Abstract
Purpose
Newly developed extra-mammary multiple primary cancers (MPCs) are an issue of concern when considering the management of breast cancer survivors. This study aimed to investigate the prevalence of MPCs and to evaluate the implications of MPCs on the survival of breast cancer patients.
Materials and Methods
A total of 8204 patients who underwent surgery at Severance Hospital between 1990 and 2012 were retrospectively selected. Clinicopathologic features and survival over follow-up periods of ≤5 and >5 years were investigated using univariate and multivariate analyses.
Results
During a mean follow-up of 67.3 months, 962 MPCs in 858 patients (10.5%) were detected. Synchronous and metachronous MPCs were identified in 23.8% and 79.0% of patients, respectively. Thyroid cancer was the most prevalent, and the second most common was gynecologic cancer. At ≤5 years, patients with MPCs were older and demonstrated significantly worse survival despite a higher proportion of patients with lower-stage MPCs. Nevertheless, an increased risk of death in patients with MPCs did not reach statistical significance at >5 years. The causes of death in many of the patients with MPCs were not related to breast cancer. Stage-matched analysis revealed that the implications of MPCs on survival were more evident in the early stages of breast disease.
Conclusion
Breast cancer patients with MPCs showed worse survival, especially when early-stage disease was identified. Therefore, it is necessary to follow screening programs in breast cancer survivors and to establish guidelines for improving prognosis and quality of life.
Keywords: Breast cancer, metachronous, multiple primary neoplasms, survival, synchronous
INTRODUCTION
Breast cancer is the most common malignancy among women globally. In the United States, more than 200000 new cases of invasive breast cancer and more than 60000 new cases of in situ breast cancer were expected among US women in 2013.1,2 Approximately 40000 US breast cancer patients were expected to die in 2013; however, breast cancer death rates decreased by 34% from 1990 to 2010.2 Similarly, the incidence rate of breast cancer is continuously increasing in Korea, and it is the second most common cancer among Korean women.3 The Korean Breast Cancer Society reported that the number of newly-diagnosed breast cancer patients was more than 16000 and that the crude age-specific incidence rate of women aged 40 to 49 years was the highest, with approximately 148 cases per 100000 women in 2010.4 The 5-year relative survival rate of female breast cancer patients diagnosed in 2006-2010 improved from 78.0% to 91.0% compared to those diagnosed in 1993-1995.3
Increasing survival rates in breast cancer patients are attributed to both early detection of malignancies and improvements in treatment.2,3 With increasing incidence and survival rates of such malignancies, the number of cancer survivors continues to increase in Korea. Although many cancer survivors return to normal daily activities after the completion of primary treatment, the cancer itself and related treatments may also result in a wide range of chronic, long-term physical and psychological problems.5 Of these issues, second or multiple primary cancers (MPCs) are both disastrous and lethal in this patient population. An increased risk of MPCs in cancer survivors compared to the general population has been associated with complex factors including genetic predisposition, host factors, environmental determinants, gene-environment interactions, shared lifestyle factors (e.g., tobacco use or excessive alcohol intake), and the late effects of cancer treatments (e.g., cytotoxic, radiation, or hormonal therapies).6
MPCs are generally defined according to the criteria of Warren and Gates7 as follows: 1) each tumor must have clear evidence of malignancy on histologic examination, 2) each tumor must be geographically separate and distinct, and 3) the possibility of a metastatic lesion having spread from a prior cancer must be excluded. Recent rules for classifying MPCs have been modified based on the cancer site of origin, date of diagnosis, histology, tumor behavior (i.e., in situ versus invasive), and laterality of the paired organ.8 International coding rules for MPCs are more restrictive, and MPCs occurring at the same site or on different sides of a paired organ are usually considered to be first primary unless the two tumors are of completely different histology.8,9
Trends of cancer incidence and mortality vary by age and between nations. Considering that the crude age-specific incidence rates of Korean breast cancer patients are different from those of Western countries,4 risks for and survival of MPCs should be clarified in Korean breast cancer patients. However, limited information is available on this subject.5,10 This study aims to investigate patterns of MPCs in Korean breast cancer patients treated at a single institution and to examine the characteristics and survival rates of these patients after diagnosis according to the presence or absence of MPCs.
