Abstract
Purpose
We aimed to discover clinical and angiographic predictors of microvascular dysfunction using the index of microcirculatory resistance (IMR) in patients with ST-segment elevation myocardial infarction (STEMI).
Materials and Methods
We enrolled 113 patients with STEMI (age, 56±11 years; 95 men) who underwent primary percutaneous coronary intervention (PCI). The IMR was measured with a pressure sensor/thermistor-tipped guidewire after primary PCI. The patients were divided into three groups based on IMR values: Low IMR [<18 U (12.9±2.6 U), n=38], Mid IMR [18-31 U (23.9±4.0 U), n=38], and High IMR [>31 U (48.1±17.1 U), n=37].
Results
The age of the Low IMR group was significantly lower than that of the Mid and High IMR groups. The door-to-balloon time was <90 minutes in all patients, and it was not significantly different between groups. Meanwhile, the symptom-onset-to-balloon time was significantly longer in the High IMR group, compared to the Mid and Low IMR groups (p<0.001). In the high IMR group, the culprit lesion was found in a proximal location significantly more often than in a non-proximal location (p=0.008). In multivariate regression analysis, age and symptom-onset-to-balloon time were independent determinants of a high IMR (p=0.013 and p=0.003, respectively).
Conclusion
Our data suggest that age and symptom-onset-to-balloon time might be the major predictors of microvascular dysfunction in STEMI patients with a door-to-balloon time of <90 minutes.
Keywords: Microvascular dysfunction, ST-segment elevation myocardial infarction, index of microcirculatory resistance, doorto-balloon time, symptom-onset-to-balloon time
INTRODUCTION
Primary percutaneous coronary intervention (PCI) is an established treatment for patients with ST-segment elevation myocardial infarction (STEMI). However, after successful PCI many patients continue to have microvascular dysfunction documented by various invasive and non-invasive tests.1,2,3 Impaired microcirculation after successful reperfusion therapy correlates strongly with a poor prognosis.4,5,6,7
The index of microcirculatory resistance (IMR) is a simple and standard method of assessing microvascular integrity by using a pressure sensor/thermistor-tipped guidewire, and it is a strong predictor of microvascular damage, especially 3 months after STEMI.8 Using contrast-cardiac magnetic resonance imaging, it has also been shown that IMR can independently predict left ventricular function and infarct volume following STEMI.9,10 In a recent study, an elevated IMR measured immediately after primary PCI was a strong predictor of poor long-term outcomes.11
Multiple clinical-, angiographic-, and procedure-related factors could affect microcirculation in STEMI patients prior to or during primary PCI; however, data on which of these factors has the most influence on microvascular integrity are limited. In the present study, we used IMR to assess microvascular integrity in STEMI patients who underwent primary PCI. We aimed to assess the clinical and angiographic predictors of microvascular dysfunction in STEMI patients.
MATERIALS AND METHODS
Study design
The present study is a retrospective cohort study designed to find out the clinical and angiographic determinants of microvascular dysfunction in STEMI patients. We enrolled STEMI patients who underwent primary PCI and coronary physiologic measurements from 2011 to 2014 at Inha University Hospital. IMR, a parameter of hyperemic microvascular resistance, was measured with a pressure sensor/thermistor-tipped guidewire immediately after successful primary PCI. A transthoracic echocardiogram (TTE) was obtained within 24 hours. This study was approved by Inha University Hospital's Institutional Review Board, and written informed consent was obtained from each patient.
Study population
We enrolled 113 patients with STEMI who underwent primary PCI. The IMR was measured immediately after successful primary PCI. The patients were divided into three groups based on IMR values as tertiles: Low IMR (<18 U, n=38), Mid IMR (18-31 U, n=38), and High IMR (>31 U, n=37). Patients with STEMI were diagnosed based on symptoms of myocardial ischemia in association with ST-segment elevation and subsequent release of cardiac biomarkers. ST elevation was defined as new ST elevation at J point of ≥2 mm (0.2 mV) in men or ≥1.5 mm (0.15 mV) in women in at least two contiguous leads on electrocardiography.12 We excluded patients with prior myocardial infarction in order to focus on de novo coronary microvascular resistance, and also excluded patients with cardiogenic shock, Killip Class IV, and atrioventricular block, as they were contraindicated to an invasive coronary physiologic study using adenosine.13
Angiographic analysis
Lesion length and reference diameter (RD) were measured using an automated edge-detection algorithm (CASS 5.7.1, Pie Medical Imaging Systems, Maastricht, the Netherlands). Automated distance calibration was used to determine pixel size. Angiographic views with the least foreshortening and the best depiction of the stenosis were used. Thrombolysis in Myocardial Infarction (TIMI) grade and TIMI myocardial perfusion grade (TMPG) were obtained using a grading scale of 0-3. Lesions of the coronary artery were classified by the American College of Cardiology/American Heart Association (ACC/AHA) grading system as type A, B1, B2, and C.
