Abstract
Objective:
To examine the existing evidence on gender differences in the prevalence, treatment, and quality of life of patients with chronic rhinosinusitis (CRS).
Methods:
Review of the literature and expert opinion.
Results:
From a sociologic standpoint, women have historically been considered more likely to report symptoms, seek medical care, and give poorer self-evaluation of health, which may bias data toward increased prevalence and a greater effect of CRS on quality of life in women. However, the influence of gender seems to be restricted primarily to the evaluation of general quality of life, whereas the disease-specific health-related quality of life is not different between genders. Furthermore, migraine headaches, which are more common among women, may be misdiagnosed as CRS, which contributes to gender differences in the prevalence of CRS. The degree to which reported differences in prevalence and health utilization represent biologic or physiologic differences between genders is not known; however, differences in anatomic size, tobacco susceptibility, and hormonal factors have been speculated to increase the overall susceptibility to CRS in women compared with men.
Conclusions:
Focused research that examines the effect of gender on the development, treatment, and outcomes of CRS is warranted.
Keywords: chronic rhinosinusitis, quality of life, gender, surgical outcomes, burden of disease, migraine headaches, State Ambulatory Surgery Database
Epidemiology studies reported that women have nearly double the rate of chronic rhinosinusitis (CRS) when compared with men,1,2 whereas other studies found no difference.3,4 Quality of life (QOL) studies reported that women have significantly lower QOL for the same objective level of disease,5,6 whereas other studies found no difference when controlling for depression or analyzing only disease-specific variables.7,8 Complicating these analyses is the distinction between these terms: sex refers to a biologic difference, gender refers to a socially based phenomenon.9 This commentary aims to explore reported differences between genders in the epidemiology, QOL, surgical outcomes, sociology, biology, and physiology of patients with CRS to highlight areas in need of further investigation.
METHODS
A literature review of the National Library of Medicine's online database was performed with a focus on gender variables in rhinosinusitis research. Keywords included the following: gender, female, women, CRS, QOL, prevalence, hormone. Additional articles were found by reviewing the citations of previously published articles and position papers, such as the European Position Paper on Rhinosinusitis and Nasal Polyps. Discussion among the authors, including a pulmonologist, immunologists, and otolaryngologists, revealed additional relevant sources. The Northwestern University Institutional Review Board reviewed the use of State Ambulatory Surgery Database data and deemed the project exempt.
DISCUSSION
Epidemiology
Although the overall prevalence of CRS is a matter of debate, large national surveys from the North America report that CRS is approximately twice as common in females as in males.1,2 According to 2010 National Health Interview Survey age-adjusted data, in the United States, females (15.5%) were more likely than males (9.8%) to report that they had ever been told by a physician or other health professional that they had sinusitis.1 Furthermore, females accounted for 63% of sinusitis reported in the 2010 National Health Interview Survey.1 Similarly, a population study of Olmstead County, Minnesota, found that, among patients given an International Classification of Diseases 9 diagnosis code for chronic sinusitis, 67.7% were female.10
Studies from North America and England also report that men have a higher prevalence of CRS with nasal polyposis (CRSwNP), whereas women have higher rates of CRS without nasal polyposis.5,11,12 In a prospective study on the incidence of symptomatic CRSwNP, Larsen and Tos13 found an estimated incidence of 0.86 per thousand per year for males and 0.39 per thousand per year for females, which reached a peak with age of 1.68 and 0.82 patients per thousand per year, respectively, among those 50–59 years old. In a cross-sectional study of 1 year of sinus surgery in England, two-thirds of all the patients undergoing polypectomy were male compared with less than half of patients undergoing surgery for CRS.14 Similarly, Tan et al.15 examined a large cohort of primary care patients over a 10-year period and found that 54% of patients diagnosed with CRSwNP were males compared with 41.8% of patients diagnosed with CRS without nasal polyps. Although the marked male preponderance of nasal polyposis has been widely reported in large series of patients, no convincing mechanisms or pathophysiologic explanations are offered to account for this.11–13
In contrast, studies from Europe, Korea, and Taiwan show no differences in CRS prevalence by sex.3,4,16,17 The GA(2)LEN network reported no significant variations in CRS prevalence by gender in their European international multicenter prevalence study of CRS based on diagnostic criteria from the European Position Paper on Rhinosinusitis and Nasal Polyps.3 Two studies from Korea present conflicting results on gender association with CRS. In one study, participants in Korea were specifically asked about CRS; the prevalence was 1.01%, with no difference between men and women.4 In another study, based on the Korea National Health and Nutritional Examination Survey, the prevalence of CRS was higher in males compared with females.16 A study that used the Longitudinal Health Insurance Database of Taiwan found that 49% of the 5849 subjects diagnosed by an otolaryngologist with CRS were males compared with 51% females.17 Although these apparent contradictions may illustrate the potential effect of differences in study methodology, sampling, or disease definitions, alternatively, they may suggest intrinsic differences in sex-specific prevalence of CRS in different parts of the world.
