Abstract
Background:
Interleukin 13 (IL13) is directly involved in the secretion of total serum immunoglobulin E (IgE), which plays a major role in the asthma pathogenesis.
Objective:
One of the polymorphic receptor of IL13 is IL13Rα1, which after binding to IL13, initiates signal transduction that results in mucin secretion, airway hyperreactivity, fibrosis, and chitinase up-regulation, which increases asthma risk.
Methods:
In the present study, the role of IL13Rα1 +1398A/G gene polymorphisms in asthma was detected with a total of 964 individuals, including 483 healthy controls and 481 asthma patients from a North Indian population using polymerase chain reaction-restriction fragment length polymorphism method.
Results:
Statistical analysis revealed that the mutant allele (G) is predominant in asthma patients (42.7%) than the controls (38.2%), which shows an increased risk toward asthma with odds ratio = 1.21, 95% confidence interval (1.00–1.45), χ2 = 4.10 and p = 0.043. Furthermore, the phenotypic characteristics also reveal a significant association with the disease (p < 0.05).
Conclusions:
This is the first study conducted in India and +1398A/G polymorphism in noncoding region of IL13Rα1 confer risk toward asthma in the studied population.
Keywords: Asthma, interleukin 13Rα1, North India, polymorphism, total immunoglobulin E
Over the past three decades, asthma problems have increased considerably worldwide, which leads to disability and the use of maximum health resources of a country. The risk of disease occurs due to interactions between more than 100 susceptibility genes and multiple environmental factors.1,2 Asthma has strong genetic components, because its heritability has been estimated to vary from 40% to 60%.3 Thus, it is important to identify the gene variants that play important role in the pathogenesis of asthma. Although interleukin (IL)-13 gene is studied extensively and found to be associated with asthma, IL13Rα1 association with asthma is still not well characterized.
The Th2 cells secrete various cytokines, such as IL-4, IL-5, IL-6, IL-9, IL-13, that are important regulator of inflammatory immune responses contributing atopy and immunity to parasites.4 IL13 is directly involved in total immunoglobulin E (IgE) secretion by stimulating B cells.5 It contains two receptors, IL13Rα1 and IL13α2,6 and IL13Rα1 is a 65- to 70-kDa glycosylated protein that binds to IL13 along with IL4Rα, which initiates signal transduction that results in mucin secretion, airway hyperreactivity, fibrosis, and chitinase up-regulation, which increases asthma risk.7,8 IL13Rα1 is expressed on nearly every cell, except human T cells.9 The definite roles of IL13Rα1 in the IL13 signaling and response are still not very clear. Recent study conducted on a mouse model establishes the critical role of IL13Rα1 in aeroallergen-induced mucous secretion, IgE production, and eosinophil recruitment through chemokines production, which leads to the development of the disease.10
The human IL13Rα1 gene is located on the chromosome Xq24. Because IL13s play a central role in the pathogenesis of asthma,4,5 the genetic variation in their receptors may also leads to the development of the asthma severity. Structure analysis found that the IL13Rα1 gene contains 88-bp coding sequence followed by 13-kbp intron and +1398A/G polymorphism lies in the noncoding region of IL13Rα1, which is associated with the increased IgE levels.11 Literature refers very few studies conducted on the role of +1398A/G polymorphism in asthma phenotypes.12 We are the first to report the impact of IL13Rα1 polymorphism in the pathogenesis of asthma in the North Indian population.
MATERIALS AND METHODS
Ethical Clearance
This study was conducted after receiving ethical clearance from Ethics Committee, Postgraduate Institute of Medical Education and Research, Chandigarh, India, vide Approval Memo No. PG-1Trg-10 on 9/21/2010. After doctors' diagnosis and the patients who fulfilled the criteria of Global Initiative for Asthma, guidelines were recruited for the study.
Inclusion/Exclusion Criteria
Recruitment of patients for this study are from different states of North India, including Chandigarh, Punjab, Haryana, Himachal Pradesh, Uttaranchal, Jammu and Kashmir, Rajasthan, Uttar Pradesh, and New Delhi. A total of 481 patients were enrolled as cases visiting Out Patient Department, Pulmonary Medicine at Postgraduate Institute of Medical Education and Research, Chandigarh. The other recruits were 483 age-matched, healthy controls, without any symptoms of atopic, pulmonary disease, any other comorbid disease or smoking habits were recruited as controls.
