Abstract
Roux-en-Y gastric bypass (RYGB) typically leads to substantial, long-term weight loss (WL) and diabetes remission, although there is a wide variation in response to RYGB among individual patients. Defining the pathways through which RYGB works should aid in the development of less invasive anti-obesity treatments, whereas identifying weight-regulatory pathways unengaged by RYGB could facilitate the development of therapies that complement the beneficial effects of surgery. Activation of serotonin 2C receptors (5-HT2CR) by serotonergic drugs causes WL in humans and animal models. 5-HT2CR are located on neurons that activate the melanocortin-4 receptors, which are essential for WL after RYGB. We therefore sought to determine whether 5-HT2CR signaling is also essential for metabolic effects of RYGB or whether it is a potentially complementary pathway, the activation of which could extend the benefits of RYGB. Diet-induced obese male mice deficient for the 5-HT2CR and their wild-type littermates underwent RYGB or sham operation. Both groups lost similar amounts of weight after RYGB, demonstrating that the improved metabolic phenotype after RYGB is 5-HT2CR independent. Consistent with this hypothesis, wild-type RYGB-treated mice lost additional weight after the administration of the serotonergic drugs fenfluramine and meta-chlorophenylpiperazine but not the nonserotonergic agent topiramate. The fact that RYGB does not depend on 5-HT2CR signaling suggests that there are important WL mechanisms not fully engaged by surgery that could potentially be harnessed for medical treatment. These results suggest a rational basis for designing medical-surgical combination therapies to optimize clinical outcomes by exploiting complementary physiological mechanisms of action.
The high prevalence of obesity and the clinical impact of its related comorbidities have created an urgent need for more effective weight-loss therapies. Gastrointestinal weight loss procedures, such as Roux-en-Y gastric bypass (RYGB), remain the most effective treatments for patients with severe obesity, producing substantial long-term weight loss and frequent remission of diabetes (1–3). The clinical improvements seen after RYGB are brought about by changes in metabolic physiology, though the precise cellular and molecular mechanisms of action, have only begun to be elucidated (4). Dissecting the energy regulatory pathways underlying surgical weight loss, and differentiating those pathways engaged by RYGB from those that are not, could reveal new and effective anti-obesity targets, thus facilitating the development of less invasive therapies that mimic the therapeutic benefits of surgery. Alternatively, identifying weight and metabolism regulatory pathways that are not used by RYGB may reveal therapeutic targets that complement surgery and thus enhance clinical outcomes after surgical intervention. The weight-loss response to RYGB in human patients is highly variable. It displays a normal distribution and is largely biologically driven, demonstrated by the heritability and genetic predicators of weight-loss response (5, 6). Given this wide variation in outcomes and the invasive nature of surgery, means of enhancing weight loss in those patients who experience less complete responses would be highly valuable.
With respect to metabolic pathways that are required for RYGB action, we have recently shown in animals models that weight loss and decreased fat mass after RYGB are dependent on intact signaling through the melanocortin-4 receptor (MC4R) (7). Recent clinical cases have confirmed this observation in human patients homozygous for mutations in the MC4R gene interfering with the efficacy of RYGB and another bariatric operation, vertical sleeve gastrectomy (Kaplan, L.M., unpublished data). MC4R-containing neurons in the hypothalamus receive signals from the proopiomelanocortin (POMC) neurons that regulate their activity. Several recent studies have demonstrated that activation of the serotonin 2C receptors (5-HT2CR) located on these POMC neurons strongly influence energy balance and glucose regulation (8–12). For example, mice lacking the 5-HT2CR only in the POMC neurons develop obesity when fed a high-fat diet and display glucoregulatory defects, demonstrating that 5-HT2CR-bearing melanocortinergic signaling neurons are critical for normal energy balance and glucose regulation (8). More generally, mice lacking the 5-HT2CR globally have increased body weight compared with wild-type mice (13).
It has been long known that stimulation of serotonin receptors can suppress food intake and reduce weight, making these receptors the target of several anti-obesity agents (14). Unfortunately, despite their efficacy, the nonselective nature of several earlier serotonergic anti-obesity medications, including fenfluramine and dexfenfluramine, has been associated with adverse cardiovascular effects that have made them clinically unacceptable (15, 16). One of the few pharmacological agents currently available to treat patients with obesity is lorcaserin, a selective agonist for the 5-HT2CR (17–19). Activation of this receptor has been shown to cause weight loss and improve insulin sensitivity independent of weight, without the adverse cardiovascular effects observed with the nonspecific agonists (9, 19).