Measurement of IMR
After successful primary PCI, a pressure sensor/thermistortipped guidewire (Radi Medical System, Uppsala, Sweden) was calibrated outside the body, equalized to the guiding catheter, and advanced to the distal two-thirds of the culprit vessel. Three bolus injections of 3 mL room temperature saline were administrated at the culprit vessel, and the mean transit time was obtained using a thermodilution technique.14 After intravenous adenosine (140 µg/kg/min) was administered to induce maximal hyperemia; the hyperemic mean transit time (hTmn) was measured again using the same method above. Simultaneously, mean aortic pressure (Pa) and mean distal pressure (Pd) were measured during the resting and maximal hyperemic state. The IMR value was calculated as Pd×hTmn.2 Fractional flow reserve (FFR) was derived from the ratio of Pd to Pa during maximal hyperemia.15 In addition, thermodilution coronary flow reserve (CFR) was calculated by dividing the resting mean transit time by the hTmn.16
Echocardiographic analysis
A TTE was obtained within 24 hours after the index PCI. Left ventricular ejection fraction (LVEF) was measured using the modified Simpson method. As recommended by the American Society of Echocardiography, the wall motion score index (WMSI) was assessed in a 16-segment model.17 An experienced cardiologist blinded to the IMR values scored segmental wall motion and the WMSI.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, USA). Data are presented as mean±SD for continuous variables and as proportions for categorical variables. Continuous variables were compared using the Student's t-test. Analysis of categorical variables was performed using the chi-square test. Continuous variables were compared using one-way analysis of variance (ANOVA) and Fisher's exact test as a post hoc test for each IMR group. Univariate correlations between variables were assessed by Pearson's correlation coefficients (r). Linear regression analyses were performed to assess the relationships between IMR and clinical, angiographic, and anatomical factors. Univariate regression analysis was used to identify relationships between each clinical and angiographic factor and increased IMR. The clinical and angiographic predictors of impaired microvascular function were assessed using multivariate logistic regression analysis. Figures were created by using GraphPad Prism v.5.01 (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
Patient characteristics between IMR groups
The mean age of the 113 study population was 56±11 years; 95 patients (84.1%) were men. The mean IMR in the study population was 28.2±17.8 U (range, 7.3-98.4 U). To determine the predictive factors for microvascular dysfunction, the study population was classified into three groups based on IMR values: Low IMR [<18 U (12.9±2.6 U), n=38], Mid IMR [18-31 U (23.9±4.0 U), n=38], and High IMR [>31 U (48.1±17.1 U), n=37] (Table 1).
Table 1. Clinical Characteristic and Laboratory and Echocardiographic Findings of Patients in the Different IMR Groups.
| Total (n=113) | Low IMR (n=38) | Mid IMR (n=38) | High IMR (n=37) | p value | |
|---|---|---|---|---|---|
| Age, mean±SD, yr | 56±11 | 51±9 | 57±11† | 61±10† | 0.031 |
| Male (%) | 95 (84.1) | 36 (94.7) | 31 (81.6) | 28 (75.7) | 0.069 |
| BMI, mean±SD, kg/m2 | 24.87±2.61 | 25.24±2.36 | 24.38±2.77 | 24.98±2.68 | 0.343 |
| SBP, mean±SD, mm Hg | 129±21 | 129±17 | 127±25 | 133±20 | 0.410 |
| DBP, mean±SD, mm Hg | 82±14 | 83±12 | 78±16 | 84±12 | 0.083 |
| HR, mean±SD, beats/min | 75±15 | 75±12 | 78±17 | 72±15 | 0.212 |
| Door-to-balloon time, mean±SD, min | 73.9±29.4 | 65.7±14.6 | 74.8±19.8 | 82.0±44.6 | 0.068 |
| Symptom-onset-to-balloon time, mean±SD, min | 221.2±145.2 | 172.2±80.1 | 197.4±104.1* | 299.8±195.1* | <0.001 |
| Hypertension, n (%) | 50 (44.2) | 16 (42.1) | 15 (39.5) | 19 (51.4) | 0.555 |
| Diabetes, n (%) | 28 (24.8) | 12 (31.6) | 8 (21.1) | 8 (21.6) | 0.503 |
| Dyslipidemia, n (%) | 31 (27.4) | 12 (31.6) | 9 (23.7) | 10 (27.0) | 0.741 |
| Smoker, n (%) | 80 (70.8) | 31 (81.6) | 25 (65.8) | 24 (64.9) | 0.199 |
| Medication history, n (%) | |||||
| CCB | 27 (23.9) | 8 (21.1) | 9 (23.7) | 10 (27.0) | 0.823 |
| ACEi or ARB | 15 (13.3) | 6 (15.8) | 7 (18.4) | 2 (5.4) | 0.212 |
| b-blocker | 4 (3.5) | 1 (2.6) | 2 (5.3) | 1 (2.7) | 0.790 |