Asthma patients with any other history of respiratory illness like chronic obstructive pulmonary disease, tuberculosis, pneumonia, or bronchitis were excluded in this study. Apart from these, any other comorbid illness such as diabetes mellitus, hypertension, or pregnant females were also excluded as cases from this study.
Pulmonary Function Test
Spirometry measures several aspects of lung function that are important in determining both the severity and prevention of asthma. Spirometry tests were performed strictly in accordance with the Association of Respiratory Technician and Physiologists guidelines13 for generating pneumotachographs, helpful in assessing conditions such as asthma, chronic obstructive pulmonary disease, etc. using device Spiro 233 (PK Morgan, Rainham, Kent, United Kingdom). Out of 481 asthma patients, spirometry was done in 377 asthmatics, and the frequency of mild obstruction and AA genotype was found to be higher in the studied population.
Allergy Screening Tests
Allergic diseases, including asthma, are characterized by an increase of total IgE levels. It initiates and propagates the inflammatory cascade that leads to allergic responses in asthma. Total IgE was measured using ImmunoCAPs with device Phadia 100 IDM version 5.43 (Thermo Fisher Scientific Inc.) in serum samples of both control and asthma patients to screen allergy. Skin Prick Test and serum-specific IgE against Aspergillus fumigatus were also done and only negative Skin Prick Test patients with specific IgE less than 0.35 KUA/L were recruited in the study (Table 1). Certain allergens (cow's milk, peanut, wheat, molds, pollens, pet dander, dust mite, etc.) are diluted and applied with a prick using a very thin needle on the surface of the skin (intradermal). After 15 minutes, the area of the skin was observed to see the reaction. If a raised, red, itchy bump develops, it indicates the presence of the allergy antibody. The larger the bump, the greater the sensitivity for that allergen in the individual.
Table 1.
Characteristic of studied population
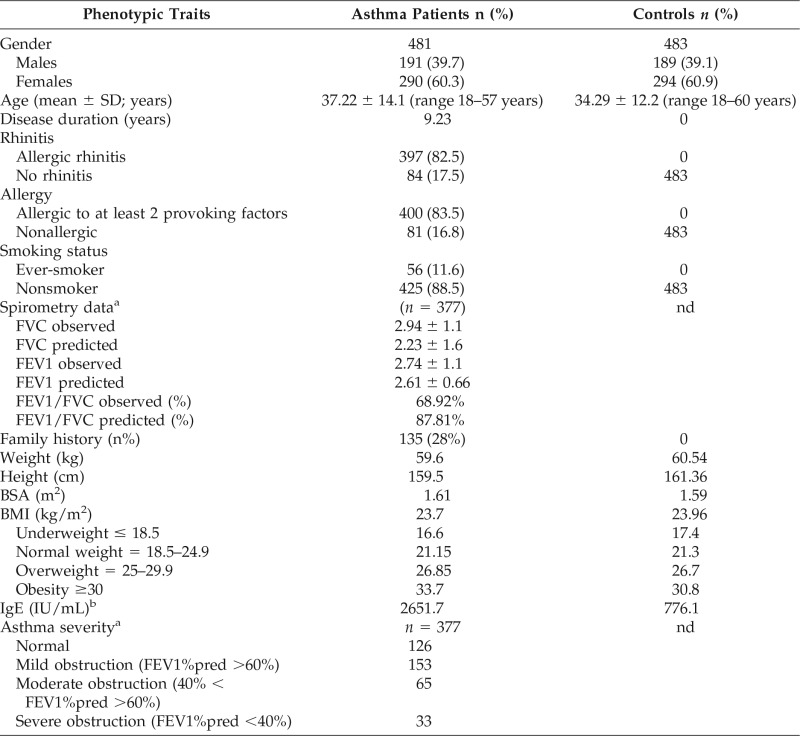
n = number of subjects sampled; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; BSA = body surface area; nd = not done; % = frequency; pred = predicted; SD = standard deviation; BMI = body mass index; IgE = Immunoglobulin E.
aSpirometry test was conducted for 276 asthma patients.
bIgE levels were confirmed for 213 asthma patients and 125 controls and given as average in IU/mL.
