Abstract
Exposure to di-(2-ethylhexyl) phthalate (DEHP) has been linked to male reproductive abnormalities. Here, we assessed transgenerational actions of DEHP on several behaviors and stress responses. We used 2 doses of DEHP (150- and 200-mg/kg body weight) and a treatment regimen previously shown to produce transgenerational effects on male reproduction. Mice, 3 generations removed from DEHP exposure (F3), were tested for social behavior and anxiety on the elevated plus maze. We collected blood and pituitaries from undisturbed and restrained mice. Body weights, anogenital distances, and reproductive organ weights were collected at killing. In social interaction tests juvenile males from the DEHP lineage (200 mg/kg) displayed more digging and less self-grooming than did controls. Interestingly, 150-mg/kg lineage males, killed in early puberty, had smaller seminal vesicle weights than their controls. However, the 200-mg/kg males (killed on average 10 d later) did not show this effect. Females from a DEHP lineage had lower corticosterone concentrations than controls after restraint stress. We also found sex- and DEHP-specific mRNA expression changes in the pituitary in 2 of the 6 stress-related genes we measured. In particular, Gnas mRNA was elevated by the combination of DEHP lineage and stress. Thus, transgenerational effects of DEHP are noted in male behavior, and in females, DEHP had transgenerational effects on levels of corticosterone. Both of these results may be related to transgenerational modifications in the expression of several pituitary hormones involved in the hypothalamic-pituitary-adrenal axis.
Endocrine disrupting chemicals are abundant in the environment. One of the most prevalent of these compounds is di-(2-ethylhexyl) phthalate (DEHP) with yearly production in the United States estimated at 1–4 million tons (1, 2). Recently, levels of DEHP and its metabolites (primarily Mono-(2-ethylhexyl) phthalate (MEHP)) have been higher than previously estimated in certain individuals (3–5), and careful examination of conversion from animal to human doses indicates relevance with doses similar to the one used here (6). DEHP is found in flexible plastic products, specifically polyvinyl chloride, food packaging, medical equipment, pill coatings, and synthetic flooring (7). Exposure in humans is variable depending on age and environment (8) but occurs on a daily basis; the vast majority of people tested have measurable levels of DEHP and/or MEHP in their urine. Furthermore, DEHP crosses the placenta (9), is absorbed through the skin (10), and metabolites are found in breast milk (11). Daily human intake levels have been estimated between 6 and 21 mg/kg per day, with children at the higher end of this range.
Pregnancy levels of MEHP are correlated, in male infants, with decreased levels of testosterone, estradiol, progesterone, inhibin B, and insulin-like factor 3. High DEHP exposure in utero has also been linked with decreased play behavior in boys (12), and concentrations of DEHP metabolites in urine are higher in autistic as compared with control individuals (13). Gestational DEHP exposure affects anxiety and depression-like behavior in animals (14, 15), and DEHP metabolites inhibit the RNA expression and activity of the corticosterone-inactivating enzyme 11-β-hydroxysteroid dehydrogenase 2 (16, 17). In 40-day-old rats, oral administration of 750-mg/kg DEHP for 4 days increases serum ACTH and corticosterone (18). These data indicate that DEHP acts on several levels of the hypothalamic-pituitary-adrenal (HPA) axis. Transgenerational effects of DEPH, including disturbed germ cell association, lower sperm counts, and decreased motility, have been noted in mice 3 generations removed from initial exposure (19). Here, using similar doses and the same treatment regimes that produced transgenerational outcomes, we evaluated behavior and stress responses in F3 mice from DEHP lineages along with their controls.
