Abstract
In type 1 diabetes (T1D), there is a specific destruction of the insulin secreting pancreatic β cell. Although the exact molecular mechanisms underlying β cell destruction are not known, sera from T1D patients have been shown to promote Ca2+-induced apoptosis. We now demonstrate that apolipoprotein CIII (apoCIII) is increased in serum from T1D patients and that this serum factor both induces increased cytoplasmic free intracellular Ca2+ concentration ([Ca2+]i) and β cell death. The apoCIII-induced increase in [Ca2+]i reflects an activation of the voltage-gated L-type Ca2+ channel. Both the effects of T1D sera and apoCIII on the β cell are abolished in the presence of antibody against apoCIII. Increased serum levels of apoCIII can thus account for the increase in β cell [Ca2+]i and thereby β cell apoptosis associated with T1D.
Research over the last 30 years has established that type 1 diabetes (T1D) is an autoimmune disease, but the mechanisms/events that trigger the initiation and progression of the disease are still not identified. Genetic, immunological, and environmental factors are involved in the pathogenesis of T1D and most likely the events involved differ between different patients. Voltage-gated L-type Ca2+ channels have an important physiological role in pancreatic β cell signal transduction (1). These channels constitute an essential link between transient changes in membrane potential and insulin release. Changes in cytoplasmic free intracellular Ca2+ concentration ([Ca2+]i) in the β cell are associated with the activation of a spectrum of intracellular signals and are strictly regulated because prolonged high [Ca2+]i is harmful to the cells. Sera from newly diagnosed T1D patients have been shown to increase the activity of voltage-gated L-type Ca2+ channels in β cells, resulting in increased [Ca2+]i upon depolarization and β cell apoptosis, effects that can be prevented by Ca2+ channel blockers (2). The key question has been what factor in T1D serum that is responsible for the changes in [Ca2+]i. In the present study, we have revealed the identity of a key factor in T1D sera that increases [Ca2+]i as well as promotes apoptosis and found it to correspond to apolipoprotein CIII (apoCIII). The fact that not all sera from T1D patients affected [Ca2+]i indicates that T1D is not caused by a single factor like apoCIII, which is in agreement with clinical observations, suggesting that different factors can act in concert with the autoimmune abnormalities resulting in β cell destruction.
Methods
Media. The basal medium used both for isolation of cells and for experiments was a Hepes buffer (pH 7.4), containing: 125 mM NaCl, 5.9 mM KCl, 1.3 mM CaCl2, 1.2 mM MgCl2, and 25 mM Hepes. BSA was added to the medium at a concentration of 1 mg/ml. For cell culture, RPMI medium 1640 was supplemented with 100 μg/ml streptomycin, 100 units of penicillin, and 10% FCS, normal human, or diabetic serum.
Preparation of Cells. Adult mice from a local colony (3) were starved overnight. Pancreatic islets were isolated by a collagenase technique, and cell suspensions were prepared as described (4, 5). Cells were seeded onto glass coverslips and cultured at 37°C in a humidified atmosphere of 5% CO2 in air.
Preparation and Purification of Sera. Sera from T1D patients and control subjects were collected, identically sterile-processed, and stored frozen at -20°C until used. The sera were heat-inactivated by incubation at 56°C for 30 min. Thereafter, β cells were incubated overnight in RPMI culture medium 1640 with 10% of the sera, and changes in [Ca2+]i were recorded, subsequent to depolarization with 25 mM KCl. The five T1D sera that induced an enhanced [Ca2+]i response were centrifuged, and the supernatant was passed through a 0.45-mm sterile filter. Samples were loaded on Sep-Pak C18 (Waters) preconditioned with 0.1% triflouroacetic acid (TFA). After a wash with 0.1% TFA, proteins were eluted with 60% acetonitrile in 0.1% TFA and were thereafter lyophilized. Batches of 1 mg of the lyophilized sample were dissolved in 500 μl of 0.1% TFA, centrifuged, and injected into an RP-HPLC with a Vydac C18 (0.46 × 25 cm) column (Grace Vydac, Hesperia, Ca). The separation was made by using a linear gradient of 20–60% acetonitrile in 0.1% TFA for 40 min at 1 ml/min. Fractions of 1 ml were collected and lyophilized.
