32, 49–52), it is conceivable that decreased reciprocal connections among KNDy cells in prenatal T animals might result in reduced coordination of synchronous activity among KNDy cells and hence disrupted GnRH/LH pulses. However, there is no evidence that GnRH or LH pulses are compromised in prenatal T animals; rather, the responsiveness of GnRH neurons to gonadal hormone feedback control is reduced (53), resulting in deficits in both negative and positive feedback (6, 53). A hallmark of PCOS women that is mimicked in prenatal T sheep is a marked reduction in responsiveness of the GnRH pulse generator to the inhibitory effects of progesterone (8). In this regard, it is worthwhile to consider the role that dynorphin, coexpressed in ARC kisspeptin (KNDy) cells, plays in mediating progesterone negative feedback in the ewe. As noted above, dynorphin, acting via the κ-opioid receptor, mediates the inhibitory influence of progesterone on GnRH/LH pulse frequency during the luteal phase of the ovine estrous cycle (38). Evidence suggests that PRs in KNDy cells of the ARC are responsible for this effect; local microimplants of the PR antagonist, RU486, in the ovine ARC are sufficient to block progesterone negative feedback (53), PR is contained in more than 95% of ovine KNDy cells (28), and progesterone induces dynorphin release into the third ventricular cerebrospinal fluid (54). Thus, decreased numbers of KNDy-KNDy inputs, and KNDy inputs to GnRH cells, in prenatal T sheep may contribute to the reduced ability of progesterone, signaling through dynorphin, to inhibit GnRH and LH pulse frequency in these animals (38). Consistent with this hypothesis is the observation that dynorphin but not kisspeptin content is reduced in the ARC of prenatal T sheep (27), as well as recent evidence documenting the presence of immunoreactive κ-opioid receptor on the cell surface of both KNDy and GnRH neurons in the ovine brain (55).
An alternate possibility is that the synaptic changes seen in inputs to both kisspeptin and GnRH neurons may be partly responsible for defects seen in the GnRH surge, which is delayed and severely dampened, in prenatal T females (7). In the sheep, both ARC (KNDy) and POA kisspeptin populations have been implicated in the surge: both populations are activated at the time of the LH surge (34). Moreover, preliminary findings suggest that in ovary-intact sheep, there is an increase in the number of synaptic inputs onto ARC kisspeptin neurons (KNDy) at the time of the preovulatory GnRH surge (44). Similarly, GnRH cells in both the POA and MBH receive a greater number of inputs during the follicular compared with luteal phase in normal cycling ewes (44), and activation of both POA and MBH GnRH cells is seen during the LH surge (44, 56). Thus, the reduced numbers of inputs to KNDy and POA kisspeptin cells, and their connections to GnRH cells, in prenatal T sheep may contribute to a reduced responsiveness of the KNDy-GnRH circuit to the positive feedback effects of E2 responsible for the GnRH surge, leading to defects either in surge amplitude or its timing. Interestingly, we have observed no differences between prenatal T and control ewes in the expression of ERα in the ARC (87), suggesting that the defect in E2 positive feedback is either due to an interruption of signaling downstream to ERα, or changes in afferent neurons or signals that control the responsiveness of KNDy or other cells to E2.
Which neuropeptides/transmitters in KNDy or kisspeptin cells might be functionally involved in control of the surge, and thus affected by the decreased synaptic inputs seen in prenatal T animals? Kisspeptin has been implicated in control of the GnRH surge (57–64) but, as mentioned previously, prenatal T treatment in sheep does not alter kisspeptin peptide content in either the ARC (KNDy) or POA kisspeptin population. On the other hand, prenatal T treatment leads to a reduction in NKB content in KNDy cells (27) as well as decreased NK3R (67). Although NKB was initially believed to be inhibitory to GnRH/LH secretion (40, 65), studies in sheep, primates, and rodents have shown this tachykinin can be stimulatory to GnRH/LH release (32, 66, 67). In the sheep, an agonist to the NKB receptor (NK3R) causes a surge-like secretion of LH when administered intraventricularly or locally into the retrochiasmatic area or POA during the follicular phase of the estrous cycle (66). Moreover, retrochiasmatic administration of an antagonist to NK3R partially suppressed the estrogen-induced LH surge in ewes (68). Thus, endogenous NKB signaling appears to be an important component of the neural mechanisms generating the LH surge in ewes, although the source of the NKB remains to be determined. In humans, NKB is also critical for normal reproductive function, because mutations of NKB or its receptor (termed tachykinin 3 and tachykinin 3 receptor, respectively) lead to hypothalamic hypogonadism (69, 70).
