Abstract
Purpose
Resistant hypertension (HTN) occurs in 15-20% of treated hypertensive patients, and 70-80% of resistant hypertensive patients have obstructive sleep apnea (OSA). The characteristics of resistant HTN that predispose patients to OSA have not been reported. Therefore, we aimed to determine the clinical, laboratory, and polysomnographic features of resistant HTN that are significantly associated with OSA.
Materials and Methods
Hypertensive patients (n=475) who underwent portable polysomnography were enrolled. The patients were categorized into controlled (n=410) and resistant HTN (n=65) groups. The risk factors for the occurrence of OSA in controlled and resistant hypertensive patients were compared, and independent risk factors that are associated with OSA were analyzed.
Results
Out of 475 patients, 359 (75.6%) were diagnosed with OSA. The prevalence of OSA in resistant HTN was 87.7%, which was significantly higher than that in controlled HTN (73.7%). Age, body mass index, neck circumference, waist circumference, and hip circumference were significantly higher in OSA. However, stepwise multivariate analyses revealed that resistant HTN was not an independent risk factor of OSA.
Conclusion
The higher prevalence and severity of OSA in resistant HTN may be due to the association of risk factors that are common to both conditions.
Keywords: Resistant hypertension, sleep apnea, polysomnography, obesity, body mass index
INTRODUCTION
Resistant hypertension (HTN) is defined as either the failure to achieve the target blood pressure (BP; <140/90) with three antihypertensive drugs of different classes, including diuretics, or the achievement of BP by using four or more antihypertensive drugs.1 It is associated with a significant elevation of cardiovascular risk.2,3,4 Therefore, early recognition and rigorous management are necessary to reduce cardiovascular mortality.5 The prevalence of resistant HTN has been reported to be as high as 30% in hypertensive patients.4 There are many causes of resistant HTN, including high sodium-containing diets, alcohol, non-adherence to drug treatments, inadequate medications, obesity, and secondary HTN.6 Among the causes of secondary HTN, obstructive sleep apnea (OSA) is strongly associated with resistant HTN.7 Surprisingly, the prevalence of OSA in resistant hypertensive patients ranges from 64%8 to 83%,9 which is significantly higher than its prevalence (4%) in the general population.10
OSA is characterized by the partial or complete collapse of the upper airway during sleep, and it is diagnosed when the apnea-hypopnea index (AHI), as detected by polysomnography (PSG), is greater than 5. OSA affects 2-4% of adults and is associated with increased cardiovascular risks and mortality.11 It is very common in hypertensive patients, and the prevalence has been reported to be as high as 56%.12,13 Resistant HTN and OSA are commonly associated with various physiological conditions, such as hyperaldosteronism, increased sympathetic activity, endothelin- and hypoxia-mediated vasoconstriction, and obesity.14 Furthermore, BP control is critical for reducing cardiovascular morbidity and mortality,15 and the treatment of OSA with continuous positive airway pressure (CPAP) modestly diminishes BP in patients with various types of HTN,16 including resistant HTN.17 The modest effect of CPAP on BP reduction in resistant hypertensive patients suggests that the high prevalence of OSA in these patients may not entirely be explained by sleep apnea which exacerbates blood pressure elevation, but also be due to common associations with risk factors. Therefore, OSA and resistant HTN do not appear be causally related. In this study, we aimed to determine the clinical, laboratory, and polysomnographic features of resistant HTN that are significantly associated with OSA. Also, we determined the association of resistant HTN with OSA after controlling for risk factors that are related to both conditions.