Purification of Isoforms of ApoCIII. ApoCIII was purified from human serum by adsorption to a lipid emulsion and delipidation, followed by chromatography of the lipid-associated proteins under denaturing conditions in guanidinium chloride and urea, respectively, as described (6). The apoCIII isoforms were dialyzed against ammonium bicarbonate and were lyophilized before use.
Measurements of [Ca2+]i. Cells attached to coverslips were pretreated with the different compounds as described in Results and Discussion and were thereafter incubated in basal medium with 2 μM fura-2AM (Molecular Probes) for 30 min. The coverslips were mounted as the bottom of an open chamber and cells were perifused with medium. Fluorescence signals were recorded with a SPEX Fluorolog-2 system connected to an inverted Zeiss Axiovert epifluorescence microscope. The excitation and emission wavelengths were 340/380 and 510 nm, respectively. The results are presented as 340/380 excitation ratios, directly representative of [Ca2+]i (7).
Patch Clamp. Whole-cell Ca2+ currents were recorded by using the perforated-patch variant of the whole-cell patch-clamp recording technique to eliminate the loss of soluble cytoplasmic components. Electrodes were filled with: 76 mM Cs2SO4, 1 mM MgCl2, 10 mM KCl, 10 mM NaCl, and 5 mM Hepes (pH 7.35), as well as amphotericin B (0.24 mg/ml) to permeabilize the cell membrane and allow low-resistance electrical access without breaking the patch. Pancreatic β cells were incubated in RPMI medium 1640 with apoCIII (10 μg/ml) or vehicle overnight. The cells were bathed in a solution containing: 138 mM NaCl, 10 mM tetraethylammonium chloride, 10 mM CaCl2, 5.6 mM KCl, 1.2 mM MgCl2, 5 mM Hepes, and 3 d-glucose (pH 7.4). Whole-cell currents induced by voltage pulses (from a holding potential of -70 mV to several clamping potentials from -60 to 50 mV in 10-mV increments, 100 ms, 0.5 Hz) were filtered at 1 kHz and recorded. All recordings were made with an Axopatch 200 amplifier (Axon Instruments, Foster City, CA) at room temperature (≈22°C). Acquisition and analysis of data were performed by using the software program pclamp6 (Axon Instruments).
Protein Characterization. Primary sequence was obtained in ABI 494C and cLC sequencers. Protein molecular weights were determined by electrospray MS (AutoSpec hybrid tandem mass spectrometer, Micromass, Manchester, U.K). For recording of positive-ion conventional-ES spectra, samples (16 pmol/ml) were introduced into the ES interface by infusion or loop injection at a flow rate of 3 ml/min. To determine the position of the glycosylation, the native protein was digested with trypsin 1:10 wt/wt (Promega). The resulting fragments were separated by HPLC using a Vydac C8 (2.1 × 150 mm) and a gradient of 0–50% B in 50 min (buffer A, 5% acetonitrile/0.1% TFA; buffer B, 80% acetonitrile/0.1% TFA). The fragments separated were applied to mass analysis.
Quantification of ApoCIII. Sera were collected and prepared as described above. The relative amounts of apoCIII in T1D serum and control serum, respectively, were evaluated by comparisons of the peak area corresponding to apoCIII in the second RP-HPLC.
Flow Cytometric Analysis of Cell Death. RINm5F cells were cultured for 36 h in the presence of 10% control serum, control serum and 40 μg/ml apoCIII, or T1D serum with or without 100 or 200 μg/ml anti-apoCIII. The whole-cell population was collected and stained with enhanced GFP-conjugated annexin V and propidium iodide (BD Pharmingen) and analyzed on a FACscan by using cellquest acquisition software (Becton Dickinson Immunocytometry Systems, San Jose, CA). Fluorescence-activated cell sorter gating, based on forward and side scatter, was used to exclude cellular debris and autofluorescence, and typically, 10,000 cells were selected for analysis.
Statistical Analysis. Statistical significance was evaluated by Student's t test and P values <0.05 were considered significant. Data are expressed as means ± SEM.