Given the broad requirement of the MC4R for RYGB-induced metabolic improvements, it is likely that one or more inputs into the melanocortin pathway are also necessary to achieve RYGB outcomes. In this study, we sought to determine the role of the 5-HT2CR in these effects. We theorized that if 5-HT2CR signaling was an essential input into the melanocortin system after RYGB, then mice deficient for the 5-HT2CR would exhibit attenuated weight loss and metabolic improvement after RYGB. In addition, if the 5-HT2CR acts primarily to influence critical MC4R-bearing cells, then adding serotonergic agents to wild-type (WT) mice after RYGB should not improve metabolic outcomes because these critical signaling pathways are already engaged in response to surgery. Surprisingly, we found no difference in the effect of RYGB on energy balance or metabolic function in 5-HT2CR-deficient mice, suggesting that 5-HT2CR signaling is not essential for the therapeutic effects of RYGB. Rather, activators of 5-HT2CR signaling appear to selectively complement the effects of RYGB.
Materials and Methods
Animals
Mice deficient for the 5-HT2C receptor were previously generated by introducing a nonsense mutation into exon 5 of the X-linked gene, thereby interrupting translation of the receptor (13). 5-HT2CR-deficient and WT C57BL/6 diet-induced obese (DIO) mice were obtained from Jackson Laboratories. At weaning, the 5-HT2CR-deficient mice and WT littermate controls were fed a high-fat diet (HFD; 60% kcal from fat; Research Diets) ad libitum. All mice were housed in a barrier animal facility with a 12-hour light, 12-hour dark cycle. All studies were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Gastrointestinal surgery and experimental design
At 24 weeks of age, male DIO 5-HT2CR-deficient and WT mice underwent RYGB or sham operations, as described previously (7, 20, 21). Overall, there was a 12% mortality rate in sham-operated mice and a 33% mortality rate in RYGB-operated mice, which is within the mortality rate observed in our previous experience. Sham-operated mice were treated in a manner similar to that of RYGB-operated animals, except that the operation included only a laparotomy with repair. All operated mice were provided with a liquid diet (40% Vital AF 1.2 Cal; Abbott Laboratories) ad libitum during the 2 weeks immediately after the surgery and were gradually weaned back onto the HFD during this time. Mice continued to be fed the HFD after surgery to control for any independent or confounding effect of diet. Body weight was measured weekly after surgery. Food intake was measured during postoperative weeks 6–8. Analyses of glucose metabolism, including oral glucose tolerance tests and blood collection for fed glucose and insulin measurements, were performed during postoperative weeks 9–12.
Body composition
Thirteen weeks after surgery, body composition was analyzed using a Bruker Minispec nuclear magnetic resonance analyzer (Bruker). Animals were placed in a clear, plastic cylinder (50 mm diameter) and kept immobile by gentle insertion of a plunger. The tube was then lowered into the nuclear magnetic resonance instrument for the duration of the scan (less than 2 min).
Glucose regulation analyses
For glucose tolerance testing, mice were fasted overnight (16–18 h), and blood glucose was measured by placing a drop of tail blood on a glucometer (AlphaTRAK; Abbott Laboratories). An oral gavage of glucose (1g/kg of 20% dextrose) was given, and blood samples were taken from the tail immediately before the time of gavage (0 min) and at 10, 20, 30, 60, 90, and 120 minutes after gavage. On a separate day, the oral glucose gavage procedure was repeated, and blood was collected at the 0-, 7-, 15-, and 30-minute time points to measure plasma insulin concentrations by using a mouse ultrasensitive insulin ELISA (ALPCO). Insulin resistance was estimated by using the homeostasis model assessment-insulin resistance (HOMA-IR) calculated from the overnight-fasted glucose and insulin (ie, 0 min) values. For fed glucose and insulin measurements, blood was collected from the tail at 1:00 pm, 2 hours after the removal of food to allow blood glucose concentrations to stabilize.
Pharmacology experimental design
Beginning at 7 weeks after surgery, WT mice were given a 3-week course of fenfluramine [10 mg/kg body weight (BW; Sigma-Aldrich], topiramate (40 mg/kg BW; Johnson & Johnson), or saline (5 μL/g BW) via daily ip injections. Doses were specifically chosen to generate similar weight loss in unoperated mice and were determined by a previously performed dose-response study (22–24). Body weight was measured daily and food intake was measured weekly during the 3 weeks of drug administration.
