Abstract
Kisspeptin, encoded by Kiss1, stimulates GnRH neurons to govern reproduction. In rodents, estrogen-sensitive kisspeptin neurons in the anterior ventral periventricular nucleus and neighboring periventricular nucleus are thought to mediate sex steroid-induced positive feedback induction of the preovulatory LH surge. These kisspeptin neurons coexpress estrogen and progesterone receptors and display enhanced neuronal activation during the LH surge. However, although estrogen regulation of kisspeptin neurons has been well studied, the role of progesterone signaling in regulating kisspeptin neurons is unknown. Here we tested whether progesterone action specifically in kisspeptin cells is essential for proper LH surge and fertility. We used Cre-lox technology to generate transgenic mice lacking progesterone receptors exclusively in kisspeptin cells (termed KissPRKOs). Male KissPRKOs displayed normal fertility and gonadotropin levels. In stark contrast, female KissPRKOs displayed earlier puberty onset and significant impairments in fertility, evidenced by fewer births and substantially reduced litter size. KissPRKOs also had fewer ovarian corpora lutea, suggesting impaired ovulation. To ascertain whether this reflects a defect in the ability to generate sex steroid-induced LH surges, females were exposed to an estradiol-positive feedback paradigm. Unlike control females, which displayed robust LH surges, KissPRKO females did not generate notable LH surges and expressed significantly blunted cfos induction in anterior ventral periventricular nucleus kisspeptin neurons, indicating that progesterone receptor signaling in kisspeptin neurons is required for normal kisspeptin neuronal activation and LH surges during positive feedback. Our novel findings demonstrate that progesterone signaling specifically in kisspeptin cells is essential for the positive feedback induction of normal LH surges, ovulation, and normal fertility in females.
In female mammals, the preovulatory LH surge, which triggers ovulation, is governed by GnRH neurons. Throughout the rodent estrous cycle, low levels of ovarian-derived estradiol (E2) inhibit GnRH secretion via negative feedback until proestrus, when elevated E2 exerts positive feedback on neural circuits to induce a preovulatory GnRH surge and hence LH surge (1–5). Additionally, progesterone (P4) and its receptor (PR) are also important contributors to the LH surge because PR knockout mice are infertile and unable to produce LH surges (6, 7). As with estrogen receptor (ER)-α, PR is not readily expressed in GnRH neurons, suggesting that P4 acts on upstream reproductive brain circuitry to regulate the GnRH/LH surge. Whereas E2-induced local synthesis of P4 in the hypothalamus is critical for the LH surge (8–10), the specific neural population that P4 targets for this process remains unknown.
The neuropeptide kisspeptin, encoded by Kiss1, regulates puberty and fertility by potently and directly stimulating GnRH (and hence LH) secretion (11–14). Kisspeptin signaling is essential for the LH surge in rodents: knockout mice lacking Kiss1 or its receptor fail to exhibit E2-induced GnRH activation or LH surges (15, 16). Two populations of kisspeptin-synthesizing neurons exist in the hypothalamus, one in the arcuate nucleus and one more rostrally in the anterior ventral periventricular nucleus (AVPV) and neighboring periventricular nucleus (PeN) (17–19). E2's positive feedback effects on GnRH/LH secretion are proposed to be mediated specifically by AVPV/PeN Kiss1 neurons, because E2 elevates Kiss1 levels in the AVPV/PeN, AVPV/PeN Kiss1 neurons express ERα, and AVPV/PeN Kiss1 neuronal activation increases during the LH surge, coincident with GnRH neuronal activation (19–24). Lastly, kisspeptin neurons in the AVPV/PeN are sexually dimorphic in cell number (greater in females than males), correlating with the sexually dimorphic nature of the rodent LH surge (19, 25).
Despite the above findings, it remains undetermined whether P4, which is also critical for the LH surge, similarly acts on AVPV/PeN kisspeptin neurons to facilitate positive feedback. AVPV/PeN kisspeptin neurons coexpress PR (15, 22, 26), suggesting they are key targets for P4 action. Here we used transgenic mice to test whether P4 signaling directly in kisspeptin cells is important for proper LH surge induction and fertility.