MATERIALS AND METHODS
Study population
This retrospective study was approved by the Institutional Review Board (IRB) at Yonsei University College of Medicine (IRB No. 4-2014-0132). We enrolled hypertensive patients who visited the Cardiology Department at Yonsei University College of Medicine between 2010 and 2013 who completed the PSG procedure, sleep questionnaires, and basal blood tests. PSG was performed using Embletta X100 (Natus Embla Systems LLC, Ontario, Canada),18 which is a type 2 full-unattended portable system by the American Academy of Sleep Medicine.19 This type 2 portable monitor incorporates a minimum of seven channels, including two electroencephalography (EEG) channels. This allows for the AHI to be accurately calculated and is an improvement over the rough estimates that are obtained by type 3 or 4 portable systems.20 The inter- [95% confidence interval (CI), 0.993-0.999; p<0.001] and intra-class correlation coefficients (95% CI, 0.993-0.999; p<0.001) for the inter-observer reliability of the AHI were checked. OSA was diagnosed when the AHI was greater than 5. Mild, moderate, and severe OSA were defined by indices of 5-14, 15-29, and >30, respectively. BP was measured twice either with a mercury sphygmomanometer or an aneroid sphygmomanometer (Welch Allyn Silver Series DS45, Skaneateles Falls, NY, USA), and the average value was used.
Anthropometric measurements and metabolic assessments
Anthropometric measurements were performed by a skilled examiner. Height was defined as the length from the top of the head to the end of the first toe. Neck circumference (NC) was measured at the thyroid cartilage level with a ruler. Waist circumference (WC) was measured at the midway point between the inferior costal margin and the superior border of the iliac crest. Hip circumference (HC) was measured at the largest lateral extension of the hips.
Blood samples were collected after overnight fasting, and the levels of total cholesterol, triglycerides (TG), high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, and blood glucose were measured.
Statistics
Independent two-sample t-tests were used for the comparison of continuous variables. The stepwise multivariate logistic regression analysis was used to determine risk factors that were associated with OSA. Discrete variables were compared using the chi-square test. The criterion for entering or removing variables in the multivariate regression model was a p-value of 0.25. The variables that were initially entered in the stepwise analysis were chosen from the preliminary univariate analyses with p<0.01. Statistical analyses were performed by SPSS version 20 (IBM Corporation, Armonk, NY, USA) and SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Flowchart of patients
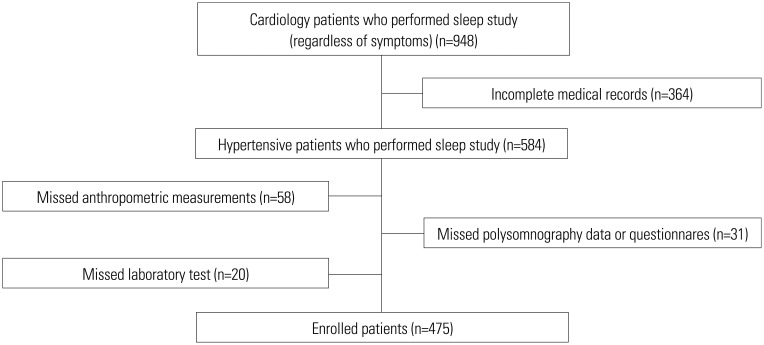
This was a retrospective analysis of the sleep apnea registry of the Cardiology Department at Yonsei University. During a 3-year period, 948 hypertensive patients underwent PSG for the evaluation of secondary HTN and/or OSA-related symptoms. Among these subjects, 364 patients were excluded due to incomplete medical records, and 109 patients were excluded due to missing values in anthropometric measurements, laboratory tests, PSG data, or sleep questionnaires. Finally, 475 patients with complete data were enrolled in the study for analysis (Fig. 1).
Fig. 1. Flowchart of patients. Hypertensive patients were asked to perform the sleep study (including polysomnography, sleep questionnaires, laboratory tests, and anthropometric measurements), and 475 patients with complete data were enrolled in this retrospective study.
Medical history of controlled and resistant hypertensive patients
The enrolled patients were identified as having controlled (n=410) or resistant HTN (n=65). We reviewed the anti-hypertensive medications that were used by patients and categorized them as diuretics, angiotensin-converting enzyme inhibitors, angiotensin-II receptor blockers, calcium channel blockers, α- and β-receptor blockers, and vasodilators (Table 1). The average numbers of medications that were used by patients with controlled and resistant HTN were 2.01±0.98 and 4.27±0.95, respectively.
