Abstract
In this fMRI study, we investigated the development between adolescence and adulthood of the neural processing of social emotions. Unlike basic emotions (such as disgust and fear), social emotions (such as guilt and embarrassment) require the representation of another’s mental states. Nineteen adolescents (10–18 years) and 10 adults (22–32 years) were scanned while thinking about scenarios featuring either social or basic emotions. In both age groups, the anterior rostral medial prefrontal cortex (MPFC) was activated during social versus basic emotion. However, adolescents activated a lateral part of the MPFC for social versus basic emotions, whereas adults did not. Relative to adolescents, adults showed higher activity in the left temporal pole for social versus basic emotions. These results show that, although the MPFC is activated during social emotion in both adults and adolescents, adolescents recruit anterior (MPFC) regions more than do adults, and adults recruit posterior (temporal) regions more than do adolescents.
INTRODUCTION
Adolescence is a period of social and psychological development during which social awareness and behavior undergo profound change (Brown, 2004; Eisenberg & Morris, 2004). As well as alterations in hormone levels and social environment, another possible cause of these changes in social behavior could be the anatomical development in brain areas involved in social cognition, including the medial prefrontal cortex (MPFC), the superior temporal cortex, and the temporo-parietal junction (TPJ) (Shaw et al., 2008; Gogtay et al., 2004; Giedd et al., 1999; Sowell et al., 1999). In addition, a number of recent functional neuroimaging studies have shown that activity within brain regions associated with social cognition changes between adolescence and adulthood (Blakemore, den Ouden, Choudhury, & Frith, 2007; Moriguchi, Ohnishi, Mori, Matsuda, & Komaki, 2007; Pfeifer, Lieberman, & Dapretto, 2007; Wang, Lee, Sigman, & Dapretto, 2006; see Blakemore, 2008 for a meta-analysis). These previous developmental functional neuroimaging studies have focused on neural activity associated with the attribution of mental states such as intentions and beliefs (“mentalizing”). The current fMRI study was designed to investigate whether a similar developmental change in activation pattern occurs for mentalizing in an emotional context. To this end, we investigated changes in brain activity between adolescence and adulthood during social emotion processing. Social emotions are defined here as emotions that require the representation of mental states. Examples are embarrassment, guilt, shame, and pride. In order to feel embarrassment, for example, you must represent someone else’s belief that you have acted foolishly. In contrast, basic emotions such as disgust and fear only require the awareness of one’s own somatic state.
It has consistently been observed in adults that mentalizing tasks, which require participants to attribute beliefs, intentions, or desires, activate a set of brain regions including the anterior rostral MPFC, the posterior superior temporal sulcus (pSTS)/TPJ, and the temporal poles (Frith, 2007; Frith & Frith, 2003; Saxe & Kanwisher, 2003). When adults reflect upon social emotions such as guilt and embarrassment, components of the “mentalizing network,” including the anterior rostral MPFC, are active (Moll, Zahn, de Oliveira-Souza, Krueger, & Grafman, 2005; Takahashi et al., 2004; Berthoz, Armony, Blair, & Dolan, 2002; Moll et al., 2002; Shin et al., 2000). Olsson and Ochsner (2008) recently discussed the overlap between regions of the brain involved in social cognition and in emotion processing, and they suggest that mental state attribution plays a role in learning about and understanding emotions.
Recent developmental fMRI studies of social cognition have consistently found differential activity within the mentalizing network in adolescents and adults (see Blakemore, 2008). Thinking about how one’s own intentions would lead to specific actions was found to recruit the anterior rostral MPFC more strongly in adolescents (aged 12–18 years) than in adults (aged 22–38 years) (Blakemore et al., 2007). In contrast, adults activated posterior regions (right STS) more than did adolescents when thinking about intentions. A similar developmental shift in brain activity was found with a task based on decoding communicative intentions (Wang et al., 2006). When adolescents (aged 9 to 14 years) and adults (aged 23–33 years) judged whether a series of ironic communications were sincere or not, adolescents showed stronger activation of the anterior rostral MPFC than did adults. Adults activated posterior regions including the superior temporal and fusiform gyri more. In another developmental study that focused on the processing of self-related sentences, children (aged 9.5–10.8 years) and adults (aged 23–31.7 years) read phrases about academic skills and social competence (Pfeifer et al., 2007). In the self condition, participants were asked to indicate whether the phrases accurately described them. In the other condition, they were asked to indicate whether the phrases accurately described a fictional, familiar other person (Harry Potter). The MPFC and the ACC were more active in children than in adults during self-knowledge retrieval compared with other-knowledge retrieval. The authors suggested that, compared with adults, adolescents might rely more on “on-line” self-reflective processing performed by the MPFC. Thus, these studies have consistently shown that activity in the MPFC during mental state understanding decreases between adolescence and adulthood, whereas activity in the temporal lobes shows the opposite developmental pattern. The current study was designed to investigate whether the same developmental shift in activation pattern occurs for social emotion processing.
Displays of social emotions such as guilt and embarrassment are seen within the first 3 years of life (see Eisenberg, 2000 for a review). The ability to describe situations in which a social emotion will be experienced emerges at around age 7 (Harris, Olthof, Terwogt, & Hardman, 1987) and, by adolescence, the experience of social emotion permeates everyday social exchange (Zeman, Cassano, Perry-Parrish, & Stegall, 2006; Elkind & Bower, 1979). However, at around puberty, children become increasingly aware of and concerned with people’s opinions of them (Parker et al., 2006; Adams & Berzonsky, 2003; Elkind, 1967), and the self-concept depends more and more upon perceived social reputation (Davey, Yücel, & Allen, 2008). This increase in self-consciousness after puberty, as well as the increased concern with others’ (especially peers’) opinions, might result in an increase in the frequency and intensity of the experience of social emotions (Zeman et al., 2006). At the same time, socialization experiences with parents and peers mean that adolescence is a key time for learning about how these emotions should be expressed in different social contexts (Zeman & Shipman, 1997).
It is unknown whether the neural correlates of social emotion processing develop between adolescence and adulthood. To investigate this question, we scanned 19 adolescents (aged 10–18 years) and 10 adults (aged 23–32 years) as they read a series of sentences that were designed to elicit either a social emotion (guilt or embarrassment) or a basic emotion (disgust or fear). We predicted that thinking about social versus basic emotion scenarios would activate components of the social brain network, including the anterior rostral MPFC, in both age groups (Moll, de Oliveira-Souza, et al., 2005; Moll, Zahn, et al., 2005; Takahashi et al., 2004; Moll et al., 2002). We further predicted that adolescents would activate the MPFC more for social compared with basic emotion than adults would, as has been found in previous developmental studies of mentalizing (Blakemore et al., 2007; Wang et al., 2006).
