Abstract
Ovulation is one of the cornerstones of female fertility. Disruption of the ovulatory process results in infertility, which affects approximately 10% of couples. Using a unique model in which the dominant follicle is collected across the periovulatory period in women, we have identified a leukocyte chemoattractant, chemokine ligand 20 (CCL20), in the human ovary. CCL20 mRNA is massively induced after an in vivo human chorionic gonadotropin (hCG) stimulus in granulosa (>10 000-fold) and theca (>4000-fold) cells collected during the early ovulatory (12–18 h) and late ovulatory (18–34 h) periods after hCG administration. Because the LH surge sets in motion an inflammatory reaction characterized by an influx of leukocytes and CCL20 is known to recruit leukocytes in other systems, the composition of ovarian leukocytes (CD45+) containing the CCL20 receptor CCR6 was determined immediately prior to ovulation. CD45+/CCR6+ cells were primarily natural killer cells (41%) along with B cells (12%), T cells (11%), neutrophils (10%), and monocytes (9%). Importantly, exogenous CCL20 stimulated ovarian leukocyte migration 59% within 90 minutes. Due to the difficulties in obtaining human follicles, an in vitro model was developed using granulosa-lutein cells to explore CCL20 regulation. CCL20 expression increased 40-fold within 6 hours after hCG, was regulated partially by the epithelial growth factor pathway, and was positively correlated with progesterone production. These results demonstrate that hCG dramatically increases CCL20 expression in the human ovary, that ovarian leukocytes contain the CCL20 receptor, and that CCL20 stimulates leukocyte migration. Our findings raise the prospect that CCL20 may aid in the final ovulatory events and contribute to fertility in women.
As early as the 1980s, it was proposed that an inflammatory reaction, characterized by an influx of leukocytes, sets in motion the events necessary for follicular rupture and oocyte release (1). This proposal has been supported by numerous investigators including a report that the number of leukocytes infiltrating the rodent ovary prior to ovulation increases 5-fold within 6 hours after human chorionic gonadotropin (hCG) (2). These leukocytes potentially secrete various chemokines and cytokines that in turn stimulate components of the ovulatory pathway, such as prostaglandins and matrix metalloproteinases, that aid in the breakdown of the follicular wall and the extrusion of the oocyte (1, 3, 4). Support for the role of leukocytes in the ovulatory process is forthcoming from reports that the addition of leukocytes to perfused rat ovaries increased the number of oocytes released approximately 3-fold (4), whereas depletion of leukocytes from the blood decreased the number of eggs released (3, 5). Furthermore, progesterone production increased when leukocytes were added to cultured granulosa cells (6, 7). These observations suggest that the influx of leukocytes plays a key role in the normal periovulatory processes associated with follicular rupture. The importance of leukocytes in the ovulatory process is counterbalanced by limited data regarding the signals, which set in motion this inflammatory cascade.
Chemokines are a diverse family that is responsible for leukocyte recruitment, adhesion, activation, and chemotaxis (3). Chemokine ligand 20 (CCL20) is a chemokine that was discovered by three groups simultaneously in different organ systems and has been ascribed different names: LARC (liver activation regulated chemokine), MIP3α (macrophage inflammatory protein-3), and Exodus-1 (8). CCL20 shows low sequence similarity with the other human chemokines (20%–31%) and is known to attract immature dendritic B cells and T cells and harbors some antimicrobial properties (9). CCL20 conveys its actions via a specific receptor, CCR6. This receptor was discovered in 1996 and was initially named STLR22. Studies have overwhelmingly demonstrated that CCR6 is activated only in the presence of CCL20 (10). The fact that CCL20 selectively binds to only the CCR6 receptor is in stark contrast to other chemokines and their receptors that display a promiscuity of interactions (8). The presence of CCR6 changes, depending on the different phases of leukocyte development and proliferation (8, 11). Studies examining the function of CCR6 have demonstrated that the major role of CCR6 is the regulation of chemotaxis; however, CCR6 has also been implicated in calcium mobilization and adhesion (10).
There are limited reports investigating the expression and role of CCL20 and CCR6 in the ovary. The data that do exist indicate that CCL20 is present in human follicular fluid and is correlated with oocyte maturation (12). CCL20 has also been identified as one of the genes overexpressed in the cumulus cells in polycystic ovarian syndrome (PCOS) patients (13), indicating a potential association in the manifestation of PCOS. However, virtually nothing is known about CCL20 in the normal ovary and its expression across the periovulatory period or its role in recruiting leukocytes and ovulation. In the present study, we have explored the expression of CCL20 and CCR6 in a unique physiological model of in vivo human ovulation. We have then determined the regulatory pathways and function of CCL20 after hCG administration in the human.
Materials and Methods
Cells, media, and reagents
Unless otherwise noted, all chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. Culture media, superscript reverse transcriptase (SuperScript III), oligodeoxythymidine, deoxynucleotide triphosphates, and RNaseOUT were purchased from Invitrogen Life Technologies.
Human tissue collection: in vivo ovulatory follicles
Human ovarian granulosa and theca cells were collected from the dominant follicle of patients across the periovulatory period as previously described (14). The collections were carried out at the Division of Gynaecology and Reproductive Medicine at Sahlgrenska University Hospital. The Human Ethics Committee of the Sahlgrenska Academy at the University of Gothenburg approved the protocol, and all participants had given their informed written consent before participating. The cohort of 30- to 38-year-old women met the following criteria: were healthy, had previous proven fertility, exhibiting regular menstrual cycles, undergoing laparoscopic sterilization, and had not been on any hormonal contraceptives for at least 3 months prior to their enrollment in the study. The patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics
| Patient Characteristics |
Patient Hormone Levels |
Sample Collection |
|||||
|---|---|---|---|---|---|---|---|
| Age | Cycle Length | Progesterone | Estradiol | Follicle Size, mm | Surgery After hCG, h | Cycle Day at Surgery, d | |
| PO phase | 35.4 ± 1.2 (31–38) | 29 ± 0.6 (28–35) | 0.4 ± 0.04 (0.3–0.5) | 0.5 ± 0.1 (0.2–1.0) | 15.9 ± 0.7 (14–17) | 14 ± 0.3 (13–15) | |
| EO phase | 33.8 ± 1.4 (30–38) | 27.2 ± 0.5 (26–28) | 1.2 ± 0.22 (0.5–1.9) | 0.6 ± 0.1 (0.4–0.9) | 17.1 ± 0.8 (15–19) | 14 ± 0.9 (12–18) | 12.6 ± 0.5 (11–14) |
| LO phase | 37.4 ± 1.4 (36–38) | 29.4 ± 0.9 (28–32) | 2.1 ± 0.3 (1.5–3.0) | 0.7 ± 0.1 (0.6–0.9) | 17.2 ± 0.8 (15–20) | 21.9 ± 0.8 (19–24) | 13 ± 0.6 (11–15) |
| Postovulatory phase | 34.2 ± 0.5 (33–36) | 28.4 ± 0.5 (27–30) | 3.1 ± 1.5 (1.3–9.5) | 0.3 ± 0.1 (0.21–0.6) | 16.7 ± 0.5 (15–18) | 49.1 ± 0.6 (44–70) | 13.1 ± 0.6 (11–15) |
Women were monitored by transvaginal ultrasound for two to three menstrual cycles before surgery to ascertain cycle regularity and monitor the size of the dominant follicle. Surgery was performed to enable retrieval of follicles at four predetermined periovulatory phases to cover the period from before the LH surge until after follicular rupture. Thus, there were four experimental groups with four to five patients per group. In the preovulatory phase (PO), surgery was performed prior to the spontaneous LH surge when the follicle was between 14 and 17.5 mm (these patients were not given hCG).
