Abstract
Introduction
Thromboangiitis obliterans or Buerger’s disease is a nonatherosclerotic, segmental, inflammatory vasculitis that is strongly associated with tobacco products and commonly affects the small- and medium-sized arteries of the upper and lower extremities. However, the disease can, rarely, involve large central or visceral arteries. We report here the case of end stage renal disease due to renal artery thrombosis caused by thromboangiitis obliterans.
Case presentation
A 51-year-old Korean man who had previously required amputation of both great toes due to thromboangiitis obliterans presented with left flank pain and oliguria. Both his renal arteries were occluded on contrast-enhanced abdominal computed tomography and abdominal angiography. He also had abdominal angina. He had no risk factor of thromboembolism from cardiac origin, atherosclerosis except for tobacco abuse, collagen diseases or hypercoagulable disorders. Renal failure and mesenteric ischemia associated with thromboangiitis obliterans progression was diagnosed.
Conclusions
Renal failure due to renal artery thrombosis and mesenteric ischemia represents an unusual manifestation of thromboangiitis obliterans. But once it occurs, it can be life-threatening. When we care for a patient with thromboangiitis obliterans, we should pay attention to this rare disease course, and encourage cessation of the smoking of tobacco products.
Keywords: End stage renal disease, Infarction, Kidney, Mesenteric ischemia, Thromboangiitis obliterans
Introduction
Thromboangiitis obliterans (TAO) or Buerger’s disease is a nonatherosclerotic, segmental, inflammatory vasculitis that is strongly associated with tobacco products and commonly affects the small- and medium-sized arteries of the upper and lower extremities. It was first described and established in the English literature in 1908 as a clinicopathologic entity distinct from atherosclerosis [1]. TAO usually occurs in young male patients and is associated with tobacco consumption; it presents with a highly cellular thrombus with relative sparing of the blood vessel wall and an absence of elevated acute-phase reactants or immunological markers. It is reported that the prevalence of TAO among all patients with peripheral arterial disease is higher in East Asia (16−66%) than Western Europe (0.5−5.6%) [2]. Although it is rare, the disease can involve large central or visceral arteries and cause intestinal ischemia or renal infarction.
We report a case of end stage renal disease caused by renal artery thrombosis and abdominal angina associated with TAO.
Case presentation
A 51-year-old Korean man was admitted to our hospital because of severe left flank pain, hematuria, and oliguria for 3 days. Additional complaints included epigastric discomfort and generalized weakness, but he denied fever or emesis. He had a medical history of hypertension for 1 year and TAO for 10 years with intermittent claudication. He had undergone amputation of both of his great toes 10 years prior because of gangrenous change due to TAO. At that time, lower extremity angiography showed that the flow of the right distal portion of the popliteal artery and the proximal portion of the tibiofibular artery were remarkably decreased by occlusion. The left superficial femoral artery was also occluded from its origin, at which collateral arteries had developed (Fig. 1). He took beraprost for TAO but had not stopped smoking tobacco products. He had smoked approximately 1 pack per day for 30 years. Four years later, he underwent repeat angiography of his abdominal aorta and lower extremities because of worsening claudication. Occlusion of his left superficial femoral artery, bilateral tibial, and peroneal arteries had progressed. He had never been diagnosed with diabetes mellitus, collagen disease or cardiac disease.
Fig. 1.
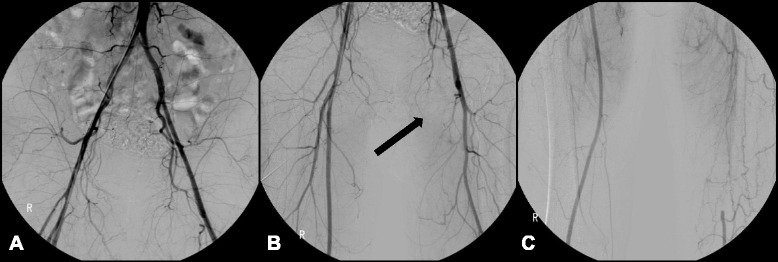
Lower extremity angiography (2004). a Both common iliac arteries showed patent flow without stenosis or occlusion. b The left superficial femoral artery was occluded at its origin (arrow). c The left distal superficial femoral artery was reconstituted by an abnormal corkscrew collateral blood flow from the left deep femoral artery
Upon presentation, his blood pressure was 180/100mmHg, and his body temperature was 36.4°C. He complained of severe tenderness in his left costovertebral angle area. Raynaud’s toe, skin nodules and phlebitis were not observed.
