Abstract
The minichromosome maintenance (MCM) 2-7 helicase complex functions to initiate and elongate replication forks. Cell cycle checkpoint signaling pathways regulate DNA replication to maintain genomic stability. We describe four lines of evidence that ATM/ATR-dependent (ataxia-telangiectasia-mutated/ATM- and Rad3-related) checkpoint pathways are directly linked to three members of the MCM complex. First, ATM phosphorylates MCM3 on S535 in response to ionizing radiation. Second, ATR phosphorylates MCM2 on S108 in response to multiple forms of DNA damage and stalling of replication forks. Third, ATR-interacting protein (ATRIP)-ATR interacts with MCM7. Fourth, reducing the amount of MCM7 in cells disrupts checkpoint signaling and causes an intra-S-phase checkpoint defect. Thus, the MCM complex is a platform for multiple DNA damage-dependent regulatory signals that control DNA replication.
Genotoxic stress evokes multiple responses including cell cycle arrest, enhanced DNA repair, changes in transcription, and apoptosis. The coordination of these responses is achieved through signal transduction pathways that sense DNA lesions and stalled replication forks. The phosphoinositide-3-kinase-like kinases family of protein kinases including ATM (ataxia-telangiectasia mutated) and ATR (ATM- and Rad3-related) are the principal regulators of these signaling pathways (1, 2). ATM is activated by DNA double-strand breaks, and ATR signals in response to a wide variety of lesions and stalled replication forks. ATR binds ATRIP, which recruits ATR to stalled replication forks by binding to single-stranded DNA coated with replication protein A (3, 4).
One of the key functions of ATM and ATR is to regulate DNA replication (5). Saccharomyces cerevisiae ATR (Mec1) slows DNA replication in response to genotoxic stress by regulating origin firing (6). The Dbf4/Cdc7 kinase controls origin firing and is inhibited by the Mec1-dependent kinase Rad53 (7, 8). Mec1 promotes replication fork fidelity when a fork stalls (6, 9–11). Mec1- or Rad53-deficient cells exhibit irreversible fork collapse in the presence of DNA damaging agents such as methyl methane sulfonate, and also accumulate long stretches of single-stranded DNA, hemireplicated DNA, and Holliday junctions (9). The targets of these kinases in this response are unknown.
The phenotypes associated with loss of ATM/ATR function are consistent with the checkpoint pathway targeting proteins at initiating and elongating replication forks. The MCM2–7 helicase complex functions in both the initiation and elongation of replication (12, 13). MCM association with chromatin, nuclear localization, and activity is all regulated. MCM proteins are substrates of at least two cell cycle-regulated kinases. MCM3 is also acetylated (14). MCMs physically associate with replication proteins including CDC45 and MCM10 (15–21). The retinoblastoma protein, cyclin D, and papillomavirus E6 protein also bind to MCM7 (22–24). The MCM complex is also an indirect target of checkpoint signaling via modulation of cyclin-dependent kinase-mediated MCM4 phosphorylation (25).
MCM proteins are attractive candidates for regulation by checkpoints. MCM proteins must be retained at stalled replication forks to resume DNA replication. If they dissociate, it is unclear how they could be reassembled because replication licensing is not allowed once S phase has begun (26). Continued MCM activity at a stalled replication fork may also be deleterious because it would expose extensive regions of single-stranded DNA, precisely the phenotype observed in S. cerevisiae checkpoint mutants treated with hydroxyurea (HU) (9). Furthermore, short stretches of single-stranded DNA that might accumulate at stalled forks because of MCM action may be essential to recruiting the ATR-ATRIP complex and activating the S-phase checkpoint. In this study, we found four direct connections between the ATR/ATM checkpoint pathways and the MCM proteins.
Methods
Antibodies and Mass Spectrometry. The serine/glutamine 2 (SQ2) antibody was made by Bethyl Laboratories (Montgomery, TX). Large-scale immunoprecipitates were performed from HeLa cell nuclei exposed to 1 mM HU for 20 h. Nuclei were lysed in radioimmunoprecipitation assay buffer (50 mM Tris, pH 7.5/150 mM NaCl/1% Igepal CA-630/0.5% SDS plus protease and phosphatase inhibitors). After preclearing the lysate and immunoprecipitation with the SQ2 antibody, proteins were identified by mass spectrometry as described in ref. 27.
MCM2 antibodies were obtained from Transduction Laboratories (Lexington, KY). MCM3, MCM7, and ATR antibodies were from Santa Cruz Biotechnology. MCM2 phosphoserine (P-S) 108 antibody was made by Bethyl Laboratories. Chk1 P-S345 and Chk2 P-T68 antibodies were obtained from Cell Signaling Technology (Beverly, MA). P-S1981 ATM antibody was from Rockland (Gilbertsville, PA).
In Vitro Kinase Assays. Kinase assays were performed with Flag-tagged recombinant ATM or ATR proteins produced in human embryonic kidney (HEK) 293 cells as described in ref. 28. Recombinant MCM2 and MCM3 proteins were produced in bacteria.
