Abstract
Introduction:
Epithelial myoepithelial carcinoma (EMC) of the nasal cavity is a rare tumor, and here we describe the first case of EMC of the nasal cavity presenting with epiphora. A case presentation and review of the literature is provided.
Methods:
A case report is described of a 63-year-old man who presented with unilateral epiphora and was found via a thorough history and physical examination to have a nasal tumor. The physical examination consisted of an ocular examination, including probing and irrigation, and a detailed nasal examination (anterior rhinoscopy, nasal endoscopy). The nasal examination was prompted by the patient's report of concurrent nasal symptoms during history taking. Immunohistochemistry subsequently identified the nasal tumor as EMC. A literature search was performed to gain insights into similar malignancies of the nasal cavity.
Results:
Eight cases of EMC of the nasal cavity were identified in the literature, none of the patients presented with epiphora. The case presented here resulted in resolution of the patient's symptoms and no evidence of disease after surgical excision.
Conclusion:
Epithelial myoepithelial is a rare salivary gland malignancy that can arise in the nasal cavity. Unilateral epiphora with concurrent nasal symptoms should prompt nasal cavity examination for the possibility of an obstructive tumor.
Keywords: Anterior skull base, epithelial cell, skull base, endoscopic minimally invasive skull-base, endoscopic skull base surgery
Epiphora results from an imbalance between tear production and drainage.1 Epiphora is four times more common in women than in men,2 and its prevalence ranges from 9% to 10% at 50 years old to 35–40% at 90 years old.3 The differential diagnosis for epiphora is broad and includes reflex tearing from dry eye, with compensatory hypersecretion,4 poor tear distribution due to altered blink dynamics and lid malposition,5 and defective tear drainage from stenosis of the nasolacrimal drainage system.6 The etiology of nasolacrimal duct obstruction may be primary (idiopathic)7 or secondary, due to a variety of causes, including local inflammation or fibrosis (e.g., iodine-131 therapy,8 glaucoma drops,9 rhinosinusitis10), systemic inflammatory disease (e.g., Wegener granulomatosis,11 sarcoidosis12), facial trauma,13 previous sinonasal surgery,14–18 or nasolacrimal system19,20 or sinonasal20 neoplasia.
Sinonasal neoplasms comprise <1% of all malignancies and <3% of all malignant tumors of the upper aerodigestive tract,21 and they have been estimated to occur at an incidence of 0.556 cases per 100,000 population per year.22 Epithelial myoepithelial carcinoma (EMC) is a rare type of tumor that predominantly occurs in the salivary glands23 but also occurs rarely in the nasal cavity. Previous cases of EMC of the nasal cavity have been described as presenting only with nasal symptoms.24–31 Herein, we reviewed the previously reported cases of EMC of the nasal cavity and present, to our knowledge, the first reported case of a patient with EMC presenting as unilateral epiphora.
CASE REPORT
A 63-year-old man was referred to our department of otolaryngology with a 2-year history of epiphora and “sandy” feeling in the left eye. He reported a 1-year history of intermittent nasal crusting and bleeding that had become persistent. On ophthalmology examination, the left puncta appeared patent but small compared with the right puncta. Lower lid ectropion and blepharitis were noted bilaterally. Probing and irrigation demonstrated a 100% blockage of the left nasolacrimal system, with normal canaliculi, which indicated a distal obstruction. On nasal endoscopy, the nasal cavities were patent, but a friable mass was noted in the left inferior meatus (Fig. 1).
Figure 1.
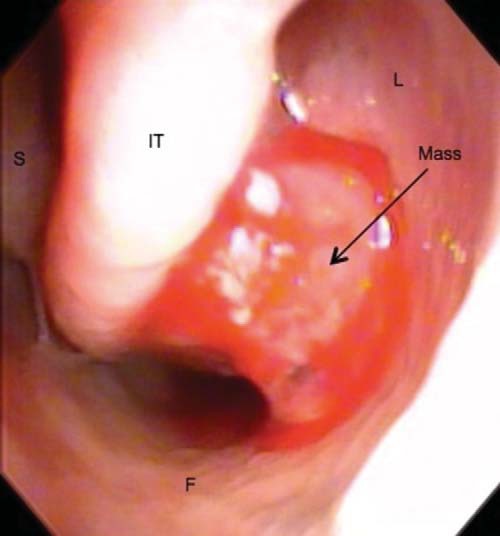
Mass noted in the left inferior meatus during nasal endoscopic examination. S = nasal septum; IT = inferior nasal turbinate; L = lateral wall of the nasal cavity; F = floor of nasal cavity.
