Abstract
Toll-like receptors (TLRs) are pattern-recognition receptors responible for triggering cells of innate immunity. In this study we investigated the expression and function of TLRs 3 and 9 in human natural killer (NK) cells. In the presence of IL-12, freshly isolated NK cells responded to double-stranded RNA or unmethylated CpG DNA and expressed CD69 and CD25 activation markers. Because both markers were expressed by virtually all NK cells, this would suggest that most of them can be triggered by TLRs. Remarkably, NK cell stimulation also resulted in the induction of their functional program as revealed by IFN-γ and tumor necrosis factor-α release and by up-regulation of cytolytic activity against tumor cells. IL-8 could efficiently substitute IL-12 in supporting NK cell responses to TLR-mediated stimulation. Importantly, freshly isolated NK cells acquired the ability to lyse immature dendritic cells after stimulation with double-stranded RNA and IL-12. However, responses to these stimuli were not restricted to fresh NK cells, because significant responses were also detected in polyclonal NK cells cultured in the presence of exogenous IL-2 for several weeks. The analysis of NK cell clones revealed some degree of heterogeneity in the ability to respond to TLR stimulation also among NK clones derived from a single donor. These data suggest that stimuli acting on TLR not only activate immature dendritic cells to release IL-12 but also render NK cells capable of receiving triggering signals from pathogen-associated molecules, thus exerting a regulatory control on the early steps of innate immune responses against infectious agents.
Toll-like receptors (TLRs) are pattern-recognition receptors that trigger innate immune responses providing both immediate protection against various pathogens and instructing the adaptive immune system by the induction of dendritic cell (DC) recruitment and maturation (1–4).
Ten TLRs have been described in humans, and for most of them specific ligands have been identified (5). Thus, TLR1, TLR2, and TLR6 mediate cell triggering after interaction with peptidoglycan and other microbial products (6, 7), TLR3 with double-stranded RNA (dsRNA) (8), TLR4 with lipopolysaccharide (9), TLR5 with flagellin (10), TLR7 and TLR8 with imidazoquinolines (5, 11), and TLR9 with unmethylated CpG DNA (12–15). TLRs are differentially expressed in DCs of different origin and monocytes (16–18). Thus, monocytes and myeloid DCs express all TLRs except TLR7 and TLR9, whereas these receptors are selectively expressed by plasmacytoid DCs. The expression of TLRs can be regulated by cytokines. For example, IFN-γ up-regulates TLR4 expression in human phagocytes/immature DCs (iDCs), thus enhancing their ability to respond to lipopolysaccharide (19). The induction of DC maturation by TLRs represents an important functional link between innate and adaptive immune responses (1, 20), rendering DCs capable of efficiently interacting with T cells.
NK cells have been shown to interact with DCs and provide a possible mechanism by which DCs are selected on the basis of their ability to express optimal amounts of MHC class I molecules required for efficient antigen (Ag) presentation to T cells (21). Indeed, NK cells, recruited together with DCs at the site of inflammation, are capable of potent cytolytic activity against iDCs that express low levels of HLA-E at the cell surface (22). In this context, we showed recently that responsible for this effect are primarily the NKG2A+ NK cells, whereas little or no cytotoxicity is mediated by killer Ig-like receptor (KIR)+ NK cells (22). The final result of this selection would be the quality control of DCs that, after Ag encountering, undergo maturation (23). On the other hand, DCs that have captured the Ag are able to promote NK cell proliferation and increase their cytotoxic activity. A soluble factor that may play a role in this process is IL-12, released by iDCs after stimulation with pathogens. In turn, NK cells can provide iDCs with signals favoring their progression toward mature DCs (23–25). Notably the ability to kill iDCs and promote their maturation seems to be confined to NK cells preactivated in vitro by cytokines such as IL-2 (21, 22). However, the physiological signals required for NK cell activation in inflamed tissues in the absence of cytokines released by cells of the adaptive immunity are still undefined.
We report here that human NK cells express functional TLR3 and TLR9 that allow responses to microbial products such as dsRNA (virus-derived) and CpG (of bacterial origin). After culture in the presence of synthetic form of these products, peripheral blood NK cells became capable of producing IFN-γ and tumor necrosis factor α (TNF-α) and acquired cytolytic activity against iDCs. Our data suggest that, during the early steps of cell recruitment at inflammatory sites, NK cells are triggered by the same stimuli that act on iDCs. TLR-induced stimulation of both DCs and NK cells would also play a crucial role in inducing NK cells to select the best-fit iDCs and to facilitate their maturation.
Methods
mAbs. The following mAbs were used in this study: JT3A (IgG2a anti-CD3), c218 (IgG1 anti-CD56), Z199 (IgG2b anti-NKG2A), XA141 (IgM anti-KIR2DL1/S1), Y249 (IgM anti-KIR2DL2/L3/S2), FS172 (IgG2a anti-KIR2DS4), AZ158 (IgG2a anti-KIR3D), c227 (IgG1 anti-CD69), and MAR93 (IgG1 anti-CD25) (all produced in our laboratory); and CD14 mAb (IgG2a) [purchased from Immunotech (Beckman Coulter, Marseille, France)].
D1.12 (IgG2a anti-HLA-DR) and HP2.6 (IgG2a anti-CD4) mAbs were kindly provided by R. S. Accolla (Università di Pavia, Pavia, Italy) and P. Sanchez-Madrid (Hospital de la Princesa, Madrid), respectively.
