Abstract
Background:
Mometasone furoate-releasing implants have been approved for use in the ethmoid sinuses following endoscopic sinus surgery (ESS) to reduce the need for medical and surgical intervention postoperatively. Outcomes have not yet been studied when these implants are utilized in other paranasal sinuses after ESS.
Objective:
To present a case in which bioabsorbable steroid-eluting implants were used to maintain patency and to decrease inflammation in the frontal and maxillary sinuses after revision ESS.
Methods:
52-year-old male with lifelong allergic rhinitis, chronic allergic fungal rhinosinusitis, and inflammatory bowel disease had previously undergone four endoscopic sinus surgeries, subcutaneous injection immunotherapy, and topical therapy with budesonide and amphotericin sinus irrigations. In July, 2012, during revision left frontal sinusotomy and right maxillary antrostomy (the fifth ESS), two bioabsorbable steroid-releasing implants were placed in the left frontal recess and the right maxillary sinus respectively and followed clinically, endoscopically, and radiographically for two years.
Results:
Two year followup demonstrated near complete clinical, endoscopic, and radiographic resolution of the patient's signs and symptoms of chronic rhinosinusitis.
Conclusions:
The steroid-releasing implants during the critical phase of wound-healing appear to have allowed the patient, now over two years postoperatively, to achieve a healthier state and to allow more successful management than the preceding 15–20 years.
Keywords: Chronic rhinosinusitis, steroid-releasing implant, nasal polyposis
Chronic rhinosinusitis with nasal polyposis (CRSwNP) and chronic rhinosinusitis without nasal polyposis (CRSsNP) remain difficult for physicians to manage effectively despite advances in endoscopic sinus surgery (ESS), immunotherapy, and topical pharmacologic therapy. These patients tend to have recurrent symptoms during or after intervention due to both the chronic nature of the underlying disease state and the adverse postoperative outcomes, such as ostial stenosis, synechiae formation, or lateralization of the middle turbinate.1,2
Various postoperative dressings and packing materials have been developed and evaluated for the ability to prevent or minimize surgical complications.3 Oral or parenteral corticosteroids and antibiotics, and topical steroid, antibiotic, and antifungal regimens are also widely used to control CRSwNP and CRSsNP postoperatively, with varying efficacy.4
Bioabsorbable mometasone furoate–releasing sinus implants (PROPEL and PROPEL Mini, Intersect ENT, Menlo Park, CA) have been approved by the U.S. Food and Drug Administration for deployment into the ethmoid cavity after ESS (but not other paranasal sinuses), based on statistically significant demonstrated safety and reductions in the need for medical and surgical intervention after ESS.5–9 These implants each contain 370 μg of mometasone furoate on a polylactide-co-glycolide backbone. Once deployed into the ethmoid cavity, the implant self-expands into a tubular conformation at the extent of the ethmoid cavity, medializing the middle turbinate and adhering to the mucosal lining of the ethmoid sinus cavity. The steroid is gradually released into the ethmoid mucosa over 30 days, whereas the polylactide-co-glycolide backbone biodegrades.
Outcomes have not yet been studied when these devices are used in other paranasal sinuses after ESS. I report here 2-year follow-up results after placement of PROPEL in the maxillary and frontal sinuses during revision ESS in a 52-year-old man with lifelong allergic rhinitis, chronic allergic fungal rhinosinusitis, and inflammatory bowel disease.
CASE REPORT
A 50-year-old white man with long-standing allergic rhinitis, chronic allergic fungal sinusitis with nasal polyposis, and inflammatory bowel disease presented in July 2012 with daily left frontal and right maxillary pain and pressure, copious purulent material in both nasal cavities, and a large antrochoanal polyp that emanated from the right maxillary sinus through the previously created maxillary antrostomy into the nasal cavity and anterior right ethmoid cavity. These signs and symptoms dramatically reduced the patient's quality of life and his attendance and performance as a software engineer at a large international telecommunications company.
The patient had been previously managed by two otolaryngologists for 15–20 years, including four previous ESS procedures, three of which occurred in consecutive years (2005, 2006, and 2007). After his 2007 operation, he was managed with long courses of daily budesonide and amphotericin nasal irrigations, and a full course of immunotherapy via subcutaneous injections, with mediocre improvement in quality of life and employment functionality. Nasal endoscopy and stereotactic localization computed tomography of the sinuses (Fig. 1) in July 2012 after a 12-day prednisone taper confirmed mucosal stenosis of the left frontal sinus outflow tract and complete soft tissue opacification of the right maxillary sinus and right anterior ethmoid cavity, despite patent bony outflow tracts. Revision ESS was recommended by both otolaryngologists.