Meta-chlorophenylpiperazine (mCPP) experimental design
Five weeks after surgery, WT mice were sc implanted with an osmotic pump (Alzet model 2001; Durect) delivering mCPP (36 mg/kg · d; Sigma-Aldrich) or saline for 7 days. Body weight and food intake were monitored during the period of drug administration.
Statistics
Oral glucose tolerance tests and glucose-stimulated insulin secretion were analyzed by area under the curve analysis. The statistical analysis was performed using GraphPad Prism software (Prism 5 for Windows). Data are presented as mean ± SEM. Data were compared using Student's t tests or an ANOVA, as appropriate. Significance is defined as P < .05.
Results
Weight loss after RYGB is independent of 5-HT2CR signaling
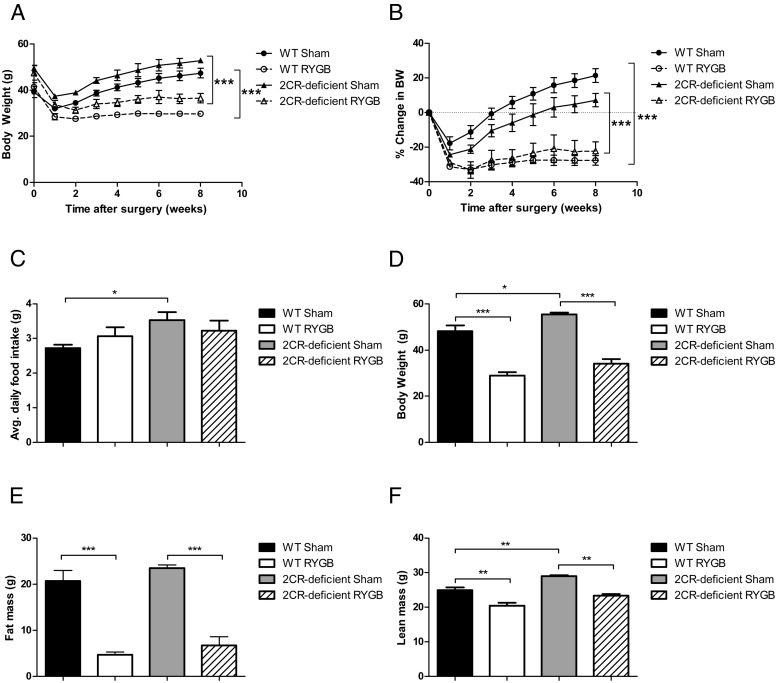
To determine whether signaling through the 5-HT2CR is necessary for weight loss after RYGB, DIO WT and 5-HT2CR-deficient mice underwent RYGB or sham operation at 24 weeks of age. The 5-HT2CR-deficient, sham-operated mice maintained a higher body weight throughout the study than WT, sham-operated animals (Figure 1A), a result that was expected based on previous studies of these mice (13, 25). Despite the differences in initial weight, the 5-HT2CR-deficient RYGB mice exhibited similar percentage body weight loss as WT RYGB mice (∼25%; Figure 1, A and B). The fat mass differences between the RYGB and sham animals in both genotype groups were also similar (Figure 1E), and we observed no significant differences in average daily food intake between RYGB and sham mice in either the WT or 5-HT2CR-deficient groups (Figure 1C). These observations indicate that signaling through the 5-HT2CR was not essential for weight loss, decreased adiposity, or diminished food intake after this operation.
Figure 1.
RYGB weight loss is independent of 5-HT2CR signaling. Body weight (A) and percentage change in body weight (B) in diet-induced obese male mice after RYGB or sham operations. Average daily food intake (C) measured during postoperative weeks 6–8. Body weight was measured at postoperative week 13 (D). Fat mass (E) and lean mass (F) were measured by Bruker Minispec at postoperative week 13 (n = 4–6 per group). *, P < .05; **, P < .01; ***, P < .001. Values represent the mean ± SEM.