Table 1. Review of Enrolled Patients' Blood Pressure Medications.
| Controlled HTN (n=410) | Resistant HTN (n=65) | |
|---|---|---|
| Number of medications | 2.01±0.98 | 4.27±0.95 |
| Diuretics (%) | 32 | 86 |
| ACEI (%) | 7 | 14 |
| ARB (%) | 61 | 77 |
| CCB (%) | 56 | 89 |
| α-blocker (%) | 1 | 29 |
| β-blocker (%) | 30 | 78 |
| Vasodilator (%) | 11 | 31 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; CCB, calcium channel blocker; HTN, hypertension.
Severity of OSA in patients with controlled or resistant HTN
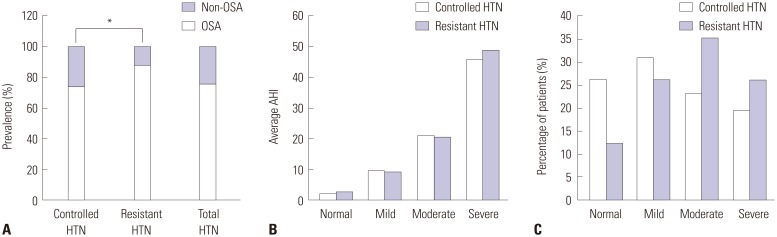
OSA, which was characterized by an AHI≥5, was diagnosed in 73.7% (302/410) and 87.7% (57/65) of patients in the controlled and resistant HTN groups, respectively (Table 2, Fig. 2A). Overall, 359 hypertensive patients (75.6%) were diagnosed with OSA (Table 2, Fig. 2A). The prevalence of OSA in resistant hypertensive patients was significantly higher than that in controlled hypertensive patients (p=0.015) (Fig. 2A). When we used the AHI to categorize the severity of OSA as mild, moderate, or severe, the resistant HTN group had a higher percentage of moderate (35.4% vs. 23.2%) and severe (26.1% vs. 19.5%) OSA patients, compared to the controlled HTN group (Fig. 2B and C).
Table 2. Severity of OSA Based on the AHI in Patients with Controlled or Resistant HTN.
| Controlled HTN (n=410) | Resistant HTN (n=65) | Total (n=475) | ||||
|---|---|---|---|---|---|---|
| Average AHI | Number (%) | Average AHI | Number (%) | Average AHI | Number (%) | |
| Non-OSA (0-4.9) | 2.10 | 108 (26.3) | 2.87 | 8 (12.3) | 2.15 | 116 (24.4) |
| Mild (5-14.9) | 9.55 | 127 (31.0) | 9.17 | 17 (26.2) | 9.50 | 144 (30.4) |
| Moderate (15-29.9) | 20.94 | 95 (23.2) | 20.67 | 23 (35.4) | 20.88 | 118 (24.8) |
| Severe (30+) | 45.63 | 80 (19.5) | 49 | 17 (26.1) | 46.21 | 97 (20.4) |
| Total | 17.26 | 410 (100) | 22.87 | 65 (100) | 18.03 | 475 (100) |
HTN, hypertension; AHI, apnea-hypopnea index; OSA, obstructive sleep apnea.
Fig. 2. Severity of OSA in HTN. The apnea-hypopnea index (AHI) was used to determine the prevalence and severity of OSA in controlled and resistant hypertensive patients. (A) The prevalence of OSA in controlled, resistant, and total hypertensive patients was compared. (B) The average AHIs of non-OSA, mild, moderate, and severe degrees of OSA are represented as mean±standard deviation in the controlled and resistant HTN groups. (C) The percentages of patients with non-OSA, mild, moderate, and severe OSA were compared between the controlled and resistant HTN groups (*p<0.05). OSA, obstructive sleep apnea; HTN, hypertension.