Gender Differences in QOL and Surgical Outcomes
The potential reasons for gender differences in the incidence and prevalence of CRS have not been identified, and only speculation exists at this point.2 From a sociologic standpoint, women have historically been considered more likely to report symptoms and to give poorer self-evaluation of health,18 which may bias self-reported data toward increased prevalence of disease in women and a greater affect of CRS on QOL.
In multiple studies, women with CRS compared with men with CRS reported higher levels of symptoms despite similar or less-extensive disease, and this may be due to a systemic difference in response styles (Table 1).5,6,8 Baumann and Blumenstock19 used the German version of the Short Form 36 Health Survey and found that women had lower results of Health Related Quality of Life for the preoperative state, despite comparable degrees of CRS by objective criteria. Three months post-operatively no significant differences between men and women were found on 7 of 8 scales.19 A further study by Baumann et al. administered a German adapted version of the Sino-Nasal Outcome test 20 to 202 patients with CRS before and after endoscopic sinus surgery.8 By using this method, they again found that women had significantly lower overall scores and general QOL scores than men preoperatively but equivalent scores postoperatively, despite similar levels of disease severity on preoperative cross-sectional imaging. However, there was no difference between men and women on the disease-specific scores of Primary Nasal Symptoms and Secondary Rhinogenous Symptoms scores pre- or postoperatively.8 They concluded that the influence of gender seems to be restricted primarily to the evaluation of aspects of general Health Related Quality of Life, whereas the disease-specific Health Related Quality of Life was not judged differently by men and women.19
TABLE 1.
Comparison of articles that involved gender differences in QOL and surgical outcomes
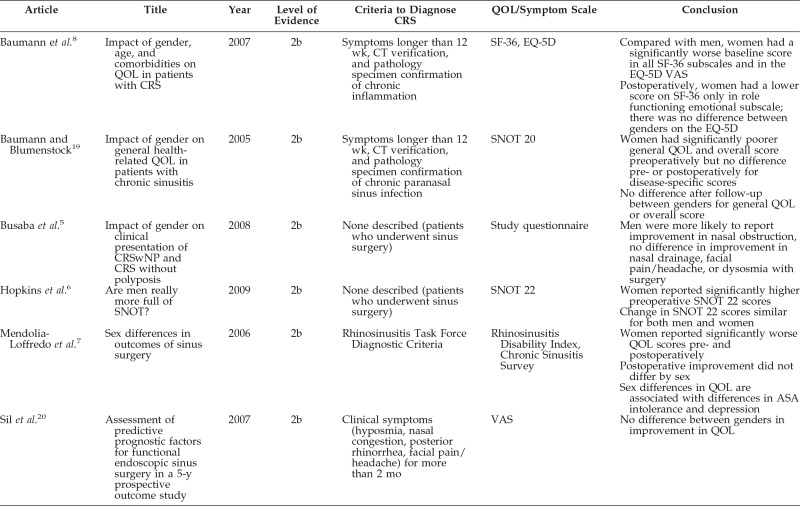
CRS = chronic rhinosinusitis; CT = Computed tomography; SF = Short Form; EQ-5D = European QOL–5 Dimensions; SNOT = Sino-Nasal Outcome Test; VAS = visual analog scale; ASA = acetylsalicylic acid.