The remaining patients received a sc injection of 250 μg recombinant hCG (Ovitrelle; Merck Serono) to mimic the endogenous LH surge. These patients were divided into one of the three predetermined periovulatory phases: early, late, and postovulatory. Patients underwent surgery between 12 and 18 hours or less after hCG to harvest early ovulatory phase (EO) follicles, between 18 and 34 hours or less for the late ovulatory phase (LO), and between greater than 44 to 70 hours or less for the postovulatory phase. The dominant follicle was excised using laparoscopic scissors and diathermy was not used. Once excised, the follicle was either fixed and processed for immunostaining or bisected and the inside of the follicle scraped to collect the granulosa cells, and the theca layer was then separated. We have previously examined the contamination of granulosa cells by theca cells and found no detectable contamination of white blood cells after granulosa cell isolation, suggesting minimal thecal cell contamination of the granulosa cells (15). Granulosa and theca cell samples were frozen for subsequent mRNA expression analysis.
Human tissue collection: in vitro fertilization (IVF) granulosa-lutein cells (GLCs)
Due to the difficulty in acquiring in vivo timed human samples, CCL20 expression and regulation were also examined in cultured human GLCs from women undergoing IVF. The protocol for GLC collection was approved by the Institutional Review Board of the University of Kentucky Office of Research Integrity. Women undergoing IVF for fertility intervention at the Bluegrass Fertility Center (Lexington, Kentucky) were administered recombinant human FSH to induce ovarian hyperstimulation. After 9–11 days of FSH treatment, patients were given 10 000 U hCG and follicles aspirated 36 hours after hCG. Oocytes were extracted and the remaining GLCs were subjected to a Percoll gradient to separate red blood cells. The isolated GLCs were then cultured in OptiMEM media containing 10% fetal bovine serum for 6–7 days, media were changed every 24 hours, and/or cells and media were collected each day. This 6- to 7-day acclimation allowed the cells to regain responsiveness to hCG after being desensitized by the ovulatory dose of hCG. After 6–7 days, these cells were serum starved for 1 hour and then treated with or without hCG. For the inhibitor experiments, cells were treated with one of the following for 1 hour prior to treatment with vehicle or hCG for 6 hours: progesterone receptor antagonist RU486 (1 μM); selective prostaglandin endoperoxide synthase (PTGS)-2 inhibitor NS-398 (1 μM); epithelial growth factor receptor tyrosine kinase inhibitor AG1478 (1 μM); or the inhibitor of PTGS1 and PTGS2, indomethacin (200 nM). Cells were processed for RNA expression as described below.
Immunostaining
Follicles were fixed in 4% formaldehyde, embedded in paraffin, sectioned (7 μm), and processed for immunostaining as previously described (16). The sections were incubated in a humidified chamber at 4°C overnight with CCL20 primary antibodies (AF360; R&D Systems) as shown in Table 2 at a concentration of 10 μg/mL. Biotinylated secondary antigoat antibody was used at a dilution of 1:500. The immunostaining was visualized using Vulcan Fast Red according to the manufacturer's protocol (Biocare). The sections were counterstained with hematoxylin. The negative control slides were prepared in an identical manner and processed without primary antibody.
Table 2.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|
| CCL20 | Antihuman CCL20/MIP-3α antibody | R&D Systems, AF360 | Goat | 10 μg/mL |
| CD3 | PE-Cy5 mouse antihuman CD3 | BD561007 | Mouse | 20 μL |
| CD14 | APC mouse antihuman CD14 | BD561708 | Mouse | 20 μL |
| CD56 | BV mouse antihuman CD56 | BD562779 | Mouse | 5 μL |
| CD45 | PE mouse antihuman CD45 | BD557059 | Mouse | 20 μL |
| CD196/CCR6 | PE-Cy 7 mouse antihuman CCR6 | BD560620 | Mouse | 5 μL |
| CD66b | FITC antihuman CD66b | Biolegend305104 | Mouse | 20 μL |
| CD19 | APC-Cy 7 mouse antihuman CD19 | BD561743 | Mouse | 5 μL |
RNA isolation and mRNA analysis
To examine CCL20 mRNA expression, in vivo or in vitro human RNA was isolated using an RNeasy kit from QIAGEN as per the manufacturer's protocol. One microgram of RNA was reverse transcribed using SuperScriptIII for use with real-time PCR according to the manufacturer's protocol as previously described (Invitrogen Life Technologies) (17). The mRNA expression was analyzed by real-time PCR using 20XTaqMan gene expression assay primers from Applied Biosystems for CCL20 (Hs00355476_m1), CCR6 (Hs01890706_s1), amphiregulin (AREG) (Hs00950669_m1), PTGS2 (Hs00153133_m1), progesterone receptor (PGR) (Hs01556702_m1), and CD45 (Hs04189704_m1) (Supplemental Table 1). The thermal cycling steps included the following: 2 minutes at 50°C to permit AmpErase uracil-N-glycosylase optimal activity, denaturation step for 10 minutes at 95°C, 15 seconds at 95°C, and 1 minute at 60°C for 50 cycles, followed by 1 minute at 95°C for 30 seconds at 58°C and 30 seconds at 95°C for ramp dissociation. The relative amount of mRNA in each sample was calculated following the 2−ΔΔCt method and normalized to glyceraldehyde-3-phosphate dehydrogenase.
Flow cytometry
Human GLCs were collected as described above. After Percoll gradient separation, cells were washed and treated with 1× ammonium chloride and potassium solution for 1 minute to remove red blood cells. About 2 × 105 cells were filtered through a 70-μm mesh to remove clumps of cells. Cells were incubated with 100 μL PBS with albumin and 0.02% NaN3 containing either 20 μL mouse antihuman CD45 (BD557059) or 5 μL mouse antihuman CCR6 (BD560620) or a combination of the two for 20 minutes in the dark on ice. Cells were centrifuged, washed, and fixed using 5% neutral buffered formalin for 20 minutes. Cells were washed and resuspended in 200 μL PBS and stored in the dark at 4°C until flow cytometry analysis (i-Cyt synergy). For determining the leukocyte subtypes in CD45+ and CCR6+ cells, the following antibodies were used: CD56 for natural killer (NK) cells (BD562779), CD3 for T cells (BD561007), CD66b for neutrophils (Biolegend305104), CD14 for monocytes (BD561708), and CD19 for B cells (BD561743). Antibodies used are shown in Table 2.