Purification of Peripheral Blood NK Cells and Generation of Polyclonal or Clonal NK Cell Populations. Peripheral blood mononuclear cells (PBMCs) were derived from healthy donors by Ficoll/Hypaque gradients. PBMCs were depleted of plastic-adherent cells and incubated with anti-CD3, anti-CD4, anti-CD14, and anti-HLA-DR mAbs (30 min at 4°C) followed by goat anti-mouse-coated Dynabeads (Dynal, Oslo) (30 min at 4°C) and immuno-magnetic depletion.
CD3-4-14-DR- cells were cultured on irradiated feeder cells in the presence of 100 units/ml recombinant human (rh) IL-2 (Proleukin, Chiron) and 1.5 ng/ml phytohemagglutinin (GIBCO) to obtain activated polyclonal NK cell populations or, after limiting dilution, NK cell clones as described (26).
TLR-Mediated Stimulation of NK Cells. CD3-4-14-DR- PBMC-derived NK cells or in vitro-activated NK cells were cultured for 20 h in 24-well plates at a concentration of 1 × 106 in 1 ml of RPMI medium 1640 supplemented with 10% FCS, 2 mM l-glutamine, 1% penicillin-streptomycin-neomycin, and the indicated cytokines in the absence or presence of one or another stimulus acting on TLRs. Purified rhIL-2 (Proleukin, Chiron), rhIL-12 (PeproTech, London), or rhIL-8 (PeproTech) were used at the final concentration of 10 units/ml, 1–2 ng/ml, and 10 ng/ml, respectively.
Polyinosinic-polycytidylic acid [poly(I·C)] (Amersham Pharmacia Biotech) was used at 50 μg/ml. The following modified CpG oligodeoxynucleotides (ODNs) were synthesized and purified by TIB MOLBIOL (CBA, Genova, Italy) and used at a final concentration of 5 μg/ml: ODN 2216 (ODN A), 5′-GGGGGACGATCGTCGGGGGG (27), and ODN 2006 (ODN B), 5′-TCGTCGTTTTGTCGTTTTGTCGTT (27, 28).
Cytokine Analysis. Supernatants collected from resting and stimulated NK cells were analyzed for the IFN-γ, TNF-α, and granulocyte/macrophage colony-stimulating factor (GM-CSF) content by using ELISA kits from BioSource International (Camarillo, CA) according to manufacturer instructions.
Phenotypic Analysis and Cytolytic Activity of NK Cells. After TLR stimulation, the phenotype of NK cells was analyzed by a FACScan (Becton Dickinson) one- or two-color fluorescence cytofluorimetric analysis (26), whereas the NK-mediated cytotoxicity was assessed as described (22, 26).
TLR3 and TLR9 RT-PCR Analysis. Total RNA was extracted from NK bulk populations and the NK92 cell line by using an RNeasy minikit (Qiagen, Valencia, CA). To exclude the chance that PCR amplifications were caused by DNA contaminations, RNA was treated with RNase-free DNase (Qiagen) and further amplified with or without retrotranscription. The two sets of primers used in this study are (i) TLR3up (5′-CAAGCAGAAGAATTTAATCAC) and TLR3down (5′-TTATTCAATCCTAAATCGATG) and (ii) TLR9up (5′-CAGCCATACCAACATCCTG) and TLR9down (5′-AAAGGACACCCTCTTTTGG). Amplifications were performed for 30 cycles: 30 sec at 95°C; 30 sec at 58°C; and 30 sec at 72°C. The amplification products were resolved in a 0.8% agarose gel, subcloned into pcDNA3.1/V5/His TOPO vector by using the eukaryotic TOPO TA cloning kit (Invitrogen), and sequenced. DNA sequencing was performed by using d-Rhodamine terminator cycle sequencing kit and a 3100 ABI automatic sequencer (PerkinElmer/Applied Biosystems).
Results
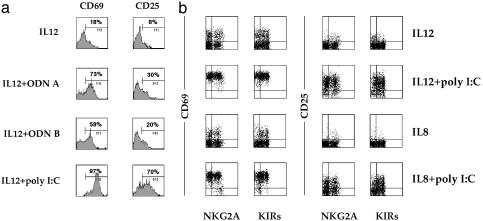
Peripheral Blood NK Cells Express Activation Markers After Triggering with poly(I·C) or ODN A/B. Previous studies (28, 29) suggested that human NK cells express mRNA for a series of TLRs including TLR3 and TLR9. Here, we investigated the functional responsiveness of resting NK cells (purified from PBMCs by depletion of cells expressing CD3, CD4, CD14, and HLA-DR surface Ags) to poly(I·C), which represents a synthetic form of dsRNA) and ODN A/B (synthetic ODNs containing unmethylated CpG motifs) acting on TLR3 and TLR9, respectively. Although treatment of resting NK cells with poly(I·C) or ODN A/B alone did not result in any detectable cell activation (data not shown), stimulation in the presence of suboptimal doses of IL-12 or IL-8 induced surface expression of activation markers including CD69 and CD25 (as assessed by cytofluorimetric analysis after 20 h of culture). These data indicate that NK cells can be triggered by poly(I·C) and ODN A/B in the simultaneous presence of proinflammatory cytokines IL-12 or IL-8.