Figure 1.
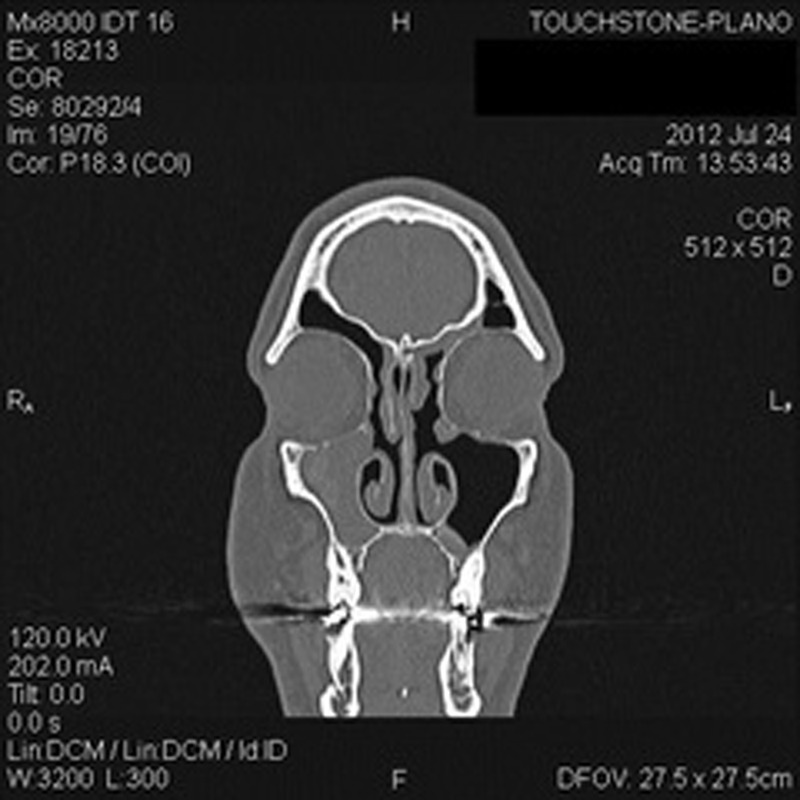
Preoperative coronal computed tomography (CT) of sinuses, demonstrating left frontal recess and right maxillary sinus.
After obtaining informed consent, including discussion of the off-label use of these implants in sinuses other than the ethmoids, the patient underwent the revision ESS procedure within a few weeks of presentation, which consisted of revision left frontal sinusotomy and revision right maxillary antrostomy, with removal of the antrochoanal polyp in the right maxillary sinus and the right nasal cavity. Ten milligrams of dexamethasone and 1 g of cefazolin were administered intravenously at the outset of the surgical procedure. At the conclusion of the surgical dissection, two bioabsorbable mometasone furoate–releasing sinus implants were deployed into the left frontal recess and into the right maxillary sinus, respectively.
No oral steroids or antibiotics were initially prescribed postoperatively. The patient underwent routine postoperative nasal endoscopy with debridement at 2- to 3-week intervals. Acute right maxillary and ethmoid sinusitis was identified 4 weeks postoperatively. Nasal endoscopy confirmed that the implant in the right maxillary sinus was absent by this time point. After culture and sensitivity were obtained, the patient was treated satisfactorily with clarithromycin and an oral prednisone taper. No further infections occurred during the early postoperative period. Limited CT of the sinuses was obtained in October 2012, which demonstrated wide patency of the left frontal recess and right maxillary antrostomy, without polypoid recurrence (Figs. 2 and 3).
Figure 2.