Materials and Methods
Animals
Mice lacking PR exclusively in kisspeptin cells were generated by crossing Kiss1-Cre mice (from Dr Carol Elias, University of Michigan, Ann Arbor, Michigan) with established PR flox mice (27). Offspring were backcrossed to PR flox mice to generate KissCre+ PRfl/fl mice (termed KissPRKO; PR knocked out in Kiss1 cells) and KissCre− PRfl/fl controls [termed wild type (WT); PR still present in all cells, including Kiss1 cells]. Mice were genotyped via PCR analysis of tail DNA. Recombination indicative of Cre-mediated excision of the PR gene was confirmed in the AVPV and ARC of KissPRKOs but not in brain areas or tissues known to lack Kiss1 expression, and PR was found to be virtually absent specifically in Kiss1 neurons (Supplemental Figures 1 and 2). All mice were tested for germline recombination and germline-recombined mice excluded from the study. Mice were housed two to three per cage under a 12-hour light, 12-hour dark cycle (lights off at 6:00 pm). All experiments were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Fertility and gonadotropin assessments
At 12 weeks of age, a blood sample was collected via retroorbital bleeding for each sex (females in diestrus stage) (n = 6–9/genotype). Blood serum LH and FSH levels were measured by the University of Virginia's Ligand Assay Core by a multiplex mouse assay with a limit of detectability for LH of 0.24 ng/mL and FSH of 2.4 ng/mL. Several days later, KissPRKO and WT mice of both sexes were paired with a C57BL6 breeder partner (∼3 mo old) to assess fertility (n = 6–9/genotype). All mice were paired for 8 weeks, after which the male was removed and the female monitored for 3 more weeks to detect any final litters. Latency to first litter, total number of litters, and litter size were recorded for the 11-week duration. A subset of mice (n = 4–5/genotype) were similarly reassessed for fertility at 6 months of age, being paired with a new C57BL6 breeder partner (∼3 mo old).
Assessment of female puberty onset and ovarian corpora lutea
Puberty onset in females (n = 8/genotype) was studied by determining the age of vaginal opening, an external measure of pubertal onset, and subsequent assessment of first estrous by analysis of daily vaginal cytology. Ovaries were collected from 12-week-old females and fixed in 11% formaldehyde/60% ETOH/10% acetic acid, embedded in paraffin and cut on a sliding microtome (10 μm), mounted onto slides, and stained with hematoxylin and eosin. The number of corpora lutea (CL) was counted for each ovary (n = 4/genotype).
Estradiol induction of LH surge
Twelve-week-old mice were ovariectomized (OVX) and given sc SILASTIC brand implants (inner diameter 0.078 in., outer diameter 0.125 in.) containing 0.625 μg E2 dissolved in sesame oil. In female mice, this E2 implant produces constantly elevated serum E2 levels of approximately 18–26 pg/mL, resembling mouse proestrus levels (16, 28), and normally produces a robust LH surge 2 days later around lights off (16, 24, 28). KissPRKO and control females treated with this E2 regimen were killed 2 days later, either in the morning (10:00 am, n = 3/group) or before lights off in the evening (5:40–6:00 pm, n = 6–7/group), and blood and brains collected. Serum LH was measured by the University of Virginia by a sensitive mouse LH RIA (limit of detectability 0.04 ng/mL). Brains were frozen on dry ice and sectioned on a cryostat into five alternating sets of 20-μm sections, thaw mounted on Superfrost-plus slides, and stored at −80°C.
Single- and double-label in situ hybridization (ISH)
For single-label ISH for Kiss1 levels in the AVPV/PeN, one set of slide-mounted brain sections encompassing the entire AVPV/PeN was assayed, using an established radiolabeled (33P) antisense Kiss1 riboprobe (0.04 pmol/mL), as previously described (29–31). For double-label ISH analysis of cfos levels in Kiss1 neurons (measure of neuronal activation), a second set of AVPV/PeN slides was used. Radiolabeled (33P) antisense cfos riboprobe (0.05 pmol/mL) and digoxygenin-labeled Kiss1 riboprobe (1:500) were used, following a previously described protocol (16, 31, 32). ISH slides were analyzed blindly with an automated grains imaging processing system (Dr Don Clifton, University of Washington, Seattle, Washington) that counts the number of silver grain clusters representing cells and the number of silver grains in each cell (a semiquantitative index of mRNA content per cell) (33). For the double-label, red fluorescent digoxygenin-containing (Kiss1) cells were identified and the grain-counting software quantified the number of silver grains (cfos mRNA) overlying each fluorescent cell. Signal to background ratios for individual cells were calculated by the program; a cell was double labeled if its ratio was 4 or greater.