It is of note that treatment of freshly derived peripheral blood NK cells with suboptimal doses of IL-12 alone can induce CD69 expression; however, this is limited to only a fraction of NK cells. In contrast, when IL-12 is used in association with poly(I·C), CD69 was expressed by all NK cells (Fig. 1a). Similar results were obtained when NK cells were stimulated with IL-12 plus ODN A or ODN B. Surface expression of CD25 was primarily detected in NK cells cultured in the presence of IL-12 and poly(I·C) (Fig. 1a). An effect similar to IL-12, although lower in magnitude, was also detected by the use of the proinflammatory cytokine IL-8. Thus, when used in association with poly(I·C) (Fig. 1b), IL-8 induced the surface expression of CD69 and CD25 in most fresh NK cells (95% and 49%, respectively). On the other hand, IL-8 alone induced only low levels of CD69 (19%) or CD25 (4%). When combined with ODN A or ODN B, IL-8 also induced NK cell activation; however, the proportion of cells expressing CD69 or CD25 was lower (data not shown). NK cells that expressed CD69 or CD25 in response to poly(I·C) or ODN A/B stimulation were not restricted to NKG2A+ or KIR+ cells, as revealed by double fluorescence and fluorescence-activated cell-sorter analysis (Fig. 1b).
Fig. 1.
ODN A, ODN B, or poly(I·C) induces the expression of CD69 and CD25 by peripheral blood NK cells. (a) Freshly isolated NK cells were cultured for 20 h in medium supplemented with suboptimal doses of IL-12 (1 ng/ml) in the absence or presence of ODN A (5 μg/ml), ODN B (5 μg/ml), or poly(I·C) (50 μg/ml), and were then analyzed by one-color immunofluorescence for the expression of CD69 and CD25. (b) Freshly isolated NK cells were cultured for 20 h in medium supplemented with suboptimal doses of IL-12 (1 ng/ml) or IL-8 (10 ng/ml) in the absence or presence of poly(I·C) (50 μg/ml), and were then analyzed by two-color immunofluorescence for the expression of CD69 and CD25 in combination with NKG2A or KIR molecules. This experiment is representative of >15 that gave similar results.
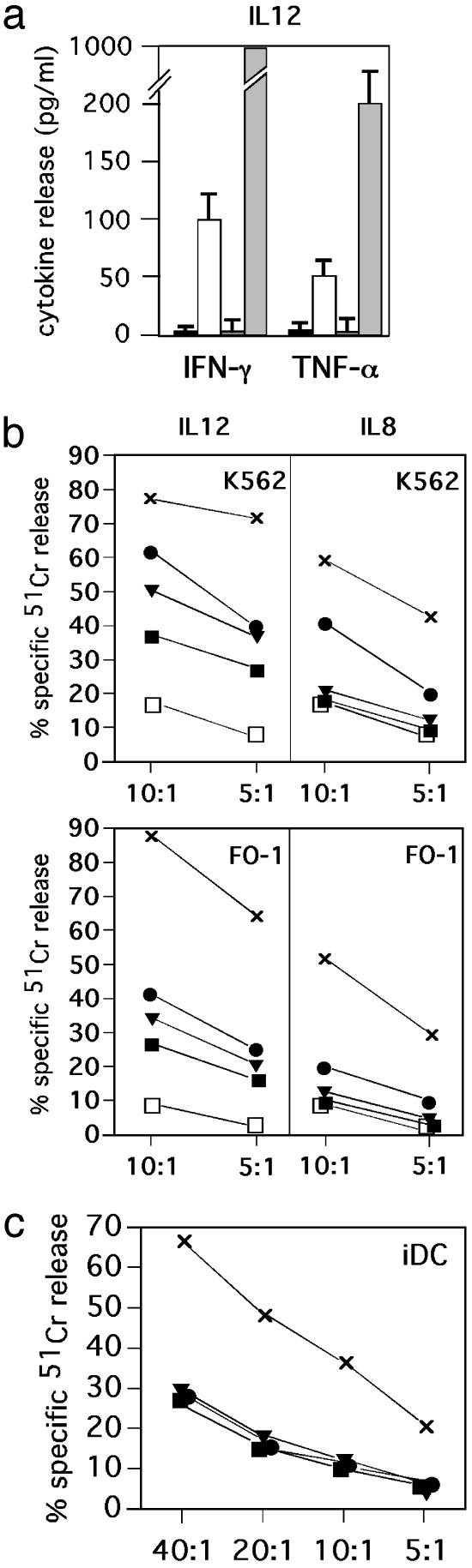
Induction of Cytokine Production in Peripheral Blood NK Cells After Stimulation by TLR. The same NK cell populations that had been cultured in the presence of either IL-12 or IL-8, either alone or in combination with either poly(I·C) or ODN A/B, were assessed next for the production of cytokines including GM-CSF, IFN-γ, and TNF-α.
As shown in Fig. 2a, high levels of IFN-γ and TNF-α were released by NK cells stimulated with IL-12 and poly(I·C). IL-12 could induce IFN-γ and TNF-α production also when used in combination with ODN A but at lower levels. Finally, stimulation with IL-12 and ODN B did not result in production of IFN-γ and TNF-α.
Fig. 2.