Materials and Methods
Mice
Animals were produced as described previously (19). The F0 ancestors of these F3 mice (C57BL/6J) were treated with DEHP via oral gavage at a dose of 150- or 200-mg/kg body weight/day during gestational days 7–14. Each animal was weighed daily and the weights used to calculate the amount of DEHP to reach the desired dose in 200 μL of corn oil. Controls received 200 μL of corn oil. Offspring (F1) were bred, and the F2 offspring bred again to create a third generation (F3) removed from initial DEHP exposure, and these are the mice we studied. Siblings were not paired. All F3, 150- and 200-mg/kg lineage mice used for organ weights and anogenital measurements were bred and maintained at Washington State University. A second cohort of F2 200-mg/kg mice and controls was bred in Washington and then shipped to Virginia and used to create F3 offspring for testing at the University of Virginia. Mice were maintained at the University of Virginia School of Medicine. The University of Virginia and Washington State University Animal Care and Use Committees approved all procedures used and described here. All animals were maintained on a 12-hour light, 12-hour dark cycle and provided with food (Harlan Teklad diet 2918) and water ad libitum.
Social interaction
Males in the 200-mg/kg DEHP lineage were used to examine juvenile social interactions. Methods were adapted from previous research (20). Mice were tested during the dark portion of the light cycle between 35 and 42 days of age with an unfamiliar partner matched for age and lineage (control males, n = 6 pairs; 200-mg/kg DEHP-lineage males, n = 12 pairs). Members of each pair were individually habituated to an empty mouse box for 10 minutes, then paired in a second empty box, and observed for 10 minutes by an observer blind to their lineage. A scan sampling method was used; every 15 seconds, the pairs were observed and behaviors recorded on a check sheet. Each pair was scored as a unit, with total behaviors from both individuals included. Social behaviors scored were: side-by-side sitting, approach, following, crawling, sniffing the other mouse, and social grooming. Nonsocial behaviors noted were exploring, self-grooming, digging, and sitting alone.
Elevated plus maze
This protocol was adapted from methods described previously (21). Mice were tested during the light portion of the light cycle (150 mg/kg between 25 and 32 d of age; 200 mg/kg between 35 and 42 d of age) on the elevated plus maze (wall height, 6 inches; arm length, 11.75 inches; and arm width, 2 inches; Columbus Instruments). An observer, blind to the group, scored each mouse for 5 minutes (numbers per group ranged from 8 to 21, see Supplemental Table 1 for exact numbers). Time spent in the open and closed arms and the center was recorded.
Restraint stress and corticosterone
Mice from both sexes in the 150-mg/kg dose DEHP lineage (and their controls) were used to assess corticosterone. Mice were housed in pairs by sex and treatment for at least 10 days before killing. One mouse in each pair was randomly assigned to the stressed or baseline condition. The mice assigned to the stressed condition were restrained in a 50-mL conical tube (with a nose hole at the tip) for 15 minutes immediately before killing (numbers per group ranged from 6 to 10). All mice were euthanized using CO2 followed by rapid cervical dislocation. Trunk blood was collected, placed on ice, centrifuged, serum collected, and frozen. Whole pituitaries were removed and immediately frozen on dry ice. The University of Virginia Ligand Core analyzed serum corticosterone concentration using RIA; samples with sufficient volume were run in duplicate, when necessary, samples were run as singlets (35 samples). Samples with percentage coefficients of variation greater than 20% (2 samples) or values outside the reportable range of 9.0–836.9 ng/mL (4 samples) were eliminated; of samples analyzed the mean coefficient of variation was 4.8 ± 0.55%.
Anogenital distances and organ weights
At killing, mice ranged between 29–30 days of age (150-mg/kg lineage) and 38–43 days of age (200-mg/kg lineage). We collected body weights, anogenital distances (AGDs), uterine, and seminal vesicle weights (numbers per group ranged from 7 to 20) (see Table 1).
Table 1.