| Statin | 22 (19.5) | 8 (21.1) | 6 (15.8) | 8 (21.6) | 0.779 |
| CRP, mean±SD, mg/dL | 0.55±1.96 | 0.24±0.37 | 0.57±1.41 | 0.86±3.07 | 0.402 |
| HbA1c, mean±SD, % | 6.34±1.09 | 6.60±1.28 | 6.32±1.11 | 6.01±0.78 | 0.161 |
| Creatinine, mean±SD, mg/dL | 1.04±0.19 | 0.99±0.18 | 1.04±0.18 | 1.07±0.20 | 0.171 |
| Total cholesterol, mean±SD, mg/dL | 191.6±40.6 | 190.1±40.9 | 184.8±34.3 | 200.1±45.4 | 0.253 |
| Triglyceride, mean±SD, mg/dL | 130.3±91.9 | 156.1±59.9 | 106.2±70.3 | 129.3±86.1 | 0.062 |
| HDL-C, mean±SD, mg/dL | 42.0±9.2 | 39.9±8.9 | 42.9±8.6 | 43.1±9.9 | 0.239 |
| LDL-C, mean±SD, mg/dL | 117.4±38.4 | 119.7±45.3 | 115.0±28.9 | 117.6±40.4 | 0.868 |
| CK peak, mean±SD, IU/L | 2576±1999 | 1962±1642 | 2190±1593 | 3502±2310* | 0.003 |
| CK-MB peak, mean±SD, ng/mL | 247.6±212.4 | 191.3±157.2 | 218.9±177.6 | 327.9±262.9* | 0.024 |
| Troponin I peak, mean±SD, ng/mL | 75.8±79.9 | 48.9±60.1 | 77.9±76.9 | 107.1±94.9* | 0.043 |
| LVEF, % | 47.34±7.66 | 48.9±6.5 | 47.1±8.7 | 45.9±7.6 | 0.246 |
| E/e' | 10.65±2.95 | 9.62±2.12 | 9.74±2.44 | 10.65±2.95 | 0.161 |
| WMSI | 1.47±0.31 | 1.40±0.29 | 1.50±0.32 | 1.52±0.32 | 0.162 |
IMR, index of microcirculatory resistance; SD, standard deviation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; CCB, calcium channel blocker; ACEi, angiotensin angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; b-blocker, beta blocker; CRP, C-reactive protein; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CK, creatine kinase; CK-MB, creatine kinase-myocardial band; LVEF, left ventricular ejection fraction; E, early diastolic velocity of the mitral annulus; E/e', ratio of E velocity to e'; WMSI, wall motion score index.
*p<0.05 versus Low IMR by post hoc test by analysis of variance (ANOVA), Fisher's exact.
Clinical and laboratory findings
The mean age of the Low IMR group was significantly lower than that of the Mid and High IMR groups (51±9 vs. 57±11 vs. 61±10, p=0.031) (Table 1). There were no significant differences in the presence of hypertension, diabetes, dyslipidemia, or smoking history between the three IMR groups.
The door-to-balloon time was <90 minutes for all patients. The High IMR group tended to have longer door-to-balloon times, compared to the Low and Mid IMR groups, although the difference was not statistically significant (65.7±14.6 min vs. 74.8±19.8 min vs. 82.0±44.6 min, p=0.068). However, the symptom-onset-to-balloon time of the high IMR group was significantly longer than that of the Low IMR and Mid IMR groups (172.2±80.1 min vs. 197.4±104.1 min vs. 299.8±195.1 min, p<0.001) (Table 1). The levels of cardiac enzymes, including creatine kinase (CK), creatine kinase-myocardial band (CK-MB), and troponin I, were significantly higher in the high IMR group, compared to the other groups (Table 1). The baseline LVEF of the High IMR group was lower than that of the Low and Mid IMR groups, although the difference was not significant (p=0.246). The high IMR group tended to have higher WMSI values than the Low and Mid IMR groups, although the difference in the values was not significant (p=0.162) (Table 1).
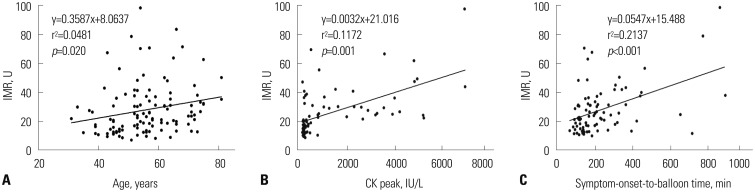
In correlation analysis, significant association was found between IMR values and age (r=0.219, p=0.020), CK level (r=0.342, p=0.010), and the symptom-onset-to-balloon time (r=0.463, p<0.001) (Fig. 1).
Fig. 1. Relations of age (A), CK peak (B), and symptom-onset-to-balloon time (C) to increasing IMR. Solid lines represent linear regression lines. IMR, index of microcirculatory resistance; CK, creatine kinase.
Angiographic findings
The culprit artery and coronary intervention including thrombus aspiration, direct stenting, the use of glycoprotein IIb/IIIa inhibitors, and lesion characteristics, including length, RD, and ACC/AHA lesion classification, were not significantly different between the IMR groups (Table 2). Culprit lesions located in the proximal portion of the target vessel were more frequent in the High IMR group than the Low IMR group (28.9% vs. 59.5%, p=0.008). The initial TIMI 0/1 flow before PCI was seen more frequently in the High than in the Low IMR group (55.3% vs. 81.1%, p=0.008). The achievement of a TIMI 3 (86.8% vs. 56.8%, p=0.015) and TMPG 3 (68.4% vs. 27.0%, p=0.008) after primary PCI was observed more frequently in the Low IMR than the High IMR group (Table 2).