ODN A, ODN B, or poly(I·C) induces functional responses by freshly isolated human peripheral blood NK cells. (a) Peripheral blood NK cells were cultured in the presence of suboptimal doses of IL-12 (1 ng/ml) in either the absence (black boxes) or presence of the following stimuli: ODN A (white boxes), ODN B (dark-gray boxes), or poly(I·C) (light gray boxes). After 20 h of culture, supernatants were harvested and assessed for IFN-γ and TNF-α content by specific ELISA (n = 3, mean ± SD). (b) Freshly isolated peripheral blood NK cells were cultured for 20 h in the presence of suboptimal doses of IL-12 (1 ng/ml) or IL-8 (10 ng/ml) in either the absence (black boxes), or presence of the following stimuli: ODN A (black circles), ODN B (black triangles), or poly(I·C) (×) and were then analyzed for cytotoxicity against the K562 target or FO-1 melanoma cell line at two different E/T ratios. As a control, the cytolytic activity of cells cultured in medium without cytokines is shown (white boxes). (c) Peripheral blood NK cells were cultured for 20 h with the stimuli discussed above in the presence of IL-12 (2 ng/ml) and were then analyzed for cytotoxicity against iDCs at various E/T ratios. Each value represents the mean of triplicate experiments. The SD did not exceed 4% in the cytotoxicity assays. Results are representative of at least 10 different experiments.
On the contrary, no cytokine production could be detected when the same NK cells were cultured in the presence of IL-8 (data not shown). No GM-CSF production could be detected in any of the various culture conditions analyzed.
Up-Regulation of NK Cell Cytotoxicity in Response to Stimuli Acting on TLRs. We next evaluated whether the NK cell triggering induced by IL-12 or IL-8 in combination with poly(I·C) or ODN A/B had any effect on the NK-mediated cytotoxicity against tumor target cells. To this end, NK cells freshly purified from PBMCs were stimulated as described above. After 20 h of culture, NK cells were analyzed for their ability to lyse K562 and FO-1 target cells. K562 is the typical target for fresh, unstimulated NK cells, whereas FO-1 melanoma is relatively resistant to fresh NK cells but is efficiently killed by IL-2-cultured NK cell lines (30). Although the NK cell-mediated killing of K562 and FO-1 target cells was partially enhanced by IL-12 alone, this effect was amplified significantly by the addition of stimuli acting on TLR (Fig. 2b). Thus, poly(I·C) stimulation resulted in relevant increments of cytotoxicity, whereas ODN A and ODN B were slightly less efficient. Unlike NK cells cultured in the presence of IL-12 alone, those cultured with IL-8 alone did not display any up-regulation of cytotoxicity. However, NK cells cultured in the presence of combinations of IL-8 and poly(I·C) significantly increased their cytolytic activity. A less marked effect was detected in cells cultured with IL-8 plus ODN A, whereas IL-8 plus ODN B had no effect.
poly(I·C)-Stimulated NK Cells Acquire the Ability to Kill iDCs. The induction of antitumor cytotoxicity in TLR-stimulated NK cells prompted us to analyze whether the same stimuli could render NK cells capable of killing iDCs. To this end, NK cells that had been stimulated for 20 h as described above were assessed for cytotoxicity against monocyte-derived iDCs. As shown in Fig. 2c, NK cells cultured in the presence of IL-12- and poly(I·C)-lysed iDCs. This effect was strictly IL-12-dependent, because no lysis could be detected in NK cells cultured with IL-8 and poly(I·C) (data not shown). On the contrary, NK cells cultured with ODN A/B did not acquire the ability to kill iDCs in either the presence of IL-12 or IL-8. These data support the concept that the degree of NK cell activation required to kill iDCs can be reached in the presence of poly(I·C), i.e., of dsRNA released in tissues infected by certain viruses. Thus, viral dsRNA can directly prime NK cells in the presence of IL-12 released by DCs in response to the same stimulus. In turn, activated NK cells become capable of selecting DCs on the basis of their susceptibility/resistance to killing. The latter has been shown to reflect the degree of maturation and, in particular, the amounts of HLA class I molecules expressed at the DC surface (21, 22, 31).
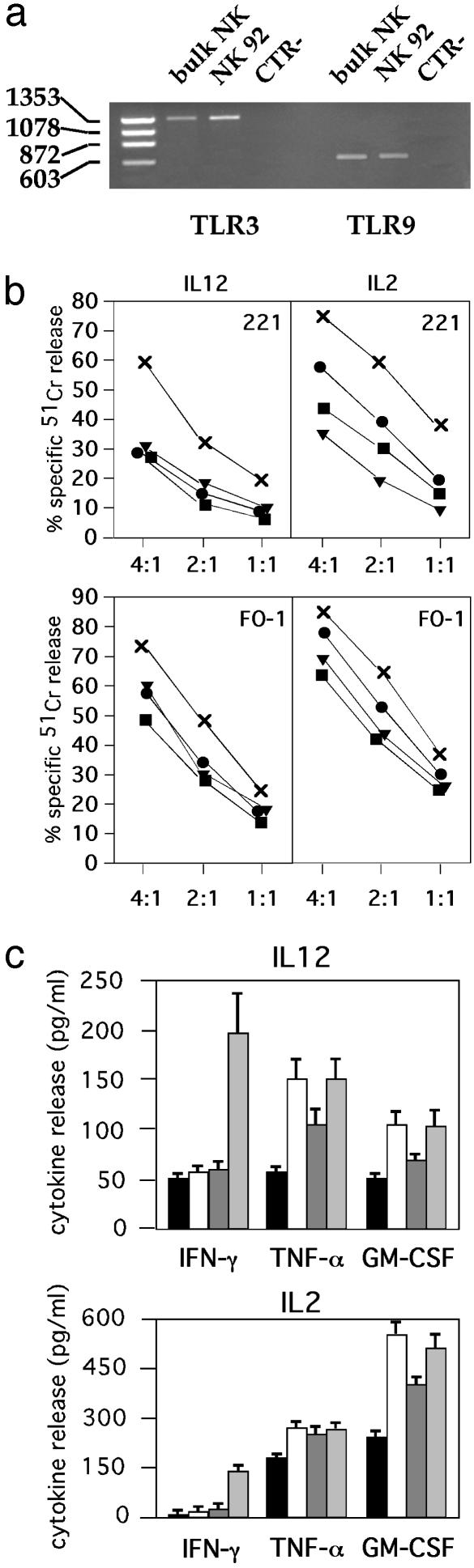