Body Weights, Anogenital Distances, Anogenital Indices, and Reproductive Organ Weights Collected From Male and Female F3 Mice From Either the 150-mg/kg or the 200-mg/kg DEHP and Their Controls
| 150-mg/kg Control-Lineage Males (n = 13) | 150-mg/kg DEHP-Lineage Males (n = 15) | 150-mg/kg Control-Lineage Females (n = 19) | 150-mg/kg DEHP-Lineage Females (n = 20) | 200-mg/kg Control-Lineage Males (n = 11) | 200-mg/kg DEHP-Lineage Males (n = 7) | |
|---|---|---|---|---|---|---|
| Body weight (g) | 15.51 ± 0.28 | 15.15 ± 0.48 | 13.32 ± 0.18 | 12.63 ± 0.39 | 24.63 ± 4.32 | 18.50 ± 0.78a |
| Anogenital distance (mm) | 11.28 ± 0.36 | 12.14 ± 0.33 | 5.74 ± 0.15 | 5.84 ± 0.32 | 13.67 ± 1.02 | 14.34 ± 0.74 |
| Anogenital index (mm/g) | 0.73 ± 0.03 | 0.81 ± 0.02a | 0.43 ± 0.01 | 0.45 ± 0.02 | 0.74 ± 0.02 | 0.77 ± 0.03 |
| Seminal vesicle weight (mg) | 7.15 ± 1.70 | 3.09 ± 0.34a | X | X | 5.14 ± 0.56 | 4.90 ± 0.68 |
| Uterine weight (mg) | X | X | 22.04 ± 1.10 | 20.22 ± 1.25 | X | X |
Data were collected from mice ages 25–32 (150-mg lineages) and 35–42 (200-mg lineages).
P < .05, significantly different from the same sex control group.
Quantitative real-time PCR
We assessed mRNA concentrations in pituitaries from mice in the DEHP (150 mg/kg) and control lineages for the following genes: CRH receptor (Crhr1), guanyl nucleotide-binding protein, α-stimulating (Gnas), proopiomelanocortin-α, Lhb, Fshb, and glycoprotein hormones, α-subunit. Real-time PCR primers (Supplemental Table 2) were designed using Primer Express software version 2.0 (Applied Biosystems). cDNA was synthesized from 80 ng of RNA using SS Vilo cDNA Synthesis Master Mix (Life Technologies) and used as template for real-time PCR assays with an ABI 7500 Fast Real-Time PCR System (Life Technologies). The real-time RT-PCR was conducted on animal RNA preps, 3 per group, with each sample done in duplicate or triplicate. Threshold values for the gene of interest and a reference gene, ribosomal S2 were determined using 7500 Software version 2.0.1 (Life Technologies). The expression level of the gene of interest was evaluated using the 2−(ΔΔCt) method (22).
Statistical analyses
Reproductive organ weights, mRNA concentrations, and AGDs were analyzed with one- or two-way ANOVA. Behavioral data were analyzed with either one- or two-way ANOVAs as appropriate following an Arcsine transformation of proportional social behavior data. Corticosterone data were analyzed with three-way ANOVA for all groups and two-way ANOVA for separate analysis of stressed and baseline corticosterone levels. We used Fisher's Least Significant Difference post hoc test for pair test analyses.
Results
Social interaction tests
Males from the 200-mg/kg DEHP lineage spent more time digging and less time self-grooming (F1,18 = 9.67, 6.41, respectively; P < .05) than control males. None of the other behavioral measures were significantly different (Figure 1).
Figure 1.
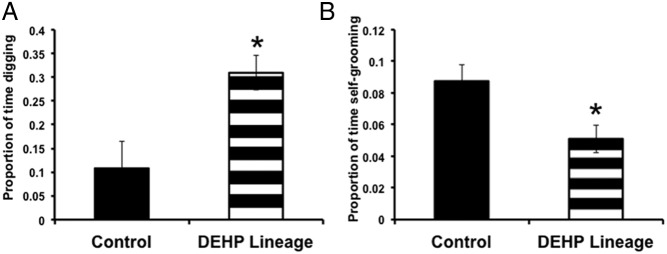
Mean (±SEM) proportion of time spent (A) digging and (B) self-grooming. Males from the DEHP (200-mg lineage) displayed more digging and less self-grooming than their controls; *, P < .05 (control males n = 6 pairs, 200-mg/kg DEHP-lineage males n = 12 pairs).
Elevated plus maze (EPM)
There were no significant effects of treatment or sex on behavior in the EPM (Supplemental Table 1).