Cell Culture. HEK 293, U2OS, and HeLa cells were cultured in DMEM with 10% FBS. HCT116 cells were cultured in McCoy's 5A medium with 10% FBS. Deletion of ATR from ATRflox/- cells was performed as described in ref. 3.
Small Interfering RNA (siRNA) Sequences and Transfection. MCM2, MCM3, and MCM7 siRNA oligonucleotides were purchased as Smart Pools from Dharmacon (Boulder, CO). ATR (CCUCCGUGAUGUUGCUUGAdTdT) and ATM (GCCUCCAGGCAGAAAAAGAdTdT) siRNAs were purchased from Dharmacon.
DNA Damage and Radio-Resistant DNA Synthesis (RDS) Assay. IR was administered by using a 137Cs source, and UV-C light was administered by using a Stratalinker crosslinker (Stratagene). The RDS checkpoint assay was performed as described in ref. 29.
Results
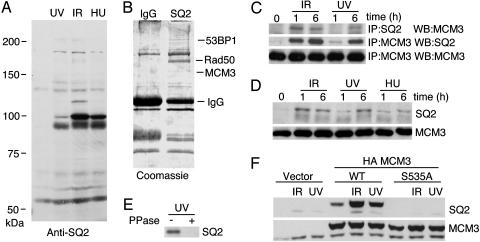
Identification of MCM3 as an ATM/ATR Substrate. ATM and ATR phosphorylate proteins on serines followed by glutamines (SQ). We reasoned that a phosphopeptide-specific antibody that had high affinity toward phospho-SQ-containing peptides would be useful as an affinity reagent to identify ATM/ATR substrates. Through serial injections of different phosphopeptides into rabbits and purification of phosphopeptide-specific antibodies, we were able to produce one antibody (SQ2) that recognized multiple proteins in a genotoxic stress-dependent manner (Fig. 1A). We used the SQ2 antibody to immunoprecipitate proteins from cell lysates derived from HU-treated HeLa cells. 53BP1, Rad50, and MCM3 were identified in the SQ2 immunoprecipitates by mass spectrometry (Fig. 1B). 53BP1 and components of the Rad50/Mre11/Nbs1 complex were previously shown to be direct substrates of ATM, validating this approach to substrate identification (29–34).
Fig. 1.
MCM3 is phosphorylated on S535 in response to genotoxic stress. (A) HeLa cells were exposed to UV light (60 J/m2) or IR (30 Gy) and then incubated for 2 h or treated with HU (2 mM) for 24 h. Cell lysates were blotted with the SQ2 antibody. (B) HeLa cells were treated with HU for 20 h and harvested, and nuclei were prepared. The nuclei were lysed in radioimmunoprecipitation assay buffer and immunoprecipitated with control IgG or the SQ2 antibody. Immunoprecipitated proteins were stained with Coomassie blue and identified by mass spectrometry. (C) HeLa cell lysates prepared from IR-treated (10 Gy) or UV-light-treated (50 J/m2) HeLa cells that were then grown for 1 or 6 h were immunoprecipitated with either SQ2 or MCM3 antibodies. Immunoprecipitates were blotted as indicated. (D) HeLa total cell lysates prepared from cells exposed to IR (10 Gy), UV light (50 J/m2), or HU (1 mM) and incubated for 1 or 6 h were blotted with the SQ2 antibody. The membrane was then stripped and reprobed for MCM3. The protein recognized by the MCM3 antibody comigrated with the upper, distinct, SQ2-recognized protein. (E) HeLa cell lysate from UV-light-treated cells was incubated with λ phosphatase or mock-treated and blotted with the SQ2 antibody. (F) HEK 293 cells were transfected with empty vector, HA-tagged WT MCM3, or HA-tagged MCM3 S535A. Cells were treated with IR (10 Gy) or UV light (50 J/m2) and lysed. Cell lysates were separated by SDS/PAGE and blotted with antibodies as indicated. The bottom band is endogenous MCM3, and the top band is HA-MCM3.
MCM3 could be immunoprecipitated because it is phosphorylated or because it is associated with a protein recognized by the SQ2 antibody. To confirm that MCM3 itself is phosphorylated, we immunoprecipitated MCM3 and blotted with the SQ2 antibody (Fig. 1C). The SQ2 antibody only blotted and immunoprecipitated MCM3 from cells exposed to DNA damage. The SQ2 antibody recognizes MCM3 on Western blots of cells exposed to IR, UV light, and HU (Fig. 1D), and binding is prevented by phosphatase treatment (Fig. 1E).
Human MCM3 has six SQ sites and two TQ sites. Each site was mutated to alanine to determine which is recognized by the SQ2 antibody. The SQ2 antibody binds hemagglutinin (HA)-tagged MCM3 expressed in HEK 293 cells (migrating slower in SDS/PAGE gels than in the endogenous protein) but does not recognize S535A MCM3 (Fig. 1F). Thus, S535 of MCM3 is inducibly phosphorylated in response to genotoxic stress in cells. It should be noted that the overexpressed WT HA-MCM3 protein is phosphorylated on S535 even without damaging cells (Fig. 1F). This damage-independent phosphorylation could be due to either endogenous DNA lesions, or the overexpression of HA-MCM3 may cause problems at replication forks that activate checkpoint pathways.