Differences in regard to self-reported symptom severity and QOL are not consistent across studies. Similar to Baumann et al.,8 Mendolia-Loffredo et al.7 found that, despite similar computed tomography scan and endoscopy findings, females consistently scored worse than males on disease-specific QOL preoperatively. However, unlike the findings by Baumann et al.,8 women in their study did not show a convergence of scores with those of men postoperatively but did show the same improvement as men between pre- and postoperative scores.7 Multiple other studies show comparable improvement after endoscopic sinus surgery in both men and women.5,6,20 In the study by Mendolia-Loffredo et al.,7 if patients with depression or aspirin sensitivity are removed from the sample, then the statistically significant gender differences go away. The increased prevalence of depression in women is well documented and may be confounding the relationship between gender and QOL.7
Gender Differences in Decisions to Seek Medical Care and Diagnosis
Gender-specific differences in the prevalence of CRS may also be due to decisions to seek medical care. Because the diagnosis of CRS is heavily dependent on patient reported symptoms, differential rates of pursuing medical care and differences in symptom reporting may play a role in the observed prevalence difference of CRS. Women tend to account for a higher percentage of claims (72%)21 and office visits for acute rhinosinusitis (ARS) (66%)22 and CRS (60%),23 but males tend to develop complications from ARS more frequently than females.24,25 Our own unpublished analysis of the State Ambulatory Surgery Databases, Health Care Cost and Utilization Project, Agency of Health Care Research and Quality shows that, among the 33,000 patients in California, Florida, Maryland, and New York, in 2011, who underwent functional endoscopic sinus surgery, women were ∼1.5 years younger on average compared with men (Fig. 1) (women, 48.1 years; men, 49.6 years; p < 0.001).26 The difference in complications of ARS and in age at the time of surgery may be due to a delay in men seeking medical care, but further research is necessary to explore this hypothesis.
Figure 1.
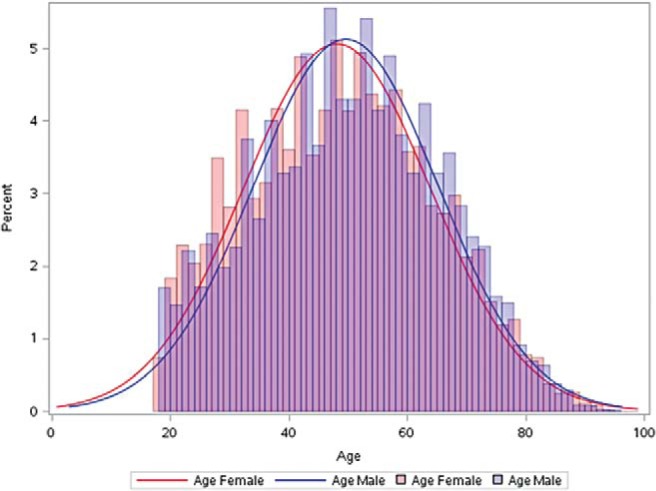
Comparison between gender and age at time of functional endoscopic sinus surgery in California, Florida, Maryland, and New York Ambulatory Surgery Centers in 2011. Women, median age 48.1 years; men, median age, 49.6 years; p < 0.001, Wilcoxon rank sum test.
Moreover, when women seek care, they may be misdiagnosed as having CRS, which further contributes to differences in observed prevalence of CRS. Busaba et al.5 found that female patients with inflammatory paranasal sinus disease were more likely than male patients to mention headache on presentation. Migraine is a common disorder that occurs in three times as many women as men,9,27,28 and patients with migraines are often misdiagnosed as having “sinus” headaches.29–31 Schreiber et al.30 screened 2991 patients with a history of self- or physician-described “sinus” headaches, of whom 77% were women, and 88% met International Headache Society criteria for migraines. Within the study population, 84% of the 2991 patients reported facial pressure, 82% sinus pain, 63% nasal congestion, and 40% rhinorrhea.30 Similarly, previous studies found that 46% of all patients with migraine reported at least one unilateral symptom of nasal congestion, rhinorrhea, or ocular redness or lacrimation due to the trigeminal-autonomic reflex,32 and 82% of patients with self-reported sinus headaches have a significant response to triptans.29 There may be significant symptomatic overlap, as Hsueh et al.33 found that, even among patients with a computed tomography–confirmed diagnosis of CRS, 28.2% met the International Headache Society diagnosis criteria for migraines. These studies show that the presenting symptom or a comorbidity may be gender dependent and may contribute to an overdiagnosis of rhinosinusitis among women.