The usually accepted upper limit is between 150 and 300 IU/mL, but it can range from 150 to 1000 IU/mL.14 These variations in the range are because of changes in the diet, genetic background, geographical location, and other factors.15
Body Mass Index (BMI)
Increased BMI exaggerates the risk of acquiring asthma. BMI is a measure of human body shape based on an individual's weight and height, which indicate underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (>30 kg/m2). Apart from this, weight (kg), height (cm), and body surface area were also provided.
Blood Sample Collection
Approximately 5-mL blood was taken in EDTA-coated vials for genomic DNA extraction using saline sodium citrate buffer method16 and checked on 0.8% agarose gel by electrophoresis.
Genotyping
The amplification of the IL13Rα1 +1398A/G polymorphism was done using polymerase chain reaction-restriction fragment length polymorphism method17 using MseI (New England BioLabs, Hitchin, United Kingdom) (Fig. 1). The primer sequences were: Forward 5′-TCAGTGATGGAGATAATTTA-3′ and Reverse 5′-TGAGCTGCCTGTTTATAAAT-3′.
Figure 1.
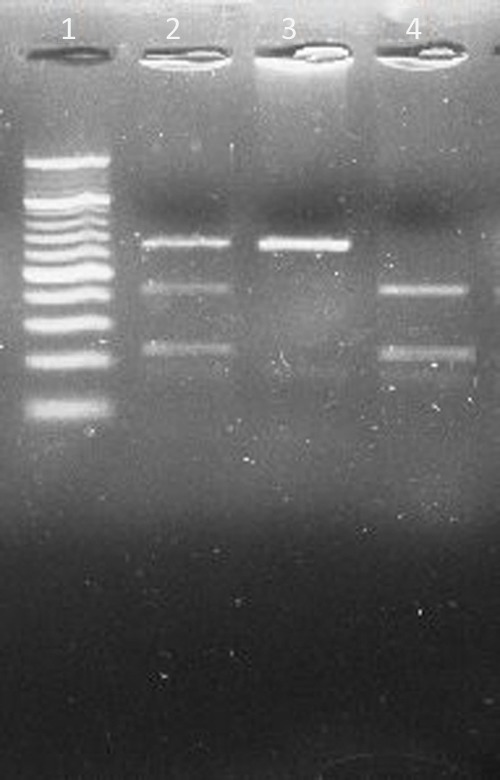
Restriction digestion (MseI) products of IL13Rα1 +1398A//G polymorphism on 3% Agarose gel. Lane 1: 20 bp ladder. Lane 2: heterozygous AG genotype (130, 85, and 45 bp). Lane 3: homozygous mutant GG genotype (130 bp). Lane 4: homozygous wild AA genotype (85 and 45 bp).
Statistical Analysis
All the statistical analyses were performed using the SPSS software version 20.0 (SPSS, Inc., Chicago, IL) and Epi Info version 3.4.7 (CDC, Atlanta, GA). χ2 analysis was used to check the deviation from Hardy-Weinberg equilibrium and to compare the genotype and allele frequency between asthma and control groups. Odds ratio (OR) and 95% confidence interval (CI) were used for the assessment of risk factors, and p < 0.05 was considered as statistically significant.
RESULTS
Study Population Characteristics
In the present study, IL13Rα1 +1398A/G polymorphism was genotyped in a total of 964 subjects, including 483 healthy controls and 481 asthma patients. Apart from total IgE, other variables such as smoke exposure, family history, spirometry diagnosis, BMI, etc. were also examined, which has a significant role in asthma severity. The mean age for asthma patients was 37 years (range 18–57 years), and age-matched healthy controls with mean age 34 years (range 18–60 years) were compared (Table 1). Mean disease duration was more than nine years in patients and 28% of asthmatic patients had a family history of asthma. Average of total IgE was higher in the asthmatics (2651.7 IU/mL) than the controls (776.1 IU/mL). Spirometry data were only available for asthma patients so we are unable to apply the statistics.
Prevalence of IL13Rα1 +1398A/G Polymorphism in the Indian Population
Comparing the statistical analysis of the results, it was found that the overall distribution of the wild A allele in IL13Rα1 +1398A/G polymorphism has increased trends among the controls (61.8%) as compared with the asthmatics (57.4%), whereas the mutant G allele was slightly more prevalent among the asthmatics (42.7%) than in control subjects (38.2%), posing a significant risk toward asthma with OR = 1.21, 95% CI (1.00–1.45) and p = 0.043 (Table 2).