Results and Discussion
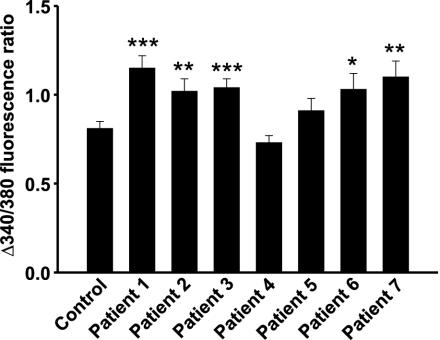
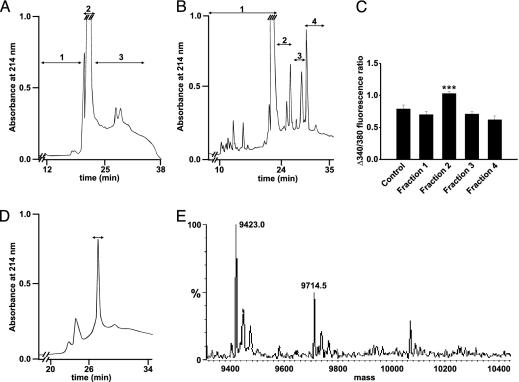
We have previously shown that T1D serum can activate voltage-gated L-type Ca2+ channels in the pancreatic β cell, resulting in increased [Ca2+]i, and thereby β cell apoptosis (2). To identify the factor in T1D serum responsible for these effects, we have now screened sera from seven newly diagnosed T1D patients (Table 1), by using increases in [Ca2+]i as a readout. Mouse pancreatic β cells were cultured overnight with 10% sera from the actual patients or normal subjects. Sera from five of the patients induced a significantly higher increase in [Ca2+]i, when cells were depolarized with 25 mM KCl, leading to an opening of voltage-gated L-type Ca2+ channels, than sera from healthy blood donors (Fig. 1). Positive sera were pooled, concentrated, and fractionated by RP-HPLC. When fractions were tested on isolated mouse pancreatic β cells, one fraction (fraction 3, Fig. 2A) eluting between 52–60% acetonitrile, induced a more pronounced increase in [Ca2+]i when cells were depolarized with high concentrations of K+. After further purification of the component(s) in this fraction by repeated RP-HPLC runs (Fig. 2 B and D), all fractions obtained were tested for effects on [Ca2+]i by incubation with mouse β cells overnight. The results from this second purification (Fig. 2B) showed a higher activity in fraction 2 (Fig. 2C). The protein that induced an increase in [Ca2+]i, indicated by the bar in Fig. 2D, was determined. Sequence information was obtained both by C- and N-terminal degradations. The sequences were identical to those of human apoCIII for 20 N-terminal and five C-terminal amino acid residues.
Table 1. Characterization of the T1D patients.
| Patient | Sex | Age, years | Duration of T1D, weeks | Medication* | ICA | GAD | IA-2 |
|---|---|---|---|---|---|---|---|
| 1 | M | 32 | <1 | No | Pos | Pos | Pos |
| 2 | F | 32 | 12 | No | Neg | Pos | Neg |
| 3 | F | 31 | <1 | No | Pos | Pos | Pos |
| 4 | F | 23 | <1 | No | Pos | Neg | ND |
| 5 | M | 19 | <1 | No | Neg | Neg | Pos |
| 6 | F | 35 | <1 | No | Pos | Pos | Pos |
| 7 | F | 33 | 28 | No | Pos | Pos | ND |
F, female; M, male. The presence (Pos), absence (Neg), or no data available (ND) of antibodies to islet cells (ICA), glutamic acid decarboxylase (GAD), and tyrosine phosphatase IA2 (IA-2) are marked. Healthy blood donors, all negative for ICA, GAD, and IA-2, served as sources of control sera.
Insulin was the only medication administered
Fig. 1.
Changes in [Ca2+]i in pancreatic β cells from mice exposed to T1D or control sera. Five of seven T1D sera induced an enhanced increase in [Ca2+]i, when the cells were depolarized with high concentrations of K+ to open the voltage-gated Ca2+ channel, compared with cells that had been exposed to normal serum (n = 29, 28, 32, 47, 21, 27, 31, and 18, respectively), ***, P < 0.001; **, P < 0.01; *, P < 0.05 versus control.
Fig. 2.