Laboratory findings showed the following: white blood cell count (WBC) 9700/uL, hemoglobin (Hb) 12.7g/dL, platelets 201×103/uL, serum creatinine 14.02mg/dL, creatinine clearance 3.6ml/minute/1.73m2 according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, β2 microglobulin 19.80mg/L, phosphorus 5.48mg/dL, intact parathyroid hormone (PTH) 329.5pg/mL, creatine phosphokinase (CPK) 74U/L and lactate dehydrogenase (LDH) 1687IU/L. Urine sediment contained 0 to 2 WBC and 3 to 5 red blood cells (RBC) per field. Urine protein electrophoresis revealed no paraprotein bands. The blood lipid profile, coagulation tests, protein C, protein S activity, complement fractions, antinuclear antibodies, rheumatoid factor, anti-Scl-70, anticardiolipin and antiphospholipid, and antineutrophil cytoplasmic antibodies were all negative or within normal limits. Electrocardiography and echocardiography were normal.
Contrast-enhanced abdominal computed tomography (CT) demonstrated left kidney enlargement (9.3cm) with a multifocal infarcted area and a shrunken right kidney (7.6cm). Neither renal artery was visualized (Fig. 2).
Fig. 2.
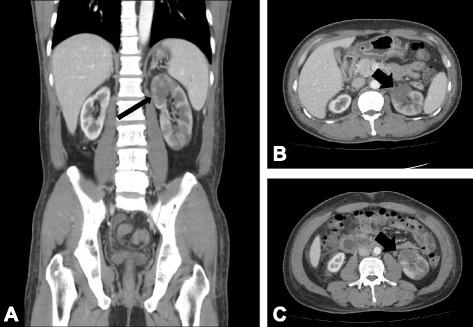
Contrast-enhanced abdominal computed tomography. a Coronal and (b, c) transverse scans showed left kidney enlargement with a multifocal infarcted area (arrows). Neither renal artery was traced from the proximal part on computed tomography
Abdominal and lower extremity angiography was performed to examine his abdominal aorta and lower extremity arteries. The vascular status in both lower limbs and the viscera had worsened. His superior mesenteric artery, inferior mesenteric artery, both renal arteries, left common iliac artery, and left superficial femoral artery were not visualized, and the arteries below both his knees were occluded. Collateral vessels were well developed in his lower extremities. During examination, a stent was inserted into his left common iliac artery (Fig. 3). Upper extremity angiographic CT showed no abnormal findings. His ankle-brachial index was 0.82 on the right and 0.61 on the left.
Fig. 3.