Computed tomography of the paranasal sinuses showed a 1.5 × 1.1 × 1.6 cm (anteroposterior × transverse × cranial caudal) anterior soft-tissue mass in the left nasal cavity located between the inferior turbinate and the medial wall of the maxillary sinus abutting the orifice of the nasolacrimal duct. The mass was associated with medial displacement of the inferior turbinate and slight underlying bony thinning of the medial wall of the maxillary sinus (Figs. 2–4).
Figure 2.
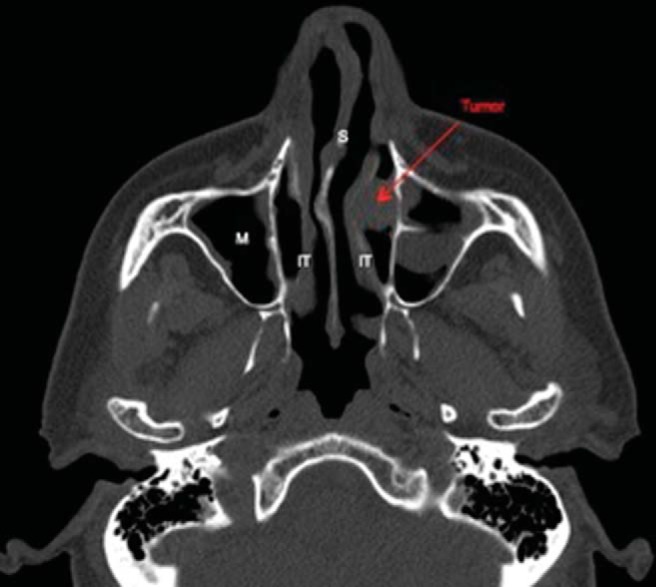
Axial computed tomography of the paranasal sinuses, showing a tumor abutting the nasolacrimal duct. S = nasal septum; IT = inferior nasal turbinate; M =maxillary sinus.
Figure 4.
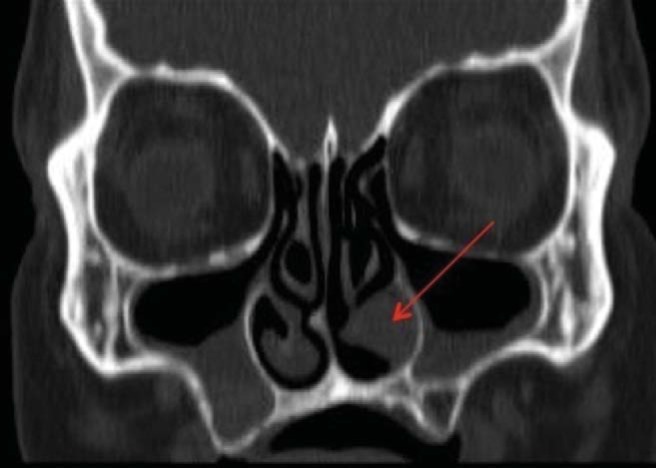
Coronal computed tomography of the paranasal sinuses, showing a tumor underneath the inferior turbinate.
Figure 3.
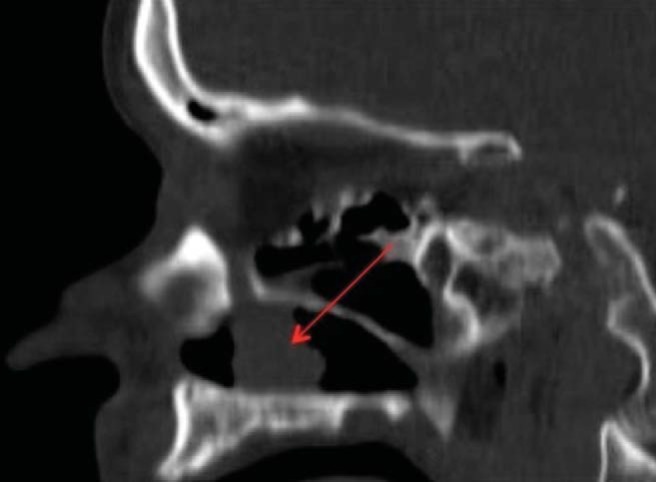
Sagittal computer tomography of the paranasal sinuses, showing a tumor abutting the nasolacrimal duct.