Another transmitter that may contribute to the role of KNDy and POA kisspeptin cells in the LH surge in sheep is glutamate. Levels of glutamate in the POA are increased during the E2-induced LH surge and at the time of puberty in female rats and monkeys (71–74) and iv infusion of NMDA increases GnRH mRNA levels in the POA of rats (75). Moreover, preliminary studies in cycling female sheep found an increase in the number of vGlut2 positive terminals apposing KNDy cells and GnRH neurons at the time of the GnRH/LH surge (44). Because changes in glutamate across the estrous cycle within the KNDy cells and their connections may act as a permissive signal, allowing the positive feedback actions of E2 to generate a GnRH surge (28, 31), it is possible that the decreased number of KNDy-KNDy connections (containing vGlut2 and kisspeptin), as well as the decrease in single-labeled vGlut2 inputs to KNDy cells (Figure 5), may contribute to a diminished ability of E2 to elicit a GnRH/LH surge acting via KNDy cells. However, the decrease in synaptic inputs onto POA kisspeptin cells we observed in prenatal T sheep was not reflected by a change in either kisspeptin/vGlut2 inputs (originating from KNDy cells) or single-labeled vGlut2 inputs; hence, other transmitter systems are also likely involved given the activation of both populations of kisspeptin neurons during the surge.
Finally, recent findings from a rodent model of PCOS, the prenatal androgenized (PNA) mouse, suggest that altered GABAergic inputs to GnRH neurons, arising from the ARC, may be an important contributor to the increased LH pulse frequency seen in this model and in PCOS women (76). However, in contrast to our results in prenatal T sheep, PNA mice show dramatically increased GABAergic inputs to GnRH neurons, with no change in glutamatergic inputs to GnRH cells (76). Although a subset of ARC kisspeptin cells in the mouse contain GABAergic markers (31), in the sheep, there appears to be little no colocalization of glutamic acid decarboxylase within ARC kisspeptin cells in the sheep (31); thus, the changes in synaptic inputs arising from KNDy cells reported here are unlikely to represent GABAergic inputs to GnRH cells. Nonetheless, the overall decrease in number of inputs to GnRH cells reported here for prenatal T sheep, although consistent with sex differences in sheep and other species (see below), is limited to axo-somatic inputs and may underestimate the complete array of inputs to GnRH neurons just as for KNDy cells. In this regard, it is notable that observations of increased inputs to GnRH neurons in PNA mice (76) was based on a transgenic approach that allowed a complete assessment of inputs along the entire GnRH dendrite. Comparable work in the future using the sheep as a model will depend on either viral vector injections that produce complete and cell-specific filling of GnRH dendrites and/or generation of transgenic sheep with those cell-specific markers.