Table 2.
IL13Rα1 + 1398A/G genotypic and allelic frequencies
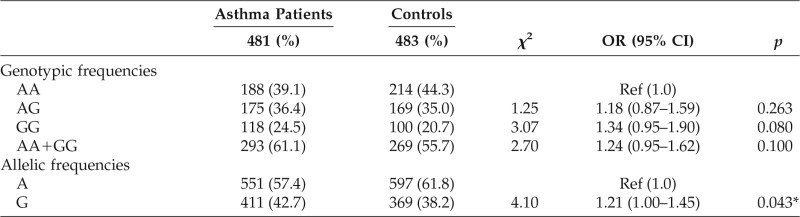
Ref = reference; OR = odds ratio; CI = confidence interval; % = frequency; * = significant.
The genotypic frequencies revealed that the homozygous wild AA genotype has increased frequency in the controls (44.3%) than in the asthmatics (39.1%) (Table 2). The heterozygous genotype AG has increased trends among the asthmatics (36.4%) as compared with the controls (35.0%), and the homozygous mutant GG genotype has also increased frequency in the asthmatics (24.5%) than in the control subjects (20.7%) with nonsignificant p > 0.05.
Further categorizing the asthma patients based on the phenotypic characteristics of the disease (Table 3), as obtained from their detailed proforma, such as sex (male/female), occurrence (seasonal/perennial), severity (wheeze on exertion/wheeze at rest), family history (positive/nil), rhinitis (positive/nil), allergy to at least two provoking factors (positive/nil), smoking status (nonsmoker/ever-smoker), long lasting cough for three to eight weeks (positive/nil), and pneumonia (childhood pneumonia/nil), statistical significant risk was found between IL13Rα1 +1398A/G polymorphism and female sex with OR = 1.32, 95% CI (1.04–1.68) and p = 0.019, perennial occurrence with OR = 1.29, 95% CI (1.00–1.66) and p = 0.039, wheeze on exertion with OR = 1.52, 95% CI (1.14–2.03) and p = 0.003, positive family history with OR = 1.31, 95% CI (0.99–1.74) and p = 0.049, and patients without childhood pneumonia with OR = 1.28, 95% CI (1.05–1.56) and p = 0.014.
Table 3.
Phenotypic characteristics and IL13Rα1 + 1398A/G polymorphism
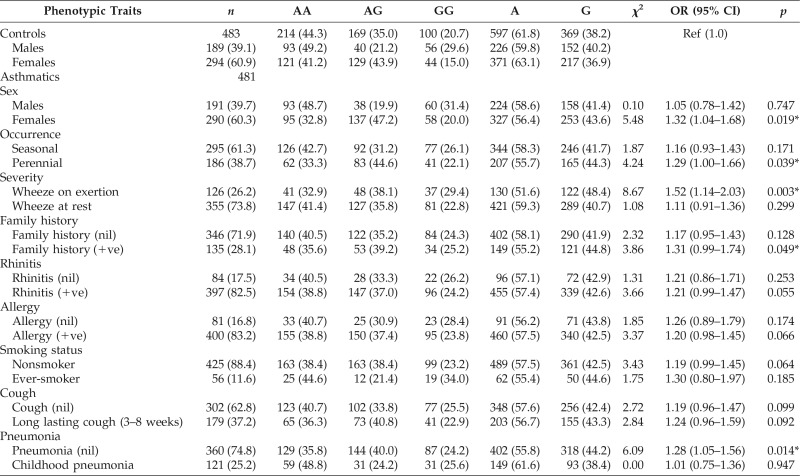
Ref = reference; OR = odds ratio; CI = confidence interval; % = frequency; * = significant.
DISCUSSION
With the completion of the human genome project, the field of genetics has evolved over the past decade to better understand the function of the human genome, its regulation, and contribution of sequence variants to diseases.18 To our knowledge, this is the first study to report the IL13Rα1 polymorphism as a risk factor toward asthma in the North Indian population. Using polymerase chain reaction-restriction fragment length polymorphism method, it was observed that the mutant G allele posing a significant risk toward asthma with significant p = 0.043 (Table 2). Although it is a weak association, some of the phenotypic parameters of the disease and the elevated level of total IgE strongly support the above finding.