Statistical analyses
All data are expressed as the mean ± SEM for each group. Group differences within each sex were analyzed by a one-way ANOVA, except for the percentage of animals breeding or showing LH surges, which was analyzed with a χ2 test. Statistical significance was set at P < .05.
Results
Fertility is significantly compromised in female, but not male, KissPRKO mice
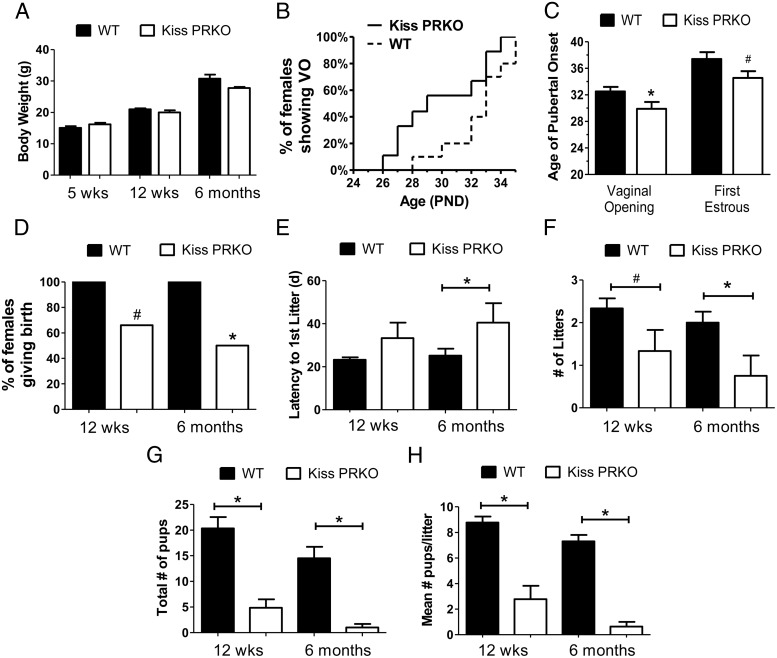
Female KissPRKO mice weighed the same as WT control females at 5 weeks, 12 weeks, and 6 months of age (Figure 1A). Mean day of puberty onset (vaginal opening) was significantly earlier in KissPRKO females (P < .05 Figure 1, B and C) and there was a nonsignificant trend for earlier first estrous as well (P = .07; Figure 1C). In adulthood, fertility in KissPRKO females was substantially impacted at both 12 weeks and 6 months. Whereas 100% of WT females bred successfully at 12 weeks and 6 months, the percentage of KissPRKO females successfully breeding was reduced at both ages (Figure 1D). The latency to first litter was normal at 12 weeks old, but at 6 months of age, KissPRKO females displayed longer latencies to give birth (P < .05, Figure 1E), and there were fewer litters produced at 6 months (P < .05; nonsignificant trend at 12 weeks, P < .08; Figure 1F). Strikingly, at both ages, KissPRKO females displayed a dramatic reduction in total number of pups produced (P < .05, Figure 1G) and a similar robust reduction in average litter size (P < .05; Figure 1H).
Figure 1.
Fertility is impacted in KissPRKO female mice at 12 weeks and 6 months of age. A, Mean body weights of WT and KissPRKO females at young and older ages. B, Percentage of females in each genotype displaying vaginal opening (VO; a measure of female puberty onset) at each postnatal age. C, Mean age of VO and first estrous (a measure of late puberty) are earlier in KissPRKO females. D, Percentage of females successfully giving birth during the 11-week fertility assessment period at 12 weeks and 6 months of age. E, Latency to deliver first litter after pairing with male partner. F and G, Mean number of litters, pups, and pups per litter (litter size) during the 11-week fertility assessment at each age. *, significant genotype difference (P < .05); #, nonsignificant trend for genotype difference (P < .08). PND, postnatal day.
