Abstract
To investigate the antigen specificity of regulatory T cells capable of preventing transplant rejection, we have developed two different strategies to achieve tolerance to fully mismatched skin grafts in euthymic mice. A combination of nondepleting Abs targeting CD4, CD8, and CD154 (CD40 ligand) induces dominant transplantation tolerance to fully mismatched skin allografts. Such tolerance is antigen-specific, mediated by regulatory T cells, and can be extended through linked suppression to naïve lymphocytes. The same protocol, when combined with allogeneic bone marrow, enables the development of mixed hematopoietic chimerism and deletional tolerance. Although we cannot exclude that some regulatory T cells may persist in chimeric mice, these cells are insufficient to mediate linked suppression. CD4+CD25+ T cells, whether taken from naïve mice or from mice tolerized through either treatment protocol, were always able to prevent rejection of skin grafts by naïve CD4+ T cells, and did so with no demonstrable specificity for the tolerizing donor antigens. Such data question whether CD4+CD25+ regulatory T cells alone can account for the antigen specificity of dominant transplantation tolerance.
Natural CD4+CD25+ regulatory T cells are known to prevent autoimmunity and gut immunopathology (1–3). They are presumed to interact with endogenous antigen, but such antigens are not yet characterized. Adoptive transfer studies have also shown that CD4+CD25+ T cells can prevent graft rejection (4). This finding has led to the claim that these same cells are responsible for dominant transplantation tolerance after therapeutic intervention. If so, we would expect CD4+CD25+ T cells from animals demonstrating dominant tolerance to show evidence of priming and specificity for the tolerated antigen. By the same token, such cells should not be detectable in animals tolerized through deletional mechanisms, where dominant tolerance has been excluded. We have used an Ab mixture of nondepleting CD4, CD8, and CD154 (CD40-ligand) Abs to produce both forms of tolerance to MHC-mismatched transplants. Tolerance induced to skin grafts as the tolerogen exhibited features of dominant tolerance. Tolerance induced to the combination of skin and donor bone marrow (BM), was associated with mixed hematopoietic chimerism and showed no linked suppression, indicating deletion as the likely mechanism. CD4+CD25+ T cells from any of the two groups of tolerized animals behaved no differently from CD4+CD25+ T cells obtained from naïve animals in being able to prevent graft rejection by naïve CD4+ T cells, yet lacking specificity for the donor alloantigens. We cannot therefore implicate CD4+CD25+ T cells alone as responsible for the exquisite donor antigen specificity of dominant transplantation tolerance. It is possible that interactions with other regulatory T cells are required.
Methods
Mice. CBA/Ca (CBA, H-2k), RAG1-/--CBA/Ca (RAG1-/-, H-2k), BALB/c (H-2d), and C57BL/10 (B10, H-2b) mice were bred and maintained in specific-pathogen-free facilities at the Sir William Dunn School of Pathology. Procedures were conducted in accordance with the Home Office Animals (Scientific Procedures) Act of 1986.
Skin Grafting. Mice were anesthetized with a mixture of 10 mg/ml hypnodil and 2 μg/ml sublimaze (Janssen, Tilburg, The Netherlands). A total of 0.12 ml per 20 g of body weight was injected i.p. Skin grafting was performed as described elsewhere (5). In short, skin grafting was conducted by transplanting full thickness tail skin (1 × 1 cm) on the lateral flank. Grafts were observed on alternate days after the removal of the bandage at day 8 and considered rejected when no viable donor skin was present. Statistical analysis of graft survival was made by the log rank method.
BM Transplantation (BMT). BM donors were depleted of T cells with an i.p. injection of 1 mg of the anti-CD8 mAbs YTS156 and YTS169, and the anti-CD4 mAbs YTS191 and YTA3.1 5 days before BM collection (6). BM was collected by flushing the femurs and tibias with R10 medium. The cells were counted, resuspended in PBS, and injected i.v. into recipient mice.