Asthma and other allergy-related diseases are hypersensitivity reactions that are mediated by IgE, therefore it is essential to find the factors that control IgE production.19 There is strong evidence from various studies that IL13 mediates their action after binding with IL4Rα and IL13Rα1 for signal transduction initiation.4,7,20 Heinzmann et al.11 revealed that IL13R is predominantly expressed in bronchial tissues and induces hypertrophic changes with increased production of IL13 in bronchial smooth muscle, subepithelial fibrosis, and goblet cell hyperplasia in asthmatic patients. IL13Rα1 is essential for signaling and has been also proved by an experiment in which truncated murine IL13Rα1 lacking the intracellular domain was not able to mediate IL13-induced signals21 that activate signal transducer and activator of transcription 6 (STAT 6) that further activate the transcription of IgE switching.22 This IgE through cross-linking of their surface receptors Fc ε receptor I activate different leukotrienes and Ca2+ channel, which finally results in mast cell degranulation and releases different mediators that increases allergic asthma risk.
Overall scenario of the studies conducted on IL13Rα1 +1398A/G polymorphism and total IgE support our findings, but neither of them proposed a significant association with asthma in their populations. In our study, asthmatic patients have a higher level of serum total IgE, which is in agreement with a recent study conducted on a total of 669 Korean children with asthma (n = 544 atopic and n = 125 nonatopic). They found that the IL13 and IL13Rα1 polymorphisms show synergistic effects on increased total serum IgE levels.23 However, in the present study, only IL13Rα1 polymorphism was studied, so we are unable to link the IL13 and IL13Rα1 role in total IgE production. Total IgE was also reported to be higher in the Korean children with atopic asthma with heterozygous or homozygous for the risk alleles as compared with homozygous for common alleles with p = 0.017. A similar result was observed in the asthmatic group with p = 0.034,17 and males did not differ significantly with asthma severity, which is consistent with our findings. In addition to this, present studies confer that the adult females asthmatics are at significant risk of occurrence of disease with p = 0.019. This result is consistent with the study conducted on United Kingdom population that found IL13Rα1 +1398A/G polymorphism has borderline association with raised total serum IgE among female asthmatics.12
In our study, we have also investigated the role of IL13Rα1 +1398 A/G polymorphism with various phenotypic characteristics of asthma, and our findings show that the patients having wheezing after exertion (p = 0.003), perennial occurrence of asthma symptoms (p = 0.039) were significantly associated with the disease. Other parameters, including family history (p = 0.049) and without childhood pneumonia (p = 0.014), were also at risk of asthma.
In contrast to our findings, a study conducted on atopic Egyptian children proposed that the IL13Rα1 +1398A/G polymorphism does not contribute to asthma or allergic rhinitis susceptibility, yet there was a significant increase in serum total IgE in atopic asthma, nonatopic asthma, and allergic rhinitis groups (p < 0.001 for each).24 Another study conducted on the same population on a total of 105 atopic patients, they also revealed a nonsignificant association between 1398A/G and atopy. They suggest that +1398A/G polymorphism might be involved in the raised total serum IgE levels in all three atopic groups (p = 0.001).25 In a study of Heinzmann et al.,11 +1398A/G polymorphism is associated with three-fold risk in males, which is inconsistent with our results, in which no association was found between asthma severity and male gender. These controversial findings in IL13Rα1 +1398A/G polymorphism might be due to ethnic differences, sample size, and different experimental scenario. Moreover, asthma severity is due to a complex interplay of genetic variants with environmental allergens that might lead to these results.
CONCLUSIONS
From the present study, we conclude that IL13Rα1 +1398A/G polymorphism poses a significant risk toward asthma in the studied North Indian population. However, further studies are needed to evaluate the impact of IL13Rα1 and its pathways that will further determine the susceptibility to asthma severity.