KNDy inputs to GnRH cells. Effects of prenatal T compared with sexual differentiation
The effects of prenatal T on synaptic inputs to GnRH cells in this animal model are similar to those previously observed for sex differences in GnRH inputs for sheep and other species (77, 78). For example, in the rat (78) and sheep (77), GnRH cell bodies and dendrites (in the sheep) receive roughly twice as many inputs in females compared with males. In the sheep, prenatal T treatment masculinizes synaptic input to GnRH cell bodies and dendrites (77), and thus, the reduction in inputs observed in this study are consistent and in the same direction of the effects of prenatal T during normal sexual differentiation. However, a difference between the prenatal T model and normal sexual differentiation is that at least some sex differences observed in the KNDy cell population are not mimicked by the effects of prenatal T. Specifically, there are sex differences in each of the 3 KNDy peptides, kisspeptin, NKB, and dynorphin, in the ovine ARC, with females having greater numbers of cells than males (27). By contrast, although NKB and dynorphin cell numbers are decreased by prenatal T, the number of kisspeptin cells does not differ between control and prenatal T sheep (27), in either the ARC (KNDy cells) or POA. Thus, the critical periods for sexual differentiation of the 3 peptides coexpressed in the same cells appear to be distinct, with dynorphin and NKB being dependent on androgen exposure during days 30–90 of gestation, and kisspeptin being dependent on either prolonged or later steroid exposure. It remains to be determined whether the changes seen in synaptic inputs to GnRH cells observed in prenatal T animals are completely comparable with normal sex differences, or whether they may differ in magnitude or in the transmitter phenotype of the inputs. It is also notable that previous studies of sex difference in inputs to GnRH cells in sheep have exclusively focused on GnRH cells in the POA (77), whereas the current results reveal prenatal T-induced differences in inputs to GnRH cells located in both their rostral (POA) and caudal (MBH) portions of their distribution. Interestingly, GnRH cells in both the POA and MBH are activated during the LH surge, so that a decrease in inputs to both GnRH populations is consistent with a defect in the generation of the LH surge in prenatal T females.
Changes in KNDy somal size
Prenatal T-treated ewes exhibited a significantly increased KNDy somal size compared with control females. It is notable that similar changes in the hypothalami of postmenopausal women were first reported by Rance et al (42) in the 1990s, where a population of neurons in the infundibular (arcuate) nucleus homologous to KNDy cells, expressing Kiss-1, NKB, substance P, dynorphin and ERα mRNA, were shown to display an increase in somal size (39, 41, 42, 78). Interestingly, these changes appear to be limited to KNDy neurons (as they are in T-treated ewes) because nearby cells containing other peptides did not appear to be hypertrophied in postmenopausal women (42). There was considerable variation in the magnitude of this effect (29%–100% increase), depending on the marker, but the 41% increase in size of kisspeptin-positive cells observed in prenatal T-treated ewes is within this range and slightly larger than the 27% increase observed in soma size of kisspeptin neurons in postmenopausal women (78). In women, this hypertrophy is believed to be a secondary response to ovarian failure (42, 79), and is associated with declining levels of circulating E2 concentrations (80, 81). Paradoxically, levels of E2 in prenatal T ewes are high, not low, possibly leading to desensitization (82). Prenatal T-treated ewes do show progressive loss of cyclicity from first breeding season to second (9, 10, 15, 16), suggesting that prenatal T treatment leads to premature reproductive failure. It would thus be of interest to determine whether similar changes in cell size occur in women with PCOS or other animal models of this condition. In OVX mice, E2 administration induces an increase in the percentage of anteroventral periventricular nucleus Kiss1 firing neurons/neuronal activity and this is linked with an increase cell surface area, suggesting that the increased somal size observed in our KNDy neurons may serve as an indicator of increased neuronal activity (83). Eghlidi et al (84) found that in monkeys, only long term ovariectomy (∼4 y) significantly increased Kiss-1 and NKB expression, and that this was reversed by E2 administration, further linking these changes to menopausal ovarian failure. As seen in postmenopausal women and ovariectomized monkeys, a decline in E2 concentrations (or perhaps a decreased sensitivity to E2), causes an increase in Kiss-1 and NKB expression (39, 41, 42, 79). However, the number of kisspeptin-positive cells do not change in prenatal T-treated sheep and thus, an increase in protein production is unlikely to fully explain the increase in cell size. Analysis of the ARC POMC and AgRP somal size provides additional confirmation that the KNDy cell hypertrophy seen in prenatal T-treated ewes is a change occurring exclusively in the KNDy population. There was no effect of prenatal T treatment on the somal size of AgRP or POMC neurons even though previous work has shown that prenatal T-treated ewes have nearly twice the number of AgRP-immunoreactive neurons as controls (88). Thus, the changes in somal size reported here are specific to KNDy neurons and may be independent of alterations in intracellular peptide levels. The precise functional significance of the hypertrophy of KNDy cells in prenatal T-treated female sheep and postmenopausal women remains an interesting, albeit unanswered question.