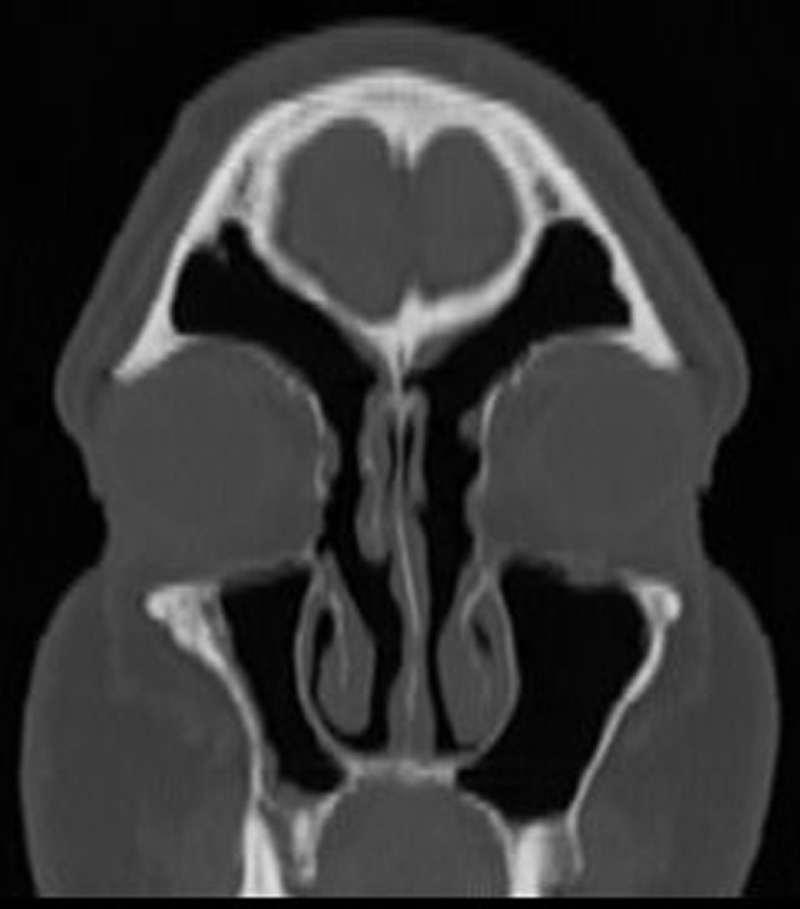
Postoperative coronal computed tomography (CT) of the left frontal recess 2 months after revision ESS.
Figure 3.
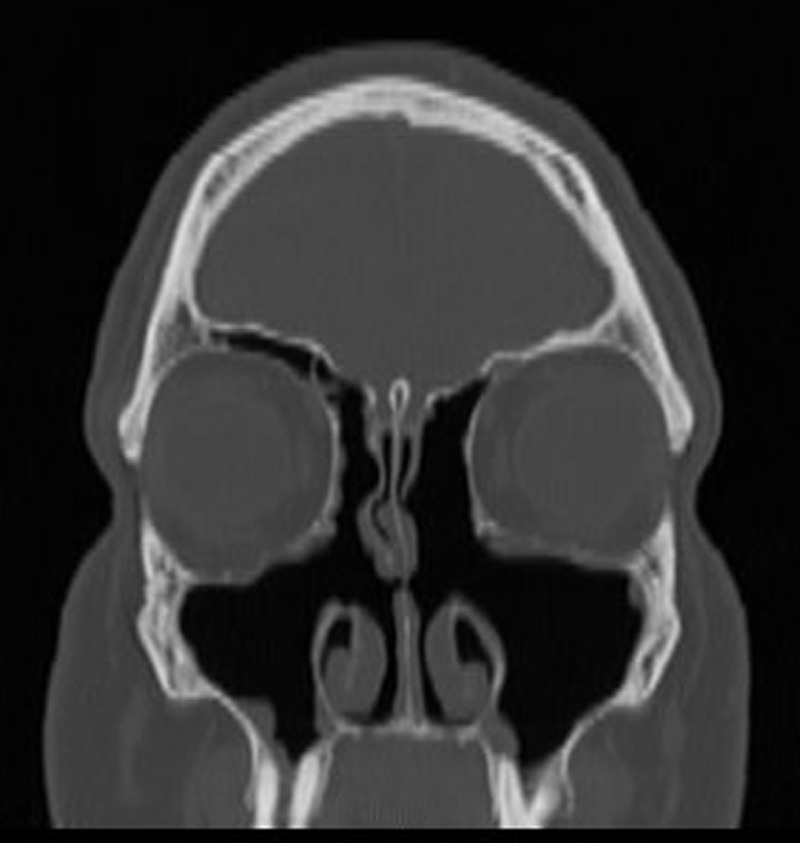
Postoperative coronal computed tomography (CT) of the right maxillary sinus 2 years after revision ESS.