We next examined whether ATM or ATR could phosphorylate MCM3 directly. Bacterial recombinant MCM3 protein was used in in vitro kinase assays with Flag-tagged ATM or ATR. WT ATM and ATR both phosphorylate MCM3 in vitro, whereas kinase-inactive ATM and ATR do not (Fig. 2A). MCM3 is a relatively poor ATR substrate. Mutation of S535 to alanine reduced ATM/ATR phosphorylation by ≈50% compared with WT MCM3 (Fig. 2B). These data indicate that ATM and ATR phosphorylate S535 directly but that additional residues on MCM3 are also phosphorylated.
Fig. 2.
MCM3 is phosphorylated by ATM. (A) GST-MCM3 was incubated with WT or kinase-dead (KD) Flag-ATM or Flag-ATR in the presence of [γ]-[32]ATP. The kinase reaction was separated by SDS/PAGE. An autoradiogram and picture of the Coomassie blue-stained MCM3 protein is shown, as well as an anti-Flag Western blot showing the levels of ATM and ATR protein. (B) WT GST-MCM3 or S535A-mutated GST-MCM3 was subjected to kinase reactions with ATM or ATR. An autoradiogram of the 32P incorporation into the MCM3 proteins is shown, as well as a picture of the Coomassie-stained gel. (C) AT22IJE-T (homozygous ATM mutant cells, A-T) or AT22IJE-T cells containing a vector encoding ATM were left untreated or exposed to 10 Gy of IR and harvested after 1 or 6 h. Protein from these cells was fractionated by SDS/PAGE and immunoblotted with either the SQ2 or MCM3 antibody. (D–F) Lysates were prepared from either ATR+/+ or ATRflox/- cells after infection with adenovirus encoding either Cre or GFP and treatment with HU (D), UV light (E), or IR (F) and harvested after the indicated number of hours. Proteins were fractionated by SDS/PAGE, blotted, and probed with either the SQ2 or MCM3 antibody. (G) U2OS cells were transfected with siRNA targeting ATM and/or ATR, exposed to UV light (50 J/m2), incubated for 2 h, and then harvested. Protein lysates were fractionated by SDS/PAGE, blotted, and probed with antibodies as indicated.
We next analyzed whether MCM3 S535 phosphorylation required ATM or ATR in vivo. ATM-deficient cells complemented by an ATM cDNA or an empty vector were exposed to IR, incubated for 1 or 6 h, then lysed and analyzed for MCM3 phosphorylation. ATM is required for MCM3 phosphorylation shortly after IR but is not required at later times (Fig. 2C). Furthermore, ATM is not required for S535 MCM3 phosphorylation in response to UV light or HU (data not shown). This pattern is similar to many other ATM substrates that are also phosphorylated by ATR. Therefore, we examined MCM3 phosphorylation in ATR-deficient cells by using the ATRflox/- conditional HCT116 cell line (3). MCM3 S535 was still phosphorylated after treatment with UV light and HU in the ATR-depleted cells. In fact, S535 of MCM3 was hyperphosphorylated in the absence of ATR compared with the control ATR-proficient cells (Fig. 2 D–F). ATR is required for Chk1 S345 phosphorylation under the same conditions (data not shown).
Loss of ATR in these cells promotes double-strand break formation after treatment with UV light and HU and therefore leads to ATM activation (35). Thus, we examined whether ATM was required for MCM3 phosphorylation in the absence of ATR by using siRNA in U2OS cells. UV-light treatment of control or ATM-depleted cells causes a slight increase in MCM3 S535 phosphorylation 2 h after treatment, whereas treatment of ATR-depleted cells causes a robust phosphorylation that is diminished by simultaneous ATM depletion (Fig. 2G). The phosphorylation is not completely eliminated under these conditions, presumably because only a subset of cells receives both siRNA duplexes and the knockdown is not completely efficient. We eliminated the possibility that DNA-PK could be serving as the MCM3 kinase after treatment with UV light because wortmannin (a DNA-PK and ATM inhibitor) does not reduce MCM3 phosphorylation and MCM3 phosphorylation is normal in DNA-PK-deficient cells (see Fig. 5, which is published as supporting information on the PNAS web site). Thus, ATM is the major kinase responsible for MCM3 S535 phosphorylation in cells depleted of ATR.
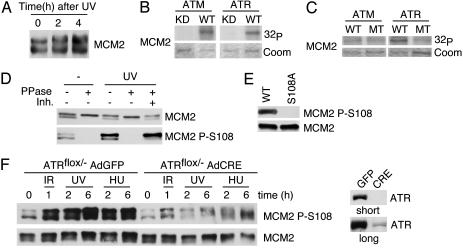
MCM2 Is Phosphorylated on S108 by ATR. Because MCM proteins form a six-subunit helicase, we analyzed the other subunits for potential DNA damage-regulated phosphorylation. MCM2 migrates as a doublet on Western blots of lysates from proliferating cells. The faster-migrating form corresponds to MCM2 that is phosphorylated by cell cycle-regulated kinases (36, 37). After exposure to UV light, we observed a rapid alteration in gel migration of MCM2 (Fig. 3A). Therefore, we tested whether MCM2 is also a kinase substrate in vitro. WT ATM and ATR phosphorylate recombinant MCM2 isolated from bacteria (Fig. 3B).