Tolerance Induction and mAbs. Tolerance was induced in CBA mice by treatment with 1 mg of YTS177.9 (7), 1 mg of YTS105.18 (7), and 1 mg of MR1 (5) at days 0, 2, and 4 after B10 skin transplantation. All mAbs were produced in our laboratory by culture in hollow fiber bioreactors, purified from culture supernatants by 50% ammonium sulfate precipitation, dialyzed against PBS, and the purity was checked by native and SDS gel electrophoresis (PhastGel, Amersham Pharmacia).
Cell Sorting and Adoptive Cell Transfer. Cells were collected from spleens of adult CBA mice. A single-cell suspension was obtained by passing the splenocytes through a 70-μm cell strainer (BD Biosciences, Oxford, U.K.), and the erythrocytes were depleted by water lysis. Cells were counted, diluted in PBS, and were injected i.v. into the tail vein. CD4+CD25+ and CD4+CD25- T cells were sorted as described (8). In brief, spleen cells were depleted of CD8+, B cells, and antigen-presenting cells by incubating them with the mAbs M5/114, 187.1, and YTS156, followed by incubation with goat anti-rat IgG Dynabeads (Dynal Biotech, Oslo). The enriched population of CD4+ T cells was stained with the biotinylated anti-CD25 mAb 7D4 (BD Biosciences), and positively selected with streptavidin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) by using an autoMACS (Miltenyi Biotec). The CD4+CD25- T cells were isolated from the CD25-depleted fraction by using positive selection with anti-CD4 microbeads (Miltenyi Biotec). CD4+ T cells were sorted from spleens of CD8-depleted mice, by using anti-CD4 microbeads (Miltenyi Biotec). CD4+CD25+ and CD4+CD25- T cells from chimeric mice were selected by flow cytometry using a MoFlo cell sorter (DakoCytomation, Fort Collins, CO), from splenocytes labeled with CD4-FITC, CD25-PE, and H2-Db-biotin with a secondary step with streptavidin-CyCr (all from BD Biosciences). Typically, the purity of the CD4+CD25+ cells sorted with magnetic beads was between 85% and 95%. The purity of other cell populations was typically ≈95%.
Flow Cytometry. Hematopoietic chimerism was quantified by staining peripheral blood with mAbs specific for H2-Db, H2-Kk, and H2-Kd (all from BD Biosciences). Cells were analyzed with a FACScalibur (BD Biosciences) and cellquest software (BD Biosciences).
Results
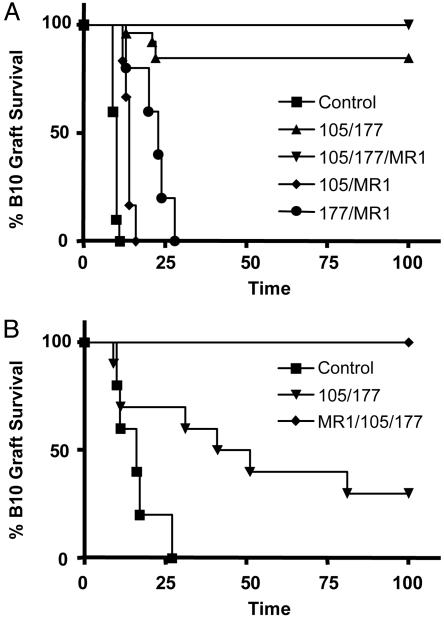
A CD4, CD8, and CD154 mAb Mixture Enables Tolerance to Fully Mismatched Skin Grafts. A brief treatment with a combination of nondepleting CD4, CD8, and CD154, but not a combination of any two of the mAbs, is capable of inducing tolerance to fully mismatched skin grafts in CBA mice. Adult euthymic mice were transplanted with B10 skin and treated with a short course of nondepleting CD4 (YTS 177), CD8 (YTS 105), and CD154 (MR1) mAbs. Mice treated with a combination of the three mAbs accepted the tolerated skin grafts indefinitely, as did most of the animals treated with anti-CD4 plus anti-CD8 mAbs. All mice treated with other mAb combinations readily rejected the skin grafts (Fig. 1A).
Fig. 1.