Restraint stress and corticosterone
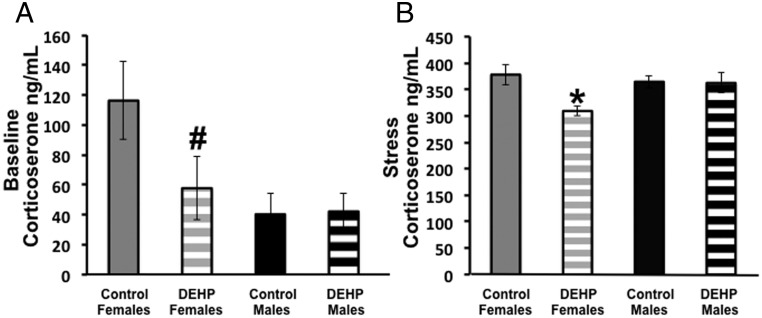
There was an overall effect of condition at the time of blood collection (F1,68 = 534.46; P < .00001) and of lineage (F1,68 = 6.77; P < .01) on corticosterone. Significant interactions between condition and sex (F1,68 = 6.97; P < .01) and sex and lineage (F1,68 = 6.96; P < .05) were noted. Because the effect of restraint stress (as predicted) was so large, to focus on the effects of sex and lineage we analyzed the stressed and baseline groups independently. Under baseline condition, we observed a significant effect of sex (F1,35 = 5.81; P < .05) where females had higher baseline corticosterone than males overall, and there was trend for an interaction between sex and lineage (F1,35 = 2.96; P < .10). When analyzing data from the stressed mice, we observed a significant effect of treatment (F1,33 = 5.05; P < .05) and an interaction between sex and treatment lineage (F1,33 = 4.44; P < .05). Post hoc analyses showed that F3 females from the DEHP lineage had lower serum corticosterone levels as compared with F3 control females and F3 control and DEHP-lineage males (Figure 2).
Figure 2.
Mean (±SEM) corticosterone levels (ng/mL) measured in plasma from 150-mg/kg DEHP-female and control F3 mice. (A) Under baseline conditions DEHP-lineage mice tended to have lower corticosterone levels than control females (#P = .10). (B) After 15 minutes of restraint stress, DEHP-lineage females had lower corticosterone than control females; *, P < .05 (control-lineage females stressed, n = 10; control-lineage females baseline, n = 9; DEHP-lineage females stressed, n = 8; DEHP-lineage females baseline, n = 10; control-lineage males stressed, n = 6; control-lineage males baseline, n = 6; DEHP-lineage males stressed, n = 8; DEHP-lineage males baseline, n = 7).
Anogenital index and organ weights
Because sex differences in these measures are expected and are large, we analyzed each sex separately. In males, anogenital indices (AGD/body weight) were significantly longer in 37- to 43-day-old males from the 150-mg/kg DEHP lineage, as compared with their control males (F1,30 = 7.44; P < .02). DEHP-lineage 200-mg/kg males had significantly lower body weights than controls (F1,28 = 6.27; P < .02). Seminal vesicle weights in F3 control mice were significantly heavier than in the DEHP (150-mg/kg dose) group (F1,28 = 6.25; P < .02) (Table 1), but no differences were noted in the 200-mg/kg groups. Females, regardless of lineage, had similar body weights, uterine weights, AGDs, and anogenital indices (Table 1).