Fig. 3.
MCM2 is phosphorylated by ATR. (A) Protein from mock- or UV-light-treated HCT116 cells was fractionated by SDS/PAGE, blotted, and probed with MCM2 antibody. (B) GST-MCM2 was purified from bacteria and subjected to an in vitro kinase assay with either WT or mutant ATM or ATR. (C) WT or S108A MCM2 mutant protein purified from bacteria was subjected to an in vitro kinase assay with WT ATM or ATR. The autoradiogram displaying the relative amount of 32P incorporated into MCM2, and an image of the Coomassie blue-stained MCM2 protein is shown. (D) Lysates from untreated or UV-light-treated cells were treated with λ phosphatase in the absence or presence of phosphatase inhibitors (Inh.). The lysates were fractionated by SDS/PAGE and blotted. (E) His-tagged WT or mutant (S108A) MCM2 was purified from transfected HEK 293 cells by using Ni-agarose. The purified protein was blotted as indicated. (F) ATRflox/- cells infected with adenovirus encoding either Cre or GFP were treated with IR, UV light, or HU and incubated for the indicated number of hours before harvesting. Lysates were separated by SDS/PAGE, blotted, and probed with the MCM2, phospho-S108 MCM2, or ATR antibody. The lower ATR blot (long) is a longer exposure of the upper blot (short) showing the residual ATR left in the cell population.
MCM2 has one TQ motif and three SQ motifs. To determine which site(s) ATM and ATR targeted, we mutated each serine or threonine to alanine. Mutation of S108 to alanine significantly reduced ATR phosphorylation but only had a mild effect on ATM phosphorylation (Fig. 3C). The other mutations had very little effect on ATM or ATR-dependent phosphorylation in vitro (data not shown). S108 is highly conserved throughout vertebrate species but not in yeast.
To confirm S108 MCM2 phosphorylation in vivo, we generated phosphopeptide-specific antibodies to P-S108. These antibodies recognize proteins that perfectly comigrate with the MCM2 doublet even in undamaged cells (Fig. 3D). Treating the extracts with phosphatase collapses MCM2 into a single band on SDS/PAGE gels and abolishes recognition by the P-S108 antibody. Furthermore, treatment of cells with UV light before harvesting lysates caused an increase in the amount of phosphorylated MCM2. P-S108 is the epitope for this antibody because an S108A mutation abolished recognition of MCM2 (Fig. 3E). Thus, MCM2 is phosphorylated on S108 in proliferating cells, and S108 phosphorylation is increased in cells exposed to genotoxic agents.
We next examined the dependence of MCM2 S108 phosphorylation on ATM and ATR in vivo. No obvious defect was observed in ATM-deficient cells (data not shown), a finding that is consistent with the lack of affect of S108A mutation on the ability of ATM to phosphorylate MCM2 in vitro (Fig. 3C). To examine ATR dependency, we exposed the ATRflox/- cells that had been infected with either control virus or Cre-expressing virus to IR, UV light, and HU. MCM2 S108 phosphorylation after all of these treatments largely depended on ATR (Fig. 3F). There was a slight induction of phosphorylation in the ATR-deficient cells 1 h after IR treatment and 6 h after HU treatment. However, there was no increase in phosphorylation after treatment with UV light or early times after HU treatment in ATR-deficient cells compared with the robust phosphorylation in the ATR-proficient cells. Furthermore, there was an ≈50% reduction in the levels of MCM2 S108 phosphorylation in ATR-deficient cells in the absence of genotoxic agents. The residual MCM2 phosphorylation may be due to another phosphoinositide-3-kinase-like kinase or the small amount of ATR that remains in this cell population after the Cre recombination (Fig. 3F). Thus, ATR appears to be the major in vivo kinase responsible for S108 MCM2 phosphorylation in response to genotoxic stress.
Chromatin association and phosphorylation of MCMs are cell cycle-regulated. We found that both soluble and chromatin-associated MCM2 is phosphorylated after treatment with UV light and that there is no change in the distribution of MCM2 (see Fig. 6, which is published as supporting information on the PNAS web site).