Improved glucose regulation after RYGB is independent of 5-HT2CR signaling
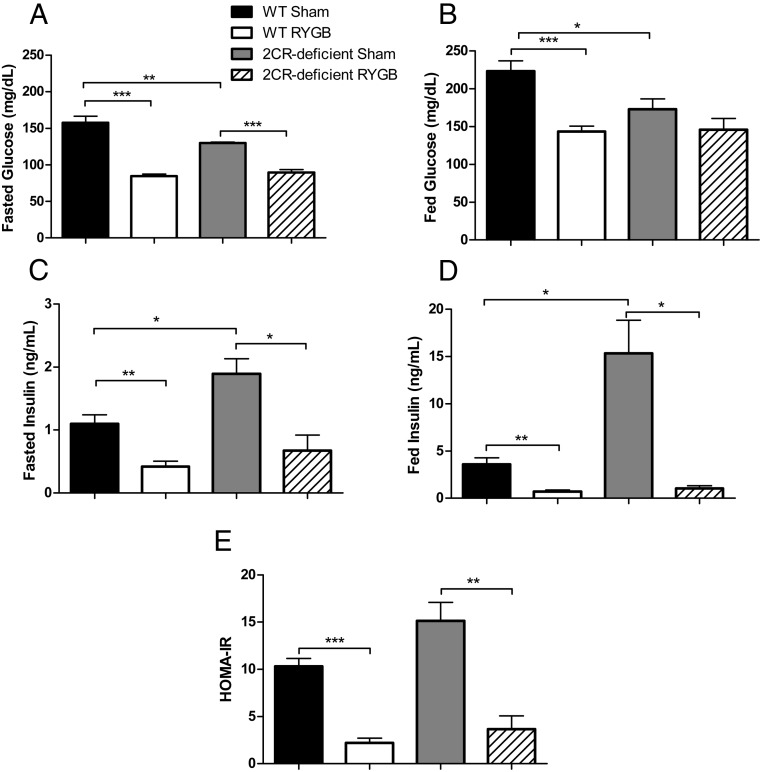
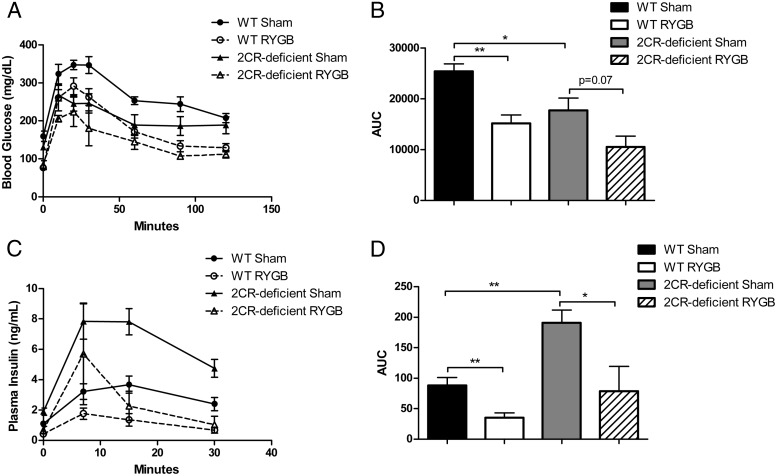
Because activation of the 5-HT2CR has been shown to have weight-independent effects on glucose homeostasis (8–10), we sought to determine whether there was any difference in glucose regulation after RYGB between the WT and 5-HT2CR-deficient mice and assessed glucose tolerance during postoperative weeks 9–12. In the sham-operated mice, we observed that 5-HT2CR-deficient mice exhibited higher concentrations of insulin under both fed and fasting conditions (Figure 2, C and D). Under overnight fasting conditions, we observed that RYGB reduced fasting blood glucose and plasma insulin concentrations significantly and to a similar degree in WT and 5-HT2CR-deficient mice (Figure 2, A and C). These effects reflect a similar improvement in insulin sensitivity in both WT and 5-HT2CR-deficient RYGB-treated mice, as estimated by HOMA-IR (Figure 2E). Oral glucose tolerance testing (1 g/kg BW of glucose) also demonstrated that RYGB significantly and similarly improved glucose tolerance in WT and 5-HT2CR-deficient mice (Figure 3, A and B). Plasma insulin concentrations during a separate oral glucose tolerance test revealed that 5-HT2CR-deficient sham-operated mice had increased insulin excursion after glucose stimulation compared with WT sham-operated mice (Figure 3, C and D). Within each genotype, however, RYGB caused a similar decrease in insulin secretion, suggesting that the effects of this operation were independent of genotype.
Figure 2.