Gender Differences in Biology and Physiology
Differences in self-reported symptom severity and QOL may exist due to differences in pathophysiology. In a prospective study of 514 patients with CRSwNP or CRS without nasal polyposis by Busaba et al.,5 facial pain and headache were more prevalent presenting symptoms among women, whereas nasal obstruction was more prevalent among men, which may be because a higher percentage of men had nasal polyposis. There were no significant differences in other presenting symptoms, the prevalence of environmental allergy, asthma, psychiatric illness, or anatomic variants that obstruct the osteomeatal unit between genders.5
However, there is a paucity of data on gender differences in biology and physiology that pertain to CRS, which might explain why men have a higher prevalence of polyps and women have a higher prevalence of disease without polyposis. The association of asthma and nasal polyps is strongest in women, in a series by Drake-Lee et al.12 of 200 patients, 41% of 49 women were asthmatic compared with 25% of the 151 men. The female preponderance of patients with asthma is remarkable because nasal polyposis occurs twice as often in men.13 Likewise, a retrospective study by Hulse et al.34 found that 64% of women with CRSwNP were asthmatic compared with only 45% of men with CRSwNP, but, interestingly, the prevalence of asthma was not different between men and women who had nonpolypoid CRS. Similarly, in a series by Collins et al.,35 of patients with nasal polyposis, women were 1.6 times more likely to be asthmatic and 2.7 times more likely to have allergic rhinitis than were men. Men were 2.25 times more likely to be smokers and 2.48 times more likely to have been exposed to chemicals and dust than women.
In addition to asthma, aspirin sensitivity also occurs more commonly among women. Hulse et al.34 found that 65% patients with aspirin-exacerbated respiratory disease were women, although only 35% of patients with CRSwNP were women. Similarly, Mendolia-Loffredo et al.7 found that patients with aspirin intolerance were 4.0 times more likely to be female than patients without aspirin intolerance. Further research is necessary to determine why more female patients with CRSwNP have asthma and aspirin sensitivity, and how these comorbidities relate to QOL and surgical outcomes.
Differences in anatomic size, tobacco susceptibility, and hormonal factors have been speculated to increase the overall susceptibility to rhinosinusitis in women. Women may be more susceptible to obstruction and subsequent infection due to smaller sinus ostia.2 Moreover, CRS is increasingly recognized to be a chronic inflammatory disease rather than an entirely infectious disease process. The increased responsiveness of the female immune system may be a factor in female predominance of CRS. Most autoimmune diseases are more prevalent and/or severe in women than in men, and estrogen is known to play a role in augmenting inflammatory responses. Recent reports noted elevated levels of autoantigen-specific antibodies in sinus tissues of patents with CRS, therefore, it is possible that similar sex-specific autoimmune disease drivers may function in CRS.36–39 Interestingly, the study by Hulse et al.34 also found that women with CRSwNP and with asthma had the highest levels of autoantigen-specific immunoglobulin G in their polyp tissue compared with men. These women with asthma and with CRSwNP also had the highest levels of eosinophil cationic protein, a marker of eosinophilic inflammation, in their polyps compared with men,34 which indicates that women with CRSwNP, especially those with comorbid asthma, have more-severe inflammation.
A hormonal component may be contributing to the higher prevalence of CRS among women. Upper airway congestive symptoms during pregnancy have been recognized since the late 19th century.40 Physiologic changes during pregnancy, with presumed hormonal etiology, account for a distinct condition known as “rhinitis of pregnancy” as well as worsened underlying sinonasal disease.40,41 However, even though rhinitis of pregnancy is a well-known entity,42–50 most CRS epidemiologic studies do not explore this potential link between hormonal factors and disease prevalence. Tan et al.15 found that pregnancy may be protective against a diagnosis of CRS without nasal polyps, even after adjusting for age and sex, pregnancy was associated with decreased odds of CRS diagnosis (patients with CRS without nasal polyps versus control subjects, odds ratio 0.7 [95% confidence interval 0.6–0.9]). Further epidemiologic studies are necessary to determine if there is a hormonal relationship between age-related prevalence of CRS and gender.