Endoscopic excisional biopsy of the primary mass was performed with the patient under general anesthesia. On gross examination, the primary tumor consisted of tan hemorrhagic tissue. Hematoxylin and eosin staining showed an uncapsulated epithelial neoplastic proliferation that infiltrated the submucosa. The mass consisted of a tubular glandular growth composed of a dual population of cells: inner epithelial-appearing cells and outer myoepithelial-appearing cells with a clear cytoplasm. Oncocytic cytoplasmic changes were observed in some of the epithelial-appearing cells and clusters of predominantly myoepithelial cells without tubular glandular structures were noted. There was infiltration of the tumor into the submucosa without perineural or lymphovascular invasion. Immunohistochemistry definitively confirmed the identity of the epithelial- and myopithelial-appearing cells. Both cell populations were positive for cytokeratin 5/6 (Fig. 5) and cytokeratin 903, and variably positive for S-100. The epithelial cells were identified by dedicated CAM 5.2 staining, whereas the myoepithelial cells were identified by dedicated α smooth muscle actin (Fig. 6), p63, and calponin staining. Ki67 staining showed a proliferation rate of 5–10%. Overall, these findings were consistent with an epithelial-myoepithelial carcinoma.
Figure 5.
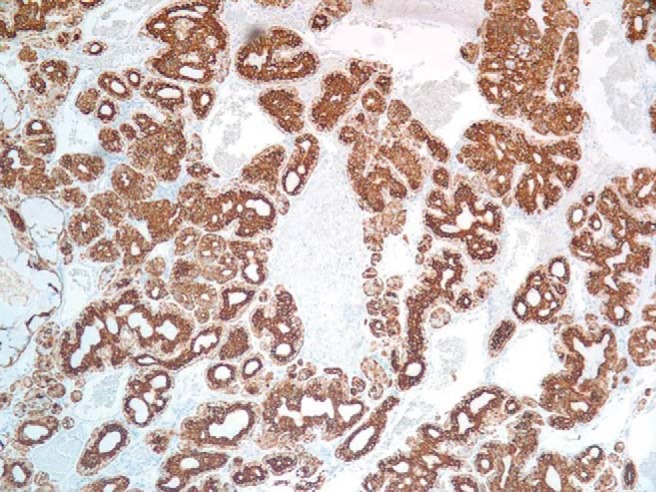
Inner luminal cells and outer myoepithelial cells positive for cytokeratin 5/6 (magnification×100).
Figure 6.
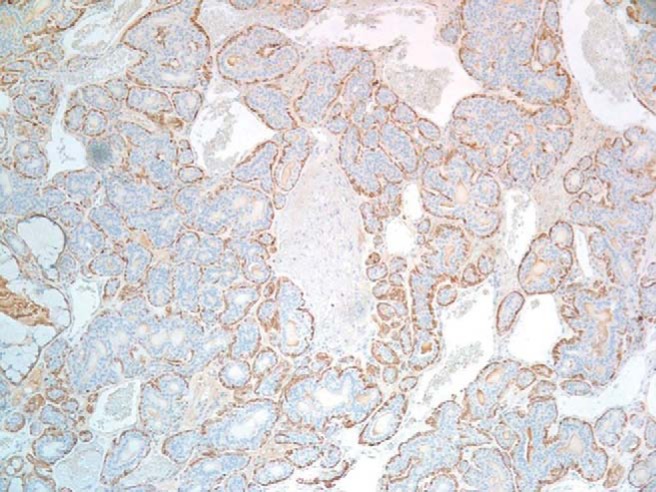
Outer myoepithelial cells stain positive for α smooth muscle actin (magnification×100).