Quantitative real-time PCR
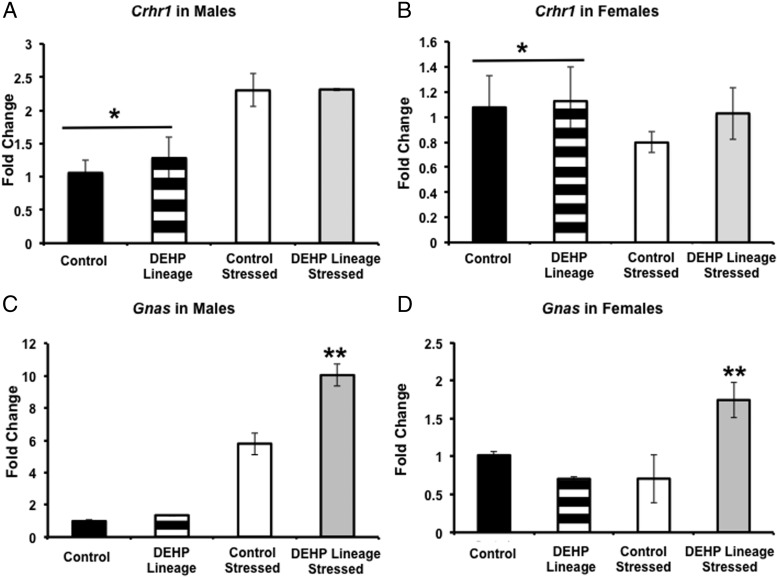
To evaluate gene expression in the pituitary, we analyzed baseline and stressed conditions separately. Males and females were also analyzed separately, because only females exhibited a significant change in serum corticosterone. In the baseline condition, we found a sex difference where by males had lower Crhr1 expression than females (F1,11 = 5.39; P < .05). There were no other significant differences in Crhr1 by sex or stress. In the stressed condition, there was a sex difference where by males had higher Gnas expression than females (F1,11 = 86.34; P < .0001). Separating the analysis by sex, we found an interaction between stress and DEHP lineage in Gnas males (F1,11 = 9.00; P < .05) and females (F1,11 = 9.01; P < .05). Post hoc tests revealed that in both sexes the stressed animals from DEHP lineage had higher Gnas expression than nonstressed DEHP animals and both control groups (Figure 3). To follow up on the differences in seminal vesicle weights in control and F3 DEHP-lineage males, we measured the expression levels of Fshb, Lhb, and the common α-subunit (glycoprotein hormones, α-subunit). Sex differences in Fshb and Lhb were noted (F1,11 = 9.62, 17.49, respectively; P < .02 or less), and in both cases, males had more gene expression than females. However, we did not detect any effects of lineage or condition.
Figure 3.
Mean (±SEM) mRNA expression levels in pituitaries from 150-mg/kg DEHP-lineage and control mice. Males that were not stressed had lower Crhr1 (A) than females that were not stressed (B) (*, P < .05). C, In males, stressed mice from the DEHP-lineage had higher Gnas mRNA than all other groups (**, P < .05). D, Likewise, restraint stressed females from the DEHP-lineage had higher Gnas expression that all other groups (**, P < .05). Overall, stressed males had higher Gnas than stressed females (#, P < .05) (n = 3 per group).
Discussion
These data indicate, for the first time, that DEHP has transgenerational actions in both sexes on the neuroendocrine axes and behaviors. Intriguingly, the data suggest that the transgenerational target of DEHP is the HPA axis. In both sexes, expression in the pituitary of Gnas message was elevated by the combination of restraint stress and DEHP lineage. Mice from the DEHP lineage had normal basal levels; in males, a 10-fold increase was found after restraint stress, and in females, we noted a smaller but still significant increase of 2-fold. In F3 females from the DEHP lineage, restraint stress produced lower levels of corticosterone as compared with control mice. It is very possible that corticosterone was also modified by lineage in males, which we may have missed because only 2 collection time points were assessed.
In addition to these results, we also observed lighter seminal vesicles in males from the 150-mg/kg DEHP lineage, which suggests they have lower testosterone levels as compared with peripubertal control-lineage males. Because we did not find this same difference in males from the higher dose group, and these animals were killed at an older age, we speculate that the DEHP-lineage males experienced delayed puberty. Interestingly, anogenital indices in F3 males from the 150-mg/kg DEHP lineage were longer than in the controls, although anogenital indices from the 200-mg/kg DEHP lineage were not changed.