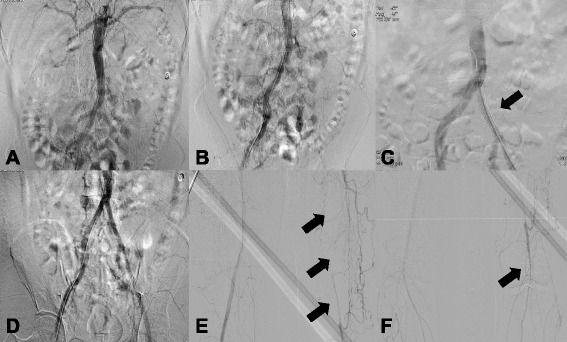
Abdominal and lower extremity angiography (2014). a Renal angiography could not identify either renal artery due to total occlusion. b Lower extremity angiography showed a chronic total obstruction lesion of the left common iliac artery due to progression of chronic thrombosis. c A stent was deployed at the site of occlusion of the left common iliac artery (arrow). d Flow was recovered. e Obstruction of the left superficial femoral artery and abnormal corkscrew collateral blood supply from the left deep femoral artery was similar to that seen in 2004 (arrows). f The left tibioperoneal trunk was occluded (arrow), and blood flow below the knee was supplied by collateral vessels
Renal failure associated with TAO progression was diagnosed. He started continuous ambulatory peritoneal dialysis (CAPD) and stopped smoking tobacco products. He was discharged with a daily oral anticoagulant, warfarin. Two months after discharge, he complained of postprandial abdominal pain without muscle guarding, preventing him from eating and resulting in an approximately 10kg weight loss. Upper gastrointestinal endoscopy revealed gastric mucosal atrophy; a follow-up contrast-enhanced abdominal CT showed colitis of the hepatic flexure and transverse colon, which was consistent with ischemic colitis. His vessel status had not changed compared with that at the prior examination (Fig. 4). He changed his dialysis modality from CAPD to hemodialysis, which improved his pain. He recently reported abdominal pain with hypotension; however, after a decrease in his hypertension medications and an increase in body weight, his pain resolved.
Fig. 4.
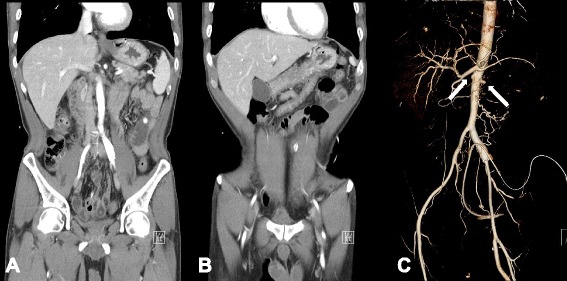
Contrast-enhanced abdominal CT and abdominal aorta CT angiography. Contrast-enhanced abdominal CT demonstrated colitis of the (a) hepatic flexure and (b) transverse colon, most likely due to ischemic colitis. c Abdominal aorta CT angiography showed total occlusion of both renal arteries (white arrows). Superior and inferior mesenteric arteries cannot be seen because the arteries were occluded from their origins
Discussion
Many kinds of diseases have been identified as causes of renal infarction, the most common of which are cardiogenic thromboembolism and atheromatous disease. Other less frequent causes include trauma, hypercoagulable state, cocaine abuse, and neoplastic and renal vascular disease [3]. Even though TAO commonly affects the small- and medium-sized arteries of the upper and lower extremities, a few cases of kidney infarction and thrombosis of visceral vessels have been reported [4–9].
In some of these cases, visceral damage is more likely to be a result of atherosclerosis or other diseases, so a broad differential diagnosis is important. The present patient had symptoms typical of TAO. Clinical criteria for the diagnosis of TAO include a cigarette smoking history, onset before age 50, infrapopliteal arterial occlusive disease, either upper limb involvement or phlebitis migrans, and the absence of atherosclerotic risk factors other than cigarette smoking [10]. The present patient had intermittent claudication and an ischemic ulcer beginning at 42 years of age. He had no atherosclerotic risk factors except for tobacco abuse. He denied any drug ingestion other than an antihypertensive agent (nifedipine) and beraprost. Although hypertension was diagnosed 1 year prior, it seemed likely that this was a result of renal artery occlusion caused by TAO. No serologic data suggested collagen disease or anticoagulation disorder. An electrocardiogram did not show atrial fibrillation that could cause distal embolization. An angiogram of his lower limb showed abrupt occlusion and a tree root pattern, findings typical of TAO. Although histological examination of arterial tissue was not possible in the present study, aortography demonstrated no atheromatous plaques in the remaining portion of the aorta. So thrombosis in his visceral arteries including both renal arteries, associated with TAO progression was diagnosed.