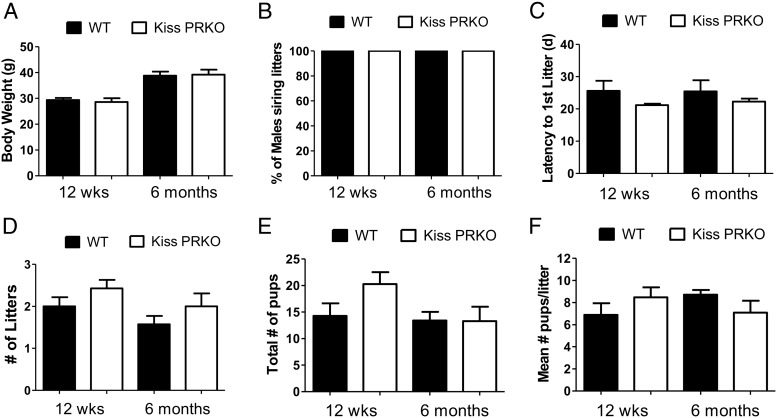
Like females, body weights of male KissPRKOs were normal at both 12 weeks and 6 months of age (Figure 2A). However, in contrast to female KissPRKOs, male KissPRKO mice displayed normal fertility at both ages. All male KissPRKO mice successfully sired offspring at both ages (Figure 2B), and there were no genotype differences in any fertility measure (Figures 2, C–F).
Figure 2.
Fertility is normal in KissPRKO male mice at 12 weeks and 6 months of age. A, Mean body weights of WT and KissPRKO males. B, Percentage of males successfully siring pups during the 11-week fertility assessment period at each age. C, Latency for female partner to deliver first litter after pairing. D–F, Mean number of litters, pups, and pups per litter (litter size) during the 11-week fertility assessment at each age. There were no genotype differences for any fertility measure between WT and KissPRKO males.
Basal gonadotropin levels are normal in KissPRKOs, but KissPRKO females have fewer CL and impaired LH surges
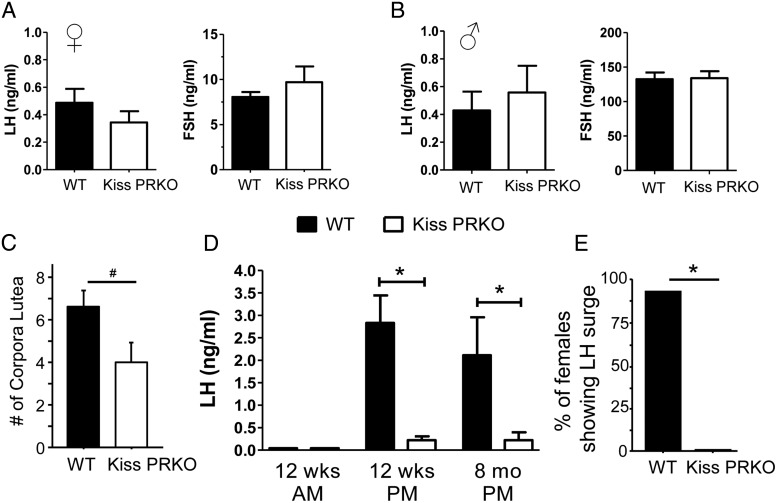
Serum basal LH and FSH were normal and not different between genotypes in either sex in 12-week-old mice (females in diestrus; Figure 3, A and B). Serum sex steroid levels, E2 in females and T in males, were also not significantly different between genotypes (not shown). Because litter size and overall fertility were notably impacted in female KissPRKOs, we examined ovaries of 12-week-old mice for signs of ovulation. KissPRKO ovaries displayed no cysts but typically exhibited fewer CL than WT females, indicating reduced ovulations (Figure 3C). To test whether this reflected a diminished ability to generate preovulatory LH surges, we used an E2-positive feedback paradigm that reliably elicits LH surges in the afternoon/evening (but not the morning, reflecting the circadian nature of the LH surge). As expected, all E2-treated WT females displayed large LH surges in the evening but not in the morning (Figure 3, D and E). In contrast, E2-treated KissPRKO females failed to display any LH surges at either 12 weeks or 8 months of age (P < .05 vs WT females; Figure 3, D and E).