MATERIALS AND METHODS
A total of 8204 patients with locoregional breast cancer were retrospectively selected from the Severance Hospital breast cancer registry. The Severance Hospital registry prospectively records clinicopathological information including past histories of cancer and details on survival outcomes. Additional information for MPCs was obtained from the Yonsei Cancer Registry of Yonsei University Health System in Seoul, Korea. All patients underwent surgery for primary breast cancer between January 1990 and December 2012. Patients with stage IV disease at the time of diagnosis were excluded. In this study, patients with bilateral breast cancer at initial diagnosis or during follow-up periods were not considered to have MPCs. In cases of simultaneous bilateral breast cancer, the side with the more advanced stage was selected, and in cases of metachronous bilateral breast cancers, the initial index tumor was considered for analysis.
In this study, the criteria of Warren and Gates7 were used to define MPCs. Synchronous MPCs were defined as a tumor diagnosed simultaneously with breast cancer or within a time interval of 6 months. Metachronous MPCs were considered to be a tumor detected more than 6 months before or after diagnosis of breast cancer. For survival analysis according to follow-up time period and time of diagnosis of MPCs, the patient cohort was subdivided into two groups as follows: patients with or without MPCs at a time point within a follow-up duration of 5 years or less (time period ≤5 years, n=8204) and those with or without MPCs among patients with a follow-up duration of more than 5 years (time period >5 years, n=3745). Therefore, patients who died after >5 years of being diagnosed with breast cancer were considered to be alive at ≤5 years. Similarly, patients diagnosed with metachronous MPCs at >5 years after being diagnosed with breast cancer were placed in the breast-cancer-alone group with a time period ≤5 years. Patients who died or were lost to follow-up within 5 years of being diagnosed with breast cancer were excluded from the analysis of the time period >5 years.
Clinical follow-up included a patient interview, physical examination, laboratory tests, and breast imaging every 6-12 months. If necessary, an abdominopelvic ultrasound, bone scan, computed tomography (CT) scan, or fluorin-18 fluorodeoxyglucose positron emission tomography/CT scan was performed. Tumor-node-metastasis (TNM) staging was based on the 6th American Joint Committee on Cancer criteria.11 Tumors with ≥1% nuclear-stained cells were considered positive for estrogen receptor (ER) and progesterone receptor (PR) according to the American Society of Clinical Oncology/College of American Pathologists guidelines.12 Human epidermal growth factor receptor 2 (HER2) staining was not available during the early 1990s, and HER2 3+ scores were considered positive.
Differences between groups were evaluated by the chi-square test. Continuous variables were compared using the independent two-sample t-test. Overall survival (OS) was calculated from the date of surgery for breast cancer to the date of the last follow-up or death from any cause. The date of the last follow-up was used as censored data in this analysis. Survival curves were plotted using the Kaplan-Meier method, and group differences in survival curve were investigated by the log-rank test. A Cox proportional hazard model was used to identify variables that were independently associated with OS. All statistical tests were two-sided and a p-value<0.05 was considered statistically significant. Categorical variables were expressed as a frequency and percentage. SPSS version 20.0 (IBM Inc., Armonk, NY, USA) was used for all statistical analyses.
RESULTS
Of the 8204 breast cancer patients with stage 0 to III disease, 858 patients (10.5%) had MPCs: 268 patients (31.2%) had metachronous MPCs alone ≥6 months before diagnosis of breast cancer, 180 patients (21.0%) had synchronous MPCs alone, 370 patients (43.1%) had metachronous MPCs alone ≥6 months after diagnosis of breast cancer, 10 patients (1.2%) had both metachronous MPCs ≥6 months before the diagnosis of breast cancer and synchronous MPCs, 14 patients (1.6%) had both synchronous and metachronous MPCs ≥6 months after the diagnosis of breast cancer, and 16 patients (1.9%) had metachronous MPCs ≥6 months both before and after the diagnosis of breast cancer. Synchronous MPCs were noted in 204 patients (23.8%), and metachronous MPCs were identified in 678 patients (79.0%). There were also 24 patients (2.8%) with both synchronous and metachronous MPCs.