Tolerance induction to fully mismatched skin grafts. (A) CBA mice were treated with three doses of 1 mg of the anti-CD4, anti-CD8, and anti-CD154 nondepleting mAbs over 1 week after B10 skin transplantation at day 0. Only mice treated with the three mAbs (▾, n = 6, MST >100 days) and anti-CD4 plus anti-CD8 (▴, n = 26, MST >100 days) showed indefinite graft survival (P < 0.05 to any other group). All grafts from mice treated with anti-CD4 plus anti-CD154 (•, n = 6, MST = 21.5 days), anti-CD8 plus anti-CD154 (♦, n = 6, MST = 14 days), and untreated controls (▪, n = 10, MST = 10 days) were rejected. (B) After 100 days, mice with surviving skin allografts received fresh B10 skin transplants. In animals treated with the three mAbs, no rejections were observed (♦, n = 6, MST >100 days). Mice treated with only anti-CD4 and anti-CD8 rejected the skin allografts (▾, n = 10, MST = 46 days, P = 0.011 vs. ♦). The control group treated with the same three mAbs (as ♦) in the absence of initial transplant rejected the skin grafts (▪, n = 5, MST = 16 days).
One hundred days after transplantation, mice with surviving skin allografts received a further donor (B10) skin graft, as did animals treated with all three mAbs in the absence of the initial donor grafts (Fig. 1B). No rejection was observed in the group rendered tolerant to B10 skin by treatment with the three mAbs. This outcome cannot be a consequence of persistence of mAbs in the circulation, because the control group injected with the same mAbs, but in the absence of an initial graft, readily rejected the transplanted skin. Mice pretreated with just the CD4 and CD8 mAbs showed extended survival of the initial allografts, but rejected both these and new donor skin transplants, albeit slowly, after the secondary graft challenge. Mice tolerized with the three mAbs remained fully competent to reject third-party BALB/c skin transplants, at a similar rate to nontolerant mAb-treated animals (data not shown).
Tolerance Induced to Transplanted Skin Is Antigen-Specific and Exhibits Linked Suppression. Linked suppression has been a constant feature of dominant transplantation tolerance induced by treatment with nondepleting CD4 or CD154 mAbs (5, 9–11). We found that the combination of the three mAbs induced a very powerful form of linked suppression across fully mismatched skin allografts.
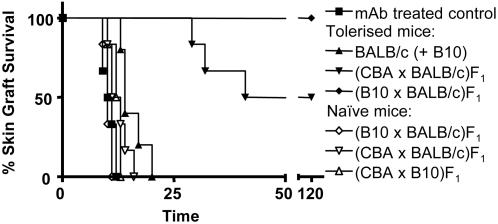
CBA mice were tolerized to B10 skin grafts as described. Fig. 2 shows graft survival for skin transplanted at 100 days. Mice grafted with (B10×BALB/c)F1 skin held their grafts indefinitely, whereas nontolerant mice rejected theirs. When mice were transplanted with both B10 and BALB/c skin in the same graft bed, BALB/c grafts were rejected, with two mice from this group subsequently rejecting the B10 grafts (on days 22 and 27, not represented in Fig. 2). In a repeat experiment, BALB/c rejection did not lead to B10 rejection in any animals (n = 5), and in all experiments where BALB/c and B10 skin grafts were transplanted sequentially, only BALB/c grafts were rejected. It is possible that in the two mice that had rejected the tolerated B10 grafts, crossreactivity of T cells recognizing antigens presented by donor antigen-presenting cells (direct presentation) overcame the effect of regulatory cells that were maintaining B10 grafts.
Fig. 2.