Stepwise separation and identification of the active fraction in T1D serum.(A) After the first RP-HPLC separation, the fraction marked 3 was found to give a higher increase in [Ca2+]i. (B) Fraction 3 (A) was rerun on RP-HPLC under identical conditions. The fractions were again tested for [Ca2+]i-stimulating activity (C), and one positive fraction (fraction 2) was identified. (C) Pancreatic β cells incubated with four fractions from RP-HPLC of diabetic sera from B (n = 6, 11, 12, 11, and 10, respectively). ***, P < 0.001 versus control. (D) The active fraction (B) was rechromatographed. The fraction, inducing a higher increase in [Ca2+]i when β cells were depolarized with high concentrations of K+, is marked with a bar. (E) The active fraction from C was analyzed by electrospray MS.
ApoCIII plays a key role in the regulation of the metabolism of triglyceride-rich lipoprotein (8). It controls the catabolism of triglyceride-rich lipoprotein by inhibiting the activity of lipoproteinlipase (9, 10), thereby inducing hypertriglyceridemia. ApoCIII also inhibits the binding of remnant lipoproteins to catabolic receptors like the low-density lipoprotein receptor-related protein (11). When the apoCIII gene was disrupted in mice, there was a 70% reduction in triglyceride levels (12). Overexpression of human apoCIII in transgenic mice results in hypertriglyceridemia (13). ApoCIII is a 79-residue, 8.8-kDa polypeptide (14) with three known isoforms that differ in terms of glycosylation, CIII0 (no sialic acid), CIII1 (one sialic acid residue), and CIII2 (two sialic acid residues), contributing ≈10%, 55%, and 35%, respectively, of total plasma apoCIII (15). Mutagenesis of the glycosylation site and expression in stable cell lines suggest that intracellular glycosylation is not required for the transport and secretion functions (16). Lack of glycosylation does not affect the affinity of apoCIII for very low- and high-density lipoprotein (16). Insulin is involved in the regulation of the apoCIII gene and induces a dose-dependent down-regulation at the transcriptional level. Overexpression of the apoCIII gene could contribute to the hypertriglyceridemia seen in T1D patients (17). Although surprising at first glance, mice transgenic for the human apoCIII gene, are neither insulin-resistant nor hyperinsulinemic (18). However, it is important to keep in mind that in T1D, we are dealing with a complex interplay between genetic predisposition, immunological changes, and environmental factors that together promote the destruction of the β cells.
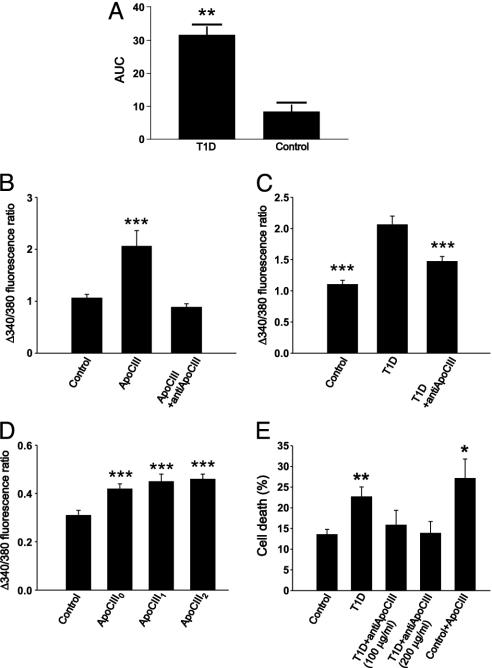
The concentration of apoCIII has previously been found to be higher in diabetic patients than in normal subjects (19–27). In insulin-deficient rats, there was no significant change in apoCIII in one study (28), whereas others have reported an increase in the proportions of the sialylated apoCIII (29, 30). We analyzed the apoCIII purified from T1D sera by MS for subcomponent identification. The major components had apparent masses of 9.423 and 9.714 kDa (Fig. 2E), corresponding to the mono- and diglycosylated forms of apoCIII (theoretical, calculated values are: CIII0 8.764 kDa, CIII1 9.420 kDa, and CIII2 9.712 kDa). To determine the positions of glycosylation, the protein was digested with trypsin and the fragments were separated by RP-HPLC. When the separated fragments were analyzed by MS, seven of the eight fragments showed masses identical to the theoretical values. The mass difference was localized to the C-terminal fragment, previously shown to be glycosylated (31). The absence of a nonglycosylated C-terminal fragment indicated that the isolated apoCIII forms were glycosylated. The relative amounts of apoCIII in T1D and control sera were evaluated by comparisons of the peak area corresponding to apoCIII in the second RP-HPLC (Fig. 3A). In T1D sera, the levels of the sialylated isoforms of apoCIII (apoCIII1 and apoCIII2) were 4-fold higher than in nondiabetic sera. The nonsialylated isoform (apoCIII0) could not be detected.