Anthropometric characteristics, PSG, and basal laboratory results in patients with controlled or resistant HTN
We evaluated the differences in anthropometric, polysomnographic, and basal laboratory features between controlled and resistant hypertensive patients (Table 3). Body weights, body mass index (BMI), NC, WC, and HC were significantly higher in the resistant HTN group, compared with the controlled HTN group (p<0.05). Based on the independent two-sample t-test, the AHI was also significantly higher in the resistant HTN group than in the controlled HTN group (p<0.05). In addition, resistant hypertensive patients had significantly worse values for polysomnographic parameters, such as average O2 saturation, lowest O2 saturation, and O2 desaturation index. No differences in the longest duration of apnea or hypopnea and average heart rate were observed between groups. Interestingly, TG levels were higher in patients with resistant HTN (p=0.050); however, there were no significant differences in the levels of glucose, total cholesterol, HDL-cholesterol, and LDL-cholesterol between groups (Table 3).
Table 3. Anthropometric Characteristics, Polysomnography, and Basal Laboratory Results in Patients with Controlled or Resistant HTN.
| Controlled HTN (n=410) | Resistant HTN (n=65) | p value | |
|---|---|---|---|
| Age (yrs) | 54.33±12.85 | 53.15±14.83 | 0.500 |
| Sex, male:female (percentage of male) | 303:107 (73.9%) | 47:18 (72.3%) | 0.786 |
| Height (cm) | 166.94±8.47 | 167.84±7.33 | 0.417 |
| Weight (kg) | 73.30±11.94 | 79.96±16.05 | <0.001 |
| BMI (kg/m2) | 26.10±3.27 | 28.05±4.18 | <0.001 |
| Neck circumference (cm) | 38.11±3.20 | 39.98±3.75 | <0.001 |
| Waist circumference (cm) | 93.05±8.93 | 99.12±9.28 | <0.001 |
| Hip circumference (cm) | 100.01±7.16 | 103.67±8.99 | 0.003 |
| ESS | 7.39±4.27 | 7.24±4.01 | 0.789 |
| Total NREM sleep duration (min) | 256.99±128.54 | 296.52±75.92 | 0.001 |
| Total REM sleep duration (min) | 71.23±35.68 | 66.01±35.40 | 0.273 |
| Percentage of NREM sleep (%) | 57.89±26.92 | 68.69±8.50 | <0.001 |
| Percentage of REM sleep (%) | 16.02±7.30 | 15.25±7.63 | 0.432 |
| Total AHI | 17.27±16.81 | 22.88±19.46 | 0.015 |
| The longest apnea duration (sec) | 29.99±20.33 | 27.79±16.39 | 0.334 |
| The longest hypopnea duration (sec) | 47.19±21.05 | 49.53±18.86 | 0.399 |
| Average O2 saturation (%) | 95.04±1.62 | 94.37±1.93 | 0.003 |
| Lowest O2 saturation (%) | 85.20±5.76 | 83.52±6.56 | 0.033 |
| O2 desaturation index | 16.05±15.89 | 22.57±19.04 | 0.003 |
| Average HR | 62.63±9.88 | 62.31±9.58 | 0.806 |
| Glucose (mg/dL) | 108.53±29.05 | 116.85±46.43 | 0.165 |
| Cholesterol (mg/dL) | 177.43±40.19 | 180.71±42.33 | 0.544 |
| TG (mg/dL) | 129.53±89.19 | 150.24±100.85 | 0.050 |
| HDL (mg/dL) | 46.64±12.03 | 43.91±10.30 | 0.084 |
| LDL (mg/dL) | 106.55±36.61 | 102.24±35.10 | 0.259 |
BMI, body mass index; ESS, Epworth sleepiness scale; REM sleep, rapid eye movement sleep; NREM sleep, non rapid eye movement sleep; AHI, apnea-hypopnea index; HR, heart rate; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HTN, hypertension.
Data with normal distribution are presented as mean±standard deviation.