RYGB improves glucose regulation independent of 5-HT2CR signaling. Fasted glucose (A), fed glucose (B), fasted insulin (C), fed insulin concentrations (D), and HOMA-IR (E) measures in diet-induced obese WT and 5-HT2CR-deficient mice 10–11 weeks after RYGB or sham operations (n = 4–6 per group). *, P < .05; **, P < .01; ***, P < .001. Values represent the mean ± SEM.
Figure 3.
RYGB improves glucose tolerance independent of 5-HT2CR signaling. Oral glucose tolerance test (1g/kg BW glucose) (A) and area under the curve calculations (B) in diet-induced obese WT and 5-HT2CR-deficient mice 9 weeks after RYGB or sham operations. Glucose-stimulated insulin secretion after an oral gavage of (1g/kg BW) glucose (C) and area under the curve calculations (D) 11 weeks after RYGB or sham operations (n = 4–6 per group). *, P < .05; **, P < .01. Values represent the mean ± SEM.
The nonspecific serotonergic agent fenfluramine augments weight loss after RYGB
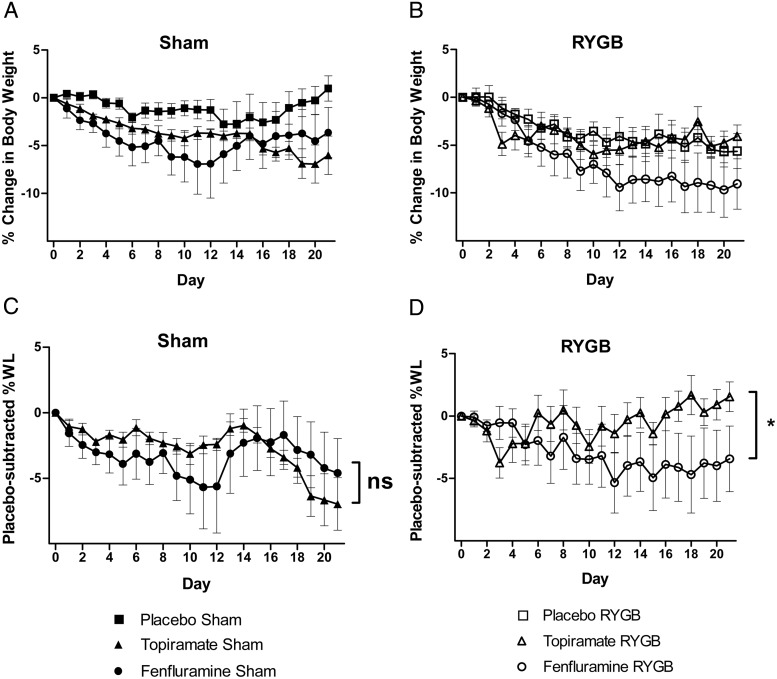
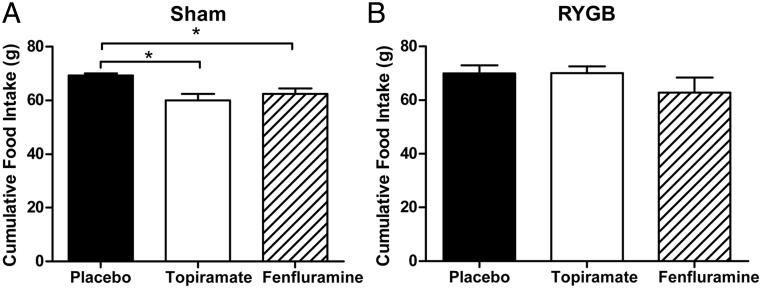
To determine whether RYGB-induced weight loss could be enhanced by 5-HT2CR activators, we administered fenfluramine or topiramate, two weight-loss agents that act via different signaling mechanisms, to mice that had previously undergone RYGB or sham operation. Fenfluramine is a nonspecific serotonergic agent that increases synaptic serotonin concentrations by blocking its degradation (26). The precise mechanisms underlying weight loss from topiramate treatment are incompletely understood but appear to involve the modulation of voltage-gated ion channels, γ-aminobutyrate neurotransmitter activity, and the activity of excitatory amino acid receptors (27). Sham and RYGB-operated DIO WT mice were administered fenfluramine (10 mg/kg BW), topiramate (40 mg/kg BW) or saline (5 μL/kg BW) by daily ip injection for 3 weeks. The doses of fenfluramine and topiramate administered had previously been shown to generate similar weight loss in nonoperated animals. Fenfluramine- and topiramate-treated, sham-operated mice lost a similar percentage of body weight (∼5%) more than placebo-treated animals. There was no significant difference in placebo-subtracted weight loss between these two groups of mice (Figure 4, A and C). Decreased food intake was observed in both fenfluramine- and topiramate-treated, sham-operated mice (∼10%; Figure 5A). In contrast, in the mice that had previously undergone RYGB, only fenfluramine treatment caused additional weight loss. Fenfluramine treatment after RYGB induced a 4.0% additional weight loss, whereas topiramate induced a 1.5% weight gain (Figure 4, B and D). In the RYGB mice, neither topiramate nor fenfluramine caused a statistically different change in food intake; however, there was a trend toward lower food intake in the fenfluramine-treated group not seen in the topiramate group (Figure 5B).