Fig. 3.
Amounts of apoCIII in T1D serum and effects on [Ca2+]i and cell death. (A) Relative levels of apoCIII1 + 2 in T1D and control serum, given as area under the curve (AUC). **, P < 0.01 (n = 5). (B) Pancreatic β cells were incubated with apoCIII or apoCIII plus antibodies against human apoCIII (n = 63, 35, and 33). ***, P < 0.001 versus control. (C) β cells were incubated with a control or a T1D serum and T1D serum plus anti-apoCIII (n = 18, 17, and 20). ***, P < 0.001 versus T1D serum. (D) Mouse β cells were incubated with apoCIII0, apoCIII1, apoCIII2, or vehicle (control; n = 54, 40, 48, and 37, respectively). ***, P < 0.001 versus control. (E) The insulin-secreting cell line RINm5F was exposed to control and T1D sera and T1D serum with the addition of two concentrations of anti-apoCIII, and finally control serum with apoCIII (n = 5). *, P < 0.05 and **, P < 0.01, versus control.
The concentration of apoCIII has been reported to be between 60–140 μg/ml in control subjects and 90–270 μg/ml in diabetics (9, 19, 20, 24–27). These variations may to a certain extent reflect the fact that various methods have been used for the determinations. In our experiments, we have used 10% T1D serum in the culture medium instead of 10% FCS normally used. Based on the levels found in diabetic patients, we have therefore chosen 10–50 μg/ml of apoCIII, a concentration range affecting intracellular Ca2+ handling. We have also tested three lower concentrations (1, 3, and 6 μg/ml), but these concentrations did not affect [Ca2+]i (data not shown).
Commercially available apoCIII (Sigma), which constitutes a mixture of apoCIII1 and apoCIII2, was tested at a concentration of 10 μg/ml and was shown to stimulate Ca2+ influx similar to the product isolated from T1D sera (Fig. 3B). Coincubation of β cells with 100 μg/ml of a polyclonal antibody against human apoCIII (Academy BioMedical, Houston) blocked the activity of both the commercial apoCIII and the T1D serum (Fig. 3 B and C). The polyclonal antibody had no activity by itself (data not shown). When testing the three isoforms of apoCIII by incubation of β cells at a concentration of 10 μg/ml, both the glycosylated (CIII1 and CIII2) and the nonglycosylated isoform caused a significantly higher increase in [Ca2+]i than cells that had been incubated with only the vehicle, 0.1% TFA (Fig. 3D). To study the effect of possible binding of apoCIII to serum lipoproteins in the culture medium, cells were incubated in basal buffer containing no serum and 10 μg/ml apoCIII1 for 2 and 6 h. There was a significantly elevated increase in [Ca2+]i upon depolarization in all of the experiments where the cells had been exposed to apoCIII1 for 6 h, but only in one of three experiments where the incubation time was only 2 h (data not shown).
There was a higher percentage of dead cells in the cell population exposed to T1D serum. This effect was prevented by the addition of anti-apoCIII (Fig. 3E). Furthermore, the addition of pure apoCIII to culture medium with control serum resulted in an increased cell death (Fig. 3E).
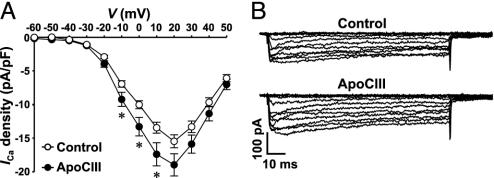
To elucidate the molecular mechanism underlying the stimulatory effect of apoCIII on [Ca2+]i, the activity of voltage-gated Ca2+ channels was analyzed in β cells incubated with 10 μg/ml apoCIII. ApoCIII-treated cells displayed larger Ca2+ channel currents than control cells during depolarizations in the range -10 to 10 mV, from a holding potential of -70 mV (Fig. 4 A and B). These data demonstrate that apoCIII modulated the activity of the voltage-gated L-type Ca2+ channel and that the effect occurred in the range of physiological depolarizations. So far, immunoblot experiments have not revealed a direct interaction of apoCIII with the Ca2+ channel (data not shown). Future experiments may clarify to what extent this may reflect limitations set by the immunoprecipitation protocol or the actual situation.