Conclusions
In summary, we report evidence of morphological changes in the size of ARC kisspeptin (KNDy) cells, and in the density and number of synaptic inputs onto KNDy, POA kisspeptin, and GnRH cells, that may in part underlie some of the reproductive neuroendocrine defects seen in female sheep as a consequence of prenatal exposure to excess T. Given the close correspondence of the defects seen in this animal model to the multifaceted symptoms of PCOS in women, it is tempting to speculate that this reproductive disease involves long-term neuroplastic changes in brain circuitry similar to those underlying a number of neuropsychiatric and neurologic disorders (20). Recent observations in a rodent model of PCOS of alterations in GABAergic inputs to GnRH cells arising from the ARC is consistent with this hypothesis, although the findings in that model were of increased rather than decreased afferent input to GnRH cells (76). Finally, it is worth noting that our observations of prenatal T-induced morphological plasticity in KNDy cells, a defined neuronal subpopulation of the ARC, build on a previous evidence for synaptic plasticity in unidentified ARC neurons related to estrous cyclicity in the female rat (85, 86). Thus, the changes seen in response to excess prenatal T in this study may be a perturbation of naturally occurring plasticity in the ARC, important for developmental and/or adult neuroendocrine function. The possibility that KNDy cells are specific cellular targets for synaptic plasticity across the estrous cycle in rodents, as they appear to be in sheep (44), remains to be explored.
Acknowledgments
We thank Dr Stanley Hileman, Dr John Connors, and Dr Casey Nestor for their excellent assistance with ovariectomy surgeries.
This work was supported by the National Institutes of Health Grant P01 HD044232 (to V.P., M.N.L., and L.M.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- agouti-related peptide
- ARC
- arcuate nucleus
- Cy5
- Cyanide 5
- DAPI
- 4',6-Diamidino-2-Phenylindole
- E2
- 17β-estradiol
- ER
- estrogen receptor
- GABA
- gamma-aminobutyric acid
- Kiss
- kisspeptin
- KNDy
- kisspeptin/NKB/dynorphin
- MBH
- medial basal hypothalamus
- NK3R
- neurokinin 3 receptor
- NKB
- neurokinin B
- OVX
- ovariectomized
- PB
- phosphate buffer
- PCOS
- polycystic ovarian syndrome
- POA
- preoptic area
- POMC
- proopiomelanocortin
- PNA
- prenatal androgenized
- PR
- progesterone receptor
- Syn
- synaptophysin
- T
- testosterone
- vGlut2
- vesicular glutamate transporter-2.
References
- 1. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84(11):4006–4011. [DOI] [PubMed] [Google Scholar]
- 2. Diamanti-Kandarakis E, Dunaif A. New perspectives in polycystic ovary syndrome. Trends Endocrinol Metab. 1996;7(8):267–271. [DOI] [PubMed] [Google Scholar]
- 3. Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16:322–353. [DOI] [PubMed] [Google Scholar]
- 4. Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853–861. [DOI] [PubMed] [Google Scholar]
- 5. Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- 6. Sarma HN, Manikkam M, Herkimer C, et al. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. [DOI] [PubMed] [Google Scholar]
- 7. Sharma TP, Herkimer C, West C, et al. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66(4):924–933. [DOI] [PubMed] [Google Scholar]
- 8. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. [DOI] [PubMed] [Google Scholar]
- 9. Taylor AE, McCourt B, Martin KA, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–2256. [DOI] [PubMed] [Google Scholar]
- 10. Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57:1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panidis D, Farmakiotis D, Rousso D, Katsikis I, Kourtis A, Diamanti-Kandarakis E. Serum luteinizing hormone levels are markedly increased and significantly correlated with Δ4-androstenedione levels in lean women with polycystic ovary syndrome. Fertil Steril. 2005;84(2):538–540. [DOI] [PubMed] [Google Scholar]
- 12. Panidis D, Koliakos G, Kourtis A, Farmakiotis D, Mouslech T, Rousso D. Serum resistin levels in women with polycystic ovary syndrome. Fertil Steril. 2004;81(2):361–366. [DOI] [PubMed] [Google Scholar]
- 13. Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007;67(3):459–467. [DOI] [PubMed] [Google Scholar]
- 14. Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147(4):1997–2007. [DOI] [PubMed] [Google Scholar]