Table 2. Angiographic and Procedural Findings of Patients in the Different IMR Groups.
| Total (n=113) | Low IMR (n=38) | Mid IMR (n=38) | High IMR (n=37) | p value | |
|---|---|---|---|---|---|
| QCA, mean±SD, mm | |||||
| RD | 2.86±0.50 | 2.86±0.43 | 2.83±0.56 | 2.88±0.52 | 0.901 |
| Lesion length | 16.82±5.26 | 16.19±4.95 | 16.81±5.26 | 17.46±5.61 | 0.584 |
| Stent diameter | 3.12±0.33 | 3.16±0.31 | 3.13±0.35 | 3.07±0.32 | 0.472 |
| Stent length | 25.37±8.60 | 23.16±6.83 | 26.80±9.43 | 26.11±9.06 | 0.143 |
| Coronary territory, n (%) | 0.266 | ||||
| LAD | 80 (70.8) | 25 (65.8) | 26 (68.4) | 29 (78.4) | |
| LCX | 9 (8.0) | 5 (13.2) | 1 (2.6) | 3 (8.1) | |
| RCA | 24 (21.2) | 8 (21.1) | 11 (28.9) | 5 (13.5) | |
| Coronary intervention, n (%) | |||||
| Thrombus aspiration | 57 (50.4) | 15 (39.5) | 20 (52.6) | 22 (59.5) | 0.212 |
| Direct stenting | 50 (44.2) | 12 (31.6) | 20 (52.6) | 18 (48.6) | 0.146 |
| Use of GP IIb/IIIa inhibitors | 18 (15.9) | 6 (15.8) | 6 (15.8) | 6 (16.2) | 0.998 |
| Lesion location | 0.008 | ||||
| Proximal lesion | 56 (49.6) | 11 (28.9) | 23 (60.5) | 22 (59.5) | |
| Non-proximal lesion | 57 (50.4) | 27 (71.1) | 15 (39.5) | 15 (40.5) | |
| ACC/AHA classification, n (%) | 0.566 | ||||
| A | 9 (8.0) | 3 (7.9) | 3 (7.9) | 3 (8.1) | |
| B1 | 16 (14.2) | 8 (21.1) | 6 (15.8) | 2 (5.4) | |
| B2 | 60 (53.1) | 20 (52.6) | 18 (47.4) | 22 (59.5) | |
| C | 28 (24.8) | 7 (18.4) | 11 (28.9) | 10 (27.0) | |
| TIMI grade and TMPG, n (%) | |||||
| Initial TIMI | 0.008 | ||||
| 0/1 | 77 (68.1) | 21 (55.3) | 26 (68.4) | 30 (81.1) | |
| 2 | 18 (15.9) | 6 (15.8) | 10 (26.3) | 2 (5.4) | |
| 3 | 18 (15.9) | 11 (28.9) | 2 (5.3) | 5 (13.5) | |
| Final TIMI | 0.015 | ||||
| 0/1 | 0 | 0 | 0 | 0 | |
| 2 | 31 (27.4) | 5 (13.2) | 10 (26.3) | 16 (43.2) | |
| 3 | 82 (72.6) | 33 (86.8) | 28 (73.7) | 21 (56.8) | |
| TMPG | 0.008 | ||||
| 0/1 | 15 (13.3) | 2 (5.3) | 5 (13.2) | 8 (21.6) | |
| 2 | 45 (39.8) | 10 (26.3) | 16 (42.1) | 19 (51.4) | |
| 3 | 53 (46.9) | 26 (68.4) | 17 (44.7) | 10 (27.0) |
IMR, index of microcirculatory resistance; QCA, quantitative coronary angiography; SD, standard deviation; RD, reference diameter; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; GP IIb/IIIa, glycoprotein IIb/IIIa; ACC/AHA, American College of Cardiology/American Heart Association; TIMI, Thrombolysis in Myocardial Infarction; TMPG, TIMI myocardial perfusion grade.
Physiologic parameters
The baseline Pa and Pd showed no differences between the IMR groups. There were significant differences in Tmn at rest (0.37±0.18 vs. 0.59±0.34 vs. 1.01±0.54, p<0.001) and hTmn (0.18±0.04 vs. 0.31±0.09 vs. 0.66±0.27, p<0.001) (Table 3). The High IMR group tended to have higher FFR and lower CFR than the Low IMR and Mid IMR groups; however, the differences were not significant (Table 3).
Table 3. Physiologic Parameters of Patients in the Different IMR Groups.
| Total (n=113) | Low IMR (n=38) | Mid IMR (n=38) | High IMR (n=37) | p value | |
|---|---|---|---|---|---|
| Pa, mean±SD, mm Hg | 84.40±17.87 | 81.61±12.71 | 88.11±18.14 | 83.46±21.57 | 0.266 |
| Pd, mean±SD, mm Hg | 77.21±16.85 | 73.29±13.53 | 81.21±16.32 | 77.14±19.72 | 0.122 |
| FFR, mean±SD | 0.92±0.06 | 0.90±0.08 | 0.92±0.05 | 0.93±0.06 | 0.093 |
| Tmn at rest, mean±SD, sec | 0.66±0.46 | 0.37±0.18 | 0.59±0.34* | 1.01±0.54* | <0.001 |
| hTmn, mean±SD, sec | 0.38±0.26 | 0.18±0.04 | 0.31±0.09* | 0.66±0.27* | <0.001 |
| CFR, mean±SD | 1.94±1.16 | 2.10±1.06 | 2.01±1.25 | 1.72±1.17 | 0.343 |
| IMR, mean±SD, U | 28.1±17.8 | 12.9±2.6 | 23.9±4.0 | 48.1±17.1 | - |
IMR, index of microcirculatory resistance; Pa, mean aortic pressure; SD, standard deviation; Pd, mean distal coronary pressure; FFR, fractional flow reserve; Tmn, mean transit time; hTmn, hyperemic mean transit time; CFR, coronary flow reserve.