Anthropometric characteristics and basal laboratory results in OSA and non-OSA hypertensive patients
To determine the common variables that are associated with both OSA and resistant HTN, we regrouped all hypertensive patients into non-OSA (n=114) and OSA (n=361) groups. The patients who had OSA were significantly older (55.89±12.46 years old) than non-OSA (48.72±13.74 years old) patients (p<0.001). Anthropometric characteristics, such as body weight, BMI, NC, WC, and HC, were also significantly higher in the OSA group than in the non-OSA group. In addition, decreased HDL-cholesterol levels were observed in the OSA group (p<0.05) (Table 4).
Table 4. Univariate Logistic Regression Analysis of Risk Factors Associated with the Prevalence of OSA.
| Variables | Non-OSA (n=114) | OSA (n=361) | OR (95% CI) | p value |
|---|---|---|---|---|
| Age (yrs) | 48.72±13.74 | 55.89±12.46 | 1.044 (1.026-1.062) | <0.001 |
| Sex, male:female (percentage of male) | 70:44 (60.5%) | 280:81 (77.9%) | 0.460 (0.293-0.722) | <0.001 |
| Height (cm) | 166.44±8.31 | 167.25±8.33 | 1.012 (0.986-1.037) | 0.367 |
| Weight (kg) | 70.66±12.59 | 75.33±12.65 | 1.032 (1.013-1.052) | <0.001 |
| BMI (kg/m2) | 25.31±3.48 | 26.70±3.41 | 1.140 (1.063-1.222) | <0.001 |
| Neck circumference (cm) | 36.97±3.44 | 38.82±3.19 | 1.194 (1.114-1.280) | <0.001 |
| Waist circumference (cm) | 89.84±10.23 | 95.17±8.48 | 1.073 (1.045-1.102) | <0.001 |
| Hip circumference (cm) | 98.62±6.53 | 101.24±7.72 | 1.051 (1.019-1.083) | 0.002 |
| Glucose (mg/dL) | 104.62±27.92 | 111.77±33.78 | 1.009 (1.000-1.018) | 0.056 |
| TG (mg/dL) | 135.31±94.76 | 154.29±101.50 | 1.002 (1.000-1.005) | 0.065 |
| HDL (mg/dL) | 48.75±11.68 | 45.48±11.79 | 0.977 (0.961-0.995) | 0.011 |
| LDL (mg/dL) | 108.75±37.21 | 105.89±35.77 | 0.997 (0.991-1.003) | 0.259 |
| Controlled:resistant (percentage of resistant HTN) | 106:8 (7.0%) | 304:57 (15.8%) | 2.484 (1.148-5.378) | 0.021 |
BMI, body mass index; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OSA, obstructive sleep apnea; HTN, hypertension; OR, odds ratio; CI, confidence interval.
Data with normal distribution are presented as mean±standard deviation.
The association of resistant HTN with OSA
Using the stepwise logistic regression analysis, waist (chisquare= 28.82, p<0.001), age (chi-square=35.01, p<0.001), gender (chi-square=16.02, p<0.001), and NC (chi-square=3.39, p=0.066) were significantly associated with OSA. However, resistant HTN was not significantly associated with OSA in the multivariate logistic regression analysis (chi-square=1.62, p=0.203) (Table 5).
Table 5. Stepwise Logistic Regression Analysis of Risk Factors Associated with the Prevalence of OSA in Hypertensive Patients.
| Step | Stepwise selection (p value at entry or stay, <0.25) | Multivariate model (c=0.767, AIC=448.79) | |||
|---|---|---|---|---|---|
| Variables | Score statistics | p value | OR (95% CI) | p value | |
| 1 | Waist circumference (cm) | 28.82 | <0.001 | 1.056 (1.018-1.095) | 0.003 |
| 2 | Age (yrs) | 35.01 | <0.001 | 1.067 (1.046-1.089) | <0.001 |
| 3 | Male sex | 16.02 | <0.001 | 1.916 (0.959-3.829) | 0.066 |
| 4 | Neck circumference (cm) | 3.39 | 0.066 | 1.108 (0.979-1.255) | 0.105 |
| 5 | Resistant HTN | 1.62 | 0.203 | 1.708 (0.744-3.923) | 0.207 |
OSA, obstructive sleep apnea; OR, odds ratio; CI, confidence interval; HTN, hypertension; AIC, Akaike Information Criterion.