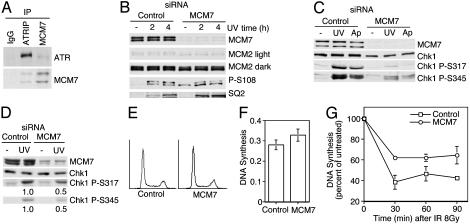
MCM7 Binds ATRIP and Participates in the S-Phase Checkpoint. In the course of studying MCM phosphorylation, we isolated MCM7 from a two-hybrid screen by using ATRIP as a bait. ATRIP binds ATR and is essential for ATR-dependent signaling (3). The minimal region of MCM7 that can bind ATRIP contained amino acids 577–719 (see Fig. 7, which is published as supporting information on the PNAS web site). Several proteins have been found to associate with the C terminus of MCM7, including retinoblastoma protein, cyclin D, MAT1, and the E6 oncoprotein (22–24, 38), raising the possibility that this region of MCM7 is either extremely important for MCM regulation or associates nonspecifically with many proteins. We confirmed that MCM7 associates with ATRIP-ATR, because ATRIP antibodies immunoprecipitated both ATR and MCM7, and MCM7 antibodies immunoprecipitated ATR (Fig. 4A).
Fig. 4.
ATR-ATRIP interacts with MCM7, and MCM7 is required for proper S-phase checkpoint control. (A) ATR-ATRIP coimmunoprecipitates with MCM7. Undamaged HeLa cell lysates were incubated with antibodies to ATRIP, MCM7, or control IgG and protein A/G agarose. The immunoprecipitates were washed, separated by SDS/PAGE, and blotted with anti-ATR and anti-MCM7 antibodies. (B–D) Knock-down of MCM7 was accomplished by two rounds of transfection into U2OS cells with siRNA targeting MCM7 or a control siRNA, using 200 nM of the duplex oligonucleotide. Cells were treated with 50 J/m2 UV light and harvested 2 or 4 h later (B) or 50 J/m2 UV light or 5 μg/ml aphidicolin (Ap) and harvested 2 h later (C). Cell lysates were prepared, separated by SDS/PAGE, blotted, and probed with antibodies. MCM2 light is a short exposure, and MCM2 dark is a long exposure of the same blot. (D–G) A single transfection with MCM7 or control siRNA oligonucleotides at 100 nM was performed in U2OS cells. (D) Cells were treated with 50 J/m2 UV light and harvested after 2 h. Cell lysates were prepared, separated by SDS/PAGE, blotted, and probed with antibodies as indicated. Quantitation of the Chk1 P-317 and Chk1 P-345 signals was normalized to total Chk1. (E) Two days after siRNA transfection, the cells were stained with propidium iodide and analyzed by flow cytometry. (F)[14C]Thymidine-labeled MCM7 or control siRNA-transfected cells were pulsed with [3H]thymidine for 15 min. DNA synthesis was measured as a ratio of [3H]thymidine incorporation compared with [14C]thymidine to control for cell number. (G) Control or MCM7 siRNA-transfected U2OS cells were tested for their ability to inhibit DNA synthesis after exposure to 8 Gy of IR. The percent of DNA synthesis of the mock-treated cells was set at 100%. The error bars represent the standard deviation.
We suspected that the ATRIP-MCM7 interaction might provide a mechanism to recruit ATR to the MCM complex and allow it to phosphorylate MCM2. Thus, we depleted MCM7 by using two successive rounds of MCM7 siRNA transfection in U2OS cells and then examined MCM2 phosphorylation after treatment with UV light or IR. This depletion decreased MCM7 protein by 80–90%. In contrast to our hypothesis, MCM2 phosphorylation was increased in MCM7-depleted cells even before DNA damage (Fig. 4 B and D). Furthermore, there was no defect in UV-light-, aphidicolin-, or IR-induced MCM2 phosphorylation. The overall levels of MCM2 were unaffected by MCM7 depletion; however, we did observe a consistent increase in the amount of the faster-migrating form of the MCM2 doublet (see light exposure of MCM2 blot in Fig. 4B). The faster-migrating form of MCM2 is phosphorylated by cyclin-dependent kinases, suggesting that the MCM7-depleted cells might be accumulating outside of the G1 phase. Flow cytometry analysis indicated that two transfections of U2OS cells with MCM7 siRNA caused an increase in the number of cells in S phase compared with controls (38% in S phase in controls vs. 46% in S phase in MCM7-depleted). This result may be due to a slowing of DNA replication in cells containing reduced MCM7 protein. The increase in MCM2 S108 phosphorylation without damage is likely due to this synchronization effect, because MCM2 S108 phosphorylation is increased in undamaged S-phase cells compared with G1-phase cells (data not shown).
We observed no difference in the basal level of phosphorylation without damage of MCM3 S535, Chk1 S345, Chk2 T68, or ATM. MCM7 depletion did lead to a slight increase in MCM3 S535 phosphorylation after treatment with UV light but not after IR (Fig. 4B; and see Fig. 8, which is published as supporting information on the PNAS web site). The only decrease in phosphorylation observed was on Chk1 S345 after treatment with UV light and aphidicolin (Fig. 4C).
To determine whether the reduction in Chk1 phosphorylation was a consequence of slowing S phase caused by a requirement for MCM7 in DNA replication or whether it reflected a separable requirement for MCM7 for checkpoint signaling, we developed conditions under which the MCM7 siRNA depletion did not affect cell cycle distribution. A single transfection of MCM7 siRNA oligonucleotides at 100 nM caused an ≈60% reduction in total MCM7 levels but did not cause any discernable cell cycle defect measured either by flow cytometry analysis or thymidine incorporation assays to measure DNA replication (Fig. 4 D–F). This result is consistent with observations that the MCM proteins are expressed far in excess of what is required to support WT levels of DNA replication (39). Under these conditions there still was a 50% reduction in Chk1 S345 and Chk1 S317 phosphorylation in response to treatment with UV light (Fig. 4D).