Figure 3.
Normal basal gonadotropin levels but impaired positive feedback induction of LH surges in KissPRKO females. Mean basal serum LH and FSH levels in 12-week-old female (A) and male (B) WT and KissPRKO mice (females in diestrus). C, Mean number of CL in ovaries of 12-week-old WT and KissPRKO females. D and E, Mean serum LH levels and percentage of females displaying an LH surge in 12-week and 8-month-old females exposed to a validated E2-positive feedback regimen known to elicit LH surge at the evening but not morning time point. *, Significantly different from each other (P < .05); #, nonsignificant trend for genotype difference (P < .10).
Positive feedback induction of kisspeptin neuronal activation is impaired in KissPRKO females
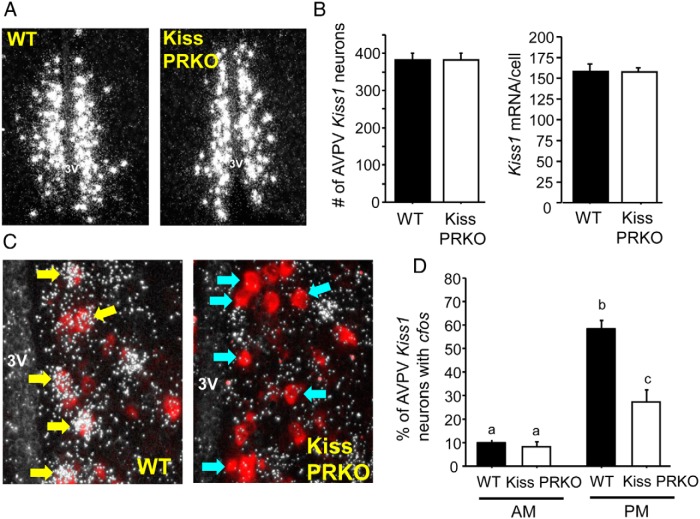
The lack of E2-induced LH surges in KissPRKO females (correlating with fewer CL and fewer pups born) suggested an absence of a preceding GnRH surge, which is thought to be triggered by AVPV/PeN kisspeptin neurons (which express ERα and PR). We hypothesized that the absent LH surges and lower ovulation rate in KissPRKO females reflected an impairment in the AVPV/PeN kisspeptin system during positive feedback. We therefore examined AVPV/PeN Kiss1 levels and Kiss1 neuronal activation in E2-treated KissPRKO females. There was no difference between WT and KissPRKOs in the number of AVPV Kiss1 neurons or in Kiss1 mRNA levels per neuron (Figure 4, A and B). However, cfos induction in Kiss1 neurons of KissPRKO females was substantially lower than in WT females in the evening, when the LH surge should occur (P < 0;05, Figure 4, C and D), demonstrating reduced Kiss1 neuronal activation in KissPRKO females.
Figure 4.
Kiss1 levels and Kiss1 neuronal activation in 12-week-old E2-treated KissPRKO females. A, Representative photomicrographs of AVPV Kiss1 expression in E2-treated WT and KissPRKO females. B, Mean number of AVPV Kiss1 neurons and relative Kiss1 mRNA levels per cell in the AVPV of 12-week-old females exposed to the E2-positive feedback regimen. C, Representative photomicrographs of cfos mRNA coexpression in AVPV Kiss1 neurons in WT and KissPRKO females exposed to an LH surge paradigm of constant elevated E2 and killed in the evening. Yellow arrows denote examples of Kiss1 cells (red fluorescence) with significant cfos (silver grains) coexpression. Blue arrows designate example Kiss1 neurons that did not have cfos induction. D, Mean percentage of AVPV Kiss1 neurons coexpressing cfos after E2-positive feedback regimen in 12-week-old females. Bars with different letters signify significantly different from each other (P < .05).