Figure 4.
The nonspecific serotonergic agent fenfluramine selectively decreases body weight after RYGB. Body weight change (A and B) and placebo-subtracted percentage body weight change (C and D) in sham and RYGB mice over 3 weeks of daily ip treatment with fenfluramine (10 mg/kg), topiramate (40 mg/kg), or saline (5 μL/g BW) (n = 4–6 per group). *, P < .05 with repeated-measures, two-way ANOVA. Values represent the mean ± SEM. WL, weight loss.
Figure 5.
Topiramate and fenfluramine decrease food intake in sham mice. Cumulative food intake during 3 weeks of treatment with ip fenfluramine (10 mg/kg), topiramate (40 mg/kg), or saline in sham-operated (A) and RYGB-operated (B) mice. *, P < .05 with unpaired Student's t test. Values represent the mean ± SEM.
Acute treatment with the 5-HT2CR-specific agonist mCPP decreased body weight and food intake after RYGB
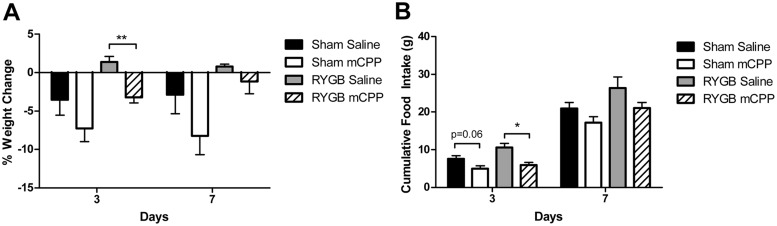
To assess whether the observed effect of fenfluramine in RYGB-treated mice was mediated specifically by the 5-HT2CR, we examined the effect of acute treatment with a selective 5-HT2CR agonist, mCPP, after RYGB. Sham- and RYGB-operated WT mice were sc implanted with osmotic pumps designed to deliver mCPP (36 mg/kg · d) or saline for 7 days. In these animals, mCPP treatment led to significantly greater weight loss than saline treatment within 3 days of treatment (3.2% weight loss vs 1.4% weight gain, respectively; Figure 6A). This reduction was accompanied by a significant decrease (43%) in food intake in the mCPP-treated RYGB mice (Figure 6B). The effect of mCPP treatment on body weight and food intake had waned by day 7 of the pump implantation.
Figure 6.
Short-term treatment with the 5-HT2CR agonist mCPP decreased body weight and food intake after RYGB. Percentage body weight change (A) and cumulative food intake (B) in sham and RYGB mice during infusion of mCPP (36 mg/kg · d) or saline via a sc osmotic pump (n = 4 per group). *, P < .05. Values represent the mean ± SEM.
Discussion
MC4R are located throughout the central nervous system, in peripheral neurons, and in intestinal enteroendocrine cells. Genetic manipulation and pharmacological studies have shown that they influence energy balance and glucose regulation (28–32). Signaling through this receptor is essential for RYGB-induced metabolic improvements, although the roles of the MC4R in specific locations have only begun to be elucidated (7, 33). Given the integrated nature of the neural circuits regulating weight and energy balance, we would anticipate that one or more inputs into the relevant melanocortin pathways are also necessary to achieve these surgical outcomes. Based on the demonstrated effects of 5-HT2CR signaling, this system is a prime candidate for regulating hypothalamic MC4R signaling, and thus, we hypothesized that it might be an essential regulator of the effects of RYGB. We undertook studies to assess this possibility using genetic and pharmaceutical approaches. We observed that mice lacking the 5-HT2CR were able to mount a weight-loss response to a degree similar to that seen in WT mice, suggesting that although the 5-HT2CR may play a role in RYGB-induced metabolic effects, signaling through this receptor is not essential for them. Thus, other activating factors, or signaling pathways, or MC4R signaling in cells outside the ventral hypothalamus are the ones required for weight loss after RYGB. This possibility is consistent with a report from Zechner et al (33), who found that MC4R located specifically in preganglionic autonomic neurons regulated energy expenditure and weight loss after RYGB, indicating a role for extrahypothalamic MC4R in response to this operation. Furthermore, Panaro et al (32) recently observed that MC4R are expressed in intestinal enteroendocrine L cells where they influence the release of glucagon-like peptide 1 and peptide YY, peptides known to regulate energy balance.