Our behavioral results were limited to males and restricted to just 2 specific nonsocial behaviors, digging and self-grooming. Males in the 200-mg/kg DEHP lineage displayed less self-grooming but more digging than controls. Increased digging and self-grooming (both nonsocial behaviors) have been used as surrogate Autism-like phenotypes in mice (23). Until recently digging was considered an anxiety behavior (24). However, we did not find any differences in EPM behavior, the “gold standard” for anxiety (25). Previous research on EPM behavior in F1 DEHP-exposed animals has reported increased time in the closed arms of the EPM in pubertal male mice and rats (14, 15). In rats, this effect is ameliorated by testosterone administration (14). DEHP induces anxiety-like behavior in adolescent female rats as well, and this effect is counteracted by exercise (26).
In humans, a few studies have linked social behaviors (27) and neurodevelopmental disorders (28) to DEHP levels in blood and urine. DEHP metabolites in utero are also associated with Autism diagnosis (13). Our hypothesis, based on data from boys who show reduced masculine play corresponding to DEHP metabolite levels in urine of pregnant mothers (12), was that male mice would spend less time than controls interacting with a novel partner. It is possible that if we had tested F1 mice we might have seen this effect. However, transgenerational effects of endocrine disruptors, in some cases, are not the same as effects noted in F1 offspring (29). Although not directly related to human neurobehavioral disorders, our data have shown that HPA axis activity, anxiety and stress responses are changed by DEHP. In vitro, MEHP regulates glucocorticoid activity by decreasing RNA levels and enzyme activity of 11-β-hydroxysteroid dehydrogenase 2, the enzyme that inhibits corticosterone inhibition (16) and presumably leads to the increased corticosterone after DEHP exposure (18). Furthermore, corticosterone and Gnas results may also cause a complex set of changes that includes regulation of other steroid hormones yet to be measured. Testosterone levels are altered by DEHP in a nonmonotonic dose-response curve in pregnant mice and their offspring (30), and prenatal/neonatal sex hormones can influence the sensitivity of the HPA axis in adulthood (31). Furthermore, adrenal aldosterone production in adult rats decreases after in utero exposure to DEHP (32). The mechanisms whereby DEHP has transgenerational effects on the HPA axis needs to be investigated.
Interestingly, we found expression changes in stress-related genes in the pituitaries of both males and females. Due to previous studies showing changes in corticosterone, we hypothesized that gene expression changes in the pituitary would reflect upstream differences in stress hormone pathways. A sex difference was noted in Crhr1 where females had higher mRNA expression than males. Although there were no significant differences between DEHP and controls when females were analyzed separately, it is possible that elevated Crhr1 is additive with Gnas and is related to sex differences specific role in corticosterone. Gnas transcribes a G protein α-subunit that couples with hormone receptors, including ACTH, through protein kinase A (33, 34). Previous research has shown that mouse adrenal cells treated with ACTH accumulate Gnas protein (35). Furthermore, Gnas is maternally imprinted in most tissues, with limited paternal imprinting. Mutation on the maternal allele leads to early lethality, obesity, and hormone resistance; paternal allele mutation precedes obesity and insulin resistance (34). We hypothesize that DEHP administration during gestational days 7–14 causes a heritable epigenetic change in Gnas. This would be an exciting discovery as the specific genes and mechanisms underlying transgenerational actions are just beginning to be examined (36).
AGD is 1 of the primary targets of DEHP, and, in this regard, the doses we used should be “antiandrogenic” and produce smaller AGDs in exposed male rodents (30, 37, 38) and humans (39, 40). Previous work in mice with similar doses shows a decrease in AGD in neonatal F1 males that is not passed on to F2 and F3 generations (19). Our results, larger anogenital distance in the F3 150-mg/kg DEHP-lineage males at puberty are unexpected, but was not observed in the F3 200-mg/kg DEHP-lineage males. Moreover, F1 and F3 effects of endocrine disrupting compounds need not be the same and in fact are often reversed, and the age could also be a factor (29).
Another androgen target tissue, the seminal vesicles, were lighter in DEHP-lineage males a finding more compatible with the presumed antiandrogenic actions of DEHP in F1 studies. These 2 measures (AGD and seminal vesicles) are indicative of androgen exposures during different critical periods, in utero vs during puberty. In fact, we only observed lineage differences in males collected during puberty, which might be indicative of differences in the timing of pubertal onset. Interestingly, none of the pituitary genes in the HPG axis that we measured were effected by lineage.