DISCUSSION
The prevalence of OSA is reported to be between 2% and 7% in middle-aged adult populations, but the prevalence of OSA without symptoms has been reported to be up to 20%.21 In patients with cardiovascular disease, the prevalence of OSA has been shown to be as high as 60%,22 and approximately 80% of resistant hypertensive patients have OSA.8,9 Although previous studies have shown that the risk for OSA is significantly increased in subjects with resistant HTN compared to those with controlled HTN,23 population studies regarding the association between resistant HTN and OSA are limited. Because resistant HTN and OSA share common risk factors, including age and obesity,1,24 we sought to determine whether resistant HTN is an independent risk factor for OSA. We also examined whether resistant HTN and OSA are causally related.
First, we compared various clinical characteristics, such as body weight, BMI, NC, WC, and HC, between controlled and resistant hypertensive patients. The results showed that these clinical features were higher in resistant HTN patients. These parameters were also significantly associated with OSA in the entire study population (Table 4). One important risk factor for OSA is obesity, as 40% of obese subjects have OSA.25 Furthermore, a 10% increase in body weight contributes to a 6-fold increase in the odds of developing OSA.26 Therefore, we suspected that these propensities for obesity might be responsible for the higher prevalence of OSA in patients with resistant HTN. This hypothesis was based on the fact that CPAP only modestly reduces BP in OSA patients. In the open-randomized HIPARCO study, which assessed the effect of CPAP in patients with resistant HTN, there was a small but significant decrease in mean BP over a 24-hour period.27 Through a meta-analysis of 16 randomized clinical trials, Bazzano, et al.28 concluded that effective CPAP treatment reduces BP, but its mean effect is modest at best. Although there are various differences in the duration of treatment, CPAP adherence, study design, and cohort size of these clinical trials, the modest effect of CPAP suggests that OSA may be a partial contributing factor to the pathophysiological mechanism of resistant HTN development. In addition, Friedman, et al.29 have demonstrated that the association between OSA and resistant HTN may be due to exaggerated fluid volume displacement from the legs to the upper airways. However, this is not supported clinically, because β-blockers, rather than diuretics, are more effective in reducing BP in hypertensive subjects with OSA.
OSA is characterized by repetitive pharyngeal narrowing during sleep. This is due to the deposition of fat in the upper airway30 and the redistribution of intravascular and interstitial fluid in the pharyngeal structure. The hypothesis of rostral fluid shift from the legs into the neck is a potential mechanism for the aggravation of OSA, and it is well established in non-obese patients.31 However, Jafari and Mohsenin32 showed that fluid shift does not significantly change the respiratory disturbance index, which is a sum of the AHI and respiratory effort-related arousal, and it does not worsen the severity of OSA.
Results from our stepwise logistic regression analyses showed that resistant HTN itself was not significantly associated with OSA, after controlling for age and indices of obesity, compared to total HTN patients (Table 5). Although resistant HTN was significantly associated with the presence of OSA in univariate analysis (Table 4), the association became non-significant when the obesity factors were adjusted by stepwise and multivariate analyses and controlled HTN was compensated (Table 5). In addition, the results in Table 3 showed that resistant HTN had more obesity than controlled HTN. Similar to our finding, it has been reported that weight loss combined with CPAP was effective for the control of blood pressure.33 Taken together, these findings suggest that the high prevalence of OSA in resistant hypertensive patients is due to the sharing of common risk factors (Table 3 and 5). Furthermore, the correction of clinical risk factors should not be overlooked in the management of resistant hypertensive patients. In particular, obesity should be emphasized for both OSA and resistant HTN. Therefore, both weight loss and CPAP usage are necessary for BP control in overweight patients with concurrent resistant HTN and OSA.