Discussion
Female fertility is dependent on sex steroid-induced positive feedback induction of GnRH/LH surges, which drive ovulation. Abundant evidence implicates the AVPV/PeN kisspeptin system in mediating positive feedback effects of E2 on the GnRH/LH surge (3, 20). AVPV/PeN kisspeptin neurons also highly coexpress PR (15, 22, 26), suggesting they are P4 targets, but whether P4, which is also critical for the LH surge (8–10), acts on AVPV/PeN kisspeptin neurons to facilitate positive feedback has remained undetermined. Here we generated transgenic mice lacking PR exclusively in kisspeptin cells to demonstrate, for the first time, that P4 signaling directly in kisspeptin cells is essential for proper LH surge induction and subsequent fertility in females but dispensable for fertility in males.
Male KissPRKO mice displayed normal gonadotropin levels and normal fertility, matching the normal fertility reported for global PRKO males. In contrast, female KissPRKO mice displayed substantially impacted fertility at both young adult and older ages. Compared with WT females, KissPRKO females demonstrated reduced occurrence of giving birth, longer latencies to give birth, a trend for fewer litters produced, and a dramatic reduction in total number of pups produced and mean litter size. The reduced fecundity was mirrored by a decrease in CL, indicating diminished ovulatory events in KissPRKO females. Thus, the infertility previously observed in global PRKO females can be attributed, at least in part, to absent PR signaling in kisspeptin cells, most likely by impacting ovulation and the LH surge. Because our KissPRKO mice were not a conditional knockout model and therefore lacked PR throughout development, it is currently unknown whether the same subfertility and absent LH surges would exist if PR was lost from Kiss1 cells just in adulthood.
Although basal gonadotropins at diestrus were normal in KissPRKO females, KissPRKO females were incapable of producing a normal E2-induced LH surge at either 12 weeks or 6 months of age. This indicates that P4 signaling specifically in kisspeptin neurons is essential for proper positive feedback induction of preovulatory LH surges. Despite the lack of observed LH surges, a few KissPRKO females exhibited some low degree of fertility. It is possible that KissPRKOs can occasionally muster a miniscule semblance of a minisurge, as indicated by slightly higher levels of LH in the evening than morning in the knockouts (Figure 3). Whereas this LH level is not high enough to be denoted a surge, it may be elevated enough to occasionally induce a few follicles to ovulate, resulting in a few pups born, as was observed.
The absence of proper LH surges in KissPRKO females likely reflects the lack of the kisspeptin induction of a GnRH surge, rather than diminished pituitary responsiveness to incoming GnRH, supported by reduced cfos-Kiss1 coexpression in E2-treated KissPRKO females, indicating that their AVPV/PeN kisspeptin neurons were not properly activated by E2-positive feedback. This finding highlights the critical importance of P4 signaling, along with E2 signaling, in positive feedback induction of LH surges and ovulation. The source of P4 in this LH surge process is thought to be neuroprogesterone, derived locally in hypothalamic glia (8, 10). Although we did not test the source of P4 in positive feedback, all of our positive feedback mice were OVX; thus, P4 would not be coming from the ovaries, supporting the possibility of neuroprogesterone in this positive feedback event. Future studies are needed to determine what P4 does mechanistically in AVPV/PeN kisspeptin neurons to influence their neuronal activation during the LH surge. In addition to PR in AVPV/PeN Kiss1 neurons, future studies will also need to determine the role, if any, of PR in arcuate Kiss1 neurons for mediating P4-negative feedback as well as any potential contribution for PR signaling in Kiss1 cells in the ovary and pituitary. The normal basal gonadotropin levels suggest that P4-negative feedback may not be impaired in adult KissPRKOs, but this requires further testing.
In summary, we generated transgenic mice to test whether P4 action specifically in kisspeptin cells is essential for proper LH surge and fertility. Although male KissPRKOs displayed normal fertility, female KissPRKOs were markedly subfertile and exhibited impaired ovulation, supported by an inability to generate E2-triggered LH surges. The compromised LH surge generation likely reflects lack of proper neuronal activation of AVPV/PeN kisspeptin neurons. Thus, P4 signaling in kisspeptin cells is essential for the positive feedback induction of normal LH surges and hence normal fertility in females.