Mechanisms for the differences reported here, in the absence of direct DEHP exposure include epigenetic changes such as DNA methylation, histone modifications or other potentially inherited regulators of gene transcription. Exposure to DEHP in utero produces increased global DNA methylation and increased expression of DNA methyltransferases in fetal testes (41). Our F1 mice were treated with DEHP during a sensitive period in embryonic development, when DNA demethylation and remethylation are taking place (42). Earlier work identifies this period of exposure to DEHP as one that results in lowered sperm count, and disrupted germ cell association in F3 mice (19). This study takes this model further by providing the first transgenerational evidence for changes in behavior and stress responses that we speculate are caused by epigenetic effects on genes such as Gnas, which are part of the HPA axis.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 ES022759 (to E.F.R.) and R01 ES019836 (to K.H.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGD
- anogenital distance
- Crhr1
- CRH receptor
- DEHP
- di-(2-ethylhexyl) phthalate
- EPM
- Elevated plus maze
- Gnas
- guanyl nucleotide-binding protein, α-stimulating
- HPA
- hypothalamic-pituitary-adrenal
- MEHP
- Mono-(2-ethylhexyl) phthalate.
References
- 1. Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;31:179–194. [DOI] [PubMed] [Google Scholar]
- 2. McKee RH, Butala JH, David RM, Gans G. NTP center for the evaluation of risks to human reproduction reports on phthalates: addressing the data gaps. Reprod Toxicol. 2004;18:1–22. [DOI] [PubMed] [Google Scholar]
- 3. Mallow EB, Fox MA. Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks. J Perinatol. 2014;34:892–897. [DOI] [PubMed] [Google Scholar]
- 4. Loff S, Kabs F, Witt K, et al. Polyvinylchloride infusion lines expose infants to large amounts of toxic plasticizers. J Pediatr Surg. 2000;35:1775–1781. [DOI] [PubMed] [Google Scholar]
- 5. Gaudin R, Marsan P, Ndaw S, Robert A, Ducos P. Biological monitoring of exposure to di(2-ethylhexyl) phthalate in six French factories: a field study. Int Arch Occup Environ Health. 2011;84:523–531. [DOI] [PubMed] [Google Scholar]
- 6. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. [DOI] [PubMed] [Google Scholar]
- 7. Latini G, Verrotti A, De Felice C. Di-2-ethylhexyl phthalate and endocrine disruption: a review. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4:37–40. [DOI] [PubMed] [Google Scholar]
- 8. Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Altern Med Rev. 2010;15:101–109. [PubMed] [Google Scholar]
- 9. Singh AR, Lawrence WH, Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci. 1975;64:1347–1350. [DOI] [PubMed] [Google Scholar]
- 10. Hopf NB, Berthet A, Vernez D, Langard E, Spring P, Gaudin R. Skin permeation and metabolism of di(2-ethylhexyl) phthalate (DEHP). Toxicol Lett. 2014;224:47–53. [DOI] [PubMed] [Google Scholar]
- 11. Kim S, Lee J, Park J, et al. Concentrations of phthalate metabolites in breast milk in Korea: estimating exposure to phthalates and potential risks among breast-fed infants. Sci Total Environ. 2015;508:13–19. [DOI] [PubMed] [Google Scholar]
- 12. Swan SH, Liu F, Hines M, et al. Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl. 2010;33:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Testa C, Nuti F, Hayek J, et al. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012;4:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carbone S, Ponzo OJ, Gobetto N, Samaniego YA, et al. R Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm Behav. 2013;63:692–699. [DOI] [PubMed] [Google Scholar]
- 15. Xu X, Yang Y, Wang R, Wang Y, Ruan Q, Lu Y. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere. 2015;124:22–31. [DOI] [PubMed] [Google Scholar]
- 16. Hong D, Li XW, Lian QQ, et al. Mono-(2-ethylhexyl) phthalate (MEHP) regulates glucocorticoid metabolism through 11β-hydroxysteroid dehydrogenase 2 in murine gonadotrope cells. Biochem Biophys Res Commun. 2009;389:305–309. [DOI] [PubMed] [Google Scholar]