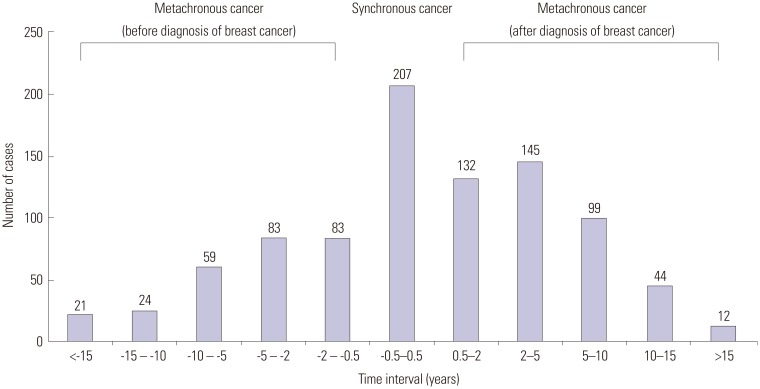
In the 858 patients with MPCs, a total of 962 primary malignancies were detected. Second primary cancer alone was noted in 762 patients (88.8%), and 96 patients (11.2%) had two or more primary malignancies other than breast cancer. Disease sites and numbers of cases are presented in Table 1. Cancers of the endocrine system, which mainly included the thyroid gland, were the most prevalent malignancy in Korean breast cancer patients, and more than two-thirds of synchronous MPCs were thyroid cancer. Subsequently, primary tumors of the gynecologic system including the ovary, cervix, and uterus were the second-most prevalent cancer. Interestingly, in patients with metachronous MPCs, primary cancers more frequently developed in respiratory and hematologic systems after, rather than before, the diagnosis of breast cancer. Fig. 1 shows the time intervals between breast cancer and MPCs. When excluding 53 metachronous cases (5.5%) that were diagnosed as MPCs before diagnosis of breast cancer and did not have information available to identify the date of cancer diagnosis, 166 cases (18.3%) had a past history of malignancy between 6 months and 5 years before diagnosis of breast cancer, and 275 cases (30.5%) developed MPCs between 6 months and 5 years after diagnosis of breast cancer.
Table 1. Site and Number of Multiple Primary Cancers.
| Site | Synchronous MPCs (n=207, %) | Metachronous MPCs | Total (n=962, %) | ||
|---|---|---|---|---|---|
| 1st cancer is not breast cancer (n=323, %) | 1st cancer is breast cancer (n=432, %) | Subtotal (n=755, %) | |||
| Thyroid gland | 147 (71.0) | 96 (29.7) | 162 (37.5) | 258 (34.2) | 405 (42.1) |
| Gynecologic system | 20 (9.7) | 90 (27.9) | 70 (16.2) | 160 (21.2) | 180 (18.7) |
| Stomach & esophagus | 9 (4.3) | 34 (10.5) | 39 (9.0) | 73 (9.7) | 82 (8.5) |
| Colon & rectum | 4 (1.9) | 31 (9.6) | 31 (7.2) | 62 (8.2) | 66 (6.9) |
| Lung & thorax | 4 (1.9) | 11 (3.4) | 30 (6.9) | 41 (5.4) | 45 (4.7) |
| Hepatobiliary & pancreas | 8 (3.9) | 16 (5.0) | 33 (7.6) | 49 (6.5) | 57 (5.9) |
| Urologic system | 2 (1.0) | 10 (3.1) | 13 (3.0) | 23 (3.0) | 25 (2.6) |
| Hematologic system | 0 (0.0) | 11 (3.4) | 26 (6.0) | 37 (4.9) | 37 (3.8) |
| Nervous system | 6 (2.9) | 12 (3.7) | 9 (2.1) | 21 (2.8) | 27 (2.8) |
| Bone & soft tissue | 3 (1.4) | 2 (0.6) | 7 (1.6) | 9 (1.2) | 12 (1.2) |
| Other | 4 (1.9) | 10 (3.1) | 12 (2.8) | 22 (2.9) | 26 (2.7) |
MPCs, multiple primary cancers.