*p<0.05 versus Low IMR by post hoc test by analysis of variance (ANOVA), Fisher's exact.
Comparison of IMR according to clinical and angiographic parameters
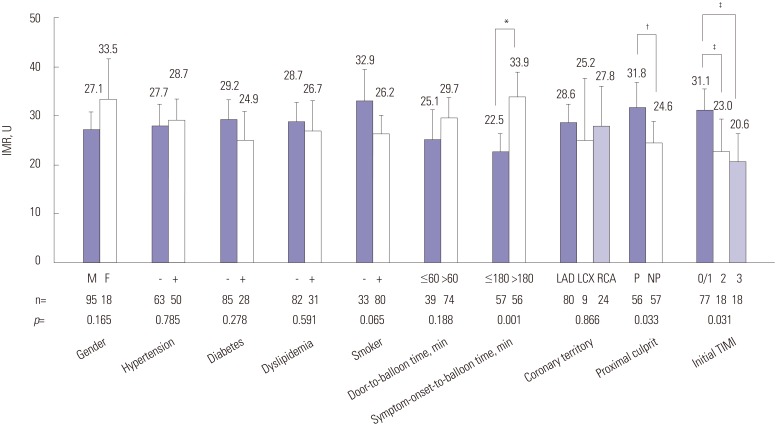
To determine whether certain parameters could influence microvascular dysfunction, we compared the IMR groups according to the presence or criteria of various parameters. As shown in Fig. 2, there were no significant differences in IMR regardless of major cardiovascular risk factors. However, our data did not show significant differences in IMR according to culprit arteries, the IMR values were significantly higher in proximal locations of the culprit lesion, as compared with non-proximal lesion (31.8±19.1 U vs. 24.6±15.8 U, p=0.033) and initial TIMI 0/1, as compared with initial TIMI 2/3 (23.0±12.8 U vs. 20.6±11.5 U, p=0.031) (Fig. 2). Since the door-to-balloon times were <90 minutes for all patients, we divided the patients into two groups (>60 minutes and ≤60 minutes). The two groups did not show a significant difference in IMR values (Fig. 2). Thereafter, the patients were divided according to a symptom-onset-to-balloon time of 180 minutes, and we observed that the IMR of those with a symptom-onset-to-balloon time of >180 minutes was significantly higher than the IMR of those with a symptom-onset-to-balloon time of ≤180 minutes (Fig. 2).
Fig. 2. Comparison of IMR according to clinical and angiographic factors. *The IMR of patients with symptom-onset-to-balloon time of >180 minutes was significantly higher than the IMR of those with a symptom-onset-to-balloon ≤180 minutes, †The IMR was significantly higher in proximal lesion than in non-proximal lesion, ‡The IMR was significantly higher in initial TIMI 0/1 group, as compared initial TIMI 2/3. IMR, index of microcirculatory resistance; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; P, proximal location of culprit artery; NP, non-proximal location of culprit artery; TIMI, thrombolysis in myocardial infarction.
Predictors of microvascular dysfunction
As shown in Table 4, age, symptom-onset-to-balloon time, cardiac biomarker, such as CK-MB and final TMPG level, showed a significant correlation with increasing IMR. Our data showed that a symptom-onset-to-balloon time of >180 minutes had a significant odds ratio (OR) for higher IMR between those with a symptom-onset-to-balloon time of >180 minutes and those with a symptom-onset-to-balloon time of ≤180 minutes [OR, 4.376; 95% confidence interval (CI): 1.851-10.347; p=0.001]. Cardiac biomarkers, such as CK-MB, was also significantly correlated to increasing IMR (Table 4). In multivariate regression analysis, age and symptom-onset-to-balloon time remained independent determinants for High IMR [Exp (β)=1.085, 95% CI: 1.016-1.158, p=0.016; Exp (β)=1.007, 95% CI: 1.001-1.014, p=0.018, respectively] (Table 4).
Table 4. Predictors Related to Increasing Index of Microcirculatory Resistance.