Progesterone assay
Progesterone levels were measured in the culture media using an Immulite1000 (Diagnostic Products Corp) as described previously (18). The assay has a sensitivity of 0.02 ng/mL. The intraassay and interassay coefficients of variation are 7 and 12, respectively.
Cell migration
Uncoated transwell assays were used to measure cell migration (Thermo Fisher). Warm serum-free RPMI 1640 media were added to the transwells to hydrate the membranes. Leukocytes were isolated from samples collected from women undergoing IVF in heparin vaccutainers (BD367871). Tubes were centrifuged, buffy coat was collected, and leukocytes were purified on a Ficoll-Paque (Life Sciences) gradient. Leukocytes were resuspended and added to the upper chamber of the transwells. CCL20 was added to the bottom (100 ng/mL in RPMI 1640 media). After 60–90 minutes, cells in the middle field of the bottom well were counted using Nikon Elements (Nikon). Data are expressed as the percentage of cells applied to the transwells that migrated through to the bottom of the transwells.
Statistical analysis
Data are presented as means ± SEM. A one- or two-way (ANOVA) with or without repeated measures was used to test differences among treatments as appropriate. Sample values were log transformed and an ANOVA was used. Tukey's post hoc test was performed to identify significant differences among treatments. Means were compared, with P ≤ .05 considered significant. Statistical analysis was performed using GraphPad Prizm (GraphPad version 5.00).
Results
CCL20 expression increases in human granulosa and theca cells collected in vivo across the periovulatory period
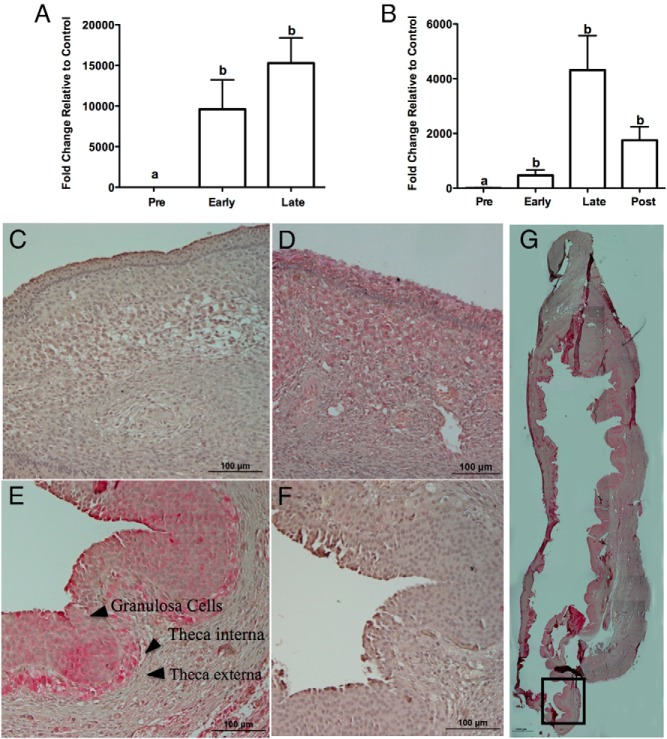
Administration of hCG resulted in a striking 10 000-fold increase in the expression of CCL20 mRNA in the granulosa cells between the PO and EO periods (Figure 1A). This hCG induction of CCL20 mRNA was sustained during the LO period. Similar to the granulosa cells, hCG induced CCL20 mRNA in the theca cells during the EO period, reaching a maximum of approximately 4000-fold at the LO (Figure 1B). To determine whether the protein expression mimicked the mRNA expression, immunohistochemistry was performed. Localization of CCL20 in sections of the dominant follicle at timed intervals throughout the periovulatory period revealed that CCL20 expression corresponds to the mRNA expression. CCL20 protein was absent in the PO (Figure 1C) and localized to granulosa and theca interna cells of follicles collected during the EO (Figure 1D) and LO stages (Figure 1E). A low-magnification image illustrating CCL20 immunostaining of the entire late preovulatory follicle is shown in Figure 1G. The staining demonstrates that CCL20 expression is found throughout the entire follicle including staining at the apex. Antibody specificity was determined using Western blot analysis, which revealed a band of the expected size (Supplemental Figure 1).
Figure 1.
CCL20 expression across the ovulatory period. CCL20 mRNA increases in the early and late ovulatory phase in human ovary granulosa (A) and theca (B) cells. Results are the means ± SEM of n = 4–5. Bars that do not share a letter designation are significantly different (P < .05). Immunostaining of CCL20 protein expression and localization in the different stages of the human ovulatory cycle is shown. CCL20 staining is shown for PO (C), EO (D), LO (E), and negative control (F). Images are representative images from three patient samples. Lower magnification composite of a late ovulatory follicle illustrates CCL20 immunostaining (G). The boxed area depicts the approximate area shown in panel E. Scale bars (C–F), 100 μm; scale bar (G), 1000 μm.