Linked suppression across fully mismatched skin transplants. CBA mice were tolerized to B10 skin grafts as described. One hundred days after tolerance induction (day 0 in the graph), mice were transplanted with (CBA×BALB/c)F1 (▾, n = 6, MST = 80.5 days), (B10×BALB/c)F1 (♦, n = 6, MST >150 days) skin grafts, or both BALB/c and B10 skin grafts onto the same graft bed (only BALB/c graft survival represented: ▴, n = 5, MST = 14 days). Ab-treated mice not transplanted with tolerizing skin were now grafted with B10 skin (▪, n = 6, MST = 10.5 days). Naïve mice were transplanted with (B10×BALB/c)F1 (♦, n = 6, MST = 10 days), (CBA×BALB/c)F1 (▵, n = 6, MST = 12 days), or (CBA×B10)F1 (▿, n = 6, MST = 12.5 days) skin grafts. All tolerized mice grafted with (B10×BALB/c)F1 skin accepted the grafts (♦, P < 0.001 to any other group except ▾ ; nonsignificant), whereas mice grafted with (CBA×BALB/c)F1 skin showed a delayed rejection with half the mice accepting the grafts indefinitely (▾, P < 0.001).
Three of six CBA mice tolerized with B10 skin allografts rejected (CBAxBALB/c)F1 skin transplants at a slower rate, and the remaining mice accepted their grafts indefinitely (Fig. 2). To confirm that this result was not due to a subtle form of immunosuppression, CBA mice treated with the same mAbs in the absence of a tolerizing graft were shown capable of rejecting (CBA×BALB/c)F1 skin grafts at the same rate as BALB/cskin grafts [n = 7; median survival time (MST) = 14 days vs. 12 days]. Naïve CBA mice readily rejected (CBA×BALB/c)F1 or (CBA×B10)F1 skin grafts. We interpret this result as a form of linked suppression due to regulatory T cells recognizing a set of indirectly processed B10 antigens shared with BALB/c but foreign to CBA (12).
One of the potential therapeutic benefits of linked suppression would be the dissemination of tolerance to third-party antigens, which can be obtained by transplanting hosts tolerant to the second party with tissues exhibiting the combination of tolerated and third-party antigens (5, 9–11). We confirmed that this was possible by transplanting BALB/c skin onto those CBA mice, pretolerized to B10 skin, where (B10×BALB/c)F1 or (CBA×BALB/c)F1 skin had survived 50 days. All of the animals accepted the BALB/c skin (data not shown). These results show how tolerance induced to fully mismatched skin grafts can be extended to third-party antigens.
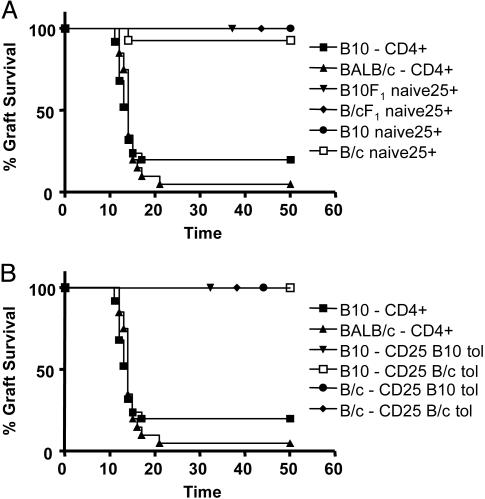
CD4+CD25+ T Cells from Naïve or Tolerized Mice Can Suppress Graft Rejection Without Any Obvious Specificity for the Tolerated Antigen. We found that CD4+CD25+ T cells from naïve mice could prevent the rejection of fully mismatched skin grafts upon transfer into lymphopenic hosts. CD4+CD25+ T cells and CD4+ T cells were sorted from the spleen of CBA naïve mice. CD4+CD25+ T cells (105) were injected together with 5 × 104 CD4+ T cells into RAG1-/- mice. The transfused mice were transplanted with BALB/c, (CBA×BALB/c)F1, B10, or (CBA×B10)F1 skin grafts (Fig. 3A). No rejection was observed in any of these experimental groups. The control mice transfused with CD4+ T cells without added CD4+CD25+ T cells readily rejected B10 and BALB/c skin grafts.
Fig. 3.