The scenarios presented to participants in the scanner pertained either to the self or to another person (the participant’s mother). We included this additional factor for two reasons. First, in adults, there is a difference in neural activity when thinking about emotion in the first-versus third-person perspective (Ruby & Decety, 2004). Second, a recent developmental fMRI study (Pfeifer et al., 2007) has shown that the neural correlates of self/other semantic knowledge retrieval (deciding whether statements such as “I like reading,” apply to the self, or to Harry Potter) differentially activate components of the mentalizing network in adults compared to children. Specifically, self versus other retrieval was associated with greater activity in the MPFC in adolescents, and greater activity in the lateral temporal cortex in adults. Because we were specifically interested in these brain regions, we decided to use self/other versions of each emotional scenario to investigate whether a similar developmental pattern would be seen for self/other processing of emotional scenarios. Our choice of participants’ mother as the protagonist in the “other” condition was motivated by a need to select an “other” who would be distinct from the self, but sufficiently familiar to participants that they would be able to adopt her emotional perspective (cf. Ruby & Decety, 2004). To investigate how similar each participant perceived herself to be to her mother, participants completed two versions (self and mother) of the NEO-V Factor Personality Inventory. The absence of group differences in this measure indicates that any group differences in brain activity between the self and other condition was not due to group differences in the perceived similarity of participants’ mothers to themselves.
We included only female participants in the study for the following reasons. First, sex differences have been observed in various measures of brain anatomy, including in brain regions involved in emotion and social cognition (Schmithorst, Holland, & Dardzinski, 2008; Lenroot et al., 2007). Specifically, there are significant differences between the sexes in terms of the age at which gray matter volume peaks in the frontal and parietal cortices (11 years in girls vs. 13 years in boys according to Giedd et al., 1999). Second, sex differences have been observed in fMRI studies of emotion in adolescents and adults (Yurgelun-Todd & Killgore, 2006; Hall, Witelson, Szechtman, & Nahmias, 2004; Killgore & Yurgelun-Todd, 2004) and in studies of social emotion processing in adults (Moll, Zahn, et al., 2005; Moll, de Oliveira-Souza, et al., 2002). Our choice of females was related to the established sex differences in expressing emotion: Females are more expressive of emotions than males (Kring & Gordon, 2007). This difference in emotion expression between the sexes becomes accentuated with age during childhood, as sex-specific, culturally defined rules for the expression of emotion are learned (see Brody, 1985, for a review). Therefore, the relationship between feeling and expressing emotions differs between the sexes and changes with age in a sex-specific way.
METHODS
Participants
Nineteen female adolescents (10.83–18.17 years; mean = 14.8 years) and 10 female adults (22.92–31.83 years; mean = 26.41 years), with no history of psychiatric or neurological disorder, took part in the study. Participants were all female, in consideration of the significant sex differences in the neuroanatomical changes that take place during adolescence (Giedd et al., 1999), which may impact on neural processing. Written informed consent was obtained prior to the study from all adult participants, and from a parent or guardian of participants younger than 18 years. The study was approved by the UCL National Hospital for Neurology and Neurosurgery Ethics Committee.
To ensure a consistent level of intelligence between groups, the Wechsler Abbreviated Scale of Intelligence (WASI, 1999) was administered to participants. Mean (±SD) full-scale IQ (FSIQ) was 115.52 (±6.63) for the adolescent group and 111.14 (±14.10) for the adult group. An independent-samples t test revealed that there was no significant difference in FSIQ between groups [t(22) = −1.052, p > .3]. Three adult participants did not complete the WASI, but because they had completed university-level education, their level of intelligence was judged to be comparable to that of the other participants.
Experimental Design
The fMRI experiment was split into two 12-min sessions. Within each session, each participant underwent 277 scans. We employed a 2 × 2 × 2 mixed factorial design, comprising within-subjects factors emotion (social vs. basic) and protagonist (self vs. other), and between-subjects factor group (adolescent vs. adult). Participants read 144 emotional sentences describing social or basic emotion scenarios pertaining either to the self or to their mother (see Table 1 for examples of scenarios). After reading each scenario, participants rated to what extent the protagonist would feel a given emotion, on a discrete rating scale from 1 (not at all) to 4 (very much), using a button box.
Table 1.
Examples of Social (Embarrassment, Guilt) and Basic (Disgust, Fear) Emotion Scenarios from the Self Condition
| Emotion | Sentence |
|---|---|
| Social: Embarrassment | “Your dad started doing rock n’ roll dances in the supermarket” |
| “You were quietly picking your nose but your friend saw you” | |
| “You tripped over in front of a boy you liked” | |
| “Your friend said you had a wet patch on your backside all the way home” | |
| “Your were eating with your friend and you dribbled down your top” | |
| Social: Guilt | “You laughed at a quiet girl you know and it made her sad” |
| “You laughed when your friend told you she was feeling upset” | |
| “You pretended to be sick so you don’t have to visit your gran” | |
| “You joined in when people were laughing at your best friend” | |
| “You lied to your dad when you wanted to go out with your friend” | |
| Basic: Disgust | “Your friend was vomiting next to you and you could smell it” |
| “You were in your friend’s garden and you put your hand in slimy cat poo” | |
| “You saw a big hairy fly laying eggs on your friend’s lunch” | |
| “Your dad told you that the fridge was infested with maggots” | |
| “You saw a pile of rotting guts near the dustbin at your friend’s house” | |
| Basic: Fear | “Your friend screamed that there was a wasp inside your jumper” |
| “An angry dog was barking and running towards you and your friend” | |
| “You suddenly woke up as someone screamed by your bed” | |
| “Your dad slammed on the brakes as a lorry hurtled towards you” | |
| “You were with your friend and a creature ran up your neck” |
Sentences from the Other condition described the same scenarios but featuring “Your Mom”.
Emotion Factor
Each scenario featured either a social emotion or a basic emotion. The social emotions were embarrassment and guilt, and the basic emotions were disgust and fear. The emotion sentences were chosen from a larger set that was presented in the form of a questionnaire to a number of adolescent and adult volunteers. These volunteers, who were different from the participants in the current study, rated their emotional response to the emotion sentences. Sentences that were rated highly by both age groups were chosen for use in this fMRI study. In addition, the sentences were designed to maximize the difference in mentalizing between social and basic conditions. Therefore, the basic emotion sentences featured immediate, visceral disgust- and fear-evoking situations.
Both social and basic scenarios featured the protagonist plus one other person. This ensured that the difference between the social and basic emotion conditions was the need to take into account another person’s mental state, not the mere presence of another person in the scenario.
Protagonist Factor
The protagonist in each scenario was either the participant (self) or the participant’s mother (other). The same emotional sentences were used for the self and other conditions. We ascertained that all participants had a living, healthy mother.
The mean (plus the range) word length, and the number of clauses, was equated between all emotion conditions and both protagonist conditions. Sentences were presented in blocks of three. Participants had 9 sec to read silently, imagine, and rate their response to each emotion sentence. The experiment was blocked by emotion and protagonist such that within a block, all three scenarios featured the same emotion (disgust, embarrassment, fear, or guilt) and the same protagonist (self or other). At the start of each block, a 1-sec cue screen informed participants which emotion and which protagonist the proceeding three sentences would feature.
Each 12-min session of the fMRI experiment contained 24 emotion blocks, each lasting 28 sec. The stimulus materials were blocked by emotion and protagonist to maximize the strength of the imagined emotions, while minimizing carryover effects between sentences featuring different emotions/protagonists. Condition order was fully randomized. In addition, there were two 28-sec visual fixation blocks per session, occurring one third and two thirds of the way through each of the two sessions. Stimulus presentation was programmed in Cogent (www.vislab.ucl.ac.uk/Cogent/index.html) running in Matlab 6.5, which recorded participant responses.