Fig. 1. Time interval between breast cancer and development of multiple primary cancers.
Table 2 summarizes the clinicopathologic characteristics associated with breast cancer according to the presence or absence of MPCs and stratified by a cutoff follow-up duration of 5 years. At ≤5 years, the mean age of patients with MPCs was 52.7 years, which was significantly older than those with breast cancer alone. Patients with MPCs showed smaller tumor size, higher node-negative disease, and lower TNM staging at the time of diagnosis of breast cancer. ER, PR, and HER2 expressions were not significantly different between groups. Similarly, at >5 years, patients with MPCs were older in age at diagnosis of breast cancer and showed smaller tumor sizes. However, node status was not significantly different between patients with and without MPCs; differences in TNM stage showed borderline statistical significance.
Table 2. Clinicopathologic Characteristics Associated with Breast Cancer According to Follow-Up Duration of 5 Years.
| Time period ≤5 yrs | Time period >5 yrs | |||||
|---|---|---|---|---|---|---|
| Breast cancer alone (n=7478, %) | MPCs (n=726, %) | p value | Breast cancer alone (n=3316, %) | MPCs (n=429, %) | p value | |
| Age at diagnosis of breast cancer (yrs, n=8204) | ||||||
| Mean±SD | 48.9±10.6 | 52.7±10.7 | <0.001* | 47.4±9.8 | 50.3±9.8 | <0.001* |
| ≤50 | 4491 (60.1) | 344 (47.4) | <0.001 | 2182 (65.8) | 220 (51.3) | <0.001 |
| >50 | 2987 (39.9) | 382 (52.6) | 1134 (34.2) | 209 (48.7) | ||
| T stage (n=8135) | <0.001 | 0.001 | ||||
| Tis-1 | 4683 (63.1) | 510 (70.9) | 1847 (56.2) | 276 (64.8) | ||
| T2-4 | 2733 (36.9) | 209 (29.1) | 1439 (43.8) | 150 (35.2) | ||
| N stage (n=8139) | 0.030 | 0.893 | ||||
| N0 | 4904 (66.1) | 504 (70.1) | 2067 (62.9) | 267 (62.5) | ||
| N1-3 | 2516 (33.9) | 215 (29.9) | 1221 (37.1) | 160 (37.5) | ||
| TNM stage (n=8137) | <0.001 | 0.053 | ||||
| Stage 0-1 | 3585 (48.3) | 409 (56.9) | 1382 (42.0) | 205 (48.0) | ||
| Stage 2 | 2830 (38.2) | 240 (33.4) | 1419 (43.2) | 161 (37.7) | ||
| Stage 3 | 1003 (13.5) | 70 (9.7) | 486 (14.8) | 61 (14.3) | ||
| ER (n=7690) | 0.796 | 0.244 | ||||
| Positive | 4784 (68.4) | 475 (68.0) | 2010 (67.7) | 255 (64.7) | ||
| Negative | 2207 (31.6) | 224 (32.0) | 961 (32.3) | 139 (35.3) | ||
| PR (n=7667) | 0.097 | 0.316 | ||||
| Positive | 4153 (59.6) | 394 (56.4) | 1851 (62.7) | 236 (60.1) | ||
| Negative | 2815 (40.4) | 305 (43.6) | 1103 (37.3) | 157 (39.9) | ||
| HER2 (n=6107) | 0.175 | 0.801 | ||||
| Positive | 1407 (25.5) | 137 (23.0) | 564 (26.5) | 76 (25.8) | ||
| Negative | 4104 (74.5) | 459 (77.0) | 1568 (73.5) | 219 (74.2) | ||
MPCs, multiple primary cancers; SD, standard deviation; is, in situ; TNM, tumor node metastasis; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
*Student's t-test.