Another risk factor for the pathogenesis of cardiovascular disease, including resistant HTN, is metabolic syndrome. Importantly, it is highly associated with OSA.34 Results from multivariate analyses show that the presence of OSA was significantly associated with metabolic syndrome in all hypertensive groups (OR=2.529, p<0.001) (Supplementary Fig. 1, only online). Although the high prevalence of metabolic syndrome may be due to common sharing of risk factors, such as obesity, OSA itself can enhance insulin resistance by increasing sympathetic nervous system activation and elevating systemic inflammation.34 This is supported by the fact that CPAP usage is associated with an improvement in lipid profiles, glycosylated hemoglobin levels, and BP.35
Our study has several limitations. First, this was a retrospective analysis of registry data with cross-sectional analysis. Second, we did not have full data regarding the etiologies of secondary HTN in the resistant HTN group. Third, portable PSG was used instead of fully attended in-laboratory PSG, which is typically used in the diagnosis of OSA. However, our device is a type 2 portable monitor with EEG channels, which enables the AHI to be accurately calculated, and this was manually scored by sleep technologists and validated by a sleep expert. Portable PSG is effective and may be used as an alternative to in-laboratory PSG, especially in patients with a high clinical probability of OSA.36,37,38 Fourth, we did not exclude subjects with white-coat hypertension. Lastly, as we utilized hospital-based registry data, the results from the study cannot be generalized to the general population. Nevertheless, the ability to analyze the association between resistant HTN and OSA in a relatively large registry is a merit of this study.
We evaluated various clinical and polysomnographic characteristics in controlled and resistant hypertensive patients who were suspected to have OSA, and found that the prevalence of OSA was higher and more severe in patients with resistant HTN than those with controlled HTN. Our results from univariate and stepwise logistic analyses showed that resistant HTN was not an independent risk factor for OSA, and that the high prevalence of OSA in resistant HTN was due to the sharing of common risk factors for both conditions.
ACKNOWLEDGEMENTS
This research was supported by a grant to H.J. Cho from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF-2013R1A1A1010151). This research was supported (in part) by the Yonsei University Future-leading Research Initiative of 2014 (2014-22-0131) to C.H. Kim and by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (2013M3A9D5072551) to C.H. Kim. This study was also supported by the Cardiovascular Research Center in Seoul, Korea, and by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0715). The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. We gratefully thank Jae Hee Kim and the Cardiology Laboratory for their help with data collection.
Footnotes
The authors have no financial conflicts of interest.
Supplementary Material
The relationship between OSA and metabolic syndrome in patients with controlled or resistant HTN. The number of patients with or without metabolic syndrome was compared among patients with resistant, controlled, and total hypertension. Each odds ratio (OR) was calculated. Metabolic syndrome was significantly associated with OSA in the controlled and total hypertensive groups (*p<0.01). OSA, obstructive sleep apnea; HTN, hypertension.