The patient underwent an endoscopic medial maxillectomy to obtain definitive margins. In addition, a prophylactic endoscopic dacryocystorhinostomy was performed to prevent potential stenosis of the nasolacrimal duct given its proximity to the initial tumor. The patient's postoperative course was unremarkable, and final pathology demonstrated no further evidence of tumor. One year after the resection, the patient was doing well, without evidence of recurrent or metastatic disease. Given the malignant nature of the disease, the patient will continue to be regularly followed up until 5 years after the resection.
DISCUSSION
History and physical examination are the keys to determine the underlying cause of epiphora.32–34 When evaluating a patient with epiphora, physicians should particularly inquire about associated symptoms (e.g., ocular or nasal symptoms, symptoms suggestive of systemic disease), history of sinus disease or surgery, facial trauma, history of obstruction of the nasolacrimal drainage system, and quality of the tears (e.g., tears tinted with blood could be a sign of neoplastic growth).33 Patients with epiphora should have a full ocular examination and a fluorescein dye retention test with probing of the canaliculi and irrigation of the nasolacrimal system. Patients with concurrent nasal symptoms should undergo endoscopic examination of the nasal cavity to exclude an obstructive mass in the distal lacrimal system.33,34
Identifying the specific or predominant cause of epiphora is essential for its proper management but can be challenging when epiphora is multifactorial, which is the case in ∼30% of patients.33 When epiphora is unilateral, excluding an obstructing neoplastic growth as a contributing factor of epiphora is critical.19,20 For example, in the present case, we described a patient who presented with bilateral lower lid ectropion and blepharitis, along with a small puncta on the side of the tumor, suggestive of punctal stenosis, all of which constitute potential independent risk factors for epiphora. Thorough history taking, which revealed epistaxis, and results of an examination, which demonstrated a total nasolacrimal duct obstruction, however, led to an endoscopic examination and then computed tomography, both of which showed the true cause of epiphora to be a neoplasm. By blocking the nasolacrimal duct, the neoplasm prevented appropriate tear drainage down the canaliculi and lacrimal sac, thereby resulting in epiphora.
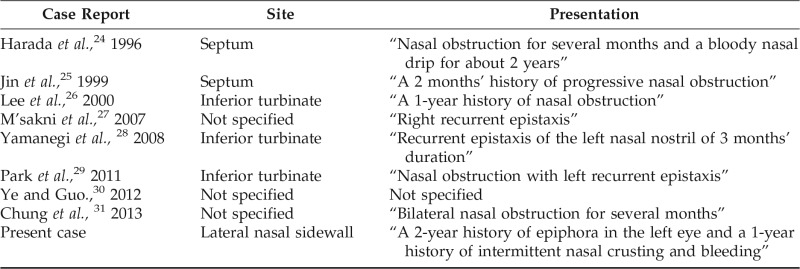
EMC obstructing the nasolacrimal duct was ultimately found to be the predominant cause of our patient's epiphora. First described in 1972 by Donath et al.,35 EMC is a rare low-grade adenocarcinoma that generally occurs in the salivary glands. Most cases of EMC originate from the parotid gland, with fewer cases occurring in the submandibular gland and the minor salivary glands.36 Rarely, EMCs have also been described in the nasal cavity. A literature search of the previous cases of EMC of the nasal cavity indicated that EMC had thus far been described as presenting only with nasal symptoms (Table 1).24–31 The present case is unique in that the patient presented primarily with epiphora in addition to nasal symptoms.
Table 1.