Prior to scanning, all participants completed a practice session consisting of four scenarios from each of the emotions. The sentences used in the practice task did not appear inside the scanner.
Participants’ Perceived Similarity to Mother
To quantify any age-related differences in participants’ perceived similarity to their mothers, which might cause between-group differences in brain activity in the protagonist condition, participants’ perceived similarity to their mothers was quantified by administering two separate versions of the NEO-V personality questionnaire (Costa & McCrae, 1991), after the scanning session. The two questionnaires were identical except that in one version participants answered personality questions about themselves, and in the other version they answered the same questions about their mother.
Data Acquisition
A 1.5-T Siemens Sonata head MRI scanner was used to acquire both 3-D T1-weighted fast-field echo structural images and multislice T2*-weighted echo-planar volumes with blood oxygenation level dependent contrast. Each functional brain volume was composed of thirty-three 3-mm axial slices with a 1.5-mm gap and in-plane resolution of 3 * 3 mm, angled at 30° to cover the whole brain and minimize signal dropout from the facial sinuses. Repetition time was 3 sec. Functional data were acquired in two scanning sessions of approximately 12 min each, in which a total of 554 volumes were acquired, or 277 scans per session. The acquisition of a T1-weighted anatomical image occurred after the two functional scanning sessions for each participant. The total duration of scanning was approximately 35 min per participant.
Data Analysis
Behavioral and fMRI data were analyzed by collapsing the four emotions—embarrassment and guilt, disgust and fear—into two emotion conditions, social and basic. This was because our hypothesis related to differential neural effects of social versus basic emotion, not to the neural effects of specific emotions.
Behavioral data (emotion ratings) were analyzed with SPSS. Main effects of emotion and protagonist in both groups, as well as two- and three-way interactions between emotion, protagonist, and group, were analyzed using mixed-model repeated measures ANOVA with within-subjects factors emotion and protagonist and between-subjects factor group. We used a significance threshold of p < .05.
Imaging data were analyzed using SPM2 (www.fil.ion.ucl.ac.uk/spm). The first six functional image volumes from each run were discarded to allow for T1 equilibrium effects, leaving 542 image volumes per participant. Preprocessing included rigid-body transformation (realignment) and slice timing to correct for head movement and slice acquisition delays. The images were then stereotactically normalized into the standard space defined by the Montreal Neurological Institute (MNI) template using the mean of the functional volumes, and smoothed with a Gaussian filter of 6 mm full width at half maximum to increase the signal-to-noise ratio and to facilitate group analyses. The time series for each participant were high-pass filtered at 128 sec to remove low-frequency drifts.
The analysis of the functional imaging data entailed the creation of statistical parametric maps representing a statistical assessment of hypothesized condition-specific effects (Friston et al., 1994), which were estimated with the general linear model. The effects of interest were the four scenario block types (2 emotion * 2 protagonist) and the visual fixation blocks. We also modeled the six realignment parameters as effects of no interest, in order to account for any group differences in head movement. Each component of the model served as a regressor in a multiple regression analysis for each participant. The resulting parameter estimates for each regressor at each voxel were then entered into a second-level analysis, where “participant” served as a random effect in a within-subjects ANOVA, enabling population inferences to be made.
The main effects and interactions between conditions were specified by appropriately weighted linear contrasts, and determined using the t statistic on a voxelby-voxel basis. Statistical analysis at the second level was performed for each group separately to examine the main effect of all scenarios versus fixation to check that expected brain regions were activated in this contrast. In order to test our main hypotheses, we first investigated the three-way interaction between emotion, protagonist, and group. A significant result then allowed us to perform a series of planned t contrasts to investigate main effects of condition for the two groups separately and two-way interactions between group and condition. Specifically, for each group separately, we looked at the main effect of emotion (social > basic, basic > social) and protagonist (self > other, other > self). We also investigated interactions between group and emotion [adult (social > basic)–adolescent (social > basic), and vice versa] and group and protagonist [adult (self > other)–adolescent (self > other), and vice versa]. We also looked at the interaction between protagonist and emotion in each group separately [adult ([self > other]–[social > basic] and vice versa; adolescent ([self > other]–[social > basic]) and vice versa].
Statistical contrasts were used to create an SPM{t}, which was transformed into an SPM{Z} and thresholded at p < .05 (corrected on the basis of the theory of random Gaussian fields for multiple comparisons across the whole brain volume examined). We report regions that survive correction at p < .05, as well as activations within regions for which we had an a priori hypothesis and which survived small-volume correction (SVC; 12 mm radius sphere, unless otherwise specified) at p < .05. These regions were the MPFC (Blakemore et al., 2007; Gilbert et al., 2006, 8 mm), the pSTS/TPJ (Aichhorn, Perner, Kronbichler, Staffen, & Ladurner, 2006; Frith & Frith, 2003), the temporal pole (Blakemore et al., 2007; 8 mm), and the precuneus (Blakemore et al., 2007) for social versus basic emotion; the anterior insula (Moll et al., 2002) and the inferior frontal gyrus (Moll, de Oliveira-Souza, et al., 2005; Moll, Zahn, et al., 2005) for basic versus social emotion; the postcentral gyrus for self versus other (Ruby & Decety, 2004); and the medial fronto-polar gyrus, the left STS, the left temporal pole, the posterior cingulate gyrus, and the right inferior parietal lobule for other versus self (Ruby & Decety, 2004).
RESULTS
Behavioral Data
Emotion Ratings
Participants rated to what extent the protagonist of each scenario would feel a given emotion, on a discrete rating scale from 1 (not at all) to 4 (very much). Mixed-design, repeated measures 2 × 2 × 2 ANOVA showed that mean emotion ratings did not differ between groups [F(1, 26) = 0.60; p > .4; see Table 2]. There were no significant two- or three-way interactions between age group and the factors emotion and protagonist (all p > .2). For both groups, basic emotion scenarios were given higher ratings than social emotion scenarios [F(1, 26) = 9.44, p < .01], and other scenarios were given higher intensity ratings than self scenarios [F(1, 26) = 4.47, p < .05].
Table 2.
Mean and Standard Deviation of the Emotion Ratings (from 1 to 4), by Group (Adult, Adolescent), Emotion (Social, Basic), and Protagonist (Self, Other)
| Emotion | Protagonist | Group | Mean | Standard Deviation |
|---|---|---|---|---|
| Social | Self | Adult | 2.98 | 0.349 |
| Adolescent | 2.94 | 0.397 | ||
| Other | Adult | 3.34 | 0.343 | |
| Adolescent | 3.07 | 0.514 | ||
| Basic | Self | Adult | 3.16 | 0.283 |
| Adolescent | 3.11 | 0.417 | ||
| Other | Adult | 3.29 | 0.395 | |
| Adolescent | 3.24 | 0.427 |
Ratings for basic emotions were significantly higher than for social emotions, and ratings for other emotions were significantly higher than for self emotions. However, there were no significant group differences and no significant interactions.