| OR | 95% CI | p value | |
|---|---|---|---|
| Univariate regression analysis | |||
| Age, yr | 1.065 | 1.022±1.108 | 0.020 |
| Female | 2.393 | 0.860±6.661 | 0.095 |
| Hypertension | 1.532 | 0.695±3.378 | 0.290 |
| Diabetes | 0.772 | 0.303±1.967 | 0.588 |
| Dyslipidemia | 0.970 | 0.401±2.345 | 0.946 |
| Smoker | 0.455 | 0.196±1.057 | 0.067 |
| Door-to-balloon time, min | 1.013 | 0.999±1.028 | 0.075 |
| Symptom-onset-to-balloon time, min | 1.007 | 1.002±1.011 | 0.002 |
| Symptom-onset-to-balloon time≤180 min | 0.229 | 0.097±0.540 | 0.001 |
| Symptom-onset-to-balloon time>180 min | 4.376 | 1.851±10.347 | 0.001 |
| Proximal location of culprit lesion | 1.812 | 0.817±4.020 | 0.144 |
| Final TMPG 3 | 0.203 | 0.060±0.693 | 0.011 |
| CK-MB peak, ng/mL | 1.003 | 1.000±1.005 | 0.019 |
| Multivariate regression analysis | |||
| Age | 1.085 | 1.016±1.158 | 0.016 |
| Symptom-onset-to-balloon time | 1.007 | 1.001±1.014 | 0.018 |
| Final TMPG 3 | 0.323 | 0.040±2.600 | 0.288 |
| CK-MB peak, ng/mL | 1.002 | 0.999±1.006 | 0.105 |
OR, odds ratio; CI, confidence interval; TIMI, thrombolysis in myocardial infarction; TMPG, TIMI myocardial perfusion grade; CK-MB, creatine kinase-myocardial band.
DISCUSSION
The main findings of the present study are as follows: First, the High IMR group was older, had a longer symptom-onset-to-balloon time, and more frequently had a culprit lesion in a proximal location. Second, age and symptom-onset-to-balloon time were independent predictors of high IMR, reflecting the presence of microvascular dysfunction in patients with STEMI.
Aging is an important factor contributing to atherosclerosis and arteriosclerosis.18 It has been reported that arterial aging is associated not only macrovascular resistance but also with impaired microcirculation due to the diminution of the capillary bed, which accompanies atrophy of subcutaneous and other tissues.18,19 The presence of structural alterations in the microcirculation may be an important link to ischemic heart disease.18,20 Most studies about IMR do not describe the relationships between age and IMR well. Our study suggests that aging may be related to impaired microcirculatory resistance, as estimated by IMR in patients with STEMI.
Coronary microvascular dysfunction in diabetes mellitus has been explained by various mechanisms of endothelial dysfunction including insulin resistance, hyperglycemia, impaired vasodilatation, autonomic dysfunction, and inflammation.21,22 Our data did not show a significant difference between individual IMR groups and diabetes mellitus. This may be due to the lack of adjusting for confounding factors such as medication, patient characteristics, and major risk factors, including hypertension, dyslipidemia, and smoking. The present data indicated that the IMR values do not significantly differ according to the presence of diabetes, as well as according to the presence of major cardiovascular risk factors, such as hypertension, dyslipidemia, and smoking. These results may be explained by the relatively small sample size of our study. In addition, as the IMR was measured shortly after the primary PCI, the angiographic- and procedure-related factors may have a greater effect on the IMR than classic cardiovascular risk factors.
Door-to-balloon time is associated with mortality in patients undergoing primary PCI for STEMI. Previous studies have shown a strong correlation between door-to-balloon time and clinical outcome.23,24,25 The ACC/AHA guidelines for the management of patients with STEMI recommend a door-to-balloon time of ≤90 minutes.26 However, it has recently been reported that, despite reducing the door-to-balloon time from 83 minutes to 67 minutes, there has been no significant change in inhospital mortality and 30-day mortality.27 In our data, the door-to-balloon times were <90 minutes in all patients, and no significant differences in door-to-balloon time were noted between the IMR groups. However, the symptom-onset-to-balloon time in the present study showed significant differences between individual IMR groups. Therefore, the IMR correlated well with the symptom-onset-to-balloon time but not the door-to-balloon time of <90 minutes. In multivariate analysis, age and symptom-onset-to-balloon time remained independent determinants for impaired microvascular resistance. This suggests that, in patients with a door-to-balloon time of <90 minutes, decreasing the absolute door-to-balloon time does not affect the microvascular resistance in patients with STEMI. Therefore, the symptom-onset-to-balloon time might be more important for determining microcirculatory resistance in STEMI patients with a door-to-balloon time of <90 minutes.
Angiographic findings have been used as the classical parameter for microvascular dysfunction. Coronary blood flow and microvascular integrity have been estimated by using TIMI and TMPG in many previous studies,4,28,29 and previous studies have shown that the final TIMI or TMPG score is related to mortality in patients with acute myocardial infarction.5 However, there are some limitations due to inter-observer variation since it is graded by visual estimation. In the present study, IMR was strongly correlated with TIMI and TMPG, as well as with the levels of cardiac biomarkers, such as peak CK, peak CK-MB, and peak troponin I. The achievement of the final TIMI 3 or TMPG 3 was much less frequent in the High IMR group than in the Low IMR group. These findings are consistent with previous studies that suggest that IMR predicts clinical outcome and prognosis.8,9,11,30,31,32 In contrast, the IMR requires less inter- and intra-observer variability than the visual estimation of the TIMI or TMPG system; therefore, IMR may be a more objective method for assessing microvascular dysfunction.
Some recent studies have shown that increased microvascular resistance and dysfunction in patients with STEMI might lead to an alteration in flow dynamics associated with a smaller pressure drop through the culprit lesion. FFR would be overestimated in this setting.33,34,35 Our data showed that the High IMR group tended to have a higher FFR than the Low IMR and Mid IMR groups, although the difference was not statistically significant.