Reported cases of epithelial myoepithelial carcinoma arising in the nasal cavity
EMCs tend to present as solitary tumors, and they can be easily diagnosed on biopsy by a combination of light microscopy and immunohistochemistry.23,36 A distinctive feature of EMC on light microscopy is a glandular structure characterized by a ductal lining, which consists of an inner layer of epithelial cells and an outer layer of clear myoepithelial cells.23 Immunohistochemistry can then be used to definitely identify these two layers; for example, CAM 5.2 is a selective epithelial cell marker and α smooth muscle actin, p63, and calponin are selective myoepithelial markers.36 Also, characteristic of EMCs is local invasion of tissues and a low-grade histology (e.g., absence of nuclear pleomorphism, low mitotic rate),23,36 with rare exceptions in which EMCs can be highly aggressive and even lethal.29,36–39
CONCLUSION
EMC is a rare salivary gland malignancy that can occur in the nasal cavity. It is a low-grade lesion but has metastatic potential, and early diagnosis and treatment are crucial for successful management. Unilateral epiphora with concurrent nasal symptoms should prompt a thorough evaluation of the nasal cavity to ensure that obstructive tumors are not present.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Bartley GB. Acquired lacrimal drainage obstruction: An etiologic classification system, case reports, and a review of the literature. Part 3. Ophthal Plast Reconstr Surg. 9:11–26, 1993. [PubMed] [Google Scholar]
- 2. Hurwitz JJ, Rutherford S. Computerized survey of lacrimal surgery patients. Ophthalmology 83:14, 1986. [DOI] [PubMed] [Google Scholar]
- 3. Dalgleish R. Idiopathic acquired lacrimal drainage obstruction. Br J Ophthalmol 51:463–468, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sibley D, Norris JH, Malhotra R. Management and outcomes of patients with epiphora referred to a specialist ophthalmic plastic unit. Clin Exp Ophthalmol 41:231–238, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Tse DT, Erickson BP, Tse BC. The BLICK mnemonic for clinical-anatomical assessment of patients with epiphora. Ophthal Plast Reconstr Surg 30:450–458, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Meyer DR. Lacrimal disease and surgery. Curr Opin Ophthalmol 4:86–94, 1993. [DOI] [PubMed] [Google Scholar]
- 7. Linberg JV, McCormick SA. Primary acquired nasolacrimal duct obstruction. A clinicopathologic report and biopsy technique. Ophthalmology 93:1055–1063, 1986. [DOI] [PubMed] [Google Scholar]
- 8. Burns JA, Morgenstern KE, Cahill KV, et al. Nasolacrimal obstruction secondary to I(131) therapy. Ophthal Plast Reconstr Surg 20:126–129, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Seider N, Miller B, Beiran I. Topical glaucoma therapy as a risk factor for nasolacrimal duct obstruction. Am J Ophthalmol 145:120–123, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Kubba H, Robson AK, Bearn MA. Epiphora: The role of rhinitis. Am J Rhinol 12:273–274, 1998. [DOI] [PubMed] [Google Scholar]
- 11. Cannady SB, Batra PS, Koening C, et al. Sinonasal wegener granulomatosis: A single institution experience with 120 cases. Laryngoscope 119:757–761, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Fergie N, Jones NS, Havlat MF. The nasal manifestations of sarcoidosis: A review and report of eight cases. J Laryngol Otol 113:893–898, 1999. [DOI] [PubMed] [Google Scholar]
- 13. Becelli R, Renzi G, Mannino G, et al. Posttraumatic obstruction of lacrimal pathways: A retrospective analysis of 58 consecutive naso-orbitoethmoid fractures. J Craniofac Surg 15:29–33, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Padovan IF, Jugo SB. The complications of external rhinoplasty. Ear Nose Throat 70:454–456, 1991. [PubMed] [Google Scholar]
- 15. Flanagan JC. Epiphora following rhinoplasty. Ann Ophthamol 10:1239–1242, 1978. [PubMed] [Google Scholar]
- 16. Unlu HH, Goktan C, Aslan A, Tarhan S. Injury to the lacrimal apparatus after endoscopic sinus surgery: Surgical implications from active transport dacryocsytography. Otol Head Neck Surg 124:308–312, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Meyers AD, Hawes M. Nasolacrimal obstruction after inferior meatus nasal antrostomy. Arch Otolaryngol Head Neck Surg 117:208–211, 1991. [DOI] [PubMed] [Google Scholar]