Acknowledgments
We thank Dr Carol Elias (University of Michigan) for the generous gift of the Kiss-Cre mice and Dr Luisa Iruela-Arispe (University of California, Los Angeles) for the kind gift of the floxed PR mice. We also thank Art Nasamran, Navi Chahal, and Ambika Munaganuru for their technical and experimental support.
This work was supported by National Science Foundation Grant IOS-1025893, National Institutes of Health (NIH) Grant R01 HD065856, and NIH Grant R01 HL074455. Additional support was provided by NIH Grant T32 HD007203 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD012303 (University of California, San Diego) and Grant U54 HD28934 (University of Virginia). K.P.T. was supported by NIH Grant F32 HD076606.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 3063
- AVPV
- anterior ventral periventricular nucleus
- CL
- corpora lutea
- E2
- estradiol
- ER
- estrogen receptor
- ISH
- in situ hybridization
- OVX
- ovariectomized
- P4
- progesterone
- PeN
- periventricular nucleus
- PR
- P4 receptor
- KissPRKO
- KissCre+ PRfl/fl mice
- WT
- wild type.
References
- 1. Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, ed. Knobil and Niell's Physiology of Reproduction. 3rd ed San Diego: Elsevier; 2006:2061–2126. [Google Scholar]
- 2. Freeman ME. Neuroendocrine control of the ovarian cycle in the rat. In: Neill JD, ed. Physiology of Reproduction. 3rd ed San Diego: Elsevier; 2006:2283–2326. [Google Scholar]
- 3. Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol 2012;24:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tolson KP, Chappell PE. The changes they are a-timed: metabolism, endogenous clocks, and the timing of puberty. Front Endocrinol (Lausanne). 2012;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chappell PE, Lydon JP, Conneely OM, O'Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–4152. [DOI] [PubMed] [Google Scholar]
- 7. Chappell PE, Schneider JS, Kim P, et al. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140:3653–3658. [DOI] [PubMed] [Google Scholar]
- 8. Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol (Lausanne). 2011;2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008;290:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. [DOI] [PubMed] [Google Scholar]
- 11. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 13. Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 2010;324:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. [DOI] [PubMed] [Google Scholar]
- 19. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. [DOI] [PubMed] [Google Scholar]
- 20. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. [DOI] [PubMed] [Google Scholar]
- 22. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 23. Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. [DOI] [PubMed] [Google Scholar]
- 24. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory GnRH/LH surge. Endocrinology. 2009;150:3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Homma T, Sakakibara M, Yamada S, et al. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81:1216–1225. [DOI] [PubMed] [Google Scholar]
- 26. Zhang J, Yang L, Lin N, Pan X, Zhu Y, Chen X. Aging-related changes in RP3V kisspeptin neurons predate the reduced activation of GnRH neurons during the early reproductive decline in female mice. Neurobiol Aging. 2014;35:655–668. [DOI] [PubMed] [Google Scholar]
- 27. Hashimoto-Partyka MK, Lydon JP, Iruela-Arispe ML. Generation of a mouse for conditional excision of progesterone receptor. Genesis. 2006;44:391–395. [DOI] [PubMed] [Google Scholar]
- 28. Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102:15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Giorgio NP, Semaan SJ, Kim J, et al. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology. 2014;155:1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim J, Tolson KP, Dhamija S, Kauffman AS. Developmental GnRH signaling is not required for sexual differentiation of kisspeptin neurons but is needed for maximal Kiss1 gene expression in adult females. Endocrinology. 2013;154:3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kauffman AS, Sun Y, Kim J, Khan AR, Shu J, Neal-Perry G. Vasoactive intestinal peptide modulation of the steroid-induced LH surge involves kisspeptin signaling in young but not in middle-aged female rats. Endocrinology. 2014;155:2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology. 1990;52:581–588. [DOI] [PubMed] [Google Scholar]