Leukocytes expressing CCR6 are present in the ovary immediately prior to ovulation
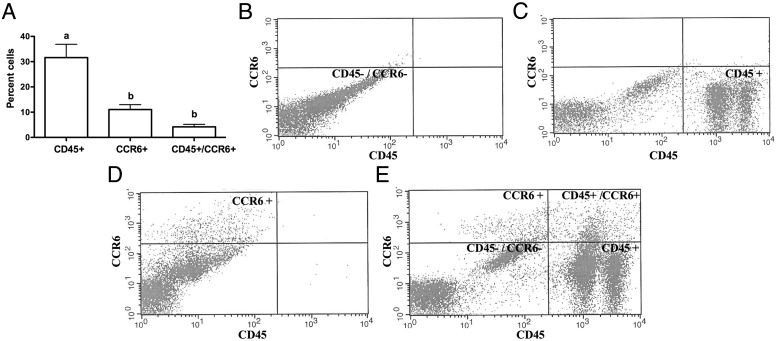
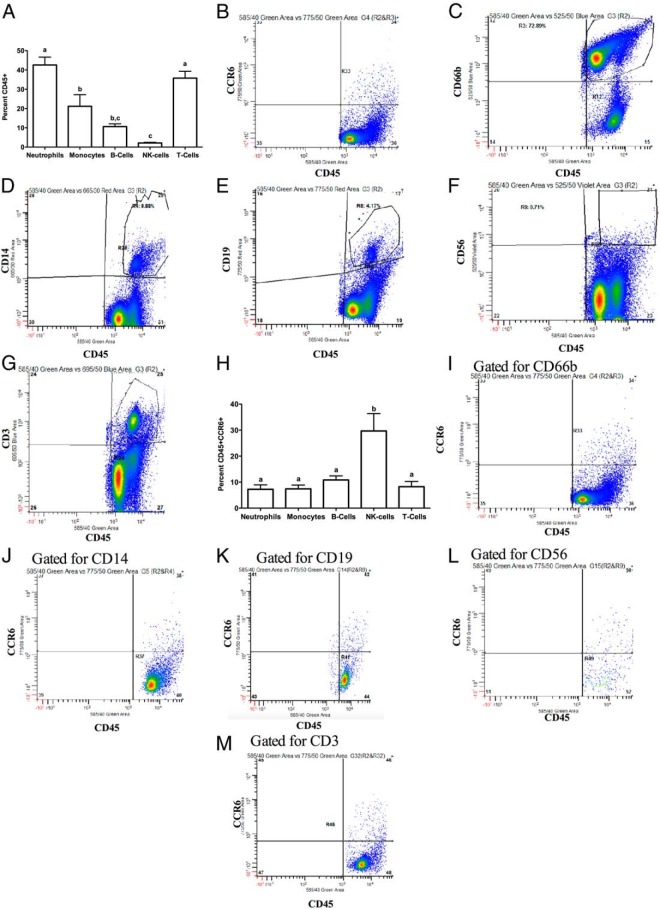
To determine the presence of leukocytes that express CCR6 immediately prior to ovulation, we determined the percentage of cells expressing CD45 as well as CCR6 in follicular aspirates obtained from our IVF clinic. By flow cytometry, approximately 30% of the cells in the aspirates express CD45 and are hence leukocytes, 10% of all the cells express only CCR6, and approximately 5% of the cells express both CD45 and CCR6 (Figure 2A). Representative histograms are shown (Figure 2, B–E). We next determined the subtypes of leukocytes in our IVF sample population (Figure 3A). Of the CD45+ cells, approximately 36% were T cells (CD3+), 42% were neutrophils (CD66b+), 21% were monocytes (CD14+), 10% were B cells (CD19+) and 2% were NK cells (CD56+). Representative histograms for flow cytometry are shown (Figure 3, B–G). Cells that were positive for CCR6 and CD45 were mainly NK cells (30%), neutrophils (7%), monocytes (7%), B cells (10%), and T cells (8%) (Figure 3H). Representative histograms for flow cytometry are shown (Figure 3, I–M). To evaluate whether CCL20 is capable of stimulating leukocyte migration, CCL20 was added to the bottom well of Boyden transwells and exogenous CCL20 stimulated leukocyte migration 59% within 60–90 minutes (Supplemental Figure 2).
Figure 2.
CCR6-positive leukocytes are present in human IVF samples. A, Number of CD45+ cells, CCR6+ cells, and double-positive (CD45+CCR6+) cells. The approach to determine these percentages is illustrated in panels B–D. Representative figure of an unstained IVF sample and one in which the gates were set (B) are shown. An IVF sample that was stained for only CD45 (C), stained for only CCR6 (D), or a representative patient sample (E) is also shown.
Figure 3.
Analysis of the leukocyte subtypes and the CCR6+ leukocyte population subtypes in human IVF samples. A, Distribution of various CD45+ leukocyte subtypes. Representative flow cytometry histograms for one patient are shown (B–H). B, Side scatter plot for cells stained for CD45+. Panels C–H depict an IVF sample that was stained for CD45 in conjunction with antibodies for one of the following: CCR6 (B), neutrophils (CD66b) (C), monocytes (CD14) (D), B cells (CD19) (E), NK cells (CD56) (F), or T cells (CD3) (G). The distribution of leukocyte subtypes that are CCR6+ is shown (H). Panels I–M are representative flow cytometry histograms for one patient. Panels depict an IVF sample that was stained for CD45 and CCR6 in conjunction with antibodies for one of the following: neutrophils (CD66b) (I), monocytes (CD14) (J), B cells (CD19) (K), NK cells (CD56) (L), or T cells (CD3) (M). Results are means ± SEM of n = 7 each. Bars that do not share a letter designation are significantly different (P < .05).
CCL20 expression increases in response to hCG administration in human GLCs in vitro
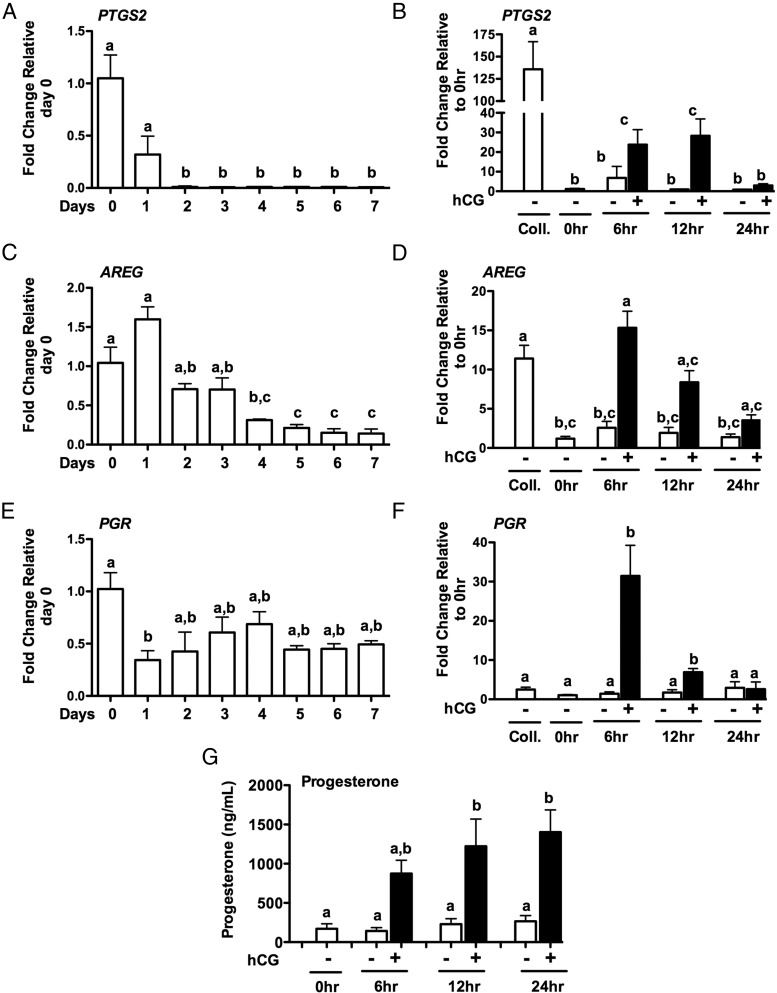
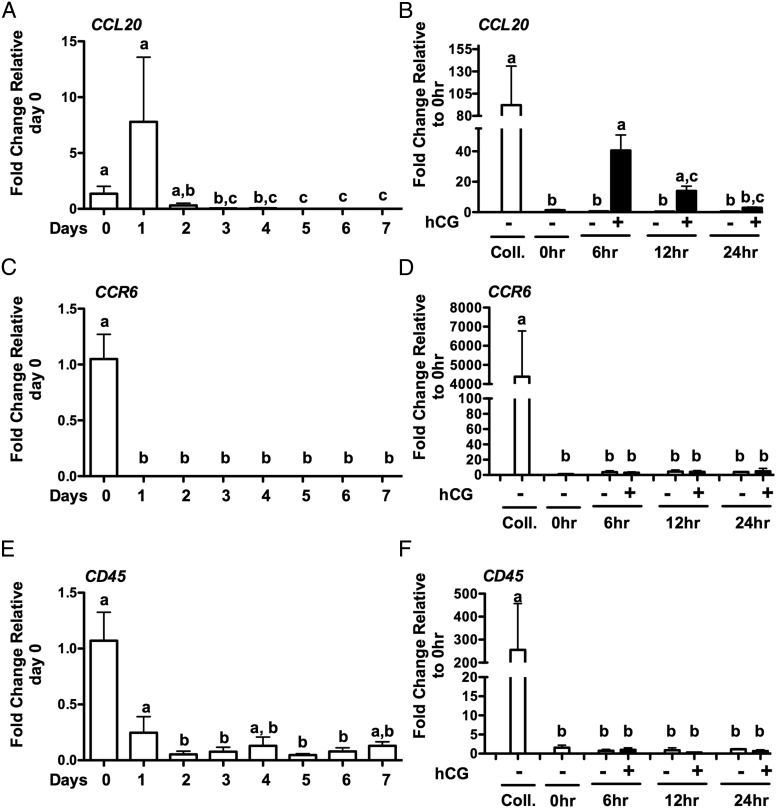