poly(I·C) and ODN A/B Induce Up-Regulation of NK Cell Cytotoxicity in IL-2-Cultured NK Cell Lines. To evaluate whether the ability to respond to stimuli acting on TLRs was a unique feature of fresh NK cells, we analyzed whether these stimuli could also act on polyclonal NK cell lines cultured in the presence of IL-2. Thus, we first verified the presence of TLR3 and TLR9 transcripts in activated NK cells by RT-PCR analysis. In agreement with the results obtained with freshly isolated NK cells (28, 29), cultured NK cell populations expressed mRNA for both TLRs analyzed (Fig. 3a). These NK cells were cultured in the presence of suboptimal concentrations of IL-12 or IL-2 and stimulated for 20 h with poly(I·C) or ODN A/B. Cells then were assessed for their cytolytic activity against two target cells (FO-1 melanoma and LCL 721.221 EBV cells) characterized by the lack of expression of HLA class I molecules and the susceptibility to lysis by IL-2-activated NK cells. As shown in Fig. 3b, a strong increment of cytotoxicity against LCL 721.221 EBV cells was observed in NK cells cultured with IL-12 plus poly(I·C). Marginal or no increments were detected in NK cells cultured with IL-12 plus ODN A or IL-12 plus ODN B. Moreover, LCL 721.221 EBV cell killing was incremented in NK cells cultured with IL-2 plus poly(I·C). Similar results were obtained when FO-1 melanoma cells were used as the source of target cells. Indeed, in the presence of either IL-12 or IL-2, the poly(I·C)-dependent NK cell stimulation resulted in higher levels of cytotoxicity as compared with ODN A or B.
Fig. 3.
Stimuli acting on TLRs promote functional responses by cultured NK cell populations. (a) RT-PCR analysis of TLR3 and TLR9 transcripts was performed on total RNA isolated from a representative NK bulk population and NK92 cell line. Molecular weights of Φx HaeIII principal bands are indicated on the left. CTR-, negative control. (b) A representative polyclonal NK cell population was cultured for 20 h with medium (black boxes), ODN A (black circles), ODN B (black triangles), or poly(I·C) (×) in the presence of IL-12 (1 ng/ml) or IL-2 (10 units/ml) and was then analyzed for cytotoxicity against two human HLA class I- target cell lines (LCL 721.221 EBV and FO-1 melanoma) at different E/T ratios. Each value represents the mean of triplicate experiments. The SD did not exceed 4% in the cytotoxicity assays. (c) A representative polyclonal NK cell population was cultured with medium (black boxes), ODN A (white boxes), ODN B (dark-gray boxes), or poly(I·C) (light-gray boxes) in the presence of IL-12 (1 ng/ml) or IL-2 (10 units/ml). After 20 h of culture, supernatants were harvested and assessed for IFN-γ, TNF-α, and GM-CSF content by specific ELISA (n = 3, mean ± SD). These data are representative of at least five different experiments.
Cytokine Production by Cultured NK Cells in Response to poly(I·C) or ODN A/B. In these experiments the production of IFN-γ, TNF-α, and GM-CSF by polyclonal NK cell lines was assessed in NK cells stimulated with poly(I·C) or ODN A/B in the presence of IL-12 or IL-2. After 20 h of culture, supernatants were harvested and assessed for cytokine content by specific ELISA. As shown in Fig. 3c, TNF-α production was detectable in NK cells cultured with IL-12. Higher levels were detected in cultures also containing poly(I·C) or ODN A/B. On the other hand, IFN-γ production was significantly up-regulated by poly(I·C) in the presence of either IL-12 or IL-2. Finally, at variance with freshly purified NK cells, GM-CSF production was increased by all different stimuli, although maximal responses were detected in the presence of IL-2.
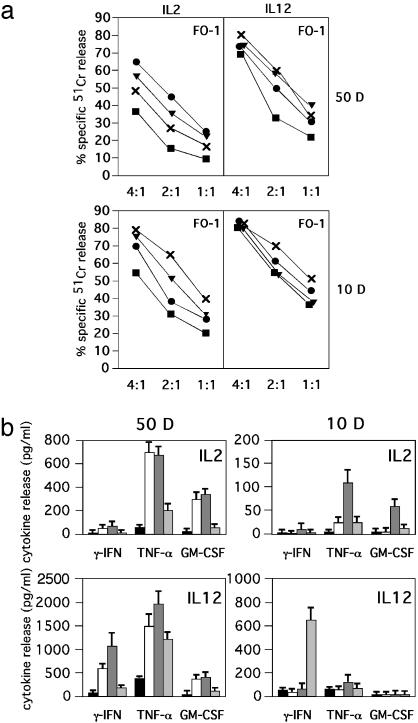
Effect of poly(I·C), ODN A, or ODN B in NK Cell Clones. In view of the phenotypic and functional heterogeneity of NK cells, we analyzed whether phenotypically distinct NK cell clones would respond differently to stimuli acting on TLR3 and TLR9. To this end, after culture (20 h) in the presence of the stimuli described above, NK clones, characterized by either the NKG2A+KIR- or NKG2A-KIR+ surface phenotype, were first assessed for surface expression of informative NK markers including the various activating receptors. Although not shown, TLR stimulation in the presence of IL-2 or IL-12 did not significantly modify the surface phenotype of the NK cell clones analyzed. On the contrary, their cytolytic activity was significantly up-regulated by the engagement of TLR in the presence of suboptimal doses of IL-2. Indeed, when the NKG2A+KIR- NK cell clone 50D was assessed for cytotoxicity against FO-1 target cells, a significant increment of lysis was detected after culture with IL-2 plus ODN A and, to a lower extent, in the presence of IL-2 and ODN B or poly(I·C) (Fig. 4a).