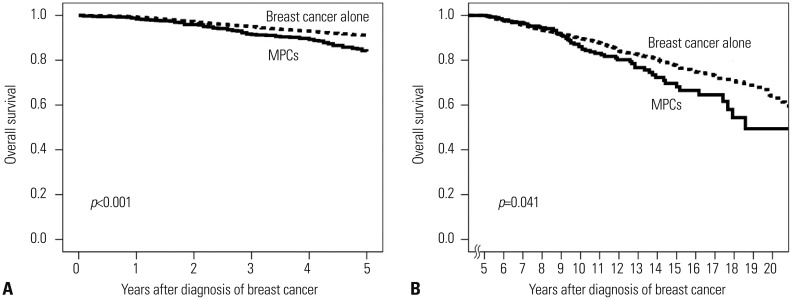
During a mean follow-up duration of 67.3 months for the whole population, the OS of patients with and without MPCs are shown in Fig. 2. Although patients with MPCs demonstrated a higher proportion of stage 0-I disease, they also showed worse survival than the breast-cancer-alone group at both ≤5 and >5 years. Table 3 shows causes of death. Of the 978 patients who died during this study, 53 (5.4%) did not have an identifiable cause of death, and most were in the breast-cancer-alone group. At ≤5 years, a significantly higher number of patients with MPCs died due to reasons not associated with breast cancer, and at >5 years, causes of death were similar to those at ≤5 years.
Fig. 2. Overall survival curve of patients with and without MPCs (A) at ≤5 years and (B) at >5 years. MPCs, multiple primary cancers.
Table 3. Survival and Cause of Death.
| Time period ≤5 yrs | Time period >5 yrs | |||||
|---|---|---|---|---|---|---|
| Breast cancer alone (n=7478, %) | MPCs (n=726, %) | p value | Breast cancer alone (n=3316, %) | MPCs (n=429, %) | p value | |
| Survival | <0.001 | 0.008 | ||||
| Alive | 6991 (93.5) | 645 (88.8) | 2969 (89.5) | 366 (85.3) | ||
| Dead | 487 (6.5) | 81 (11.2) | 347 (10.5) | 63 (14.7) | ||
| Cause of death | <0.001 | <0.001 | ||||
| Related to breast cancer | 416 (87.2) | 47 (58.0) | 267 (87.3) | 26 (42.6) | ||
| Not related to breast cancer | 61 (12.8) | 34 (42.0) | 39 (12.7) | 35 (57.4) | ||
MPCs, multiple primary cancers.
When adjusting for age, TNM stage, and ER expression at diagnosis of breast cancer, patients with MPCs were significantly associated with an increased risk of death at ≤5 years (Table 4). However, a slightly increased risk of death in patients with MPCs did not reach statistical significance at >5 years. Older age, advanced TNM stage, and ER-positive disease were independent prognostic factors at >5 years. A stage-matched subgroup analysis showed that patients with MPCs had significantly worse OS than the breast-cancer-alone group for stage 0-I disease at both ≤5 and >5 years and for stage II disease at ≤5 years (Fig. 3). Nevertheless, no statistical difference in OS was noted between stage II disease at >5 years and stage III disease irrespective of follow-up duration.
Table 4. Multivariate Analysis for Overall Survival.
| Time period ≤5 yrs | Time period >5 yrs | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | p value | Hazard ratio | 95% confidence interval | p value | |
| Age at diagnosis of breast cancer (yrs) | ||||||
| ≤50 | Ref. | Ref. | ||||
| >50 | 1.125 | 0.944-1.339 | 0.187 | 1.556 | 1.263-1.916 | <0.001 |
| TNM stage | ||||||
| Stage 0-1 | Ref. | Ref. | ||||
| Stage 2 | 2.663 | 2.060-3.444 | <0.001 | 1.824 | 1.369-2.430 | <0.001 |
| Stage 3 | 9.271 | 7.205-11.930 | <0.001 | 3.834 | 2.850-5.158 | <0.001 |
| ER | ||||||
| Positive | Ref. | Ref. | ||||
| Negative | 2.163 | 1.820-2.570 | <0.001 | 0.779 | 0.621-0.976 | 0.030 |
| Multiple primary cancers | ||||||
| Breast cancer alone | Ref. | Ref. | ||||
| MPCs | 1.927 | 1.505-2.468 | <0.001 | 1.195 | 0.897-1.593 | 0.223 |
Ref., reference; TNM, tumor node metastasis; ER, estrogen receptor; MPCs, multiple primary cancers.