Since activation of the 5-HT2CR reduces body weight, but because these receptors do not appear to be required for RYGB-induced weight loss, it appears that RYGB does not exploit all the available weight-regulatory systems in the body. We therefore hypothesized that weight loss after activation of the 5-HT2CR might be complementary to that after RYGB. This hypothesis was borne out. We observed that treatment with the nonspecific serotonin receptor activator fenfluramine augmented weight loss after RYGB but that topiramate, which acts through distinct mechanisms involving the modulation of voltage-gated ion channels and neurotransmitter activity, had no effect, suggesting that the weight-loss effect of combining medication with RYGB was serotonin signaling specific. To examine the specificity of serotonin's action to augment RYGB-induced weight loss, we administered mCPP, a selective 5-HT2CR agonist, to surgically treated animals. The demonstration that mCPP also augmented weight loss and reduced food intake after RYGB is consistent with the 5-HT2CR null mouse studies, confirming the functional distinction between the 5-HT2CR and RYGB signaling pathways. These studies thus form a rational basis for using 5-HT2CR agonists as a complementary cotherapy with or after RYGB.
The fact that topiramate did not cause additional weight loss in RYGB-treated mice suggests that the weight-loss mechanisms of topiramate and RYGB overlap in this murine model. We should note that this observation is distinct from our recent study in human patients, where topiramate did cause weight loss after RYGB. However, in that human study, we found that the post-RYGB weight-loss effect of topiramate was selectively observed in patients who experienced suboptimal outcomes characterized by inadequate weight loss or later weight regain, respectively (Ahmad, N.N., and L.M. Kaplan, unpublished observations). Unlike the mice, in these patients with an abnormal response to RYGB, it appears that topiramate's ability to complement the surgery may reflect an inadequate activation of the normal RYGB-responsive topiramate signaling systems. In this case, the topiramate is acting to rescue this deficient response, a phenomenon not needed in the normally responsive mice.
The mechanisms by which RYGB induces weight loss in mice vs human patients, although similar, exhibit some important differences. In the mouse, increased energy expenditure drives most of the weight loss after RYGB (20, 34, 35), whereas the postoperative weight loss in human patients is due primarily to decreased food intake (36, 37). Accordingly, in this study we did not find a difference in food intake between sham and RYGB mice within each genotype, confirming the primary role of energy expenditure in altering energy balance after surgery in these animals. However, when we administered the serotonergic agents fenfluramine or mCPP to RYGB-operated mice, we observed decreased food intake. It thus appears that RYGB and the serotonergic drugs have truly distinct and complementary effects on energy balance and fat mass.
Although RYGB is effective, it is invasive and associated with appreciable risk. To the degree that its beneficial effects can be augmented or made more consistent through the addition of medication, we could improve the overall risk-benefit profile of this important treatment. In this study, we present a rational basis for designing and using combination therapy to improve surgical outcomes. We report that despite its powerful effects, RYGB does not activate or require the function of all weight-loss regulatory systems. Furthermore, we found that activators of 5-HT2CR signaling can augment RYGB-induced weight loss, demonstrating that medications that act through weight-regulatory mechanisms not fully engaged by RYGB can provide complementary benefits. This study is the first example of a rational design of such complementary treatment, and it suggests both an approach to identify medications that can augment surgical and medical device-based therapies and, by extension, the design of drug-drug combinations most likely to be synergistic.
Acknowledgments
This work was supported by National Institutes of Health Grant DK088661 (to L.M.K.) and a research grant from Ethicon, Inc.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BW
- body weight
- DIO
- diet-induced obese
- HFD
- high-fat diet
- HOMA-IR
- homeostasis model assessment-insulin resistance
- 5-HT2CR
- serotonin 2C receptor
- mCPP
- meta-chlorophenylpiperazine
- MC4R
- melanocortin-4 receptor
- POMC
- proopiomelanocortin
- RYGB
- Roux-en-Y gastric bypass
- WT
- wild type.