- 15. Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146(7):3185–3193. [DOI] [PubMed] [Google Scholar]
- 16. Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144(4):1426–1434. [DOI] [PubMed] [Google Scholar]
- 17. West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol. 2001;185(1–2):51–59. [DOI] [PubMed] [Google Scholar]
- 18. Clarke IJ, Scaramuzzi RJ, Short RV. Ovulation in prenatally androgenized ewes. J Endocrinol. 1977;73(2):385–389. [DOI] [PubMed] [Google Scholar]
- 19. Yen SS, Vela P, Rankin J. Inappropriate secretion of follicle-stimulating hormone and luteinizing hormone in polycystic ovarian disease. J Clin Endocrinol Metab. 1970;30(4):435–442. [DOI] [PubMed] [Google Scholar]
- 20. Ganguly K, Poo MM. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80(3):729–741. [DOI] [PubMed] [Google Scholar]
- 21. Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol Reprod. 2005;72(3):619–627. [DOI] [PubMed] [Google Scholar]
- 22. Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140(12):5797–5805. [DOI] [PubMed] [Google Scholar]
- 23. Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998;3(2):130–140. [DOI] [PubMed] [Google Scholar]
- 24. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci USA. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 26. Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475–1483. [DOI] [PubMed] [Google Scholar]
- 27. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 30. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18(7):534–541. [DOI] [PubMed] [Google Scholar]
- 31. Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett. 2006;401(3):225–230. [DOI] [PubMed] [Google Scholar]
- 36. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374. [DOI] [PubMed] [Google Scholar]
- 37. Richter TA, Robinson JE, Lozano JM, Evans NP. Progesterone can block the preovulatory gonadotropin-releasing hormone/luteinising hormone surge in the ewe by a direct inhibitory action on oestradiol-responsive cells within the hypothalamus. J Neuroendocrinol. 2005;17(3):161–169. [DOI] [PubMed] [Google Scholar]
- 38. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. [DOI] [PubMed] [Google Scholar]
- 39. Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. [DOI] [PubMed] [Google Scholar]
- 41. Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. [DOI] [PubMed] [Google Scholar]
- 42. Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., 3rd Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71(1):79–85. [DOI] [PubMed] [Google Scholar]
- 43. García-Segura LM, Chowen JA, Párducz A, Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol. 1994;44(3):279–307. [DOI] [PubMed] [Google Scholar]
- 44. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jansen HT, Hershey J, Mytinger A, Foster DL, Padmanabhan V. Developmental programming: reproductive endocrinopathies in the adult female sheep after prenatal testosterone treatment are reflected in altered ontogeny of GnRH afferents. Endocrinology. 2011;152(11):4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147(10):4843–4851. [DOI] [PubMed] [Google Scholar]
- 47. Jackson LM, Mytinger A, Roberts EK, et al. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology. 2013;154:1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shestopalov VI, Missey H, Bassnett S. Delivery of genes and fluorescent dyes into cells of the intact lens by particle bombardment. Exp Eye Res. 2002;74(5):639–649. [DOI] [PubMed] [Google Scholar]
- 49. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–315. [DOI] [PubMed] [Google Scholar]
- 50. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297(2):E538–E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silverman AJ, Wilson R, Kesner JS, Knobil E. Hypothalamic localization of multiunit electrical activity associated with pulsatile LH release in the rhesus monkey. Neuroendocrinology. 1986;44(2):168–171. [DOI] [PubMed] [Google Scholar]
- 52. Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–260. [DOI] [PubMed] [Google Scholar]
- 53. Goodman RL, Holaskova I, Nestor CC, et al. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology. 2011;152(9):3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- 55. Witty CF, Weems PW, Goodman RL, Coolen LM, Lehman MN. κ Opioid receptors are present within a majority of KNDy neurons in the ewe. Program of the 44th Annual Meeting of the Society for Neuroscience, Washington, DC, 2014 (Abstract 543.504/NN531). [Google Scholar]