Perceived Self–Mother Difference
Data were not available for one adult participant. For the remaining participants, perceived self–mother difference (PSMD) scores were computed by calculating the square root of the summed squared self–mother differences for all five dimensions of the NEO-V “self” and “mother” personality questionnaires. This yielded PSMD scores ranging from 5.57 to 37.03 (mean ± SD = 20.34 ± 9.14). A median test of the PSMD scores revealed no difference between adult and adolescent participant groups (χ2 = 0, p > .99). Linear regression revealed no relationship between age and PSMD score (r2 = .009, p > .6).
Functional Imaging Data
Data from both imaging runs of one adolescent participant and one run of a second adolescent participant were excluded due to excessive head movement (>5 mm). After this, there were no statistical differences in mean movement between the two age groups for any of the six dimensions of movement [independent-samples t tests (df = 26): all p > .15]. We report group-level activations in hypothesized regions that survived SVC at p < .05.
Main Effect of Sentences vs. Visual Fixation
In both adult and adolescent groups, the main effect of all scenarios versus visual fixation resulted in expected activation of visual and motor areas, as well as areas involved in reading (Figure 1).
Figure 1.
Main effect of scenarios versus visual fixation in adult and adolescent groups (adult image shown at p < .05, fully corrected across the whole brain, with minimum spatial extent = 10 voxels; adolescent image shown at p < .01, fully corrected across the whole brain, with minimum spatial extent = 10 voxels).
Three-way Interaction between Emotion, Protagonist, and Group
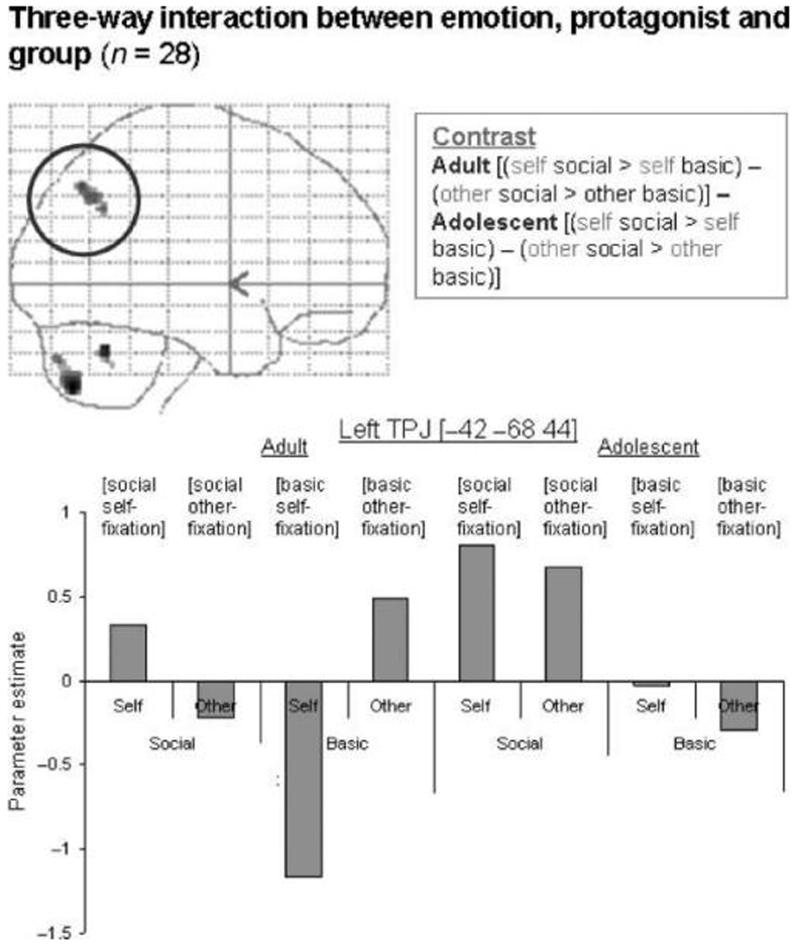
The three-way interaction between protagonist (self > other), emotion (social > basic), and group (adult > adolescent) yielded a significant region of activation in the left TPJ (MNI coordinates [−42 −68 44]; Z = 3.60, p < .05 SVC; Figure 2, top left. For illustration purposes, parameter estimates are shown relative to fixation baseline). Activity in this region was driven by a main effect of social > basic emotion in adolescents, and an interaction between emotion and protagonist in the adult group (Figure 2, bottom). The opposite contrast with respect to group (adolescent > adult, social > basic, self > other) did not yield any significant activations.
Figure 2.
Three-way interaction between emotion, protagonist, and group in the left TPJ, shown at p < .001 on a sagittal glass brain projection. Parameter estimates for each condition relative to fixation are shown for each group. This region of the left TPJ is most active in adult self-social emotions and other-basic emotions, and in adolescent self- and other-social emotions.
Main Effect of Emotion in Each Group
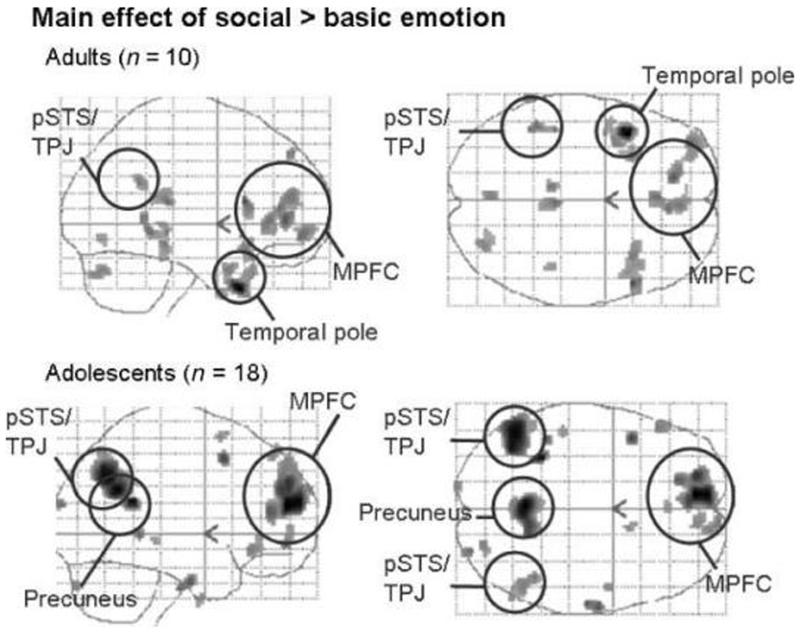
In the adult group, the main effect of social > basic emotion resulted in activation of the anterior rostral MPFC, the left pSTS/TPJ, and the left temporal pole (Table 3, Figure 3). In the adolescent group, the main effect of social emotion (social > basic) resulted in activation of the anterior rostral MPFC, the left and right pSTS/TPJ, and the precuneus (Table 3, Figure 3).
Table 3.