- 17. Zhao B, Chu Y, Huang Y, Hardy DO, Lin S, Ge RS. Structure-dependent inhibition of human and rat 11β-hydroxysteroid dehydrogenase 2 activities by phthalates. Chem Biol Interact. 2010;183:79–84. [DOI] [PubMed] [Google Scholar]
- 18. Supornsilchai V, Söder O, Svechnikov K. Stimulation of the pituitary-adrenal axis and of adrenocortical steroidogenesis ex vivo by administration of di-2-ethylhexyl phthalate to prepubertal male rats. J Endocrinol. 2007;192:33–39. [DOI] [PubMed] [Google Scholar]
- 19. Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cox KH, Rissman EF. Sex differences in juvenile mouse social behavior are influenced by sex chromosomes and social context. Genes Brain Behav. 2011;10:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor α. Genes Brain Behav. 2004;3:20–26. [DOI] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 23. Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci. 2012;14:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masuda Y, Ishigooka S, Matsuda Y. Digging behavior of ddY mouse. Exp Anim. 2000;49:235–237. [DOI] [PubMed] [Google Scholar]
- 25. Neumann ID, Wegener G, Homberg JR, et al. Animal models of depression and anxiety: what do they tell us about human condition? Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1357–1375. [DOI] [PubMed] [Google Scholar]
- 26. Wang DC, Chen TJ, Lin ML, Jhong YC, Chen SC. Exercise prevents the increased anxiety-like behavior in lactational di-(2-ethylhexyl) phthalate-exposed female rats in late adolescence by improving the regulation of hypothalamus-pituitary-adrenal axis. Horm Behav. 2014;66:674–684. [DOI] [PubMed] [Google Scholar]
- 27. Lien YJ, Ku HY, Su PH, et al. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2015;123:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tellez-Rojo MM, Cantoral A, Cantonwine DE, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ. 2013;461–462:386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolstenholme JT, Edwards M, Shetty SR, et al. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, Taylor JA. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol. 2012;34:614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have 'organizational' effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105:295–307. [DOI] [PubMed] [Google Scholar]
- 32. Martinez-Arguelles DB, Guichard T, Culty M, Zirkin BR, Papadopoulos V. In utero exposure to the antiandrogen di-(2-ethylhexyl) phthalate decreases adrenal aldosterone production in the adult rat. Biol Reprod. 2011;85:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kozasa T, Itoh H, Tsukamoto T, Kaziro Y. Isolation and characterization of the human Gs α gene. Proc Natl Acad Sci USA. 1988;85:2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinstein LS, Xie T, Zhang QH, Chen M. Studies of the regulation and function of the Gs α gene Gnas using gene targeting technology. Pharmacol Ther. 2007;115:271–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schimmer BP, Cordova M. Corticotropin (ACTH) regulates alternative RNA splicing in y1 mouse adrenocortical tumor cells. Mol Cell Endocrinol. 2015;408:5–11. [DOI] [PubMed] [Google Scholar]
- 36. Rissman EF, Adli M. Minireview: transgenerational epigenetic inheritance: focus on endocrine disrupting compounds. Endocrinology. 2014;155:2770–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. [DOI] [PubMed] [Google Scholar]
- 38. Moore RW, Rudy TA, Lin TM, Ko K, Peterson RE. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer di(2-ethylhexyl) phthalate. Environ Health Perspect. 2001;109:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35:236–244. [DOI] [PubMed] [Google Scholar]
- 41. Wu S, Zhu J, Li Y, et al. Dynamic epigenetic changes involved in testicular toxicity induced by di-2-(ethylhexyl) phthalate in mice. Basic Clin Pharmacol Toxicol. 2010;106:118–123. [DOI] [PubMed] [Google Scholar]
- 42. Durcova-Hills G, Capel B. Development of germ cells in the mouse. Curr Top Dev Biol. 2008;83:185–212. [DOI] [PubMed] [Google Scholar]