Fig. 3. Stage-matched overall survival curve (A, B, and C) at ≤5 years and (D, E, and F) at >5 years. (A and D) Stage 0-I; (B and E) stage II; and (C and F) stage III disease. The dotted line represents patients with breast cancer alone, and the solid line indicates patients with multiple primary cancers.
DISCUSSION
It is occasionally clinically difficult to distinguish new second primary malignancies from a metastatic neoplasm during the follow-up of cancer survivors. MPCs are generally considered new primary cancers when detected in a patient with a prior history of malignancy that is in a new site or tissue and subsequent to the initial cancer.5 Cancer survivors have a higher risk of developing MPCs than the general population, and studies using population-based registry datasets have demonstrated standardized incidence ratios for subsequent MPCs of 1.17 to 1.6 in female cancer survivors.5,8,13,14 Recently, these relative risks for developing MPCs among women with breast cancer were reported differently, with ratios as high as 1.96 [95% confidence interval (CI), 1.48-2.44] according to age at diagnosis of breast cancer.15,16 However, there has been no official study on MPCs from the nationwide Korean Central Cancer Registry.
In Korea, incidence rates of female thyroid cancer have increased sharply since the early 2000s, and this was found to be the most common malignancy of Korean women, with an age-standardized incidence rate of 87.4 in 2010.3 In our study, thyroid cancer was the most prevalent malignancy among breast cancer patients. Interestingly, more than two-thirds of patients with synchronous MPCs had a thyroid malignancy with breast cancer. Globally, incidence rates of thyroid cancer have increased, with the exception of countries such as Sweden, Norway, and Spain. The cause of such changing trends in the incidence of thyroid cancer may be multifactorial, although it remains unclear.17 It also remains to be determined whether our results reflect a true increase in the development of Korean thyroid cancer, an increased identification of previously undetectable subclinical thyroid disease along with improved diagnostic techniques and screening rates, or close biological connections between female breast and thyroid cancers.3,18
It has been suggested that certain types of anticancer treatments are closely linked to an increased risk of developing MPCs in cancer survivors.19,20,21 Examples include findings that cytotoxic chemotherapy was associated with an increased risk for leukemia, chest irradiation for Hodgkin's disease was related to an increased risk of breast cancer, and tamoxifen treatment for breast cancer was connected to a higher risk of endometrial cancer.15,18,20,22 By analyzing patients with MPCs among our study cohort, the proportions of patients with malignancy in the thyroid gland, lung, thorax, and hematologic system were elevated after, rather than before, the diagnosis and treatment of breast cancer. In this study, we were unable to confirm whether certain types of cancer were developed in association with anticancer therapies, and in the future, it will be necessary to further investigate this topic. In addition, given that MPCs in the gynecologic system were the second-most common malignancy and development of breast and ovarian cancer shares a close genetic linkage in deleterious BRCA 1 & 2 mutation carriers, results of the Korean Hereditary Breast Cancer (KOH-BRA) Study for the Korean population may shed further light on this topic.23
In the present study, patients with MPCs showed a higher proportion of early-stage breast cancer, especially at ≤5 years. Recent meta-analyses suggested that cancer survivors were more likely to be screened for breast, cervical, colorectal, and prostate cancer than non-cancer controls.24,25 Cross-sectional surveys involving the Korean population have shown that screening rates for breast cancer within 2 years were 46.4% (95% CI, 36.2-56.7) in cancer survivors, 36.0% (95% CI, 33.2-38.9) in non-cancer chronic disease controls, and 30.0% (95% CI, 27.8-32.2) in non-cancer non-chronic disease controls when adjusted for gender, age, marital status, education, income, working status, insurance status, smoking and drinking status, self-reported health status, and survey year.26 Higher screening rates and interest in personal health among patients with metachronous MPCs could partly explain our results; however, further investigation of the association of health screening with the first primary cancer and subsequent MPCs is required.