MNI Coordinates, Z-values, and Cluster Size (mm3) for Regions of Activation in the Three-way Interaction between Emotion, Protagonist, and Group, the Interaction between Emotion and Group, the Main Effect of Emotion in Each Group, and the Interaction between Protagonist and Group
| Contrast | Region of Activation | MNI Coordinates | Z | Size in mm3 at p < .001 |
|---|---|---|---|---|
| Interaction between emotion, protagonist, and group | Left TPJ (for contrast, see Figure 2) | −42 −68 44 | 3.60 | 264 |
| −52 −58 34 | 3.50 | 40 | ||
| Emotion: social > basic | Adult | |||
| Anterior rostral MPFC | −14 44 2 | 3.82 | 96 | |
| 4 48 18 | 3.56 | 216 | ||
| 6 42 12 | 3.28 | (part of above cluster) | ||
| Left pSTS/TPJ | −56 −62 28 | 3.48 | 32 | |
| Left temporal pole | −36 8 −30 | 3.43 | 24 | |
| Adolescent | ||||
| Anterior rostral MPFC | −10 52 18 | 4.13 | 1600 | |
| −6 62 22 | 4.03 | (part of above cluster) | ||
| −4 50 28 | 3.64 | (part of above cluster) | ||
| −18 42 16 | 3.65 | 128 | ||
| −4 52 −8 | 3.43 | 64 | ||
| −16 48 34 | 3.37 | 40 | ||
| Precuneus | −4 −56 28 | 4.64 | 1360 | |
| 14 −56 34 | 3.49 | (part of above cluster) | ||
| −4 −62 40 | 3.32 | 64 | ||
| Left TPJ | −38 −66 42 | 4.00 | 2032 | |
| −48 −62 36 | 3.98 | (part of above cluster) | ||
| −38 −62 32 | 3.62 | (part of above cluster) | ||
| Right pSTS/TPJ | 44 −48 28 | 3.31 | 40 | |
| Interaction between emotion and group | [Adult (social > basic)–adolescent (social > basic)] | |||
| Left temporal pole | −40 −6 −26 | 3.43 | 32 | |
| [Adolescent (social > basic)–adult (social > basic)] | ||||
| Left lateral anterior rostral MPFC | −16 42 20 | 3.39 | 32 | |
| Protagonist: self > other | Adolescents | |||
| Left postcentral gyrus | −24 −40 52 | 4.32 | 112 | |
| Interaction between emotion and protagonist | Adult [(self social > self basic)–(other social > other basic)] | |||
| Left TPJ | −42 −66 40 | 3.83 | 264 | |
| Anterodorsal MPFC | 14 42 36 | 3.38* | 40 | |
| Adolescent [(self social > self basic)–(other social > other basic)] | ||||
| Anterodorsal MPFC | 14 38 44 | 3.29* | 8 | |
Active at p < .001, uncorrected.
Figure 3.
Main effect of social emotion (social > basic) in both groups. Sagittal and transverse glass brains showing average group activation for adults and adolescents. Shown at p < .005; minimum spatial extent = 10 voxels; smoothed with a filter of 6 mm full width at half maximum at the second level.
Interactions between Group and Emotion
To identify differences in brain activity to social versus basic emotion between groups, we tested for significant interactions between group (adult, adolescent) and emotion (social, basic).
[Adult (social > basic)–adolescent (social > basic)]
This analysis revealed a cluster in the left temporal pole (Table 3, Figure 4). Inspection of the parameter estimates, as well as inclusive masking (with adult social > basic), showed that this region was activated more to social than to basic emotion in adults, and more to basic than to social emotion in adolescents (Figure 3).
Figure 4.
Interaction between group and emotion: activity in the left temporal pole ([−40 −6 −26]) resulting from the contrast [adult (social > basic)–adolescent (social > basic)], shown at p < .005 projected onto a sagittal T1 image; graph showing parameter estimates in the left temporal pole for social and basic emotion relative to fixation, in both groups; positive correlation between age and adjusted BOLD signal in the left temporal pole ([−40 −6 −26]) in the contrast [social > basic] (r = .572; p < .001).
[Adolescent (social > basic)–adult (social > basic)]
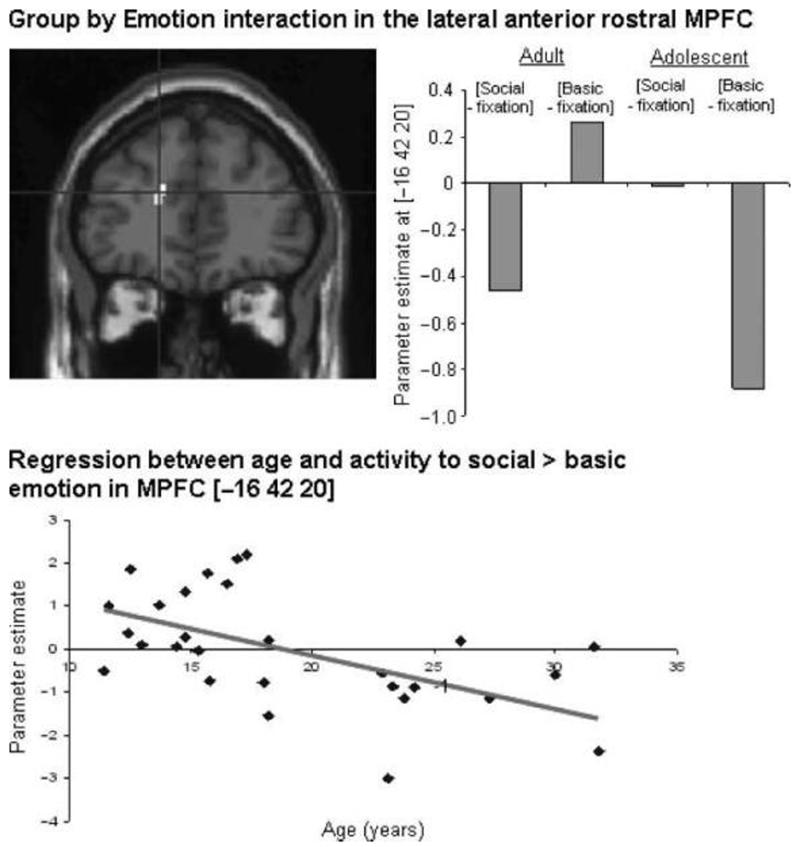
A cluster in left lateral anterior rostral MPFC (Table 3, Figure 5) was activated by the contrast [adolescent (social > basic)–adult (social > basic)]. Inspection of the parameter estimates, as well as inclusive masking (with adolescent social > basic), showed that this region was activated more to social than to basic emotion in adolescents, and more to basic than to social emotion in adults (Figure 5).
Figure 5.
Interaction between group and emotion: activity in the MPFC ([−16 42 20]) resulting from the contrast [adolescent (social > basic)–adult (social > basic)], shown at p < .005 projected onto a sagittal T1 image; graph showing parameter estimates of activity in the MPFC for basic and social emotion relative to fixation, in both groups; negative correlation between age and adjusted BOLD signal in the MPFC ([−16 42 20]) in the contrast [social > basic] (r = .541; p < .005).
Main Effect of Protagonist in Each Group
In the adult group, the main effect of self (self > other) did not yield any significant activations. In the adolescent group, the main effect of self (self > other) resulted in activation of the left postcentral gyrus (Table 3). The main effect of other (other > self) did not yield any significant activations in either the adult or the adolescent group.
Interactions between Protagonist and Group
No brain regions showed a significant interaction between protagonist and group.