In the parallel world of pulmonary disease, scientists are beginning to further explore hormonal effects on obstructive lung disease, with its increasing prevalence among women.51,52 Studies from Canada provide evidence that women are more likely than men to develop chronic obstructive pulmonary disease and asthma.53,54 Incidence rates of asthma in women are higher until the perimenopausal period, with premenstrual aggravation of symptoms in up to 40% of female patients with asthma.52 A prospective cohort study showed an increased risk of asthma with postmenopausal hormone use, which indicates that female reproductive hormones may contribute to the onset of asthma among adult women.55 Although sex hormones appear to influence airway function in asthma, and asthma is a common comorbid condition with CRS, whether sex hormones contribute to CRS pathogenesis remains unclear. The increased susceptibility of women may not be restricted to the lower respiratory tract but also may extend to the sinuses.
Implications for Practice and Future Directions
Although CRS is predominantly a disease of women, many studies are underpowered to detect gender treatment differences. Again referencing the pulmonary literature, the Euroscope study indicated that inhaled steroid use for chronic obstructive pulmonary disease was a significant predictor of reduced phlegm production in men but not in women.56 These results are compatible with findings in patients with asthma in which the magnitude of the steroid effect was significantly greater in males than in females.57 Because intranasal steroids are the mainstay of treatment for CRS, gender differences in outcomes of intranasal steroids warrant investigation. Future studies on intranasal steroids should be powered sufficiently to detect gender treatment differences. Moreover, no data exist on the effects of hormonal therapy in CRS, an important topic for future study.
Analysis of the data indicates that patterns of sex differences in morbidity are more complicated than previously believed. There is a paucity of investigations that target gender-related differences in CRS biology and physiology. Little is known about gender differences with respect to diagnosis or treatment of CRS because studies generally have not been designed to assess the dependent variable stratified by sex. As a result, there is a lack of conclusive evidence to answer whether male and female patients with RS are different, and if so, whether we should treat women with RS differently from men with RS. Focused research in this area is warranted.
Footnotes
Research for this article was done in large part while S. S. Smith was a postdoctoral fellow at the Institute for Healthcare Studies, supported by an institutional award from the Agency for Healthcare Research and Quality, T-32 HS 000078 (PI, Jane L. Holl, M.D., M.P.H.), and while S. Smith was supported by a National Institutes of Health/National Heart, Lung, and Blood Institute training grant T-32 HL 076139 (PI, Jacob Sznajder, M.D.). This work was supported by National Institutes of Health grant K23DC012067 (to B. K. Tan)
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat 10;252:1–207, 2012. [PubMed] [Google Scholar]
- 2. Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope 113:1199–1205, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe: An underestimated disease. A GA2LEN study. Allergy 66:1216–1223, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Min YG, Jung HW, Kim HS, et al. Prevalence and risk factors of chronic sinusitis in Korea: Results of a nationwide survey. Eur Arch Otorhinolaryngol 253:435–439, 1996. [DOI] [PubMed] [Google Scholar]