There have been limited data regarding the impact of MPCs on the survival of breast cancer patients. One early study of Korean breast cancer patients reported no difference in survival according to MPCs.10 However, in a recent study by the M.D. Anderson Cancer Center on 4198 patients treated with breast conservation therapy, patients with MPCs showed a worse OS than those without MPCs after excluding patients who had a past history of malignancy prior to the diagnosis of breast cancer. 27 Similarly, patients with MPCs in this study demonstrated a worse survival than those without MPCs, and most died due to diseases other than breast cancer. Although statistical significance in the multivariate analysis was not maintained at >5 years as more than half of the patients were lost to follow-up, further long-term study is needed. Stage-matched subgroup analysis revealed that the implications of MPCs on breast cancancer survival were more significantly evident among patients with early stage 0-I disease, yet not among those with advanced stage II-III disease.
Many cancer survivors do not perceive their risk of a subsequent second primary malignancy, and appropriate screening rates among Korean cancer survivors are suboptimal compared to the United States.5 Therefore, awareness and education regarding the development of MPCs and importance of screening programs are required for both cancer survivors and surgical and medical oncologists.28,29 In addition, as there are no evidence-based guidelines on screening programs for cancer survivors, it is accepted that cancer survivors should, at minimum, follow the screening guidelines for the general population until the establishment of screening guidelines for cancer survivors. Limitations of the present study include the retrospective nature of the survival analysis over a short duration of follow-up and the use of a hospital-based registry database at a single institution. More importantly, it was not possible to incorporate details of TNM stage, treatment patterns of MPCs, or environmental risk factors in the analysis.
In conclusion, Korean breast cancer patients are at a risk of second or multiple primary malignancies. Cancers most commonly occurred in the thyroid gland and gynecologic system. Breast cancer patients with MPCs exhibited worse survival, and many died due to causes unrelated to breast cancer. However, these patients were older at diagnosis and had lower breast cancer staging. The implications of MPCs on survival were more evident when patients had early-stage breast disease. Therefore, further efforts are needed to investigate the nationwide incidence of MPCs in order to discover the causes of increased risk for MPCs, to develop preventive methods for MPCs, and to increase awareness of and enrollment in screening programs for breast cancer survivors.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of Korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012;15:393–400. doi: 10.4048/jbc.2012.15.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin DW, Cho B, Kim SY, Jung JH, Park JH. Management of cancer survivors in clinical and public health perspectives: current status and future challenges in Korea. J Korean Med Sci. 2013;28:651–657. doi: 10.3346/jkms.2013.28.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10:289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 7.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–1414. [Google Scholar]
- 8.Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, et al. New malignancies among cancer survivors: SEER cancer registries, 1973-2000. Bethesda, MD: National Cancer Institute; 2006. NIH Publ. No. 05-5302. [Google Scholar]
- 9.Working Group Report. International rules for multiple primary cancers (ICD-0 third edition) Eur J Cancer Prev. 2005;14:307–308. doi: 10.1097/00008469-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Jung SH, Kwak SS, Kim SC, Park MK, Lee GS, Kim HJ, et al. Clinical characteristics of multiple primary cancer in breast cancer patients. J Breast Cancer. 2007;10:263–268. [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC cancer staging manual. 6th ed. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 12.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C, Hemminki K. Second primary neoplasms in 633,964 cancer patients in Sweden, 1958-1996. Int J Cancer. 2001;93:155–161. doi: 10.1002/ijc.1317. [DOI] [PubMed] [Google Scholar]