Ninety days after surgery, as the patient moved from the convalescent period to a prophylaxis and maintenance period, he was initially prescribed daily nasal nebulized therapy, which consisted of tobramycin and fluticasone. Both implants were absent at the 30-day endoscopy, so there was no overlap in topical steroid. This inhalational therapy was changed to mupirocin, fluticasone, and itraconazole 9 months later (1-year postoperatively), when daily mucus production subjectively increased. Symptom control has continued to be excellent overall since initiation of nasal nebulized therapy, with only mild occasional epistaxis from a posterior pinpoint septal perforation present since the patient's initial septoplasty 15–20 years ago. In October 2014, limited CT of the sinuses (Figs. 4 and 5) and nasal endoscopy of the left frontal recess (Fig. 6) and the right maxillary antrostomy (Fig. 7) were performed to objectively confirm the long-term positive postoperative outcome from ESS with deployment of the mometasone-eluting implants compared with the previous postoperative courses and periods of long-term medical management.
Figure 4.
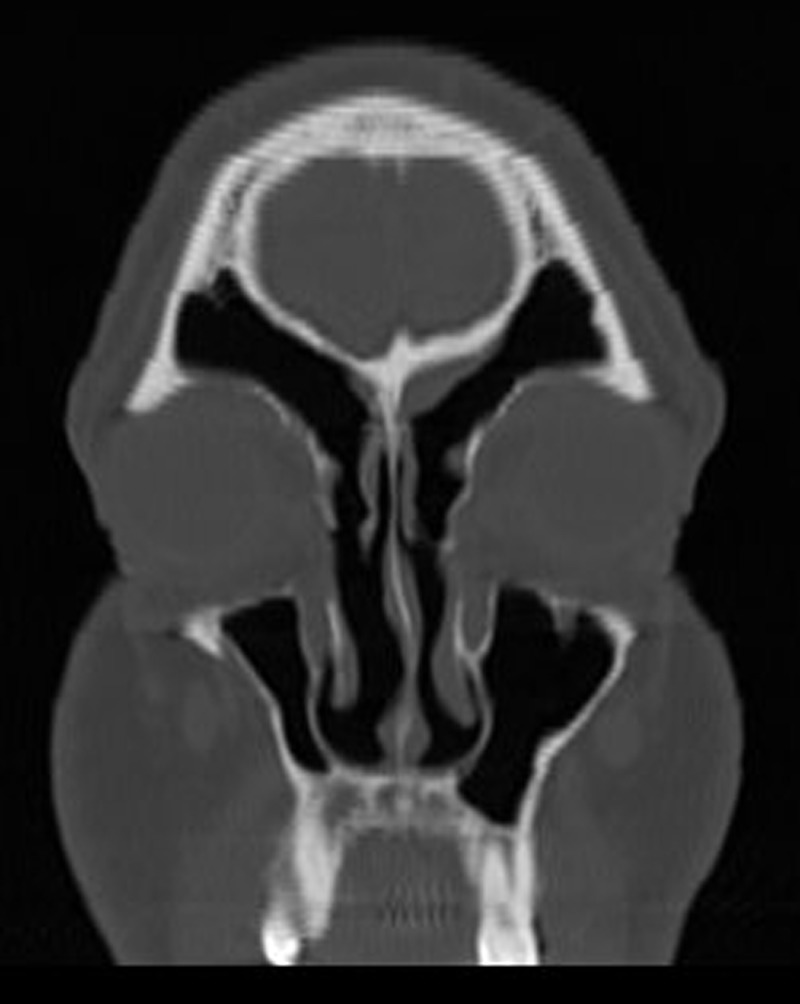
Postoperative coronal computed tomography (CT) of left frontal recess 2 years after revision ESS.
Figure 5.