To begin to understand the regulation of CCL20, we used a model in which GLCs are collected after IVF and cultured for 6–7 days to allow the cells to regain hCG responsiveness (19). This model has been previously used to examine the hCG regulation of amphiregulin (AREG) and epiregulin (EREG) (19). To confirm that the in vitro culture model is responsive to hCG, the expression of known hCG-induced genes were measured. PTGS2, AREG, and PGR mRNA expression levels were measured across days in culture (Figure 4, A, C, and E) and after treatment with hCG (Figure 4, B, D, and F). PTGS2 (Figure 4A), AREG (Figure 4C) and PGR (Figure 4E) mRNA expression decreased across days but increased in response to hCG treatment (Figure 4, B, D, and F, respectively). Progesterone production did not significantly change across days in culture but increased in response to hCG (Figure 4G). These data demonstrate that these revitalized GLCs are responsive to hCG treatment. The expression of CCL20 and CCR6 was then measured throughout the culture period. Both CCL20 and CCR6 mRNA levels decreased across days in culture (Figure 5, A and C). Likewise, expression of the leukocyte marker CD45 decreased across days of culture (Figure 5E). To determine whether the expression of these genes can be induced in GLCs in culture via hCG, mRNA induction of CCL20 (Figure 5B), CCR6 (Figure 5D), and CD45 (Figure 5F) was examined. CCL20 mRNA increased 6 hours after hCG treatment to about 40-fold (Figure 5B) while CCR6 (Figure 5D) and CD45 (Figure 5F) did not change. The level of CCL20 mRNA induced in the GLCs is not as robust as in the in vivo samples; however, the induction of CCL20 in vitro approximates levels seen in IVF GLCs collected immediately prior to ovulation. Furthermore, the hCG induction of CCL20 mRNA correlated with progesterone production compared with control GLCs treated without hCG (P < .001). However, CCL20 added exogenously did not alter progesterone production in the absence or presence of hCG, indicating that CCL20 does not impact progesterone production (data not shown).
Figure 4.
Human GLC expression of ovulatory genes in vitro. Human GLCs were cultured for 7 days and then serum starved for 1 hour in the presence or absence of hCG or vehicle control. Expression of mRNA for PTGS2 (A), AREG (C), and PGR (E) across days of culture and after temporal treatment of GLCs with hCG for PTGS2 (B), AREG (D), and PGR (F). Progesterone secretion increased after hCG treatment (G). Results are the means ± SEM of n ≥ 3. Coll, samples at the time of IVF collection. 0 hours represents samples after 6–7 days in culture at the initiation of hCG treatment. Bars that do not share a letter designation are significantly different (P < .05).
Figure 5.
Human GLC expression of target genes in vitro. Human GLCs were cultured for 7 days and then serum starved for 1 hour in the presence or absence of hCG or vehicle control. Expression of mRNA for CCL20 (A), CCR6 (C), and CD45 (E) across days of culture and after temporal treatment of GLCs with hCG for CCL20 (B), CCR6 (D), and CD45 (F). Results are the means ± SEM of n = 3. Coll, samples at the time of IVF collection. 0 hours represents samples after 6–7 days in culture at the initiation of hCG treatment. Bars that do not share a letter designation are significantly different (P < .05).
CCL20 regulation in human GLCs cells in vitro
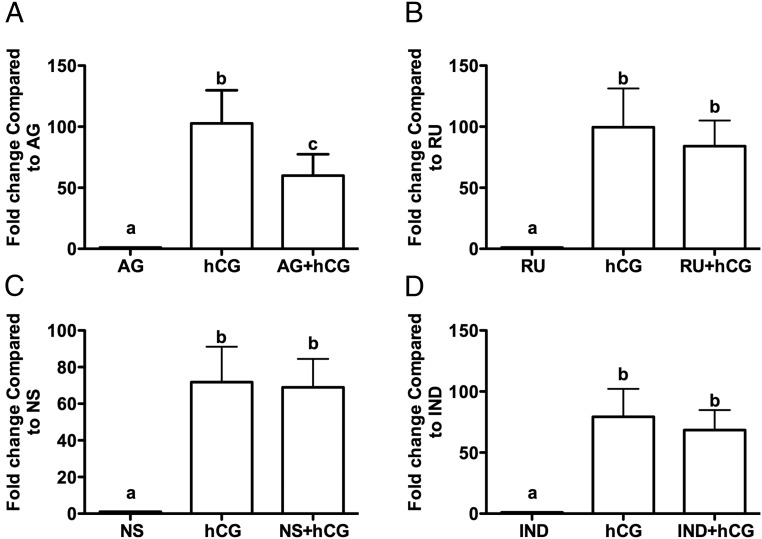
To begin to understand the regulation of CCL20, GLCs were cultured in the presence or absence of inhibitors or activators of major LH/hCG signaling pathways. The LH surge induces expression of the progesterone receptor, prostaglandin production, and the epithelial growth factor (EGF)-related peptides, such as AREG and epiregulin, all of which have been shown to play a crucial role in the ovulatory process (19, 20). Treatment of GLCs with specific pathway inhibitors revealed that hCG induction of CCL20 mRNA expression was blocked approximately 40% by AG1478, suggesting partial regulation by the EGF pathway, whereas RU486 and indomethacin were without effect (Figure 6, A–D).
Figure 6.