- 14.Tabuchi T, Ito Y, Ioka A, Miyashiro I, Tsukuma H. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci. 2012;103:1111–1120. doi: 10.1111/j.1349-7006.2012.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina-Montes E, Pollán M, Payer T, Molina E, Dávila-Arias C, Sánchez MJ. Risk of second primary cancer among women with breast cancer: a population-based study in Granada (Spain) Gynecol Oncol. 2013;130:340–345. doi: 10.1016/j.ygyno.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 16.Evans HS, Lewis CM, Robinson D, Bell CM, Møller H, Hodgson SV. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84:435–440. doi: 10.1054/bjoc.2000.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzaferri EL. Managing thyroid microcarcinomas. Yonsei Med J. 2012;53:1–14. doi: 10.3349/ymj.2012.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellemkjær L, Christensen J, Frederiksen K, Pukkala E, Weiderpass E, Bray F, et al. Risk of primary non-breast cancer after female breast cancer by age at diagnosis. Cancer Epidemiol Biomarkers Prev. 2011;20:1784–1792. doi: 10.1158/1055-9965.EPI-11-0009. [DOI] [PubMed] [Google Scholar]
- 19.Kirova YM, De Rycke Y, Gambotti L, Pierga JY, Asselain B, Fourquet A, et al. Second malignancies after breast cancer: the impact of different treatment modalities. Br J Cancer. 2008;98:870–874. doi: 10.1038/sj.bjc.6604241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arriagada R, Averbeck D, Dahl AA, Darby S, Fosså S, Friberg S, et al. OECI Workshop on late side-effects of cancer treatments. Eur J Cancer. 2009;45:354–359. doi: 10.1016/j.ejca.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Andersson M, Jensen MB, Engholm G, Henrik Storm H. Risk of second primary cancer among patients with early operable breast cancer registered or randomised in Danish Breast Cancer cooperative Group (DBCG) protocols of the 77, 82 and 89 programmes during 1977-2001. Acta Oncol. 2008;47:755–764. doi: 10.1080/02841860801978921. [DOI] [PubMed] [Google Scholar]
- 22.Oeffinger KC, Baxi SS, Novetsky Friedman D, Moskowitz CS. Solid tumor second primary neoplasms: who is at risk, what can we do? Semin Oncol. 2013;40:676–689. doi: 10.1053/j.seminoncol.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang E, Kim SW. The korean hereditary breast cancer study: review and future perspectives. J Breast Cancer. 2013;16:245–253. doi: 10.4048/jbc.2013.16.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corkum M, Hayden JA, Kephart G, Urquhart R, Schlievert C, Porter G. Screening for new primary cancers in cancer survivors compared to non-cancer controls: a systematic review and meta-analysis. J Cancer Surviv. 2013;7:455–463. doi: 10.1007/s11764-013-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh B, Shin DW, Kim SY, Park JH, Chang WY, Lim SP, et al. Mode of primary cancer detection as an indicator of screening practice for second primary cancer in cancer survivors: a nationwide survey in Korea. BMC Cancer. 2012;12:557. doi: 10.1186/1471-2407-12-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho J, Guallar E, Hsu YJ, Shin DW, Lee WC. A comparison of cancer screening practices in cancer survivors and in the general population: the Korean national health and nutrition examination survey (KNHANES) 2001-2007. Cancer Causes Control. 2010;21:2203–2212. doi: 10.1007/s10552-010-9640-4. [DOI] [PubMed] [Google Scholar]
- 27.Yi M, Cormier JN, Xing Y, Giordano SH, Chai C, Meric-Bernstam F, et al. Other primary malignancies in breast cancer patients treated with breast conserving surgery and radiation therapy. Ann Surg Oncol. 2013;20:1514–1521. doi: 10.1245/s10434-012-2774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin DW, Baik YJ, Kim YW, Oh JH, Chung KW, Kim SW, et al. Knowledge, attitudes, and practice on second primary cancer screening among cancer survivors: a qualitative study. Patient Educ Couns. 2011;85:74–78. doi: 10.1016/j.pec.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Shin DW, Kim Y, Baek YJ, Mo HN, Choi JY, Cho J. Oncologists experience with second primary cancer screening: current practices and barriers and potential solutions. Asian Pac J Cancer Prev. 2012;13:671–676. doi: 10.7314/apjcp.2012.13.2.671. [DOI] [PubMed] [Google Scholar]