Interactions between Emotion and Protagonist
To identify differences in brain activity to social versus basic emotion which differed as a function of protagonist (self, other), we tested for significant interactions between emotion (social, basic) and protagonist (self, other) for each group separately.
Adult [(self social > self basic)–(other social > other basic)]
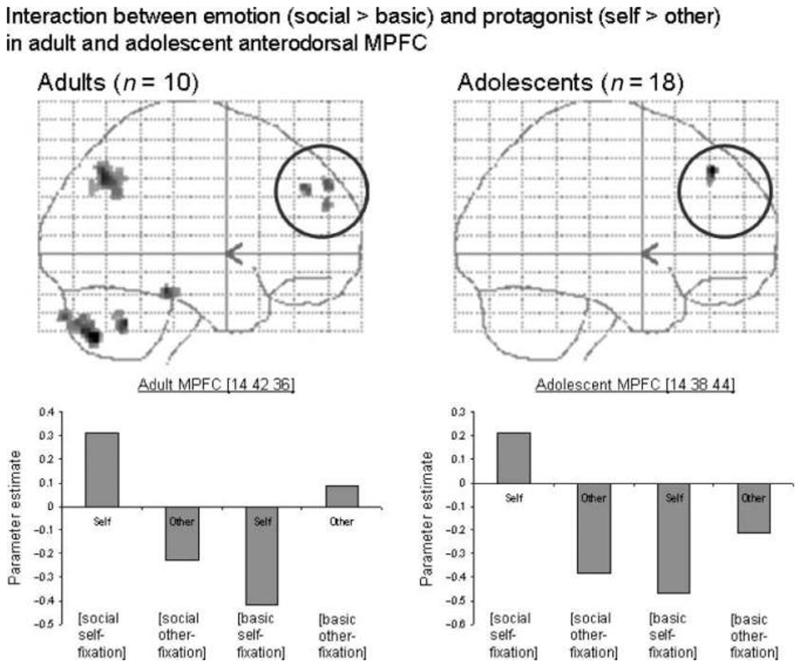
The adult group activated the left TPJ in this contrast (Table 3; Figure 2, top left). At a less stringent threshold (p < .001 uncorrected), the adult group activated an anterodorsal portion of the MPFC for this contrast (Table 3; Figure 6, top left). Inspection of the parameter estimates, as well as inclusive masking (with social > basic for self only; and basic > social for other only), showed that the MPFC region was more active in self-social emotions than in any other condition (Figure 6, bottom left). In contrast, the left TPJ was more active in self-social emotions and other-basic emotions than in the other conditions (shown in the graph in Figure 2).
Figure 6.
Adult and adolescent activity in the MPFC in the contrast [(self social > self basic)–(other social > other basic)], shown at p < .005 on sagittal glass brain projections. The adult interaction in the left TPJ, containing the region showing a three-way interaction, can also be seen. Parameter estimates are shown for both emotion and both protagonist conditions (all relative to fixation) in the MPFC, in adults and adolescents. In both groups, this anterodorsal region of the MPFC is most active in self-social emotions.
Adolescent [(self social > self basic)–(other social > other basic)]
At a less stringent threshold (p < .001 uncorrected), the adolescent group showed activity within a similarly anterodorsal region of the MPFC for this contrast (Table 3; Figure 6, top right). Inspection of the parameter estimates, as well as inclusive masking (with social > basic for self only; and basic > social for other only), showed that the MPFC region was more active in self-social emotions than in any other condition (Figure 6, bottom right).
Adult and adolescent [(other social > other basic)–(self social > self basic)]
No brain regions were significantly active for this contrast, in either group.
Regression between Age and Activation to Social > Basic Emotion
Whole-brain linear regression analysis revealed that activity to social > basic emotion was positively correlated with age in the left temporal pole (MNI coordinates [−40 −6 −26]; r = .572; p < .001; Figure 4, bottom). Activity in the contrast social > basic emotion was negatively correlated with age in the left lateral anterior rostral MPFC (MNI coordinates [−16 42 20]; r = .541; p < .005; Figure 5, bottom).
DISCUSSION
The current fMRI study investigated the neural correlates of social emotion processing in adults and in adolescents. When participants imagined social versus basic emotion scenarios featuring either themselves or their mothers, both adults and adolescents activated the anterior rostral MPFC. However, there was an interaction between group and condition in the lateral anterior rostral MPFC such that the adolescent group activated this region more than did adults for social relative to basic emotions. The adult group activated a region of the left temporal pole more for social relative to basic emotions. The absence of any significant group differences in participants’ emotion ratings to the scenarios suggests that the differences in brain activity between the adult and adolescent groups cannot be accounted for by differences in performance.
Brain Activations Associated with Social Emotion in Both Groups
Social emotions, in contrast to basic emotions, require the ability to represent people’s mental states (mentalizing). In other words, social emotions require insight into the mental states of other people—whether they are physically present, imagined, or perhaps represented by the concept of societal norms (Moll, de Oliveira-Souza, et al., 2005; Moll, Zahn, et al., 2005). For example, guilt is experienced when one believes that one’s actions warrant disapproval or punishment, or that they have caused harm to another individual. In the current study, both adults and adolescents activated brain regions known to be involved in mentalizing, namely, the MPFC and the pSTS/TPJ (Amodio & Frith, 2006; Frith & Frith, 2003; Saxe & Kanwisher, 2003), during social versus basic emotion processing. In a recent review paper, Olsson and Ochsner (2008) have drawn attention to the overlap between brain regions involved in social cognition and in social emotion. The results of the current study concur with this finding. We found that brain regions involved in social cognition were also active when imagining social emotion situations. The current results therefore support the idea that the neural systems for social emotion and mentalizing share partially overlapping neural components.
The anterior rostral MPFC, within the region defined as the mental state attribution region (MNI y-coordinates from 30 to 60; z-coordinates from 0 to 40; Amodio & Frith, 2006; Gilbert et al., 2006), was active in both groups for social versus basic emotion. Our data are also in agreement with studies of social emotion in adults, which have reported activity within the MPFC (Takahashi et al., 2004; Berthoz et al., 2002; Moll et al., 2002). This supports the notion that a cognitive process, that is, a mental state representation, is involved in imagining social emotion situations (cf. Olsson & Ochsner, 2008). It should be noted that the implication of our findings depends on what role the anterior rostral MPFC plays in mentalizing. It has been proposed that the cognitive role of the MPFC in mentalizing is to decouple mental states from physical reality (Frith, 2007). An alternative, but not necessarily incompatible, explanation of the role of the MPFC in mentalizing is that it represents the motivational relevance of social behaviors (Moll, Zahn, et al., 2005). Our study was not designed to distinguish between these possibilities and further work is needed on the precise role of the MPFC in social–emotional tasks and the implications for models of social–cognitive functioning and emotional processing.
Several previous studies have demonstrated activity within the anterior rostral MPFC in adolescents during mentalizing tasks relative to control conditions (Blakemore et al., 2007; Moriguchi et al., 2007; Wang et al., 2006). The current result extends our understanding of the neural correlates of social emotion processing to the adolescent population.