Fig. 4.
TLR stimulation up-regulates cytotoxicity and cytokine release by human NK clones. (a) Two representative NK clones, characterized by the NKG2A+KIR- (50D) or NKG2A-KIR+ (10D) phenotype, were cultured in medium supplemented with IL-2 (10 units/ml) or IL-12 (1 ng/ml) in either the absence (black boxes) or presence of ODN A (black circles), ODN B (black triangles), or poly(I·C) (×) for 20 h and were then assessed for cytotoxicity against the FO-1 target cell line at different E/T ratios. Each value represents the mean of triplicate experiments. The SD did not exceed 4% in the cytotoxicity assays. (b) The same NK clones were cultured with medium (black boxes), ODN A (white boxes), ODN B (dark-gray boxes), or poly(I·C) (light-gray boxes) in the presence of IL-2 (10 units/ml) or IL-12 (1 ng/ml). After 20 h of culture, supernatants were harvested and assessed for IFN-γ, TNF-α, and GM-CSF content by specific ELISA (n = 3, mean ± SD). These data are representative of at least five different experiments.
On the other hand, the cytolytic activity of the NKG2A-KIR+ NK cell clone 10D against the same target was up-regulated primarily after culture in the presence of IL-2 and poly(I·C) from 32% to 67% at an effector-to-target cell (E/T) ratio of 2:1. Lower increments could be detected when this clone was cultured with IL-2 and ODN B (53%) or ODN A (39%). The same stimuli were less efficient in up-regulating the cytolytic activity of NK cell clones stimulated in the presence of IL-12.
The same NK cell clones were also evaluated for cytokine release (Fig. 4b). The NKG2A+KIR- NK clone 50D produced large amounts of both TNF-α and GM-CSF after triggering with suboptimal doses of IL-2 and ODN A or ODN B. On the other hand, with suboptimal doses of IL-12, the same NK clone released also IFN-γ in response to the same stimuli. This NK clone displayed little IFN-γ release in response to poly(I·C) (in the presence of both IL-2 and IL-12). Thus, in response to poly(I·C), only a marginal increment of TNF-α production could be detected.
The KIR+NKG2A- NK clone 10D displayed marked differences in terms of cytokine release as compared with the KIR-NKG2A+ 50D clone. Thus, it was essentially characterized by a high release of TNF-α and GM-CSF when stimulated by IL-2 plus ODN B, whereas it produced a remarkable amount of IFN-γ when stimulated by IL-12 plus poly(I·C).
Altogether, these data provide direct evidence for the existence of clonal heterogeneity among human NK cells in the ability to respond to stimuli acting on TLR3 or TLR9. Moreover, the type of cytokine released in response to these stimuli was influenced by the cytokine environment existing during NK cell stimulation.
Discussion
Our present study provides experimental evidence that pathogen-associated products known to strongly activate DCs can also act on NK cells. This effect is made possible by the release of proinflammatory cytokines and chemokines by DCs or other cell types during the early events of an inflammatory response to infection.
Evidence has been provided for the existence of a remarkable crosstalk between NK cells and DCs that may serve as a control switch between innate and adaptive immune responses (23–25). It has also been suggested that NK cells may be capable of selecting the most appropriate DCs for subsequent T cell priming within secondary lymphoid compartments (23, 32). This process of “quality control” mediated by NK cells would be based on their ability to eliminate some DCs and favor maturation of other DCs after Ag capture. However, both of these functional effects require NK cell activation, because freshly isolated peripheral blood NK cells were unable to kill autologous iDCs or favor their maturation.
As shown here, human NK cells are able to respond to stimuli acting on different TLRs expressed by myeloid DCs. Thus, dsRNA as well as CpG, in the presence of IL-12, induce NK cell activation characterized by (i) de novo expression of activation markers such as CD69 and CD25, (ii) release of various cytokines including IFN-γ and TNF-α, (iii) up-regulation of antitumor cytotoxicity, and (iv) acquisition of cytotoxicity against iDCs [only in the presence of IL-12 plus poly(I·C)]. However, the ability to respond to dsRNA or CpG is not confined to fresh NK cells, because significant responses were also elicited by activated NK cell populations or NK cell clones. Thus, pathogens that induce maturation of DCs may simultaneously act on NK cells that become activated and can effectively interact with DCs. For example, viral infections, characterized by the release of dsRNA, are likely to act simultaneously on monocyte-derived iDCs and NK cells. Remarkably, both of these cell types can be recruited by chemokine gradients to inflammatory sites. As a consequence, NK cells, in the presence of IL-12 released by DCs soon after Ag uptake, would acquire the ability to eliminate those DCs that are susceptible to NK-mediated killing. These cells are likely represented by iDCs that have failed to take up Ag but would also include those DCs that after Ag uptake do not up-regulate HLA class I molecules to levels required to acquire resistance to killing mediated by KIR-NKG2A+ NK cells (22). The NK-mediated process of quality control would then positively select only those DCs that, during the process of maturation, reach a threshold of HLA-E expression sufficient to ensure resistance to NKG2A+ NK cells (22). On the other hand, the ability of NK cells to exert a regulatory control on DCs would not be based exclusively on this cytolytic mechanism but would be primarily consequent to the production of IFN-γ and TNF-α in response to dsRNA and IL-12 (released by monocyte-derived DCs after Ag uptake). In turn, IFN-γ produced by NK cells is likely to induce up-regulation of TLR expression by monocyte-derived iDCs. As a consequence, there will be an increase in the number of DCs equipped with high surface density of receptors involved in Ag uptake (19). In the case of responses to CpG, plasmacytoid rather than monocyte-derived DCs would be involved. Accordingly, the DC-derived soluble factor available for TLR9-responsive NK cells would be IFN-α instead of IL-12, which will determine a different effect on the subsequent functional responses by NK cells (33).