- 5. Busaba NY, Sin HJ, Salman SD. Impact of gender on clinical presentation of chronic rhinosinusitis with and without polyposis. J Laryngol Otol 122:1180–1184, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Hopkins C, Gillett S, Slack R. Are men really more full of SNOT? Clin Otolaryngol 34:267–268, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Mendolia-Loffredo S, Laud PW, Sparapani R, Loehrl TA. Sex differences in outcomes of sinus surgery. Laryngoscope 116:1199–1203, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Baumann I, Blumenstock G, Zalaman IM, et al. Impact of gender, age, and comorbidities on quality of life in patients with chronic rhinosinusitis. Rhinology 45:268–272, 2007. [PubMed] [Google Scholar]
- 9. Peterlin BL, Gupta S, Ward TN, Macgregor A. Sex matters: Evaluating sex and gender in migraine and headache research. Headache 51:839–842, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shashy RG, Moore EJ, Weaver A. Prevalence of the chronic sinusitis diagnosis in Olmsted County, Minnesota. Arch Otolaryngol Head Neck Surg 130:320–323, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Bernstein JM, Anon JB, Rontal M, et al. Genetic polymorphisms in chronic hyperplastic sinusitis with nasal polyposis. Laryngoscope 119:1258–1264, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drake-Lee AB, Lowe D, Swanston A, Grace A. Clinical profile and recurrence of nasal polyps. J Laryngol Otol 98:783–793, 1984. [DOI] [PubMed] [Google Scholar]
- 13. Larsen K, Tos M. The estimated incidence of symptomatic nasal polyps. Acta Otolaryngol 122:179–182, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Hopkins C, Browne JP, Slack R, et al. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clin Otolaryngol 31:390–398, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol 131:1350–1360, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim YS, Kim NH, Seong SY, et al. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy 25:117–121, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Chung SD, Hung SH, Lin HC, Lin CC. Health care service utilization among patients with chronic rhinosinusitis: A population-based study. Laryngoscope 124:1285–1289, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Macintyre S, Hunt K, Sweeting H. Gender differences in health: Are things really as simple as they seem? Soc Sci Med 42:617–624, 1996. [DOI] [PubMed] [Google Scholar]
- 19. Baumann I, Blumenstock G. Impact of gender on general health-related quality of life in patients with chronic sinusitis. Am J Rhinol 19:282–287, 2005. [PubMed] [Google Scholar]
- 20. Sil A, Mackay I, Rowe-Jones J. Assessment of predictive prognostic factors for functional endoscopic sinus surgery in a 5-year prospective outcome study. Am J Rhinol 21:289–296, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Bhattacharyya N, Grebner J, Martinson NG. Recurrent acute rhinosinusitis: Epidemiology and health care cost burden. Otolaryngol Head Neck Surg 146:307–312, 2012. [DOI] [PubMed] [Google Scholar]
- 22. Smith SS, Kern RC, Chandra RK, et al. Variations in antibiotic prescribing of acute rhinosinusitis in United States ambulatory settings. Otolaryngol Head Neck Surg 148:852–859, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee LN, Bhattacharyya N. Regional and specialty variations in the treatment of chronic rhinosinusitis. Laryngoscope 121:1092–1097, 2011. [DOI] [PubMed] [Google Scholar]
- 24. Lebovics RS, Moisa II, Ruben RJ. Sex predilection in patients with acute frontal sinusitis. Ear Nose Throat J 68:433–434, 437, 1989. [PubMed] [Google Scholar]
- 25. Bayonne E, Kania R, Tran P, et al. Intracranial complications of rhinosinusitis. A review, typical imaging data and algorithm of management. Rhinology 47:59–65, 2009. [PubMed] [Google Scholar]
- 26. Overview of the State Ambulatory Surgery and Services Databases (SASD). Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. State Ambulatory Surgery Databases (SASD). 2011; www.hcup-us.ahrq.gov/sasdoverview.jsp Accessed January 15, 2014.