Adults and adolescents also activated the pSTS/TPJ for social versus basic emotion, with activity greater on the left than on the right. The left TPJ is consistently activated during mentalizing tasks, and is thought to play a role in reasoning about the beliefs of others (Samson, Apperly, Chiavarino, & Humphreys, 2004; Frith & Frith, 2003; Saxe & Kanwisher, 2003). The pSTS/TPJ is also activated during mentalizing in adolescents (Blakemore et al., 2007; Moriguchi et al., 2007). Activity within this region in the social relative to basic emotion condition may be related to the need for representing or reasoning about other people’s beliefs when imagining social versus basic emotion situations.
Differences in Social Emotion Processing between Adults and Adolescents
Although both age groups activated the anterior rostral MPFC for social versus basic emotion, a more lateral part of the anterior rostral MPFC was activated for this contrast by the adolescent group, but not by the adult group. This region was contiguous with the region activated by adolescents in the main effect of social versus basic emotion. Activity to social emotion relative to basic emotion at this locus was also negatively correlated with age. Our results therefore suggest that, as well as showing greater activity than adults within parts of the MPFC for social versus basic emotion, adolescents also activate a greater volume of the MPFC for social emotion processing. This result is consistent with recent developmental neuroimaging studies looking at other aspects of social cognition, such as thinking about how intentions cause behavior (Blakemore et al., 2007) and understanding the intended meanings of sarcastic remarks (Wang et al., 2006). These studies demonstrated an age-related decrease in activity in social relative to control conditions in the anterior rostral MPFC, in a similarly lateral dorsal location to that found in the current study (see Blakemore, 2008 for meta-analysis). This result is also consistent with studies that report more extensive activation of the PFC in adolescents compared with adults for nonsocial tasks (e.g., Durston et al., 2006). The pattern of relative activity in the lateral dorsal MPFC is also consistent with the idea that activity to basic emotion as well as social emotion may develop during adolescence. Previous fMRI studies have shown that neural activity to facial expressions of basic emotion changes during adolescence (Yurgelun-Todd & Killgore, 2006; Monk et al., 2003).
The left temporal pole demonstrated a significant Group by Condition interaction (Figure 4) such that the adult group showed more activation in this region than did adolescents for social versus basic emotion processing. Activity to social versus basic emotion in the left temporal pole was also positively correlated with age. The temporal poles are thought to store abstract social knowledge (Frith, 2007; Zahn et al., 2007). The current result therefore suggests that adults might use social semantic knowledge when thinking about guilt and embarrassment situations.
One possible interpretation of the difference in activation pattern between the groups is that, during childhood and adolescence, a greater degree of mental state attribution (or, more effortful and less automatic mental state attribution) may be required to generate social emotions. In adulthood, perhaps mentalizing plays a less prominent role in experiencing social emotions. This might be because accumulated social experience is greater in adults so social semantic knowledge (represented in the temporal poles) can be relied upon more heavily. Alternatively, it could be the case that in adults, MPFC-based mentalizing is more automatic, leading to more efficient recruitment of this brain region (Durston et al., 2006).
It is interesting to note that the parameter estimates for the interactions between group and emotion (shown in Figures 2, 4, 5, and 6) are often negative relative to activity in the fixation baseline, particularly in the MPFC. Many studies have reported similarly higher activity in the MPFC during low-level baseline conditions than during more demanding task conditions. It has been suggested that activity within the MPFC (together with the precuneus/posterior cingulate cortex) reflects a default mode in which, in the absence of more demanding task demands, subjects are free to reflect on themselves (Gusnard & Raichle, 2001). Amodio and Frith (2006) have also suggested that during baseline participants might indulge in spontaneous mentalizing (reflecting on their own and other people’s mental states) and this causes the elevated MPFC activation. Therefore, the key result to note in the current study is that activity within certain portions of the MPFC is greater in social versus basic emotion in adolescents than in adults.
Brain Activations Associated with Self/Other Processing
In the current study, participants imagined emotion scenarios either from a first-person (self) or a third-person (mother) perspective. This additional factor was included because previous studies in adults and adolescents have shown that neural activity to emotion and semantic knowledge respectively differs between self and other perspectives (Pfeifer et al., 2007; Ruby & Decety, 2004). In the current study, thinking about emotion from a first-person versus third-person perspective resulted in activation in the right postcentral gyrus in adolescents. Activation of this region is consistent with previous studies (Ruby & Decety, 2004), and may be related to imagining the sensory consequences of emotional scenarios.
A region of the MPFC showed a significant interaction between protagonist and emotion in both groups. This region of the MPFC was similarly located in both groups, and was more anterodorsal than the main foci of activation to social versus basic emotion and self versus other processing. Inspection of the parameter estimates for this region revealed that, in both groups, the anterodorsal MPFC was most active to social emotion in the first-person perspective.
We also found a significant region of activation in the left TPJ for the three-way interaction between emotion, protagonist, and group. Although the left TPJ was more active for social versus basic emotion in the adolescent group, the adult group showed highest activation of this region for basic emotion in the third-person perspective and for social emotion in the first-person perspective. The role of this brain region in thinking about others’ beliefs has been highlighted in adult neuroimaging and lesion studies (Samson et al., 2004; Frith & Frith, 2003). The differential recruitment of the left TPJ in adults and adolescents in the current study may indicate that different cognitive strategies are being used for the attribution of social and basic emotions to self and other. For a given emotion, the left TPJ seems to differentiate better between self and other in adults than it does in adolescents. A possible interpretation of these data, which has been proposed elsewhere (Moriguchi et al., 2007), is that adolescents rely more heavily on a simulation-based strategy when imagining another person’s emotional response than adults do. It is notable that this difference in brain activity occurred despite a lack of group difference in perceived self–mother similarity (see Results).
Role of the MPFC in Social Cognitive Development
An interesting perspective on the role of the MPFC in social cognition has recently been raised in the adult lesion and neuroimaging literature. Although the MPFC is robustly activated by mentalizing tasks, and adults presenting with MPFC lesions usually show mentalizing deficits (Frith & Frith, 2003; see Frith, 2007), there is one report of an individual who suffered extensive bilateral MPFC damage during adulthood but who was unimpaired on mentalizing tasks (Bird, Castelli, Malik, Frith, & Husain, 2004). The MPFC is not specifically activated when adults make semantic discriminations among abstract social concepts, such as “brave” or “stingy” (Zahn et al., 2007). Rather, this task relies on the superior temporal poles. In addition, a small number of social emotion studies in adults fail to find MPFC activation, but do find activity within other regions of the social brain network such as the STS and the temporal poles, as well as in more ventral and/or anterior prefrontal regions such as the fronto-polar and orbito-frontal cortex (Moll, de Oliveira-Souza, et al., 2005; Moll, Zahn, et al., 2005; Takahashi et al., 2004). The current study, as well as previous studies, found greater MPFC activity in adolescents than in adults for social cognition tasks relative to control conditions (Blakemore et al., 2007; Pfeifer et al., 2007; Wang et al., 2006). This suggests that activation of the MPFC for a particular social cognition task may lessen with age. Reasons for this could include the accumulation of social experience and the use of alternative cognitive strategies.
Further work is needed on the development during adolescence of the cognitive strategies used for understanding people. For example, we do not know whether the type of mentalizing needed for social emotion understanding changes with development. It may be the case that a more explicit mentalizing process is needed to learn about social emotions initially, but that more scripted, heuristic, or intuitive strategies are employed later on (Haidt, 2001).