The capability of responding to dsRNA or CpG seems to be common to most peripheral blood NK cells, which was suggested by the fact that CD69 was induced on virtually all NK cells stimulated with dsRNA or CpG in the presence of IL-12. However, different NK cells may elicit different functional responses, as revealed by the analysis of NK cell clones. Additional studies may help to clarify whether the heterogeneity in functional responses by the two NK clones analyzed in this study (NKG2A+KIR- and NKG2A-KIR+) may be correlated with phenotypically defined NK cell subsets. It may be interesting also to evaluate to what extent different stimuli for TLRs may be capable to modulate the expression of TLR mRNA in both freshly isolated and NK cell clones. Our data on the mRNA expressed by polyclonally activated NK cells are in line with those reported previously on fresh NK cells. However, this analysis will be more informative when the same NK cells are tested comparatively before and after TLR-dependent stimulation.
Our data also provide evidence that dsRNA- or CpG-dependent NK cell stimulation can be promoted by IL-8. Although weaker as compared with IL-12, responses to TLR in the presence of IL-8 were characterized by the up-regulation of cytolytic activity (Fig. 2b) and expression of activation markers (Fig. 1b). However, no significant cytokine release occurred. We show that culture of PBMC-derived NK cells in the presence of dsRNA and IL-8 promotes potent antitumor cytotoxicity against “NK-resistant” cell lines. Because IL-8 may be produced by endothelial cells or tissue macrophages during inflammation, it seems that, in inflamed tissues, NK cells may acquire antitumor cytolytic activity even in the absence of effective DC stimulation. If this hypothesis holds true, NK cells that are recruited to tissues in response to IL-8 gradients could directly acquire effector function and eliminate tumor cells. Indeed, freshly derived NK cells were shown previously to express receptors for IL-8 (CXCR1) (23, 34) and to display migratory capability in response to this chemokine. A limiting factor in this scenario would be the availability of virus-derived dsRNA, necessary to induce cytotoxicity by NK cells responding to IL-8. Notably, however, in the presence of IL-8 (at variance with IL-12), dsRNA-responsive NK cells do not acquire the capability of killing iDCs, which further supports the notion that IL-12 represents a DC-derived factor, crucial for allowing dsRNA-stimulated NK cells to select DCs within abused tissues.
Acknowledgments
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità, Ministero della Sanità, and Ministero dell'Università e della Ricerca Scientifica e Tecnologica. The financial support of Fondazione Compagnia di San Paolo (Torino, Italy) is also gratefully acknowledged.
Abbreviations: TLR, Toll-like receptor; DC, dendritic cell; dsRNA, double-stranded RNA; iDC, immature DC; Ag, antigen; KIR, killer Ig-like receptor; TNF-α, tumor necrosis factor α; PBMC, peripheral blood mononuclear cell; rh, recombinant human; ODN, oligodeoxynucleotide; GM-CSF, granulocyte/macrophage colony-stimulating factor; E/T, effector-to-target cell; poly(I·C), polyinosinic-polycytidylic acid.