To test whether MCM7 is required for other S-phase checkpoint responses, we analyzed the ability of cells with reduced MCM7 expression levels to slow DNA synthesis after IR. These experiments were performed by using the single 100 nM siRNA transfection conditions that yielded no change in DNA replication rates or cell cycle position. In response to IR, MCM7-depeleted cells were partially defective in slowing DNA synthesis (Fig. 4G). This radio-resistant DNA synthesis phenotype is the defining characteristic of cells lacking the intra-S-phase checkpoint response. This checkpoint defect in the MCM7-depleted cells does not appear to be due to a defect in the activation of ATM or ATR, because ATM is phosphorylated on the activating S1981 site and both Chk2 and Chk1 are also phosphorylated (Fig. 8). We did not observe a significant S-phase checkpoint defect in cells transfected with siRNA to deplete MCM2 or MCM3 that caused a similar level of protein depletion (data not shown). Although these negative data are difficult to interpret conclusively, they do support the hypothesis that each MCM subunit provides unique functions to the complex and the checkpoint defect is not caused by a general defect in MCM2–7 replication activity.
Discussion
ATR and ATM Directly Phosphorylate MCM2 and MCM3 After Genotoxic Stress. Using an immunopurification/proteomics approach, we identified MCM3 as an ATM substrate. MCM3 S535 is clearly phosphorylated by ATM in response to IR. The kinase responsible for phosphorylation in response to UV light and HU is likely to be ATR. However, there were complications with this interpretation because double-strand breaks produced by treatment with UV and HU in the absence of ATR activate ATM (35), which also phosphorylates this site. Identification of MCM3 as a substrate led us to discover that MCM2 is also phosphorylated by ATR after DNA damage. In contrast to MCM3 and most ATM/ATR substrates, MCM2 is phosphorylated in the absence of exogenous DNA damage on S108, and this phosphorylation depends, at least partially, on ATR.
The functional consequences of MCM2 S108 and MCM3 S535 phosphorylation are not clear. Both MCM3 and MCM2 are phosphorylated after IR in all phases of the cell cycle, but optimal UV-light-induced phosphorylation only occurs in replicating cells (data not shown). Both chromatin-bound and soluble MCM proteins are phosphorylated, and we have seen no indication that this phosphorylation changes their localization. MCM2 and MCM3 proteins with serine-to-alanine mutations do not cause an obvious phenotype when overexpressed, even when the endogenous proteins are reduced by siRNA (data not shown). However, it is possible that we are unable to measure the true function, such as regulation of single-stranded DNA, at stalled forks. In addition, we may not have mapped all of the important phosphorylation sites on the MCM complex. Indeed, there is significant residual phosphorylation of both the MCM2 S108A and MCM3 S535A mutants by ATM and ATR in vitro (Figs. 2 and 4). Many ATM and ATR substrates have multiple, semiredundant phosphorylation sites (28, 40). Thus, identification of additional damage-dependent phosphorylation sites on the MCM complex may be required before an understanding of how phosphorylation regulates the MCM complex is possible.
MCM7 Is a Critical Regulator of the S-Phase Checkpoint. Although the functional consequences of MCM2 and MCM3 phosphorylation remain unclear, we have shown that the MCM7 subunit is essential for regulating the intra-S-phase checkpoint response. MCM7 depletion causes a significant defect in the ability to inhibit DNA replication in response to IR, even though there is no defect in ATM activation or Chk1/2 phosphorylation, suggesting that the requirement for MCM7 of an optimal checkpoint response is downstream of these events. However, depletion of MCM7 did cause a defect in Chk1 phosphorylation after treatment with UV light. Chk1 S345 phosphorylation depends on ATR and ATRIP in response to treatment with UV light (41) (D.C., unpublished data). Therefore, the requirement for normal levels of MCM7 protein for optimal Chk1 phosphorylation may indicate a need for the ATRIP–MCM7 interaction to promote ATR accessibility to or activity toward Chk1.
One possible explanation of the defects in MCM7-depleted cells is that the depletion of MCM7 decreases the amount of damage signal that is present. The MCM complex may unwind DNA in advance of the replicative polymerase. Single-stranded DNA is an essential intermediate in activation of the ATR-ATRIP. Thus, reduced MCM function could lead to a reduced number of replication forks, a reduced amount of single-stranded DNA exposed after damage, and, therefore, reduced checkpoint signaling. ATR activation in S phase requires replication (42). In yeast, MCM activity is required to establish, but not to maintain, the replication checkpoint (43). Although this model may explain the reduction in Chk1 phosphorylation in response to UV light, it is not consistent with all of our data. We did not observe any defect in replication when MCM7 was depleted to levels that caused a significant S-phase checkpoint defect and Chk1 phosphorylation defect. The MCM proteins are known to be in excess compared with that required for replication. Most importantly, there is no indication that the intra-S-phase checkpoint after IR is sensitive to the amount of DNA replication occurring in the cells. Indeed, we did not observe any defect in Chk1, ATM, or Chk2 phosphorylation after IR in the MCM7-depleted cells. Finally, we did not observe similar checkpoint defects in MCM2- or MCM3-depleted cells. Thus, the most likely explanation is that the checkpoint function of MCM7 that we observed is independent of its replication function.