Another factor which may contribute to a change in the role of the MPFC with age is anatomical brain development. Volumetric MRI studies show that gray matter volume in social brain regions such as the MPFC and the TPJ decreases during adolescence, whereas white matter volume increases (Shaw et al., 2008; Gogtay et al., 2004; Giedd et al., 1999; Paus et al., 1999; Sowell et al., 1999). These changes are thought to be due to synaptic pruning and axonal myelination, respectively (see Blakemore, 2008), and may result in increased coordination between components of the social brain network and greater processing efficiency within brain regions mediating social cognition.
Conclusion and Implications
This fMRI study shows that the neural processing of social emotion from a first- and third-person perspective develops between adolescence and adulthood. Although components of the social brain network including the MPFC were active in both groups, adolescents activated the lateral rostral MPFC more for social versus basic emotion, whereas adults did not. Adults activated the left temporal pole more for social versus basic emotion than did adolescents. These results indicate that the neural processing of social emotion continues to develop between adolescence and adulthood, such that the predominant activity moves from anterior (MPFC) to more posterior (temporal) regions with age. Further work is needed to ascertain how this is related to neuroanatomical development within social brain regions, and to changes in cognitive strategy resulting from developing social ability and experience. Our study was conducted only with female participants; whether there are gender differences in social emotion processing and its development is an empirical question.
We have shown that adolescents and adults use a similar neural network for social emotion processing, but with relative differences. What is the functional relevance of this developmental shift across adolescence? In the current experiment, our aim was not to find behavioral differences between the age groups. Indeed, it was important that performance between groups was matched because, had performance differed between groups, it would have been impossible to interpret any differences in neural activity, which might have been a cause or a consequence of differences in task performance. Very few empirical behavioral studies have reported significant behavioral development during adolescence that is specific to social cognition and which cannot be explained by general improvement in attention, concentration, memory, and so on. The reasons for this are probably multifactorial. One possibility is that, in the lab, adolescents are able to pass complicated social cognitive tasks, which in everyday life they do not accomplish successfully. More naturalistic paradigms might be useful in addressing this question. However, it is important not to try to explain all of adolescent behavior in terms of neuroanatomical changes or changes in functional activation, as this neglects important factors such as changes in hormones and social environment. A challenge for future adolescent research is to disentangle how these hormonal and social factors interact with cognitive and neuroanatomical changes to produce the unique constellation of social behaviors that characterize adolescence.
Acknowledgments
This study was funded by the Royal Society and the Wellcome Trust. S. J. B. has a Royal Society University Research Fellowship and S. B. is funded by the Wellcome Trust 4-year PhD in neuroscience at UCL.
REFERENCES
- Adams GR, Berzonsky MD. The Blackwell handbook of adolescence. Blackwell; Oxford: 2003. [Google Scholar]
- Aichhorn M, Perner J, Kronbichler M, Staffen W, Ladurner G. Do visual perspective tasks need theory of mind? Neuroimage. 2006;30:1059–1068. doi: 10.1016/j.neuroimage.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Bird CM, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on “Theory of Mind” and cognition. Brain. 2004;127:914–928. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Social Cognitive and Affective Neuroscience. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody LR. Gender differences in emotional development: A review of theories and research. Journal of Personality. 1985;53:102–149. [Google Scholar]
- Brown BB. Adolescents’ relationships with peers. In: Lerner RM, Steinberg L. Handbook of adolescent psychology. 2nd ed. Wiley; Hoboken, NJ: 2004. 363‐394. [Google Scholar]
- Costa PT, McCrae RR. NEO Five-Factor Inventory (NEO-FFI). Professional Manual. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annual Review of Psychology. 2000;51:665–697. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Morris AS. Moral cognitions and prosocial responding in adolescence. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. Wiley; Hoboken, NJ: 2004. pp. 155–188. [Google Scholar]
- Elkind D. Egocentrism in adolescence. Child Development. 1967;38:1025–1034. [PubMed] [Google Scholar]
- Elkind D, Bower R. Imaginary audience behaviour in children and adolescents. Developmental Psychology. 1979;15:38–44. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1994;2:189–210. [Google Scholar]
- Frith CD. The social brain? Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2007;362:671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalising. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, et al. Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haidt J. The emotional dog and its rational tail: A social intuitionist approach to moral judgment. Psychological Review. 2001;110:193–196. doi: 10.1037/0033-295x.108.4.814. [DOI] [PubMed] [Google Scholar]
- Hall GB, Witelson SF, Szechtman H, Nahmias C. Sex differences in functional activation patterns revealed by increased emotion processing demands. NeuroReport. 2004;15:219–223. doi: 10.1097/00001756-200402090-00001. [DOI] [PubMed] [Google Scholar]
- Harris PL, Olthof K, Terwogt MM, Hardman CC. Children’s knowledge of the situations that provoke emotion. International Journal of Behavioural Development. 1987;10:319–343. [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Perceptual and Motor Skills. 2004;99:371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: Expression, experience, and physiology. Journal of Personality and Social Psychology. 2007;74:686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallance GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiuolo PA, et al. The neural correlates of moral sensitivity: A functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002;22:2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, Ignacio FA, Bramati IE, Caparelli-Daquer EM, et al. The moral affiliations of disgust: A functional MRI study. Cognitive and Behavioral Neurology. 2005;18:68–78. doi: 10.1097/01.wnn.0000152236.46475.a7. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Mori T, Matsuda H, Komaki G. Changes of brain activity in the neural substrates for theory of mind during childhood and adolescence. Psychiatry and Clinical Neuroscience. 2007;61:355–363. doi: 10.1111/j.1440-1819.2007.01687.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Parker JG, Rubin KH, Erath SA, Wojslawowicz JC, Buskirk AA. Peer relationships, child development, and adjustment: A developmental psychopathology perspective. In: Cicchetti D, Cohen DJ. Developmental psychopathology. Vol. 1: Theory and methods. 2nd ed. Wiley; New York: 2006. 96‐161. [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: Neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective taking with social emotions. Journal of Cognitive Neuroscience. 2004;16:988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nature Neuroscience. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Human Brain Mapping. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biological Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. Neuroimage. 2004;23:967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Developmental changes in the neural basis of interpreting communicative intent. Social Cognitive and Affective Neuroscience. 2006;1:107–121. doi: 10.1093/scan/nsl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler Abbreviated Scale of Intelligence . PsychCorp, Harcourt Assessment; Texas: 1999. [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: A preliminary fMRI study. Neuroscience Letters. 2006;406:194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman J, Cassano M, Perry-Parrish C, Stegall S. Emotion regulation in children and adolescents. Journal of Developmental & Behavioral Pediatrics. 2006;27:155–168. doi: 10.1097/00004703-200604000-00014. [DOI] [PubMed] [Google Scholar]
- Zeman J, Shipman K. Social–contextual influences on expectancies for managing anger and sadness: The transition from middle childhood to adolescence. Developmental Psychology. 1997;33:917–924. doi: 10.1037//0012-1649.33.6.917. [DOI] [PubMed] [Google Scholar]