The EGF pathway is involved in the induction of CCL20 expression in GLCs in vitro. Human GLCs were cultured for 7 days and then serum starved for 1 hour in the presence or absence of the following inhibitors: EGF receptor tyrosine kinase selective inhibitor, AG1478 (AG; 1 μM) (A), RU486 (RU; 1 μM) (B), PTGS-2 inhibitor, NS-398 (NS; 1 μM) (C), or the inhibitor of PTGS-1 and PTGS-2, indomethacin (200 nM) (D). Cells were then treated with or without hCG for 6 hours. Results are the means ± SEM. Bars that do not share a letter designation are significantly different (P < .05).
Discussion
The present study is the first identification of a specific chemokine, CCL20, induced in the dominant follicle during the periovulatory period in the human ovary. One of the major strengths of this study is the use of well-defined ovarian tissues collected to identify a potential new mediator in the ovulatory process. The dominant follicle was collected across the periovulatory period from patients that had proven fertility and regular menstrual cycles and without hormonal treatments for at least 3 months, and thus, the data have direct relevance to human fertility. The present findings reveal the presence and increase of both RNA and protein for CCL20 in human tissues. The expression of CCL20 was more pronounced in the granulosa cells layer as indicated by the mRNA profiles and as illustrated with immunostaining. In addition, the staining was observed throughout the entire follicle including staining at the apex. Evidence in this manuscript is provided that leukocytes collected from the ovary at the time of ovulation contain receptors for CCL20 and that this chemokine causes leukocyte migration.
Espey (1) proposed that ovulation was an inflammatory reaction that was characterized by an influx of leukocytes. This influx of leukocytes has been reported in numerous species including human (21), ovine (22), rabbit (23), and rodent (2, 24). For example, in the rodent the number of leukocytes infiltrating the rat ovary prior to ovulation increases 5-fold after hCG (2). This influx is postulated to occur in part through LH mediated pathways that increase vasodilation and vascular permeability, allowing the transmigration of leukocytes from the blood vessels to the preovulatory follicle (25). Mice lacking genes that are responsible for the LH mediated inflammatory reaction, such as the PGR (26, 27), PTGS2 (28), or increased vasodilation, such as endothelin 2 (29), fail to ovulate or have reduced ovulatory capacity (30). However, the importance of leukocytes in the ovulatory process is evident from observations that the number of oocytes released increased approximately 3-fold when leukocytes were added to perfused rat ovaries (4), whereas depletion of leukocytes from the blood decreased the number of ovulations (3, 5). Furthermore, leukocytes were able to increase progesterone production when added to cultured granulosa cells (6, 7). However, the present findings indicate that the leukocyte induced increase in progesterone is not mediated by CCL20. These observations taken in toto suggest that the influx of leukocytes plays a key role in the normal periovulatory processes associated with follicular rupture.
An important question is what are the exact signals for leukocyte recruitment that set in motion the ovulatory inflammatory cascade, particularly in the human? There is evidence that the ovary produces a number of chemokines that may function for leukocyte recruitment, adhesion, activation, and chemotaxis (4). In the present study, the chemokine CCL20 is markedly induced after an hCG stimulus. CCL20 is known to attract immature dendritic B and T cells and selectively binds to the CCR6 receptor (10). CCL20 has also been shown to increase the capability of regulatory T cells (Th17) to migrate to inflammatory sites (11). Interestingly, the presence and elevation of regulatory T cells in the blood of patients undergoing IVF was positively correlated with pregnancy and birth rates (31). We have found that CCR6 was highly abundant on NK cells (30% CCR6 positive) and was also found on T cells (8% CCR6 positive) and B cells (10% CCR6 positive). CCR6 was also present on macrophages and neutrophils, both of which increase in the ovary at the time of ovulation and early corpus luteum formation (32). The GLC samples acquired from the IVF clinic contained approximately 35% CD45+ cells, which is similar to the findings of Wang et al (33), who observed approximately 30% of cells in IVF samples were leukocytes. The presence of CCR6 on these leukocytes can be interpreted that CCL20 may recruit these cells to the ovary at the time of ovulation because CCR6 is the sole identified receptor for CCL20 (8). This interpretation is based on the fact that CCR6 has been identified in numerous cell types in the lymphatic system in which it plays a chemotactic role (34) and our findings that CCL20 stimulates leukocyte migration. Furthermore, the interaction of CCL20 with CCR6 has been implicated in a number of inflammatory conditions such as inflammatory bowel disease (35), rheumatoid arthritis (35), asthma (36), chronic pulmonary disease (36), and tumor cell metastasis (34). Hence, the role that CCL20-CCR6 plays in inflammation in other systems, in conjunction with the hypothesis of ovulation being an inflammatory process, suggests that CCL20-CCR6 has a role in the ovulatory process.
There are limited reports on the expression of CCL20 and CCR6 in the ovary. CCL20 has been reported to be present in human follicular fluid collected at the time of IVF and its expression is correlated with oocyte maturation (12). A second report identified CCL20 as one of the genes overexpressed in the cumulus cells collected from PCOS patients (13) indicating a potential association in the manifestation of PCOS. The present study is the first report to our knowledge in which CCL20 is identified in the granulosa and theca cell compartments of the dominant human follicle across the periovulatory period. To compare CCL20 expression in other species, CCL20 was examined in the immature gonadotropin primed rat ovary. The rat ovary expressed both CCL20 and CCR6 mRNA albeit in very low amounts. The expression increased in the whole ovary at 8 hours, but when separated, the granulosa cell expression of CCL20 was low and not stimulated by hCG, whereas the theca cells expressed CCL20 at low levels but expression was increased at 8 hours after hCG (data not shown). This indicates that there are species differences in terms of the expression and regulation of CCL20 in the ovary.
To explore the hCG-mediated regulation of CCL20, we used a model whereby GLCs are cultured for 7 days to regain gonadotropin responsiveness due to the difficulty in acquiring or manipulating in vivo ovarian tissues. The validity of this revitalized in vitro system was demonstrated by the ability of hCG to induce key ovulatory genes, such as AREG, PTGS2, and PGR (37). Using this model, we were able to demonstrate that hCG induced CCL20 mRNA expression and was mediated partially through the EGF pathway. These findings are similar to data from ovarian cancer cells in which CCL20 is induced when cells are treated with EGF (38). Likewise in other systems such as human keratinocytes (39), the EGF signaling pathway regulates CCL20 expression acting through mediators such as IL-6 (40).
CCL20 has been identified using a microarray approach as one of the genes overexpressed in cumulus cells of PCOS patients and that it is differentially expressed between lean and obese PCOS patients (13). The presence of proinflammatory markers was noted in samples from PCOS patients (41). Interleukins such as IL-6 have been previously implicated in the regulation of regulatory T cells (42), which have also been shown to express CCR6 and are recruited by CCL20 (11). IL-6 has been shown to regulate the expression of CCL20 and vice versa (40, 43). IL-6 levels in a PCOS study were positively correlated with body mass index (BMI) and triglycerides (41). Thus, although IL-6 was elevated in high BMI PCOS patients, this increase was not necessarily due to PCOS but rather due to an increased BMI. This was evident when BMI-matched control IL-6 levels were measured and compared with those of PCOS high BMI patients (41). Similarly, the levels of cytokine mRNA for IL-6, IL-8, and IL-10 were not affected by the PCOS diagnosis (44). Thus, the association between IL-6, BMI, PCOS, and CCL20 remains unclear.