Conclusions. Our data point to the possibility that the MCM complex functions both upstream and downstream in checkpoint signaling pathways. Regulation of replication forks by the DNA replication checkpoint is poorly understood but critical to maintaining genomic integrity (6, 9, 10, 44–48). Our results directly link ATM and ATR/ATRIP checkpoint signaling to three subunits of the MCM helicase complex. Thus, the MCM complex provides a platform for multiple regulatory inputs, including from checkpoint kinases that regulate DNA replication.
Supplementary Material
Acknowledgments
We thank Robert Abraham and Jean Gautier for helpful discussions, Jun Qin for assistance in mass spectrometry, and Ellen Fanning for critically reading the manuscript. This work was supported by National Cancer Institute Grants K0193701 and R01CA102729 (to D.C.) and a National Institute of Health Grant (to S.J.E.). D.C. is also supported by the Pew Scholars Program in the Biological Sciences sponsored by the Pew Charitable Trusts. S.J.E. is an investigator with the Howard Hughes Medical Institute.
Abbreviations: ATM, ataxia-telangiectasia-mutated; ATR, ATM- and Rad3-related; HEK, human embryonic kidney; HA, hemagglutinin; HU, hydroxyurea; IR, ionizing radiation; MCM, minichromosome maintenance; P-S, phosphoserine; siRNA, small interfering RNA; SQ2, serine/glutamine 2.
References
- 1.Shiloh, Y. (2001) Curr. Opin. Genet. Dev. 11, 71-77. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, R. T. (2001) Genes Dev. 15, 2177-2196. [DOI] [PubMed] [Google Scholar]
- 3.Cortez, D., Guntuku, S., Qin, J. & Elledge, S. J. (2001) Science 294, 1713-1716. [DOI] [PubMed] [Google Scholar]
- 4.Zou, L. & Elledge, S. J. (2003) Science 300, 1542-1548. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg, K. A., Michelson, R. J., Putnam, C. W. & Weinert, T. A. (2002) Annu. Rev. Genet. 36, 617-656. [DOI] [PubMed] [Google Scholar]
- 6.Tercero, J. A. & Diffley, J. F. (2001) Nature 412, 553-557. [DOI] [PubMed] [Google Scholar]
- 7.Weinreich, M. & Stillman, B. (1999) EMBO J. 18, 5334-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kihara, M., Nakai, W., Asano, S., Suzuki, A., Kitada, K., Kawasaki, Y., Johnston, L. H. & Sugino, A. (2000) J. Biol. Chem. 275, 35051-35062. [DOI] [PubMed] [Google Scholar]
- 9.Sogo, J. M., Lopes, M. & Foiani, M. (2002) Science 297, 599-602. [DOI] [PubMed] [Google Scholar]
- 10.Lopes, M., Cotta-Ramusino, C., Pellicioli, A., Liberi, G., Plevani, P., Muzi-Falconi, M., Newlon, C. S. & Foiani, M. (2001) Nature 412, 557-561. [DOI] [PubMed] [Google Scholar]
- 11.Desany, B. A., Alcasabas, A. A., Bachant, J. B. & Elledge, S. J. (1998) Genes Dev. 12, 2956-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labib, K., Tercero, J. A. & Diffley, J. F. (2000) Science 288, 1643-1647. [DOI] [PubMed] [Google Scholar]
- 13.Bell, S. P. & Dutta, A. (2002) Annu. Rev. Biochem. 71, 333-374. [DOI] [PubMed] [Google Scholar]
- 14.Takei, Y., Swietlik, M., Tanoue, A., Tsujimoto, G., Kouzarides, T. & Laskey, R. (2001) EMBO Rep. 2, 119-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopwood, B. & Dalton, S. (1996) Proc. Natl. Acad. Sci. USA 93, 12309-12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou, L., Mitchell, J. & Stillman, B. (1997) Mol. Cell. Biol. 17, 553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy, C. F. (1997) Gene 187, 239-246. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki, Y., Hiraga, S. & Sugino, A. (2000) Genes Cells 5, 975-989. [DOI] [PubMed] [Google Scholar]
- 19.Izumi, M., Yanagi, K., Mizuno, T., Yokoi, M., Kawasaki, Y., Moon, K. Y., Hurwitz, J., Yatagai, F. & Hanaoka, F. (2000) Nucleic Acids Res. 28, 4769-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homesley, L., Lei, M., Kawasaki, Y., Sawyer, S., Christensen, T. & Tye, B. K. (2000) Genes Dev. 14, 913-926. [PMC free article] [PubMed] [Google Scholar]