We comprehensively reviewed the English literature reporting renal involvement in TAO in either abstract or full text form [4–9]. The clinical characteristics of six patients with TAO who showed renal artery involvement with TAO, including the present case, are summarized in Table 1. Among the seven cases, one was excluded because it was not an English report [7]. The mean age was 40.3 years, and all patients were male. TAO was diagnosed in five of six cases before renal artery involvement, except one in which the medical history could not be found in the literature. The average duration from initial diagnosis of TAO to involvement of the renal arteries was 11 years (range 7–15 years). Severe hypertension was the most common symptom, followed by flank pain caused by renal infarction. In five cases, visceral arteries including the descending aorta were involved. None of the patients stopped smoking tobacco products after they were diagnosed with TAO, highlighting the importance of tobacco smoking cessation to prevent the progression of TAO.
Table 1.
Reported cases of renal artery involvement of thromboangiitis obliterans
| Case | Age/sex | Diagnosed TAO before (duration) | Symptom | Affected visceral artery | Treatment | Reference (reported year) |
|---|---|---|---|---|---|---|
| 1 | 34/M | Yes | Severe hypertension | Right renal artery | Right nephrectomy | Malisoff and Macht (1951) [4] |
| 2 | 30/M | Yes (15 years) | Diffuse back and muscle pain | Descending aorta, celiac axis, iliac artery, femoral artery, coronary artery, left renal artery | Flesh et al. (1977) [5] | |
| 3 | 42/M | Yes (12 years) | Severe hypertension | Left renal artery, aorta below the level of renal artery | Antihypertensive medication | Gomi et al. (1978) [6] |
| 4 | 51/M | Yes | Severe hypertension, respiratory distress | Both renal arteries, descending aorta, celiac trunk, superior mesenteric artery | Hepatorenal artery bypass | Stillaert et al. (2003) [8] |
| 5 | 37/M | Yes (7 years) | Right flank pain, weakness, fever | Intrarenal branches of the right renal artery | Conservative care | Goktas et al. (2006) [9] |
| 6 | 52/M | Yes (10 years) | Left flank pain, anuria, weakness | Descending aorta, both renal arteries, superior mesenteric artery, common iliac artery | Hemodialysis | This case |
M male, TAO thromboangiitis obliterans
In the presented case, the patient complained of postprandial abdominal pain after starting peritoneal dialysis. Intestinal TAO has been rarely reported. Kobayashi et al. [11] reported a case of TAO with intestinal ischemia and reviewed the literature. They summarized 26 cases of visceral TAO including their case. The mean age of patients was 39.1, and all but two patients were male. The predominant symptom was abdominal pain, and 20 of 26 patients underwent digestive organ resection. The perioperative mortality rate was 30%, and only three patients underwent conservative treatment. The present patient experienced improvement in postprandial abdominal pain after avoiding dehydration and switching from peritoneal dialysis to hemodialysis. It is known that hemodialysis is more susceptible to intestinal ischemia than peritoneal dialysis because of its more unstable hemodynamics [12]. The patient in this case underwent peritoneal dialysis for this reason; however, even though he was not dehydrated and his blood pressure was not low while receiving peritoneal dialysis, he experienced severe abdominal pain that got worse after starting peritoneal dialysis. His pain was relieved after stopping peritoneal dialysis. We presumed that hyperglycemia of the peritoneal cavity induced several changes, including leukostasis, vasoconstriction, and a pro-inflammatory state that caused aggravation of intestinal hypoxia [13].
Conclusions
Although visceral involvement, including renal and intestinal arteries, is rare in TAO, once an internal organ is affected, the disease becomes life-threatening and usually cannot be cured. When treating a patient with TAO, we have to carefully observe unusual symptoms such as anuria, flank pain, uncontrolled hypertension, and abdominal pain and strongly encourage cessation of tobacco smoking.
Consent
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgments
This study was supported by Konyang University Research Fund of 20.