PCOS is known to present itself as a string of pearls, which is due to incomplete follicular growth and/or loss of ovulation. Hence, atresia is reported to be altered in PCOS (45, 46). The process of follicular atresia involves the removal of apoptotic debris, which requires the presence of macrophages (47). Thus, one possibility is that in PCOS, CCL20 acts to signal the influx of macrophages to regulate the process of follicular atresia.
In conclusion, the collection of unique, well-defined, and timed in vivo dominant follicles has allowed the identification for the first time a specific chemokine, CCL20, that is up-regulated in the human ovary (Figure 7). We postulate that CCL20 secreted by granulosa cells causes the recruitment of leukocytes from the blood stream and a drop in the number of leukocytes in patients (48) as has been reported in the rodent (2). These leukocytes are then activated and secrete matrix metalloproteinases that assist in the remodeling of the ovarian follicle to bring about breakdown of the follicular wall and luteal formation (3). Interestingly, CCL20 may be a possible target for ovarian hyperstimulation in which there is a notably high level of leukocytes and is correlated with early pregnancy loss (49). Thus, the present discovery opens new avenues of investigation into the processes of human ovulation and potential targets to better understand the underlying reasons behind different infertility causes.
Figure 7.
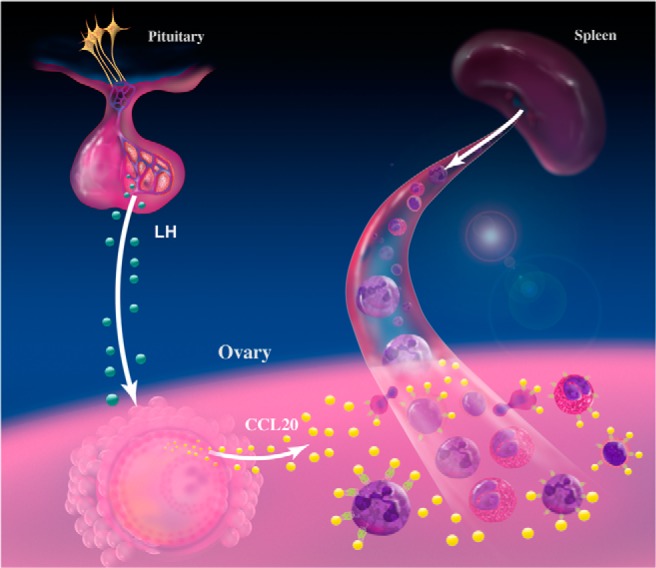
Proposed model for CCL20 action. The LH surge leads to an increased expression of CCL20 in the granulosa and theca cells of the dominant follicle. The presence of CCL20 causes leukocytes from the bloodstream that have CCR6 to migrate into the ovary to aid in the breakdown of the follicular wall leading to oocyte expulsion.
Acknowledgments
We thank Dr Misung Jo and Dr Ji Yeon Park for the critical evaluation of this manuscript. In addition, we acknowledge Dr Corrine Williams and Ms Ibitola Asaoula for their statistical assistance and Ms Carole Bryant for her assistance in determining the progesterone levels.
This work was supported by National Institutes of Health Grants HD057446 (to T.E.C.), HD071875 (to T.E.C.), and R03 HD071291 (to T.E.C.) and the Swedish Research Council Grant 11607 (to M.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AREG
- amphiregulin
- BMI
- body mass index
- CCL20
- chemokine ligand 20
- EGF
- epithelial growth factor
- EO
- early ovulatory phase
- GLC
- granulosa-lutein cell
- hCG
- human chorionic gonadotropin
- IVF
- in vitro fertilization
- LO
- late ovulatory phase
- NK
- natural killer
- PCOS
- polycystic ovarian syndrome
- PGR
- progesterone receptor
- PO
- preovulatory phase
- PTGS
- prostaglandin endoperoxide synthase.
References
- 1. Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod. 1980;22(1):73–106. [DOI] [PubMed] [Google Scholar]
- 2. Oakley OR, Kim H, El-Amouri I, et al. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology. 2010;151(9):4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bukulmez O, Arici A. Leukocytes in ovarian function. Hum Reprod Update. 2000;6(1):1–15. [DOI] [PubMed] [Google Scholar]
- 4. Brannstrom M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol. 2002;57(1–2):47–60. [DOI] [PubMed] [Google Scholar]
- 5. Brannstrom M, Bonello N, Norman RJ, Robertson SA. Reduction of ovulation rate in the rat by administration of a neutrophil-depleting monoclonal antibody. J Reprod Immunol. 1995;29(3):265–270. [DOI] [PubMed] [Google Scholar]
- 6. Emi N, Kanzaki H, Yoshida M, et al. Lymphocytes stimulate progesterone production by cultured human granulosa luteal cells. Am J Obstet Gynecol. 1991;165(5 Pt 1):1469–1474. [DOI] [PubMed] [Google Scholar]
- 7. Halme J, Hammond MG, Syrop CH, Talbert LM. Peritoneal macrophages modulate human granulosa-luteal cell progesterone production. J Clin Endocrinol Metab. 1985;61(5):912–916. [DOI] [PubMed] [Google Scholar]
- 8. Townson DH, Liptak AR. Chemokines in the corpus luteum: implications of leukocyte chemotaxis. Reprod Biol Endocrinol. 2003;1:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soboll G, Crane-Godreau MA, Lyimo MA, Wira CR. Effect of oestradiol on PAMP-mediated CCL20/MIP-3α production by mouse uterine epithelial cells in culture. Immunology. 2006;118(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14(5):409–426. [DOI] [PubMed] [Google Scholar]
- 11. Hirata T, Osuga Y, Takamura M, et al. Recruitment of CCR6-expressing Th17 cells by CCL 20 secreted from IL-1β-, TNF-α-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology. 2010;151(11):5468–5476. [DOI] [PubMed] [Google Scholar]
- 12. Kawano Y, Fukuda J, Nasu K, Nishida M, Narahara H, Miyakawa I. Production of macrophage inflammatory protein-3alpha in human follicular fluid and cultured granulosa cells. Fertil Steril. 2004;82(suppl 3):1206–1211. [DOI] [PubMed] [Google Scholar]
- 13. Kenigsberg S, Bentov Y, Chalifa-Caspi V, Potashnik G, Ofir R, Birk OS. Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod. 2009;15(2):89–103. [DOI] [PubMed] [Google Scholar]
- 14. Thoroddsen A, Dahm-Kahler P, Lind AK, et al. The water permeability channels aquaporins 1–4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J Clin Endocrinol Metab. 2011;96(4):1021–1028. [DOI] [PubMed] [Google Scholar]
- 15. Lind AK. Human ovulation: studies on collagens, gelatinases and tissue inhibitors of metalloproteinases [dissertation]. Göteborg, Sweden: The Sahlgrenska Academy at Göteborg Universit; 2006. [Google Scholar]
- 16. Rosewell KL, Li F, Puttabyatappa M, Akin JW, Brannstrom M, Curry TE., Jr Ovarian expression, localization, and function of tissue inhibitor of metalloproteinase 3 (TIMP3) during the periovulatory period of the human menstrual cycle. Biol Reprod. 2013;89(5):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lind AK, Dahm-Kahler P, Weijdegard B, Sundfeldt And K, Brannstrom M. Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloproteinase-1. Mol Hum Reprod. 2006;12(12):725–736. [DOI] [PubMed] [Google Scholar]