Prevention of graft rejection by CD4+CD25+ T cells irrespective of origin. (A) CD4+CD25+ T cells and CD4+ T cells were sorted from the spleen of CBA naïve mice. A quantity of 105 CD4+CD25+ T cells were injected together with 5 × 104 CD4+ T cells into RAG1-/- mice. The transfused mice were transplanted with BALB/c(□, n = 14), (CBA×BALB/c)F1 (♦, n = 7), B10 (•, n = 7), and (CBA×B10)F1 (▾, n = 7) skin grafts. The control mice transfused with CD4+ T cells without added CD4+CD25+ T cells readily rejected B10 (▪, n = 25) and BALB/c (▴, n = 20) skin grafts. (B) CD4+CD25+ T cells were sorted from spleens of CBA mice tolerized to B10 (▾, n = 20; •, n = 13) or BALB/c(□, n = 10; ♦, n = 21) skin grafts. A quantity of 105 of these cells were injected together with 5 × 104 CD4+ T cells from naïve mice into RAG1-/- mice that were transplanted with B10 (▾ and □) or BALB/c (• and ♦) skin grafts. Mice transfused with CD4+ T cells alone rejected B10 (▪, n = 25) or BALB/c(▴, n = 20) skin grafts.
To investigate whether tolerance induction would lead to antigen-specific regulation by CD4+CD25+ T cells, we transplanted CBA mice with either B10 skin or BALB/c skin under the cover of nondepleting CD4, CD8, and CD154 mAbs as described. Whereas full tolerance can be achieved to B10 skin, such is not the case for BALB/c grafts with most being eventually rejected (n = 23, MST = 86 days). CD4+CD25+ T cells were sorted from the spleens of these mice 100 days after transplantation and were injected together with 5 × 104 CD4+ T cells from naïve CBA mice into RAG1-/- mice (Fig. 3B). The transfused mice were transplanted with BALB/c or B10 skin. Injection of 105 CD4+CD25+ T cells from the mice initially transplanted with B10 skin was able to prevent rejection of either B10 or BALB/c skin grafts. Similar results were obtained with the same number of CD4+CD25+ T cells from mice initially transplanted with BALB/c skin, where complete tolerance had not been achieved.
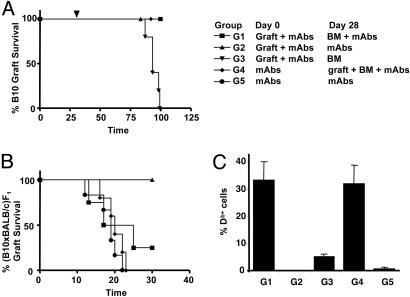
Nondepleting Abs CD4, CD8, and CD154 Facilitate the Induction of Mixed Hematopoietic Chimerism and Tolerance Without Dominant Regulation. Previous reports (13, 14) have shown that CD154 mAbs allow the development of stable mixed hematopoietic chimerism, in the absence of myeloablation, when megadoses of donor BM are transplanted. A recent report (15) has shown that the induction of peripheral tolerance can facilitate the induction of hematopoietic chimerism without myeloablation. We used our same tolerizing protocol as above, with the three mAbs targeting CD4, CD8, and CD154, to induce mixed chimerism and tolerance without myeloablative conditioning. We grafted B10 skin onto CBA mice under the cover of the three mAbs as described. One month after this initial treatment, mice were treated with 40 × 106 BM cells from T cell-depleted B10 donors with or without an additional mAb course. Pretolerization with a donor skin was not essential, because mice treated initially with mAbs in the absence of a donor graft were able to accept donor skin grafts transplanted at the time of BMT and mAb treatment (Fig. 4A and Fig. 6, which is published as supporting information on the PNAS web site). It is interesting to note that donor BM given 1 month after tolerance induction abrogated tolerance unless a further course of mAbs was combined with the BMT. Tolerance achieved with donor BM did not manifest as dominant because we could not demonstrate linked suppression [(B10×BALB/c)F1 skin grafts were rejected as shown Fig. 4B]. Flow cytometric analysis of peripheral blood confirmed that mixed chimerism was maintained in the tolerant mice 120 days after BMT (Fig. 4C). Similar results were obtained by using BALB/c mice, where the mAb treatment prolongs B10 graft survival without leading to permanent acceptance of skin grafts (Fig. 6). However, tolerance and long-term hematopoietic chimerism were achieved when donor BMT was associated with the mAb treatment.