Abbreviations
- CAPD
Continuous ambulatory peritoneal dialysis
- CT
Computed tomography
- TAO
Thromboangiitis obliterans
- WBC
White blood cell count
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HJY, DIK, KHL and SHY were the physicians who treated the patient in this report. SJL performed the radiology studies. The manuscript was prepared by HJY, WMH, SRY, and SHY. All authors participated in discussions about the manuscript and approved the final version.
Contributor Information
Hyo-Jin Yun, Email: yhj0927@kyuh.ac.kr.
Dong-Il Kim, Email: athlonpu@nate.com.
Kyung-Ho Lee, Email: dlrudgh@kyuh.ac.kr.
Seong-Joo Lim, Email: ecoizm@hanmail.net.
Won-Min Hwang, Email: hwangwm@kyuh.ac.kr.
Sung-Ro Yun, Email: sryun@kyuh.ac.kr.
Se-Hee Yoon, Email: sehei@hanmail.net.
References
- 1.Buerger L. Thromboangiitis obliterans: a study of the vascular lesions leading to presenile spontaneous gangrene. Am J Med Sci. 1908;136:567–80. doi: 10.1097/00000441-190810000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Vijayakumar A, Tiwari R, Kumar PV. Thromboangiitis obliterans (Buerger’s disease) – current practices. Int J Inflam. 2013;2013:1–9. doi: 10.1155/2013/156905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CC, Chen WL, Chen JH, Wu YL, Shiao CJ. Clinical characteristics of renal infarction in an Asian population. Ann Acad Med Singapore. 2008;37:416–20. [PubMed] [Google Scholar]
- 4.Malisoff S, Macht MB. Thromboangitic occlusion of the renal artery with resultant hypertension. J Urol. 1951;65:371–9. doi: 10.1016/S0022-5347(17)68493-3. [DOI] [PubMed] [Google Scholar]
- 5.Flesh LH, Kihm RH, Ciccio SS. Radionuclide imaging of aortic involvement in Buerger’s disease: case report. J Nucl Med. 1977;18:125–7. [PubMed] [Google Scholar]
- 6.Gomi T, Ikeda T, Yuhara M. Renovascular hypertension due to Buerger’s disease. Jpn Heart J. 1978;19:308–14. doi: 10.1536/ihj.19.308. [DOI] [PubMed] [Google Scholar]
- 7.Keller F, Gotzen R. A rare case: thromboangiitis obliterans in renal artery stenosis. Med Klin Prax. 1982;77:58–62. [PubMed] [Google Scholar]
- 8.Stillaert P, Louagie Y, Donckier J. Emergency hepato-renal artery bypass using a PTFE graft. Acta Chir Belg. 2003;103:524–7. doi: 10.1080/00015458.2003.11679483. [DOI] [PubMed] [Google Scholar]
- 9.Goktas S, Bedir S, Bozlar U, Ilica AT, Seckin B. Intrarenal arterial stenosis in a patient with thromboangiitis obliterans. Int J Urol. 2006;13:1243–4. doi: 10.1111/j.1442-2042.2006.01546.x. [DOI] [PubMed] [Google Scholar]
- 10.Dimmick SJ, Goh AC, Cauzza E, Steinbach LS, Baumgartner I, Stauffer E, et al. Imaging appearances of Buerger’s disease complications in the upper and lower limbs. Clin Radiol. 2012;67:1207–11. doi: 10.1016/j.crad.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Kurose K, Kobata T, Hida K, Sakamoto S, Matsubara J. Ischemic intestinal involvement in a patient with Buerger disease: case report and literature review. J Vasc Surg. 2003;38:170–4. doi: 10.1016/S0741-5214(02)75469-4. [DOI] [PubMed] [Google Scholar]
- 12.Zier M, Hupp T, Wiesel M, Rambausek M, Ritz E. Non-occlusive intestinal ischemia as a complication of hemodialysis treatment. Dtsch Med Wochenschr. 1993;118:1020–4. doi: 10.1055/s-2008-1059421. [DOI] [PubMed] [Google Scholar]
- 13.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]