- 18. Park ES, Choi S, Muse KN, Curry TE, Jr, Jo M. Response gene to complement 32 expression is induced by the luteinizing hormone (LH) surge and regulated by LH-induced mediators in the rodent ovary. Endocrinology. 2008;149(6):3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324(2):829–834. [DOI] [PubMed] [Google Scholar]
- 20. Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN. Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol. 1998;145(1–2):47–54. [DOI] [PubMed] [Google Scholar]
- 21. Yang Z, Kong B, Mosser DM, Zhang X. TLRs, macrophages, and NK cells: our understandings of their functions in uterus and ovary. Int Immunopharmacol. 2011;11(10):1442–1450. [DOI] [PubMed] [Google Scholar]
- 22. Murdoch WJ, McCormick RJ. Production of low molecular weight chemoattractants for leukocytes by periovulatory ovine follicles. Biol Reprod. 1989;41(1):86–90. [DOI] [PubMed] [Google Scholar]
- 23. Gerdes U, Gafvels M, Bergh A, Cajander S. Localized increases in ovarian vascular permeability and leucocyte accumulation after induced ovulation in rabbits. J Reprod Fertil. 1992;95(2):539–550. [DOI] [PubMed] [Google Scholar]
- 24. Gaytan F, Morales C, Bellido C, Tarradas E, Sanchez-Criado JE. Effects of indomethacin on ovarian leukocytes during the periovulatory period in the rat. Reprod Biol Endocrinol. 2003;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brannstrom M, Mayrhofer G, Robertson SA. Localization of leukocyte subsets in the rat ovary during the periovulatory period. Biol Reprod. 1993;48(2):277–286. [DOI] [PubMed] [Google Scholar]
- 26. Hashimoto-Partyka MK, Lydon JP, Iruela-Arispe ML. Generation of a mouse for conditional excision of progesterone receptor. Genesis. 2006;44(8):391–395. [DOI] [PubMed] [Google Scholar]
- 27. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 28. Lim H, Paria BC, Das SK, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. [DOI] [PubMed] [Google Scholar]
- 29. Cacioppo JA, OH SW, Kim HY, et al. Loss of function of endothelin-2 leads to reduced ovulation and CL formation. PLoS One. 2014;9(4):e96115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akison LK, Robker RL. The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction. Reprod Domest Anim. 2012;47(suppl 4):288–296. [DOI] [PubMed] [Google Scholar]
- 31. Zhou J, Wang Z, Zhao X, Wang J, Sun H, Hu Y. An increase of Treg cells in the peripheral blood is associated with a better in vitro fertilization treatment outcome. Am J Reprod Immunol. 2012;68(2):100–106. [DOI] [PubMed] [Google Scholar]
- 32. Brannstrom M, Pascoe V, Norman RJ, McClure N. Localization of leukocyte subsets in the follicle wall and in the corpus luteum throughout the human menstrual cycle. Fertil Steril. 1994;61(3):488–495. [PubMed] [Google Scholar]
- 33. Wang LJ, Brannstrom M, Pascoe V, Norman RJ. Cellular composition of primary cultures of human granulosa-lutein cells and the effect of cytokines on cell proliferation. Reprod Fertil Dev. 1995;7(1):21–26. [DOI] [PubMed] [Google Scholar]
- 34. Ghadjar P, Rubie C, Aebersold DM, Keilholz U. The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. Int J Cancer. 2009;125(4):741–745. [DOI] [PubMed] [Google Scholar]
- 35. Comerford I, Bunting M, Fenix K, et al. An immune paradox: how can the same chemokine axis regulate both immune tolerance and activation? CCR6/CCL20: a chemokine axis balancing immunological tolerance and inflammation in autoimmune disease. Bioessays. 2010;32(12):1067–1076. [DOI] [PubMed] [Google Scholar]
- 36. Ito T, Carson WF, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317(5):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. [DOI] [PubMed] [Google Scholar]
- 38. Son DS, Kabir SM, Dong Y, Lee E, Adunyah SE. Characteristics of chemokine signatures elicited by EGF and TNF in ovarian cancer cells. J Inflamm (Lond). 2013;10(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnston A, Gudjonsson JE, Aphale A, Guzman AM, Stoll SW, Elder JT. EGFR and IL-1 signaling synergistically promote keratinocyte antimicrobial defenses in a differentiation-dependent manner. J Invest Dermatol. 2011;131(2):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hosokawa Y, Shindo S, Hosokawa I, Ozaki K, Matsuo T. IL-6 trans-signaling enhances CCL20 production from IL-1beta-stimulated human periodontal ligament cells. Inflammation. 2014;37(2):381–386. [DOI] [PubMed] [Google Scholar]
- 41. Kucuk M, Altinkaya SO, Nergiz S, et al. Interleukin-6 levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2014;30(6):423–427. [DOI] [PubMed] [Google Scholar]
- 42. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. [DOI] [PubMed] [Google Scholar]
- 43. Tanida S, Yoshitomi H, Nishitani K, et al. CCL20 produced in the cytokine network of rheumatoid arthritis recruits CCR6+ mononuclear cells and enhances the production of IL-6. Cytokine. 2009;47(2):112–118. [DOI] [PubMed] [Google Scholar]
- 44. Wu R, Fujii S, Ryan NK, et al. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. 2007;22(2):527–535. [DOI] [PubMed] [Google Scholar]
- 45. Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15(6):707–724. [DOI] [PubMed] [Google Scholar]
- 46. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–378. [DOI] [PubMed] [Google Scholar]
- 47. Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10(2):119–133. [DOI] [PubMed] [Google Scholar]
- 48. Giuliani A, Mitterhammer H, Burda A, Egger G, Glasner A. Polymorphonuclear leukocyte function during the menstrual cycle and during controlled ovarian hyperstimulation. Fertil Steril. 2004;82(6):1711–1713. [DOI] [PubMed] [Google Scholar]
- 49. Qublan HS, Amarin ZO, Abu-Salem AN, Malkawi HY. Miscarriage and clinical correlates of leukocyte count in patients with ovarian hyperstimulation syndrome. J Obstet Gynaecol. 2009;29(4):318–321. [DOI] [PubMed] [Google Scholar]