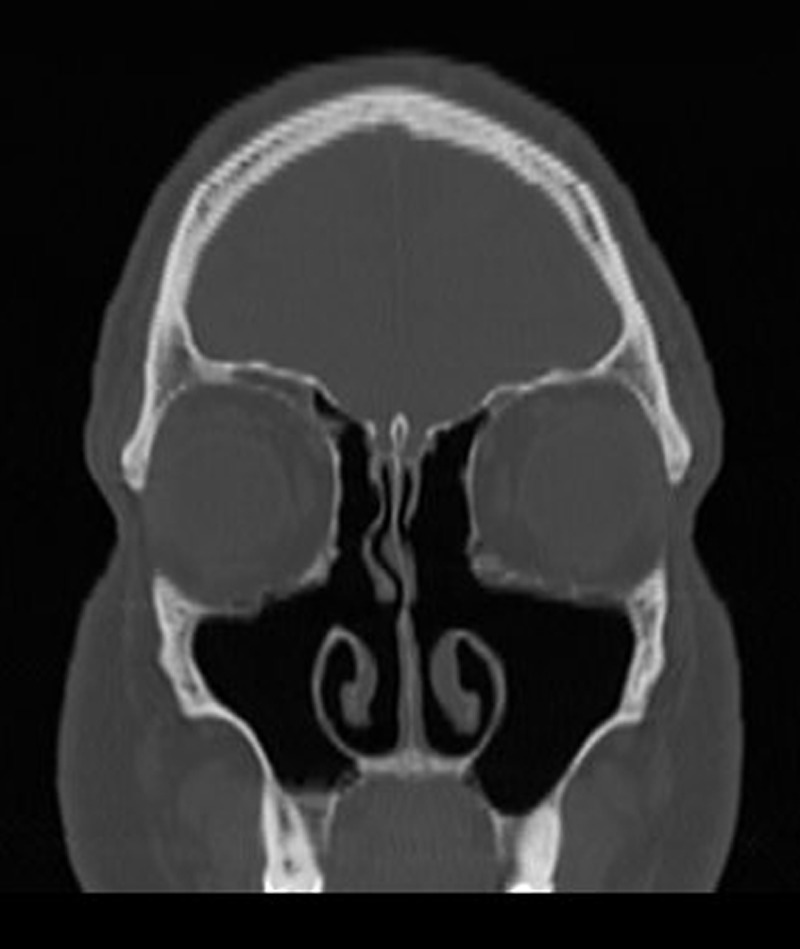
Postoperative coronal computed tomography (CT) of the right maxillary sinus 2 years after revision ESS.
Figure 6.
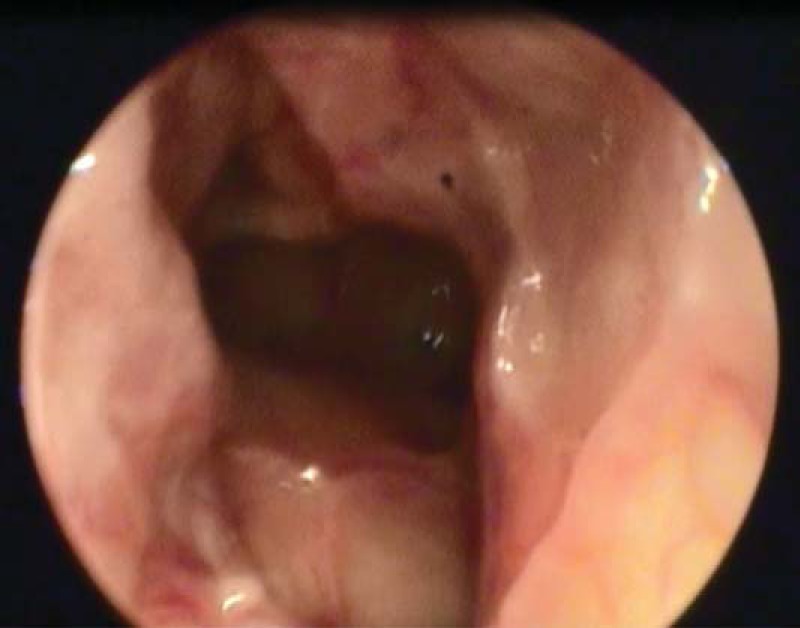
Postoperative nasal endoscopy of the left frontal recess 2 years after revision ESS.
Figure 7.
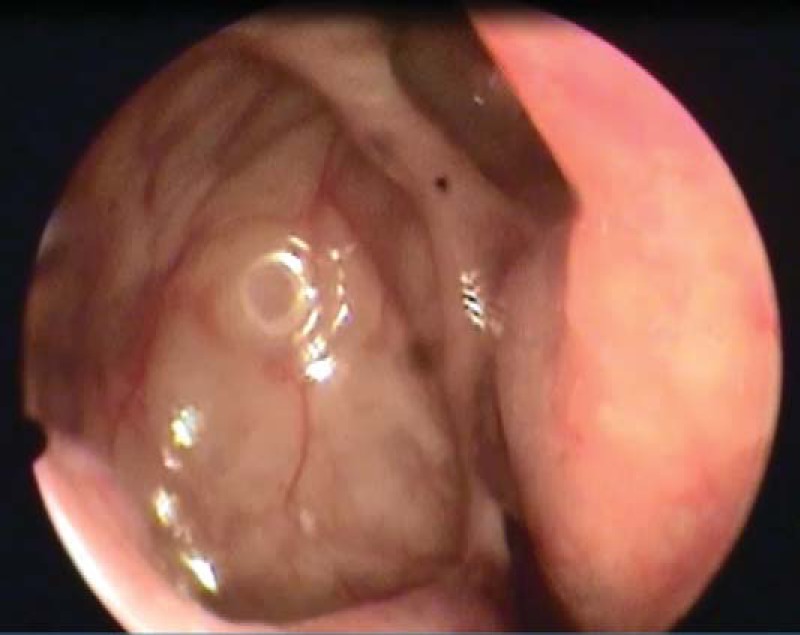
Postoperative nasal endoscopy of the right maxillary sinus 2 years after revision ESS.