Fig. 4.
Interaction of apoCIII with the voltage-gated L-type Ca2+ channel. (A) Summary graph of current density–voltage relationships. ApoCIII-treated cells (filled circles, n = 56) and control cells (open circles, n = 55) were depolarized to potentials between -60 and 50 mV, in 10-mV increments, from a holding potential of -70 mV. *, P < 0.05. (B) Sample whole-cell Ca2+ current traces from a control cell (cell capacitance: 4.3 pF) and a cell incubated with apoCIII (cell capacitance: 4.2 pF). Cells were depolarized by a set of voltage pulses (100 ms, 0.5 Hz) between -60 and 50 mV, in 10-mV increments, from a holding potential of -70 mV.
In our previous study (2), we tested T1D sera on GH3 cells, a pituitary cell line, and obtained the same effect as in primary β cells and RINm5F cells. This finding suggests that the observed effects induced by apoCIII may not be exclusive for the β cells, but rather associated with the presence of voltage-gated L-type Ca2+ channels. The sensitivity of the pancreatic β cell to the cytotoxic effect of apoCIII and resulting increase in [Ca2+]i is likely to reflect an inherent overall low tolerance to stress (32).
Our study shows that the sialylated forms of apoCIII were on average 4-fold higher in sera from newly diagnosed T1D patients than in sera from healthy subjects. ApoCIII induced both an increase in [Ca2+]i and β cell death. The molecular mechanism underlying the stimulatory effect of apoCIII on [Ca2+]i reflected an activation of the voltage-gated L-type Ca2+ channel. Addition of an antibody against apoCIII blocked the effects of both T1D serum and apoCIII on [Ca2+]i as well as on β cell death. This finding suggests that the Ca2+-dependent cytotoxic effect of T1D serum on the pancreatic β cell is mediated by apoCIII.
Acknowledgments
This work was supported by grants from Barndiabetesfonden, the Swedish Diabetes Association, the Karolinska Institutet, the Swedish Research Council, the Swedish Society for Medical Research, the Novo Nordisk Foundation, the Family Persson Foundation, Berth von Kantzow's Foundation, and Juvenile Diabetes Research Foundation International, and by National Institutes of Health Grant DK58508.
Abbreviations: ApoCIII, apolipoprotein CIII; T1D, type 1 diabetes; TFA, triflouroacetic acid; [Ca2+]i, intracellular Ca2+ concentration.
References
- 1.Efendic, S., Kindmark, H. & Berggren, P. O. (1991) J. Intern. Med. Suppl. 735, 9-22. [PubMed] [Google Scholar]
- 2.Juntti-Berggren, L., Larsson, O., Rorsman, P., Ammala, C., Bokvist, K., Wahlander, K., Nicotera, P., Dypbukt, J., Orrenius, S., Hallberg, A. & Berggren, P. O. (1993) Science 261, 86-90. [DOI] [PubMed] [Google Scholar]
- 3.Hellman, B. (1965) Ann. N.Y. Acad. Sci. 131, 541-558. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson, T., Arkhammar, P., Hallberg, A., Hellman, B. & Berggren, P. O. (1987) Biochem. J. 248, 329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lernmark, A. (1974) Diabetologia 10, 431-438. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson-Olivecrona, G. & Olivecrona, T. (1991) Methods Enzymol. 197, 345-356. [DOI] [PubMed] [Google Scholar]
- 7.Kindmark, H., Kohler, M., Efendic, S., Rorsman, P., Larsson, O. & Berggren, P. O. (1992) FEBS Lett. 303, 85-90. [DOI] [PubMed] [Google Scholar]