References
- 1.Medzhitov, R. & Janeway, C., Jr. (2000) N. Engl. J. Med. 343, 338-344. [DOI] [PubMed] [Google Scholar]
- 2.Muzio, M., Polentarutti, N., Bosisio, D., Prahladan, M. K. & Mantovani, A. (2000) J. Leukocyte Biol. 67, 450-456. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., Takeda, K. & Kaisho, T. (2001) Nat. Immunol. 2, 675-680. [DOI] [PubMed] [Google Scholar]
- 4.Aderem, A. & Ulevitch, R. J. (2001) Nature 406, 782-787. [DOI] [PubMed] [Google Scholar]
- 5.Akira, S. (2003) Curr. Opin. Immunol. 15, 5-11. [DOI] [PubMed] [Google Scholar]
- 6.Campos, M. A., Almeida, I. C., Takeuchi, O., Akira, S., Valente, E. P., Procopio, D. O., Travassos, L. R., Smith, J. A., Golenbock, D. T. & Gazzinelli, R. T. (2001) J. Immunol. 167, 416-423. [DOI] [PubMed] [Google Scholar]
- 7.Underhill, D. M., Ozinsky, A., Hajjar, A. M., Stevens, A., Wilson, C. B., Bassetti, M. & Aderem, A. (1999) Nature 401, 811-815. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature 413, 732-738. [DOI] [PubMed] [Google Scholar]
- 9.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085-2088. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., Eng, J. K., Akira, S., Underhill, D. M. & Aderem, A. (2001) Nature 410, 1099-1103. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi, H., Kaisho, T., Takeuchi, O., Sato, S., Sanjo, H., Hoshino, K., Horiuchi, T., Tomizawa, H., Takeda, K. & Akira, S. (2002) Nat. Immunol. 3, 196-200. [DOI] [PubMed] [Google Scholar]
- 12.Krieg, A. M. (2002) Annu. Rev. Immunol. 20, 709-760. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi, H., Takeuchi, O., Kawai, T., Kaisho, T., Sato, S., Sanjo, H., Matsumoto, M., Hoshino, K., Wagner, H., Takeda, K. & Akira, S. (2000) Nature 408, 740-745. [DOI] [PubMed] [Google Scholar]
- 14.Bauer, S., Kirschning, C. J., Hacker, H., Redecke, V., Hausmann, S., Akira, S., Wagner, H. & Lipford, G. B. (2001) Proc. Natl. Acad. Sci. USA 98, 9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeshita, F., Leifer, C. A., Gursel, I., Ishii, K. J., Takeshita, S., Gursel, M. & Klinman, D. M. (2001) J. Immunol. 167, 3555-3558. [DOI] [PubMed] [Google Scholar]
- 16.Jarrossay, D., Napolitani, G., Colonna, M., Sallusto, F. & Lanzavecchia, A. (2001) Eur. J. Immunol. 31, 3388-3393. [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki, N., Ho, S., Antonenko, S., Malefyt, R. W., Kastelein, R. A., Bazan, F. & Liu, Y. J. (2001) J. Exp. Med. 194, 863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krug, A., Towarowski, A., Britsch, S., Rothenfusser, S., Hornung, V., Bals, R., Giese, T., Engelmann, H., Endres, S., Krieg, A. M., et al. (2001) Eur. J. Immunol. 31, 3026-3037. [DOI] [PubMed] [Google Scholar]
- 19.Bosisio, D., Polentarutti, N., Sironi, M., Bernasconi, S., Miyake, K., Webb, G. R., Martin, M. U., Mantovani, A. & Muzio, M. (2002) Blood 99, 3427-3431. [DOI] [PubMed] [Google Scholar]
- 20.Liu, Y. J. (2001) Cell 106, 259-262. [DOI] [PubMed] [Google Scholar]
- 21.Ferlazzo, G., Tsang, M. L., Moretta, L., Melioli, G., Steinman, R. M. & Munz, C. (2002) J. Exp. Med. 195, 343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Chiesa, M., Vitale, M., Carlomagno, S., Ferlazzo, G., Moretta, L. & Moretta, A. (2003) Eur. J. Immunol. 33, 1657-1666. [DOI] [PubMed] [Google Scholar]
- 23.Moretta, A. (2002) Nat. Rev. Immunol. 2, 957-964. [DOI] [PubMed] [Google Scholar]
- 24.Piccioli, D., Sbrana, S., Melandri, E. & Valiante, N. M. (2002) J. Exp. Med. 195, 335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerosa, F., Baldani-Guerra, B., Nisii, C., Marchesini, V., Carra, G. & Trinchieri, G. (2002) J. Exp. Med. 195, 327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivori, S., Pende, D., Bottino, C., Marcenaro, E., Pessino, A., Biassoni, R., Moretta, L. & Moretta, A. (1999) Eur. J. Immunol. 29, 1656-1666. [DOI] [PubMed] [Google Scholar]
- 27.Hornung, V., Rothenfusser, S., Britsch, S., Krug, A., Jahrsdorfer, B., Giese, T., Endres, S. & Hartmann, G. (2002) J. Immunol. 168, 4531-4537. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann, G., Weeratna, R. D., Ballas, Z. K., Payette, P., Blackwell, S., Suparto, I., Rasmussen, W. L., Waldschmidt, M., Sajuthi D., Purcell R. H., et al. (2000) J. Immunol. 164, 1617-1624. [DOI] [PubMed] [Google Scholar]
- 29.Bourke, E., Bosisio, D., Golay, J., Polentarutti, N. & Mantovani, A. (2003) Blood 102, 956-963. [DOI] [PubMed] [Google Scholar]
- 30.Pende, D., Parolini, S., Pessino, A., Sivori, S., Augugliaro, R., Morelli, L., Marcenaro, E., Accame, L., Malaspina, A., Biassoni, R., et al. (1999) J. Exp. Med. 190, 1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferlazzo, G., Morandi, B., D'Agostino, A., Meazza, R., Melioli, G., Moretta, A. & Moretta, L. (2003) Eur. J. Immunol. 33, 306-313. [DOI] [PubMed] [Google Scholar]
- 32.Ferlazzo, G., Thomas, D., Lin, S. L., Goodman, K., Morandi, B., Muller, W. A., Moretta, A. & Munz, C. (2004) J. Immunol. 172, 1455-1462. [DOI] [PubMed] [Google Scholar]
- 33.Kerkmann, M., Rothenfusser, S., Hornung, V., Towarowski, A., Wagner, M., Sarris, A., Giese, T., Endres, S. & Hartmann, G. (2003) J. Immunol. 170, 4465-4474. [DOI] [PubMed] [Google Scholar]
- 34.Campbell, J. J., Qin, S., Unutmaz, D., Soler, D., Murphy, K. E., Hodge, M. R., Wu, L. & Butcher, E. C. (2001) J. Immunol. 166, 6477-6482. [DOI] [PubMed] [Google Scholar]