- 18. Habib R, Har-El G. Management of the lacrimal system during maxillectomy. Am J Rhinol 18:367–370, 2004. [PubMed] [Google Scholar]
- 19. Chaudhry IA, Taiba K, Al-Sadhan Y, Riley FC. Inverted papilloma invading the orbit through the nasolacrimal duct: A case report. Orbit 24:135–139, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Yuen HK, Cheuk W, Cheng AC, et al. Malignant oncocytoma of the lacrimal sac as an unusual cause of epiphora. Ophthal Plast Reconstr Surg 23:70–72, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Osguthorpe JD. Sinus neoplasia. Arch Otolaryngol Head Neck Surg 120:19–25, 1994. [DOI] [PubMed] [Google Scholar]
- 22. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: A historical analysis of population-based data. Head Neck 34:877–885, 2012. [DOI] [PubMed] [Google Scholar]
- 23. Simpson RH, Clarke TJ, Sarsfield PT, Gluckman PG. Epithelial-myoepithelial carcinoma of salivary glands. J Clin Pathol 44:419–423, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harada H, Kashiwagi SI, Fujiura H, et al. Epithelial-myoepithelial carcinoma: Report of a case arising in the nasal cavity. J Laryngol Otol 110:397–400, 1996. [DOI] [PubMed] [Google Scholar]
- 25. Jin XL, Ding CN, Chu Q. Epithelial-myoepithelial carcinoma arising in the nasal cavity: A case report and review of literature. Pathology 31:148–151, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Lee HM, Kim AR, Lee SH. Epithelial-myoepithelial carcinoma of the nasal cavity. Eur Arch Otorhinolaryngol 257:376–378, 2000. [DOI] [PubMed] [Google Scholar]
- 27. M'sakni I, Laabidi B, Bougrine F, et al. Epithelial-myoepithelial carcinoma of the nasal cavity [in French with English abstract]. Ann Otolaryngol Chir Cervicofac 124:228–231, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Yamanegi K, Uwa N, Hirokawa M, et al. Epithelial-myoepithelial carcinoma arising in the nasal cavity. Auris Nasus Larynx 35:408–413, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Park JO, Jung CK, Sun DI, Kim MS. An unusual presentation of aggressive epithelial-myoepithelial carcinoma of the nasal cavity with high-grade histology. J Laryngol Otol 125:1286–1289, 2011. [DOI] [PubMed] [Google Scholar]
- 30. Ye Z, Guo Y. Epithelial-myoepithelial carcinoma arising in the nasal cavity: A case report and literature review [in Chinese with English abstract]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 26:1092–1094, 2012 [PubMed] [Google Scholar]
- 31. Chung HJ, Lee BH, Hwang YJ, Kim SY. Epithelial-myoepithelial carcinoma in nasal cavity with bony destruction: A case report. J Korean Soc Radiol 69:265–268, 2013. [Google Scholar]
- 32. Williams B, Johnson D, Hurst J, Kratky V. Patterns and causes of epiphora referrals to a tertiary oculoplastic practice. Can J Ophthalmol 49:180–182, 2014. [DOI] [PubMed] [Google Scholar]
- 33. Blackmore KJ, Ainsworth G, Robson AK. Epiphora: An evidence based approach to the 12 minute consultation. Clin Otolaryngol 35:210–214, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Sibley D, Norris JH, Malhotra R. Management and outcomes of patients with epiphora referred to a specialist ophthalmic plastic unit. Clin Experiment Ophthalmol 41:231–238, 2013. [DOI] [PubMed] [Google Scholar]
- 35. Donath K, Seifert G, Schmitz R. [Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts. Epithelial-myoepithelial carcinoma of the intercalated ducts]. [Article in German] Virchows Arch A Pathol Pathol Anat 356:16–31, 1972. [PubMed] [Google Scholar]
- 36. Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: A review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol 31:44–57, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Collina G, Gale N, Visona A, et al. Epithelial-myoepithelial carcinoma of the parotid gland: Clinic, pathologic and immunohistochemical study of seven cases. Tumori 77:257–263, 1991. [DOI] [PubMed] [Google Scholar]
- 38. Corio R, Sciubba J, Brannon R, Batsakis J. Epithelial myoepithelial carcinoma of intercalated duct origin. A clinicopathologic and ultrastructural assessment of sixteen cases. Oral Surg Oral Med Oral Pathol 53:280–287, 1982. [DOI] [PubMed] [Google Scholar]
- 39. Alos L, Carrillo R, Ramos J, et al. High-grade carcinoma component in epithelial-myoepithelial carcinoma of salivary glands clinicopathological, immunohistochemical and flow-cytometric study of three cases. Virchows Arch 434:291–299, 1999. [DOI] [PubMed] [Google Scholar]