- 8.Fredenrich, A. (1998) Diabetes Metab. 24, 490-495. [PubMed] [Google Scholar]
- 9.Krauss, R. M., Herbert, P. N., Levy, R. I. & Fredrickson, D. S. (1973) Circ. Res. 33, 403-411. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg, H. N., Le, N. A., Goldberg, I. J., Gibson, J. C., Rubinstein, A., Wang-Iverson, P., Norum, R. & Brown, W. V. (1986) J. Clin. Invest. 78, 1287-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowal, R. C., Herz, J., Weisgraber, K. H., Mahley, R. W., Brown, M. S. & Goldstein, J. L. (1990) J. Biol. Chem. 265, 10771-10779. [PubMed] [Google Scholar]
- 12.Maeda, N., Li, H., Lee, D., Oliver, P., Quarfordt, S. H. & Osada, J. (1994) J. Biol. Chem. 269, 23610-23616. [PubMed] [Google Scholar]
- 13.Ito, Y., Azrolan, N., O'Connell, A., Walsh, A. & Breslow, J. L. (1990) Science 249, 790-793. [DOI] [PubMed] [Google Scholar]
- 14.Brewer, H. B., Jr., Shulman, R., Herbert, P., Ronan, R. & Wehrly, K. (1974) J. Biol. Chem. 249, 4975-4984. [PubMed] [Google Scholar]
- 15.Kashyap, M. L., Srivastava, L. S., Hynd, B. A., Gartside, P. S. & Perisutti, G. (1981) J. Lipid Res. 22, 800-810. [PubMed] [Google Scholar]
- 16.Roghani, A. & Zannis, V. I. (1988) J. Biol. Chem. 263, 17925-17932. [PubMed] [Google Scholar]
- 17.Chen, M., Breslow, J. L., Li, W. & Leff, T. (1994) J. Lipid Res. 35, 1918-1924. [PubMed] [Google Scholar]
- 18.Reaven, G. M., Mondon, C. E., Chen, Y. D. & Breslow, J. L. (1994) J. Lipid Res. 35, 820-824. [PubMed] [Google Scholar]
- 19.Briones, E. R., Mao, S. J., Palumbo, P. J., O'Fallon, W. M., Chenoweth, W. & Kottke, B. A. (1984) Metabolism 33, 42-49. [DOI] [PubMed] [Google Scholar]
- 20.Joven, J., Vilella, E., Costa, B., Turner, P. R., Richart, C. & Masana, L. (1989) Clin. Chem. (Washington, D.C.) 35, 813-816. [PubMed] [Google Scholar]
- 21.Stewart, M. W., Laker, M. F. & Alberti, K. G. (1994) J. Intern. Med. Suppl. 736, 41-46. [PubMed] [Google Scholar]
- 22.Bren, N. D., Rastogi, A. & Kottke, B. A. (1993) Mayo Clin. Proc. 68, 657-664. [DOI] [PubMed] [Google Scholar]
- 23.Nestel, P. J. & Fidge, N. H. (1982) Adv. Lipid Res. 19, 55-83. [DOI] [PubMed] [Google Scholar]
- 24.Blackett, P., Sarale, D. C., Fesmire, J., Harmon, J., Weech, P. & Alaupovic, P. (1988) South Med. J. 81, 469-473. [DOI] [PubMed] [Google Scholar]
- 25.al Muhtaseb, N., al Yousuf, A. & Bajaj, J. S. (1992) Pediatrics 89, 936-941. [PubMed] [Google Scholar]
- 26.Manzato, E., Zambon, A., Lapolla, A., Zambon, S., Braghetto, L., Crepaldi, G. & Fedele, D. (1993) Diabetes Care 16, 469-475. [DOI] [PubMed] [Google Scholar]
- 27.Reverter, J. L., Senti, M., Rubies-Prat, J., Lucas, A., Salinas, I., Pizarro, E., Pedro-Botet, J. & Sanmarti, A. (1993) Clin. Chim. Acta 223, 113-120. [DOI] [PubMed] [Google Scholar]
- 28.O'Looney, P., Irwin, D., Briscoe, P. & Vahouny, G. V. (1985) J. Biol. Chem. 260, 428-432. [PubMed] [Google Scholar]
- 29.Callow, M. J. & Redgrave, T. G. (1993) Biochim. Biophys. Acta 1168, 271-279. [DOI] [PubMed] [Google Scholar]
- 30.Bar-On, H., Roheim, P. S. & Eder, H. A. (1976) J. Clin. Invest. 57, 714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito, Y., Breslow, J. L. & Chait, B. T. (1989) J. Lipid Res. 30, 1781-1787. [PubMed] [Google Scholar]
- 32.Lenzen, S., Drinkgern, J. & Tiedge, M. (1996) Free Radical Biol. Med. 20, 463-466. [DOI] [PubMed] [Google Scholar]