- 27. Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology 44(suppl. 4):S17–S23, 1994. [PubMed] [Google Scholar]
- 28. Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R. Incidence of primary headache: A Danish epidemiologic follow-up study. Am J Epidemiol 161:1066–1073, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Kari E, DelGaudio JM. Treatment of sinus headache as migraine: The diagnostic utility of triptans. Laryngoscope 118:2235–2239, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Schreiber CP, Hutchinson S, Webster CJ, et al. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med 164:1769–1772, 2004. [DOI] [PubMed] [Google Scholar]
- 31. Eross E, Dodick D, Eross M. The Sinus, Allergy and Migraine Study (SAMS). Headache 47:213–224, 2007. [DOI] [PubMed] [Google Scholar]
- 32. Guven H, Cilliler AE, Comoglu SS. Unilateral cranial autonomic symptoms in patients with migraine. Acta Neurol Belg 113:237–242, 2013. [DOI] [PubMed] [Google Scholar]
- 33. Hsueh WD, Conley DB, Kim H, et al. Identifying clinical symptoms for improving the symptomatic diagnosis of chronic rhinosinusitis. Int Forum Allergy Rhinol 3:307–314, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hulse KE, Stevens WW, Tan BK, et al. Sex-specific differences in disease severity in patients with chronic rhinosinusitis with nasal polyps. 2014 AAAAI Annual Meeting. J Allergy Clin Immunol 133(Supp):AB169, 2014. [Google Scholar]
- 35. Collins MM, Pang YT, Loughran S, Wilson JA. Environmental risk factors and gender in nasal polyposis. Clin Otolaryngol Allied Sci 27:314–317, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 33:1–47, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee TP, Chiang BL. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev 11:A422–A429, 2012. [DOI] [PubMed] [Google Scholar]
- 38. Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 128:1198–1206.e1191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeffe JS, Seshadri S, Hamill KJ, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope 123:2104–2111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Incaudo GA. Diagnosis and treatment of allergic rhinitis and sinusitis during pregnancy and lactation. Clin Rev Allergy Immunol 27:159–177, 2004. [DOI] [PubMed] [Google Scholar]
- 41. Orban N, Maughan E, Bleach N. Pregnancy-induced rhinitis. Rhinology 51:111–119, 2013. [DOI] [PubMed] [Google Scholar]
- 42. Ellegard E, Hellgren M, Toren K, Karlsson G. The incidence of pregnancy rhinitis. Gynecol Obstet Invest 49:98–101, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Ellegard E, Karlsson G. IgE-mediated reactions and hyperreactivity in pregnancy rhinitis. Arch Otolaryngol Head Neck Surg 125:1121–1125, 1999. [DOI] [PubMed] [Google Scholar]
- 44. Ellegard E, Karlsson G. Nasal congestion during pregnancy. Clin Otolaryngol Allied Sci 24:307–311, 1999. [DOI] [PubMed] [Google Scholar]
- 45. Ellegard E, Oscarsson J, Bougoussa M, et al. Serum level of placental growth hormone is raised in pregnancy rhinitis. Arch Otolaryngol Head Neck Surg 124:439–443, 1998. [DOI] [PubMed] [Google Scholar]
- 46. Ellegard EK. Pregnancy rhinitis. Immunol Allergy Clin North Am 26:119–135, vii, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Ellegard EK. Special considerations in the treatment of pregnancy rhinitis. Womens Health (Lond Engl) 1:105–114, 2005. [DOI] [PubMed] [Google Scholar]
- 48. Ellegard EK. Clinical and pathogenetic characteristics of pregnancy rhinitis. Clin Rev Allergy Immunol 26:149–159, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Ellegard EK. The etiology and management of pregnancy rhinitis. Am J Respir Med 2:469–475, 2003. [DOI] [PubMed] [Google Scholar]
- 50. Ellegard EK, Karlsson NG, Ellegard LH. Rhinitis in the menstrual cycle, pregnancy, and some endocrine disorders. Clin Allergy Immunol 19:305–321, 2007. [PubMed] [Google Scholar]
- 51. Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: Why it matters. Am J Respir Crit Care Med 176:1179–1184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax 54:1119–1138, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen Y, Breithaupt K, Muhajarine N. Occurrence of chronic obstructive pulmonary disease among Canadians and sex-related risk factors. J Clin Epidemiol 53:755–761, 2000. [DOI] [PubMed] [Google Scholar]
- 54. Chen Y, Dales R, Tang M, Krewski D. Obesity may increase the incidence of asthma in women but not in men: Longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol 155:191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 55. Barr RG, Wentowski CC, Grodstein F, et al. Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Arch Intern Med 164:379–386, 2004. [DOI] [PubMed] [Google Scholar]
- 56. Watson L, Vestbo J, Postma DS, et al. Gender differences in the management and experience of Chronic Obstructive Pulmonary Disease. Respir Med 98:1207–1213, 2004. [DOI] [PubMed] [Google Scholar]
- 57. Convery RP, Leitch DN, Bromly C, et al. Effect of inhaled fluticasone propionate on airway responsiveness in treatment-naive individuals: A lesser benefit in females. Eur Respir J 15:19–24, 2000. [DOI] [PubMed] [Google Scholar]