Fig. 4.
Recessive transplantation tolerance induced with mAb treatment and donor BMT. (A) CBA mice were transplanted with B10 skin at day 0, and treated with nondepleting CD4, CD8, and CD154 mAbs, as described. One month after transplantation, at day 28, mice were treated with 4 × 107 T cell-depleted B10 BM cells under the cover of the same mAb treatment (G1: ▪, n = 5), mAbs alone (G2: ▴, n = 6), or BM alone (G3: ▾, n = 5). Different mice were treated initially with the mAbs in the absence of a skin graft, followed by a further treatment with mAbs at day 28 (G5: •, n = 6), or BM together with mAbs and a B10 skin graft (G4: ♦, n = 5; see treatment table in A). Group G2 (▴, MST = 93 days) was significantly different compared with all other grafted groups (MST >110 days); P < 0.001. (B) One hundred days after BM treatment, mice with surviving B10 skin grafts were transplanted with (B10×BALB/c)F1 skin. MST in days were: G1 = 21, G2 = >30, G4 = 20, and G5 = 19. Group G2 was significantly different to all other groups; P < 0.011. (C) All mice were bled 120 days after BMT to quantify the percentage of H2-Db+ T cells in their peripheral blood by flow cytometry.
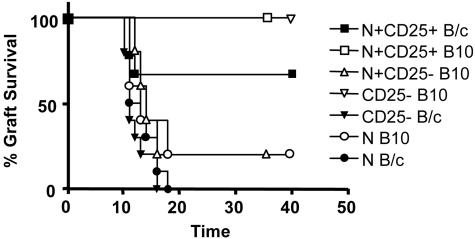
CD4+CD25+ T Cells from Mixed Chimeras Can also Suppress Graft Rejection Without Specificity for Donor Antigen. To rule out any role for CD4+CD25+ T cells in determining the donor antigen specificity of transplantation tolerance, we examined the function of such cells in CBA mice tolerized to B10 skin through mixed chimerism. Such mice show a “recessive” form of tolerance consistent with deletion of allospecific clones. Alloreactive cells could indeed be shown to be eliminated as H2-Db-CD4+CD25- T cells from the chimeric mice, once transfused into a RAG1-/- recipient, could mediate rejection of BALB/c skin grafts but not B10 grafts (Fig. 5). This outcome was not due to regulatory T cells among the CD4+CD25- population, because these cells did not interfere with rejection of B10 skin mediated by cotransferred CD4+ T cells from naïve CBA mice. Where H2-Db-CD4+CD25+ T cells from the chimeric mice were coinjected with naïve CD4+ T cells into RAG1-/- mice, rejection of both BALB/c and B10 skin grafts was prevented. Evidently, CD4+CD25+ T cells could prevent graft rejection without any obvious specificity for donor antigen in all of the systems tested. Their apparent lack of specificity for donor antigens is clearly inconsistent with the demonstrable antigen specificity of dominant tolerance.
Fig. 5.
Regulation by CD4+CD25+ T cells from chimeric mice shows no specificity for the tolerated antigen. H2-Db-CD4+CD25+ and H2-Db-CD4+CD25- T cells were sorted from the spleen of CBA tolerized 100 days before to B10 skin with donor BM and mAb treatment as described. CD4+ T cells were isolated from the spleen of naïve CBA mice. RAG1-/- mice were injected with 105 CD4+ T cells alone (•, n = 10; ○, n = 5), or together with 2 × 105 H2-Db-CD4+CD25+ (▪, n = 9; □ n = 4) or H2-Db-CD4+CD25- (▵, n = 5) T cells. In two groups of mice, 2 × 105 H2-Db-CD4+CD25- T cells were injected alone (▾, n = 10; ▿, n = 4). The transfused mice were transplanted with B10 (open symbols) or BALB/c (filled symbols) skin grafts. P values for the comparisons were as follows: ▾ vs. ▿, P < 0.002; □ vs. ○, P < 0.015; ▪ vs. •, P < 0.003.