Footnotes
J Singh was supported by the Department of Science and Technology, New Delhi under the Ministry of Science and Technology, India Research Grant SR/FT/LS-018/2008
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Umetsu DT, McIntire JJ, Akbari O, et al. Asthma: An epidemic of dysregulated immunity. Nat Immunol 3:715–720, 2002. [DOI] [PubMed] [Google Scholar]
- 2. von Mutius E. Gene-environment interactions in asthma. J Allergy Clin Immun 123:3–11; quiz 12–13, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Adcock IM, Barnes PJ. Con: Genome-wide association studies have not been useful in understanding asthma. Am J Respir Crit Care Med 184:633–636, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev 202:175–190, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Howard TD, Whittaker PA, Zaiman AL, et al. Identification and association of polymorphisms in the interleukin-13 gene with asthma and atopy in a Dutch population. Am J Resp Cell Mol 25:377–384, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Hilton DJ, Zhang JG, Metcalf D, et al. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA 93:497–501, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miloux B, Laurent P, Bonnin O, et al. Cloning of the human IL-13R α1 chain and reconstitution with the IL4R α of a functional IL-4/IL-13 receptor complex. FEBS Lett 401:163–166, 1997. [DOI] [PubMed] [Google Scholar]
- 8. Munitz A, Brandt EB, Mingler M, et al. Distinct roles for IL-13 and IL-4 via IL-13 receptor 1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci USA 105:7240–7245, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: Revisiting the hygiene hypothesis. Nat Rev Immunol 1:69–75, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Rothenberg ME, Wen T, Shik D, et al. IL-13 receptor α1 differentially regulates aeroallergen-induced lung responses. J Immunol 187:4873–4880, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heinzmann A, Mao XQ, Akaiwa M, et al. Genetic variants of IL-13 signalling and human asthma and atopy. Hum Mol Genet 9:549–559, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Konstantinidis AK, Barton SJ, Sayers I, et al. Genetic association studies of interleukin-13 receptor α1 subunit gene polymorphisms in asthma and atopy. Eur Respir J 30:40–47, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Miller MR, Hankinson J, Brusasco V, et al. , ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 26:319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Laurent J, Noirot C, Ansquer JC, et al. How to define the normal level of serum IgE in adults? Ann Med Interne (Paris) 136:419–422, 1985. [PubMed] [Google Scholar]
- 15. Chowdary VS, Vinaykumar EC, Rao JJ, et al. A study on serum IgE and eosinophils in respiratory allergy patients. Indian J Allergy Asthma Immunol 17:21–24, 2003. [Google Scholar]
- 16. Roe BA, Crabtree JS, Khan AS. DNA isolation and sequencing. In Essential Techniques Series. Rickwood D. (Ed). New York, NY: John Wiley and Sons, 85–86, 116–117, 1996. [Google Scholar]
- 17. Kim HB, Lee YC, Lee SY, et al. Gene-gene interaction between IL-13 and IL-13Rα1 is associated with total IgE in Korean children with atopic asthma. J Hum Genet 51:1055–1062, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Postma DS, Kerkhof M, Boezen HM, Koppelman GH. Asthma and chronic pbstructive pulmonary disease common genes, common environments? Am J Respir Crit Care Med 183:1588–1594, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature 402:B18–B23, 1999. [DOI] [PubMed] [Google Scholar]
- 20. Hershey GK. IL-13 receptors and signaling pathways: An evolving web. J Allergy Clin Immunol 111:677–690; quiz 691, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Orchansky PL, Ayres SD, Hilton DJ, Schrader JW. An interleukin (IL)-13 receptor lacking the cytoplasmic domain fails to transduce IL-13-induced signals and inhibits responses to IL-4. J Biol Chem 272:22940–22947, 1997. [DOI] [PubMed] [Google Scholar]
- 22. Kabesch M, Schedel M, Carr D, et al. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol 117:269–274, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Choi WA, Kang MJ, Kim YJ, et al. Gene-gene interactions between candidate gene polymorphisms are associated with total IgE levels in Korean children with asthma. J Asthma 49:243–252, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Hussein YM, El-Tarhouny SA, Shalaby SM, et al. Interleukin-13 receptor A1 gene polymorphism and IL-13 serum level in atopic and non-atopic Egyptian children. Immunol Inves 40:523–534, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Hussein YM, Ahmad AS, Ibrahem MM, et al. Interleukin 13 receptors as biochemical markers in atopic patients. J Investig Allergol Clin Immunol 21:101–107, 2011. [PubMed] [Google Scholar]