To find out the relevant factors for microvascular dysfunction in STEMI, our study population was classified into three groups based on IMR value as tertiles. A definite cut-off value of IMR to determine LV recovery and clinical outcomes in STEMI patients has not yet been established. However, it is interesting that our cut-off value (>31 U) for the High IMR group was similar to suggested IMR values in previous studies on predicting myocardial dysfunction in STEMI patients.8,30 To apply the cutoff value of IMR for predicting a clinical outcome in STEMI patients, further large-scaled studies may be needed in the future.
Murai, et al.36 reported that right coronary artery lesion location was significantly associated with increased IMR in patients with intermediate coronary artery lesions. Our data did not show significant differences in IMR according to culprit arteries. The proximal location of the culprit lesion in STEMI is associated with greater myocardial damage. It has been reported that patients with proximal culprit lesions are more likely to have a poorer clinical outcome and prognosis than patients with non-proximal culprit lesions.37 Proximal culprit lesions were found more frequently in the High IMR group than in the Low IMR group in the present study, and the mean IMR in patients with proximal culprit lesions was significantly higher than that in patients with non-proximal culprit lesions. This suggests that a proximal location of culprit lesion might play an important role in deteriorating microcirculatory resistance in patients with STEMI.
The Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS) demonstrated that aspiration of the thrombus before stenting seemed to improve 1-year clinical outcomes following primary PCI for STEMI.38 However, a recent study showed that, compared with PCI alone, routine thrombus aspiration before PCI did not reduce the 30-day mortality in patients with STEMI.39 In a recent randomized study, it was reported that thrombus aspiration as an adjunctive therapy to primary PCI for STEMI may reduce IMR and have beneficial effects on myocardial microcirculation.40 Our data showed that thrombus aspiration was more frequent in the High IMR group, compared with the Low IMR group; however, the difference was not significant, although there may be an operator-dependent bias in the thrombus aspiration, which was determined by operator at the time of the primary PCI. Although the use of glycoprotein IIb/IIIa inhibitor may reduce mortality in high-risk patients with STEMI,41 the use of glycoprotein IIb/IIIa inhibitors did not differ between the IMR groups in the present study.
Study limitations
Our study consisted of a relatively small number of STEMI patients who underwent primary PCI. Because patients with cardiogenic shock, prior myocardial infarction, and hemodynamic instability were excluded, our results may not represent all patients with STEMI. Moreover, this study is a retrospective analysis and was performed at a single center. We need more data and randomized control study to better understand determinants of microvascular dysfunction in STEMI patients. The 113 study subjects were relatively small as a study group and most of our enrolled STEMI patients had mild LV systolic dysfunction or preserved LV function with a LVEF of approximately 50%. Therefore, there were no significant differences in LVEF and WMSI among the IMR groups, although the High IMR group tended to have lower LVEF and higher WMSI than the Low and Mid IMR groups. If more patients presenting severe LV systolic dysfunction in STEMI patients were included, the differences of LVEF and WMSI might be clarified among the IMR groups. Furthermore, the present study was not performed using a randomization study protocol for interventional techniques and medications such as direct stenting, thrombus aspiration, and glycoprotein IIb/IIIa inhibitors; therefore, it is possible that an operation-dependent bias may be present in these variables. These variables might play a role as confounding factors. In the multivariate analysis, it would have been ideal to exclude all factors in order to avoid selection bias and confounding factors; however, this was not ensured in the present study, except for age, gender, comorbidities, lesion location, and symptom-onset-to-balloon time.
In conclusion, our study suggests that age, proximal location of the culprit vessel, and symptom-to-onset-balloon time are correlated with microvascular dysfunction estimated using IMR. In STEMI patients undergoing primary PCI with a door-to-balloon time of <90 minutes, age and symptom-onset-to-balloon time might be major predictors of microvascular dysfunction.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J. 2012;33:2771–2782b. doi: 10.1093/eurheartj/ehs246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, et al. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1. [DOI] [PubMed] [Google Scholar]
- 3.Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart. 2011;97:587–595. doi: 10.1136/hrt.2009.183327. [DOI] [PubMed] [Google Scholar]
- 4.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–130. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 6.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 7.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 8.Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, et al. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;51:560–565. doi: 10.1016/j.jacc.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 9.McGeoch R, Watkins S, Berry C, Steedman T, Davie A, Byrne J, et al. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2010;3:715–722. doi: 10.1016/j.jcin.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Yoo SH, Yoo TK, Lim HS, Kim MY, Koh JH. Index of microcirculatory resistance as predictor for microvascular functional recovery in patients with anterior myocardial infarction. J Korean Med Sci. 2012;27:1044–1050. doi: 10.3346/jkms.2012.27.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon WF, Low AF, Yong AS, McGeoch R, Berry C, Shah MG, et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RF, Wyche K, Christensen BV, Zimmer S, Laxson DD. Effects of adenosine on human coronary arterial circulation. Circulation. 1990;82:1595–1606. doi: 10.1161/01.cir.82.5.1595. [DOI] [PubMed] [Google Scholar]