- 21.Wohlschlegel, J. A., Dhar, S. K., Prokhorova, T. A., Dutta, A. & Walter, J. C. (2002) Mol. Cell 9, 233-240. [DOI] [PubMed] [Google Scholar]
- 22.Sterner, J. M., Dew-Knight, S., Musahl, C., Kornbluth, S. & Horowitz, J. M. (1998) Mol. Cell. Biol. 18, 2748-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladden, A. B. & Diehl, J. A. (2003) J. Biol. Chem. 278, 9754-9760. [DOI] [PubMed] [Google Scholar]
- 24.Kukimoto, I., Aihara, S., Yoshiike, K. & Kanda, T. (1998) Biochem. Biophys. Res. Commun. 249, 258-262. [DOI] [PubMed] [Google Scholar]
- 25.Ishimi, Y., Komamura-Kohno, Y., Kwon, H. J., Yamada, K. & Nakanishi, M. (2003) J. Biol. Chem. 278, 24644-24650. [DOI] [PubMed] [Google Scholar]
- 26.Diffley, J. F. (2001) Curr. Biol. 11, R367-R370. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Y., Cortez, D., Yazdi, P., Neff, N., Elledge, S. J. & Qin, J. (2000) Genes Dev. 14, 927-939. [PMC free article] [PubMed] [Google Scholar]
- 28.Cortez, D., Wang, Y., Qin, J. & Elledge, S. J. (1999) Science 286, 1162-1166. [DOI] [PubMed] [Google Scholar]
- 29.Lim, D. S., Kim, S. T., Xu, B., Maser, R. S., Lin, J., Petrini, J. H. & Kastan, M. B. (2000) Nature 404, 613-617. [DOI] [PubMed] [Google Scholar]
- 30.Rappold, I., Iwabuchi, K., Date, T. & Chen, J. (2001) J. Cell Biol. 153, 613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson, L., Henderson, C. & Adachi, Y. (2001) Mol. Cell. Biol. 21, 1719-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia, Z., Morales, J. C., Dunphy, W. G. & Carpenter, P. B. (2001) J. Biol. Chem. 276, 2708-2718. [DOI] [PubMed] [Google Scholar]
- 33.Wu, X., Ranganathan, V., Weisman, D. S., Heine, W. F., Ciccone, D. N., O'Neill, T. B., Crick, K. E., Pierce, K. A., Lane, W. S., Rathbun, G., et al. (2000) Nature 405, 477-482. [DOI] [PubMed] [Google Scholar]
- 34.Zhao, S., Weng, Y. C., Yuan, S. S., Lin, Y. T., Hsu, H. C., Lin, S. C., Gerbino, E., Song, M. H., Zdzienicka, M. Z., Gatti, R. A., et al. (2000) Nature 405, 473-477. [DOI] [PubMed] [Google Scholar]
- 35.Cortez, D. (2003) J. Biol. Chem. 278, 37139-37145. [DOI] [PubMed] [Google Scholar]
- 36.Todorov, I. T., Attaran, A. & Kearsey, S. E. (1995) J. Cell Biol. 129, 1433-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita, M., Yamada, C., Tsurumi, T., Hanaoka, F., Matsuzawa, K. & Inagaki, M. (1998) J. Biol. Chem. 273, 17095-17101. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Y., Xu, F. & Hall, F. L. (2000) FEBS Lett. 484, 17-21. [DOI] [PubMed] [Google Scholar]
- 39.Tye, B. K. (1999) Annu. Rev. Biochem. 68, 649-686. [DOI] [PubMed] [Google Scholar]
- 40.Tibbetts, R. S., Cortez, D., Brumbaugh, K. M., Scully, R., Livingston, D., Elledge, S. J. & Abraham, R. T. (2000) Genes Dev. 14, 2989-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, Q., Guntuku, S., Cui, X. S., Matsuoka, S., Cortez, D., Tamai, K., Luo, G., Carattini-Rivera, S., DeMayo, F., Bradley, A., et al. (2000) Genes Dev. 14, 1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 42.Lupardus, P. J., Byun, T., Yee, M. C., Hekmat-Nejad, M. & Cimprich, K. A. (2002) Genes Dev. 16, 2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labib, K., Kearsey, S. E. & Diffley, J. F. (2001) Mol. Biol. Cell 12, 3658-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cimprich, K. A. (2003) Curr. Biol. 13, R231-R233. [DOI] [PubMed] [Google Scholar]
- 45.Kolodner, R. D., Putnam, C. D. & Myung, K. (2002) Science 297, 552-557. [DOI] [PubMed] [Google Scholar]
- 46.Katou, Y., Kanoh, Y., Bando, M., Noguchi, H., Tanaka, H., Ashikari, T., Sugimoto, K. & Shirahige, K. (2003) Nature 424, 1078-1083. [DOI] [PubMed] [Google Scholar]
- 47.Tercero, J. A., Longhese, M. P. & Diffley, J. F. (2003) Mol. Cell 11, 1323-1336. [DOI] [PubMed] [Google Scholar]
- 48.Carr, A. M. (2002) Science 297, 557-558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.