References
- 1. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256.e5. [DOI] [PubMed] [Google Scholar]
- 2. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. [DOI] [PubMed] [Google Scholar]
- 3. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring). 2013;21(8):1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatoum IJ, Greenawalt DM, Cotsapas C, Reitman ML, Daly MJ, Kaplan LM. Heritability of the weight loss response to gastric bypass surgery. J Clin Endocrinol Metab. 2011;96(10):E1630–E1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatoum IJ, Stylopoulos N, Vanhoose AM, et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab. 2012;97(6):E1023–E1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berglund ED, Liu C, Sohn JW, et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013;123(12):5061–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6(5):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Y, Berglund ED, Sohn JW, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010;13(12):1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Y, Jones JE, Kohno D, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60(4):582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lam DD, Przydzial MJ, Ridley SH, et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149(3):1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tecott LH, Sun LM, Akana SF, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374(6522):542–546. [DOI] [PubMed] [Google Scholar]
- 14. Guy-Grand B, Apfelbaum M, Crepaldi G, Gries A, Lefebvre P, Turner P. International trial of long-term dexfenfluramine in obesity. Lancet. 1989;2(8672):1142–1145. [DOI] [PubMed] [Google Scholar]
- 15. Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. [DOI] [PubMed] [Google Scholar]
- 16. James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–917. [DOI] [PubMed] [Google Scholar]
- 17. Kushner RF. Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis. 2014;56(4):465–472. [DOI] [PubMed] [Google Scholar]
- 18. Colman E, Golden J, Roberts M, Egan A, Weaver J, Rosebraugh C. The FDA's assessment of two drugs for chronic weight management. N Engl J Med. 2012;367(17):1577–1579. [DOI] [PubMed] [Google Scholar]
- 19. Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325(2):577–587. [DOI] [PubMed] [Google Scholar]
- 20. Nestoridi E, Kvas S, Kucharczyk J, Stylopoulos N. Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology. 2012;153(5):2234–2244. [DOI] [PubMed] [Google Scholar]
- 21. Kucharczyk J, Nestoridi E, Kvas S, Andrews R, Stylopoulos N. Probing the mechanisms of the metabolic effects of weight loss surgery in humans using a novel mouse model system. J Surg Res. 2012;179(1):e91–e98. [DOI] [PubMed] [Google Scholar]
- 22. York DA, Singer L, Thomas S, Bray GA. Effect of topiramate on body weight and body composition of Osborne-mendel rats fed a high-fat diet: alterations in hormones, neuropeptide, and uncoupling-protein mRNAs. Nutrition. 2000;16(10):967–975. [DOI] [PubMed] [Google Scholar]
- 23. Heisler LK, Cowley MA, Tecott LH, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297(5581):609–611. [DOI] [PubMed] [Google Scholar]
- 24. Finger BC, Dinan TG, Cryan JF. Progressive ratio responding in an obese mouse model: effects of fenfluramine. Neuropharmacology. 2010;59(7–8):619–626. [DOI] [PubMed] [Google Scholar]
- 25. Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4(10):1152–1156. [DOI] [PubMed] [Google Scholar]
- 26. Davis R, Faulds D. Dexfenfluramine. An updated review of its therapeutic use in the management of obesity. Drugs. 1996;52(5):696–724. [DOI] [PubMed] [Google Scholar]
- 27. Verrotti A, Parisi P, Agostinelli S, et al. Weight regain after discontinuation of topiramate treatment in patients with migraine: a prospective observational study. CNS Drugs. 2015;29(2):163–169. [DOI] [PubMed] [Google Scholar]
- 28. Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8(10):1298–1308. [DOI] [PubMed] [Google Scholar]
- 29. Liu H, Kishi T, Roseberry AG, et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23(18):7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wan S, Browning KN, Coleman FH, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28(19):4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol. 2010;518(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Panaro BL, Tough IR, Engelstoft MS, et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 2014;20(6):1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zechner JF, Mirshahi UL, Satapati S, et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144(3):580–590.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring). 2009;17(10):1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bueter M, Lowenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138(5):1845–1853. [DOI] [PubMed] [Google Scholar]
- 36. Laurenius A, Larsson I, Bueter M, et al. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond). 2012;36(3):348–355. [DOI] [PubMed] [Google Scholar]
- 37. le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–785. [DOI] [PubMed] [Google Scholar]