- 14.Pijls NH, De Bruyne B, Smith L, Aarnoudse W, Barbato E, Bartunek J, et al. Coronary thermodilution to assess flow reserve: validation in humans. Circulation. 2002;105:2482–2486. doi: 10.1161/01.cir.0000017199.09457.3d. [DOI] [PubMed] [Google Scholar]
- 15.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 16.De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–2006. doi: 10.1161/hc4201.099223. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–341. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 19.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 20.Safar ME. Peripheral pulse pressure, large arteries, and microvessels. Hypertension. 2004;44:121–122. doi: 10.1161/01.HYP.0000135448.73199.75. [DOI] [PubMed] [Google Scholar]
- 21.Potenza MA, Gagliardi S, Nacci C, Carratu' MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 22.Marciano C, Galderisi M, Gargiulo P, Acampa W, D'Amore C, Esposito R, et al. Effects of type 2 diabetes mellitus on coronary microvascular function and myocardial perfusion in patients without obstructive coronary artery disease. Eur J Nucl Med Mol Imaging. 2012;39:1199–1206. doi: 10.1007/s00259-012-2117-9. [DOI] [PubMed] [Google Scholar]
- 23.Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, et al. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009;338:b1807. doi: 10.1136/bmj.b1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamara RL, Wang Y, Herrin J, Curtis JP, Bradley EH, Magid DJ, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–2186. doi: 10.1016/j.jacc.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 25.De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–1225. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- 26.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 27.Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, et al. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. doi: 10.1056/NEJMoa1208200. [DOI] [PubMed] [Google Scholar]
- 28.Gibson CM, Schömig A. Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion. Circulation. 2004;109:3096–3105. doi: 10.1161/01.CIR.0000134278.50359.CB. [DOI] [PubMed] [Google Scholar]
- 29.Angeja BG, Gunda M, Murphy SA, Sobel BE, Rundle AC, Syed M, et al. TIMI myocardial perfusion grade and ST segment resolution: association with infarct size as assessed by single photon emission computed tomography imaging. Circulation. 2002;105:282–285. doi: 10.1161/hc0302.103588. [DOI] [PubMed] [Google Scholar]
- 30.Lim HS, Yoon MH, Tahk SJ, Yang HM, Choi BJ, Choi SY, et al. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J. 2009;30:2854–2860. doi: 10.1093/eurheartj/ehp313. [DOI] [PubMed] [Google Scholar]
- 31.Byrne RA, Ndrepepa G, Braun S, Tiroch K, Mehilli J, Schulz S, et al. Peak cardiac troponin-T level, scintigraphic myocardial infarct size and one-year prognosis in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2010;106:1212–1217. doi: 10.1016/j.amjcard.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 32.Chin CT, Wang TY, Li S, Wiviott SD, deLemos JA, Kontos MC, et al. Comparison of the prognostic value of peak creatine kinase-MB and troponin levels among patients with acute myocardial infarction: a report from the Acute Coronary Treatment and Intervention Outcomes Network Registry-get with the guidelines. Clin Cardiol. 2012;35:424–429. doi: 10.1002/clc.21980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamita K, Akasaka T, Takagi T, Yamamuro A, Yamabe K, Katayama M, et al. Effects of microvascular dysfunction on myocardial fractional flow reserve after percutaneous coronary intervention in patients with acute myocardial infarction. Catheter Cardiovasc Interv. 2002;57:452–459. doi: 10.1002/ccd.10350. [DOI] [PubMed] [Google Scholar]
- 34.Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274–1281. doi: 10.1016/j.jcin.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 35.Gibson CM, Pinto D. Fractional flow reserve: a new set of lenses for the occulostenotic reflex? JACC Cardiovasc Interv. 2010;3:1282–1283. doi: 10.1016/j.jcin.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Murai T, Lee T, Yonetsu T, Iwai T, Takagi T, Hishikari K, et al. Variability of microcirculatory resistance index and its relationship with fractional flow reserve in patients with intermediate coronary artery lesions. Circ J. 2013;77:1769–1776. doi: 10.1253/circj.cj-12-1442. [DOI] [PubMed] [Google Scholar]
- 37.Harjai KJ, Mehta RH, Stone GW, Boura JA, Grines L, Brodie BR, et al. Does proximal location of culprit lesion confer worse prognosis in patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction? J Interv Cardiol. 2006;19:285–294. doi: 10.1111/j.1540-8183.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 38.Vlaar PJ, Svilaas T, van der Horst IC, Diercks GF, Fokkema ML, de Smet BJ, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 39.Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 40.Woo SI, Park SD, Kim DH, Kwan J, Shin SH, Park KS, et al. Thrombus aspiration during primary percutaneous coronary intervention for preserving the index of microcirculatory resistance: a randomised study. EuroIntervention. 2014;9:1057–1062. doi: 10.4244/EIJV9I9A179. [DOI] [PubMed] [Google Scholar]
- 41.Sethi A, Bajaj A, Bahekar A, Bhuriya R, Singh M, Ahmed A, et al. Glycoprotein IIb/IIIa inhibitors with or without thienopyridine pretreatment improve outcomes after primary percutaneous coronary intervention in high-risk patients with ST elevation myocardial infarction--a meta-regression of randomized controlled trials. Catheter Cardiovasc Interv. 2013;82:171–181. doi: 10.1002/ccd.24653. [DOI] [PubMed] [Google Scholar]