- 56. Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133(2):896–903. [DOI] [PubMed] [Google Scholar]
- 57. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. [DOI] [PubMed] [Google Scholar]
- 58. Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. Neural systems mediating seasonal breeding in the ewe. J Neuroendocrinol. 2010;22(7):674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li XF, Kinsey-Jones JS, Cheng Y, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4(12):e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus interactions with GnRH neuronal system. J Chem Neuroanat. 2008;36(3–4):131–137. [DOI] [PubMed] [Google Scholar]
- 62. Dungan HM, Gottsch ML, Zeng H, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27(44):12088–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol. 2006;18(10):806–809. [DOI] [PubMed] [Google Scholar]
- 65. Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026(2):307–312. [DOI] [PubMed] [Google Scholar]
- 66. Billings HJ, Connors JM, Altman SN, et al. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guran T, Tolhurst G, Bereket A, et al. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab. 2009;94:3633–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22(1):111–151. [DOI] [PubMed] [Google Scholar]
- 72. Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. An increase in glutamate release follows a decrease in γ aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkeys. J Neuroendocrinol. 1999;11(4):275–282. [DOI] [PubMed] [Google Scholar]
- 73. Goroll D, Arias P, Wuttke W. Preoptic release of amino acid neurotransmitters evaluated in peripubertal and young adult female rats by push-pull perfusion. Neuroendocrinology. 1993;58(1):11–15. [DOI] [PubMed] [Google Scholar]
- 74. Jarry H, Hirsch B, Leonhardt S, Wuttke W. Amino acid neurotransmitter release in the preoptic area of rats during the positive feedback actions of estradiol on LH release. Neuroendocrinology. 1992;56(2):133–140. [DOI] [PubMed] [Google Scholar]
- 75. Petersen SL, McCrone S, Keller M, Gardner E. Rapid increase in LHRH mRNA levels following NMDA. Endocrinology. 1991;129(3):1679–1681. [DOI] [PubMed] [Google Scholar]
- 76. Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci USA. 2015;112(2):596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim SJ, Foster DL, Wood RI. Prenatal testosterone masculinizes synaptic input to gonadotropin-releasing hormone neurons in sheep. Biol Reprod. 1999;61(3):599–605. [DOI] [PubMed] [Google Scholar]
- 78. Chen WP, Witkin JW, Silverman AJ. Sexual dimorphism in the synaptic input to gonadotropin releasing hormone neurons. Endocrinology. 1990;126(2):695–702. [DOI] [PubMed] [Google Scholar]
- 79. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92(7):2744–2750. [DOI] [PubMed] [Google Scholar]
- 80. Wise PM, Krajnak KM, Kashon ML. Menopause: the aging of multiple pacemakers. Science. 1996;273(5271):67–70. [DOI] [PubMed] [Google Scholar]
- 81. vomSaal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill J, eds. The Physiology of Reproduction. New York, NY: Raven Press; 1994:861–1010. [Google Scholar]
- 82. Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78(4):636–647. [DOI] [PubMed] [Google Scholar]
- 83. Frazão R, Cravo RM, Donato J, Jr, et al. Shift in Kiss1 cell activity requires estrogen receptor α. J Neurosci. 2013;33(7):2807–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151(8):3783–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Naftolin F, Mor G, Horvath TL, et al. Synaptic remodeling in the arcuate nucleus during the estrous cycle is induced by estrogen and precedes the preovulatory gonadotropin surge. Endocrinology. 1996;137(12):5576–5580. [DOI] [PubMed] [Google Scholar]
- 86. Olmos G, Naftolin F, Perez J, Tranque PA, Garcia-Segura LM. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32(3):663–667. [DOI] [PubMed] [Google Scholar]
- 87. Cheng G, Coolen LM, Jackson LM, Padmanabhan V, Lehman MN. Anti-androgen treatment partially restores the neuropeptide imbalance seen in the arcuate nucleus of sheep treated prenatally with testosterone. Society for Neuroscience (SfN) Abstract. 2008;474.13/PP1:Washington, DC. [Google Scholar]
- 88. Sheppard KM, Padmanabhan V, Coolen LM, Lehman MN. Prenatal programming by testosterone of hypothalamic metabolic control neurones in the ewe. J Neuroendocrinol. 2011;23(5):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]