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Muiesan ML, Salvetti M, Rizzoni D, Paini A, Agabiti-Rosei C, Aggiusti C, et al. Resistant hypertension and target organ damage. Hypertens Res. 2013;36:485–491. doi: 10.1038/hr.2013.30. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveras A, de la Sierra A. Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertens. 2014;28:213–217. doi: 10.1038/jhh.2013.77. [DOI] [PubMed] [Google Scholar]
- 6.Fagard RH. Resistant hypertension. Heart. 2012;98:254–261. doi: 10.1136/heartjnl-2011-300741. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 9.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 12.Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010;138:434–443. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan A, Patel NK, O'Hearn DJ, Khan S. Resistant hypertension and obstructive sleep apnea. Int J Hypertens. 2013;2013:193010. doi: 10.1155/2013/193010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drager LF, Pedrosa RP, Diniz PM, Diegues-Silva L, Marcondes B, Couto RB, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–555. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 17.Lozano L, Tovar JL, Sampol G, Romero O, Jurado MJ, Segarra A, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–2168. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 18.Chung F, Liao P, Sun Y, Amirshahi B, Fazel H, Shapiro CM, et al. Perioperative practical experiences in using a level 2 portable polysomnography. Sleep Breath. 2011;15:367–375. doi: 10.1007/s11325-010-0340-9. [DOI] [PubMed] [Google Scholar]
- 19.Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1994;17:372–377. [PubMed] [Google Scholar]
- 20.Flemons WW, Littner MR, Rowley JA, Gay P, Anderson WM, Hudgel DW, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543–1579. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 21.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 23.Gus M, Gonçalves SC, Martinez D, de Abreu Silva EO, Moreira LB, Fuchs SC, et al. Risk for Obstructive Sleep Apnea by Berlin Questionnaire, but not daytime sleepiness, is associated with resistant hypertension: a case-control study. Am J Hypertens. 2008;21:832–835. doi: 10.1038/ajh.2008.184. [DOI] [PubMed] [Google Scholar]
- 24.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–1711. [PubMed] [Google Scholar]
- 26.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-García MA, Capote F, Campos-Rodríguez F, Lloberes P, Díaz de Atauri MJ, Somoza M, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 28.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 29.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–1082. doi: 10.1161/HYPERTENSIONAHA.110.154427. [DOI] [PubMed] [Google Scholar]
- 30.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 31.Redolfi S, Yumino D, Ruttanaumpawan P, Yau B, Su MC, Lam J, et al. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am J Respir Crit Care Med. 2009;179:241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 32.Jafari B, Mohsenin V. Overnight rostral fluid shift in obstructive sleep apnea: does it affect the severity of sleep-disordered breathing? Chest. 2011;140:991–997. doi: 10.1378/chest.11-0044. [DOI] [PubMed] [Google Scholar]
- 33.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin QC, Chen LD, Yu YH, Liu KX, Gao SY. Obstructive sleep apnea syndrome is associated with metabolic syndrome and inflammation. Eur Arch Otorhinolaryngol. 2014;271:825–831. doi: 10.1007/s00405-013-2669-8. [DOI] [PubMed] [Google Scholar]
- 35.Trzepizur W, Le Vaillant M, Meslier N, Pigeanne T, Masson P, Humeau MP, et al. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest. 2013;143:1584–1589. doi: 10.1378/chest.12-1652. [DOI] [PubMed] [Google Scholar]
- 36.Polese JF, Santos-Silva R, Kobayashi RF, Pinto IN, Tufik S, Bittencourt LR. Portable monitoring devices in the diagnosis of obstructive sleep apnea: current status, advantages, and limitations. J Bras Pneumol. 2010;36:498–505. doi: 10.1590/s1806-37132010000400017. [DOI] [PubMed] [Google Scholar]
- 37.Bravata DM, Ferguson J, Miech EJ, Agarwal R, McClain V, Austin C, et al. Diagnosis and Treatment of Sleep Apnea in patients' homes: the rationale and methods of the "GoToSleep" randomized-controlled trial. J Clin Sleep Med. 2012;8:27–35. doi: 10.5664/jcsm.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrosa RP, Barros IM, Drager LF, Bittencourt MS, Medeiros AK, Carvalho LL, et al. OSA is common and independently associated with hypertension and increased arterial stiffness in consecutive perimenopausal women. Chest. 2014;146:66–72. doi: 10.1378/chest.14-0097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The relationship between OSA and metabolic syndrome in patients with controlled or resistant HTN. The number of patients with or without metabolic syndrome was compared among patients with resistant, controlled, and total hypertension. Each odds ratio (OR) was calculated. Metabolic syndrome was significantly associated with OSA in the controlled and total hypertensive groups (*p<0.01). OSA, obstructive sleep apnea; HTN, hypertension.