DISCUSSION
Topical, oral, and systemic mediations, especially corticosteroids, have been shown to improve management of CRSwNP and CRSsNP, both pre- and postoperatively. Topical steroids are generally safe due to their low amount of local, regional, and systemic bioavailability. However, effective drug delivery with these preparations varies due to both difficulty delivering therapeutic levels to the sinus mucosa and the dependence on patient compliance. Conversely, oral and parenteral steroids carry significant risks for adverse effects, such as elevation of blood glucose levels, cataract formation, mood changes, osteoporosis, and aseptic necrosis of the femoral head.4
Off-label impregnation of postoperative nasal dressings and packing material with steroids, e.g., triamcinolone, has been reported to be beneficial, but the duration of delivery of the drug to the mucosa is often very short, and the dose and local pharmacokinetics are unproven, unpredictable, and highly variable among different materials and different steroids.10 The safety and efficacy of mometasone furoate–releasing implants, when placed in the ethmoid cavity, are well established in numerous published clinical studies.5–9 These reductions in synechiae and recurrent polyposis appear to correlate with the longer duration of time that the mucosa is exposed to the mometasone furoate, perhaps allowing the surgical field adequate time to heal before complications occur that could preempt this healing.
CONCLUSIONS
Controlled topical delivery of mometasone furoate to healing ethmoid mucosa has been demonstrated to minimize adverse events and to promote healing after ethmoid ESS in a safe and effective fashion.5–8 In this patient, the steroid-eluting implants appear to have conferred similar benefits to the healing frontal and maxillary operative sites for enough duration to allow the patient to achieve a healthier state and to subsequently be managed medically to a much higher degree of success and satisfaction than the preceding 15–20 years. Further evaluation of these types of bioabsorbable, mechanically active, local drug-delivery devices in other paranasal sinuses is certainly warranted and holds great promise to improve the management of patients with CRSwNP and CRSsNP.
Footnotes
K.E. Matheny is a consultant for Intersect ENT
REFERENCES
- 1. Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl 23:3 p preceding table of contents, 1–298, 2012. [PubMed] [Google Scholar]
- 2. Kennedy DW, Wright ED, Goldberg AN. Objective and subjective outcomes in surgery for chronic sinusitis. Laryngoscope 110:29–31, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Weitzel EK, Wormald PJ. A scientific review of middle meatal packing/stents. Am J Rhinol 22:302–307, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Cope D, Bova R. Steroids in otolaryngology. Laryngoscope 118:1556–1560, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Murr AH, Smith TL, Hwang PH, et al. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent. Int Forum Allergy Rhinol 1:23–32, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Forwith KD, Chandra RK, Yun PT, et al. ADVANCE: A multisite trial of bioabsorbable steroid-eluting implants. Laryngoscope 121:2473–2480, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Marple BF, Smith TL, Han JK, et al. ADVANCE II: A prospective, randomized study assessing safety and efficacy of bioabsorbable steroid-releasing sinus implants. Otolaryngol Head Neck Surg 46:1004–1011, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Han JK, Marple BF, Smith TL, et al. Effect of steroid releasing sinus implants on postoperative medical and surgical interventions: An efficacy meta-analysis. Int Forum Allergy Rhinol 2:271–279, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Matheny KE, Carter KB, Jr, Tseng EY, Fong KJ. Safety, feasibility, and efficacy of placement of steroid-eluting bioabsorbable sinus implants in the office setting: A prospective case series. Int Forum Allergy Rhinol 4:808–815, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Cote DW, Wright ED. Triamcinolone-impregnated nasal dressing following endoscopic sinus surgery: A randomized, double-blind, placebo-controlled study. Laryngoscope 120:1269–1273, 2010. [DOI] [PubMed] [Google Scholar]