Discussion
To characterize the antigen specificity of CD4+CD25+ regulatory T cells, we developed two protocols to achieve tolerance to fully mismatched skin grafts. In one protocol, mAbs targeting CD4, CD8, and CD154 achieve dominant tolerance to skin transplants mediated by CD4+ regulatory T cells. Tolerance so induced, manifests as resistance to rejection mediated by transfused naïve lymphocytes, together with “linked suppression” directed toward third-party antigens located in a graft, which also exhibits the tolerated set. With the addition of donor BM to the mAb treatment, we achieve recessive tolerance to the donor antigens, maintained through mixed hematopoietic chimerism. We confirmed that this type of tolerance does not rely on regulatory T cells, but rather, on the inactivation of donorspecific clones (16).
It has been a long-standing assumption that BM engraftment cannot be achieved without the creation of “space” through myeloablative conditioning (17–19). This dogma has been challenged with reports showing that costimulation blockade is effective in facilitating BM engraftment when “megadoses” of donor BM are used (13, 14, 20), and a recent report showing that BM engraftment can be achieved through prior induction of peripheral tolerance with a smaller dose of BM (15). We have now shown that simultaneous targeting of costimulatory molecules (with anti-CD154 mAbs) and CD4 and CD8 coreceptors can also facilitate the induction of mixed hematopoietic chimerism in the absence of any myeloablative conditioning, and without the need for megadoses of donor BM. With this protocol, it becomes possible to achieve transplantation tolerance in “resistant” strain combinations.
Having developed two strategies to induce tolerance to fully mismatched allografts, one based on regulatory T cells and the other on deletion of alloreactive clones, it became possible to directly assess whether CD4+CD25+ T cells were wholly responsible for donor antigen-specific dominant transplantation tolerance.
The characteristics of the regulatory T cells mediating dominant transplantation tolerance are still controversial (21). We have previously shown (8), after tolerance induction to minor antigens, that the in vivo inhibitory potency of CD4+CD25+ T cells (studied as a population) increased only modestly over that achieved by naïve CD4+CD25+ T cells. Furthermore, when the regulatory potency of the CD4+CD25+ and CD4+CD25- T cell populations from tolerized mice was compared, both populations exhibited similar regulatory potency when analyzed in relation to their physiological proportions (8). In this study, we have used a ratio of regulators to effectors of 2:1. When the regulatory potency of CD4+CD25+ T cells from tolerant mice was tested, suppression was not achieved with a ratio of 1:1 (n = 5; MST = 13 days). For this reason, and in line with recent reports (22), we calculated that we were at the lowest testable ratio of regulators to effectors. Previous reports (22–24) have claimed CD4+CD25+ T cells to be alloantigen-specific. However, none of those experiments were performed in a crisscross manner, and hardly any involved a titration of regulators in relation to effectors. Where specificity was claimed it was only “detected” at one single ratio of regulators to effectors, and must therefore remain questionable (22). In contrast to our findings, some of these previous studies (23) showed no regulatory activity for CD4+CD25+ T cells from naïve mice. CD4+CD25+ T cells from naïve donors were also shown to attenuate graft-vs.-host disease without obvious host antigen specificity (25–27). It is not clear why some observers have data consistent with alloantigen specificity of these cells. An outcome implying apparent specificity could in part be explained by higher “rejectability” of the third-party graft used, or deletion by activation induced cell death of nonregulatory alloreactive cells, specific for the tolerated graft, within the CD4+CD25+ population. Alternatively, it may be that the bulk of CD4+CD25+ T cells are self-reactive, and that this activity variably obscures the regulatory effect of donor antigen-specific CD4+CD25+ cells.
Here, we unequivocally demonstrate that CD4+CD25+ T cells whether from naïve or tolerized mice (either protocol) can prevent graft rejection when injected into lymphopenic hosts regardless of the donor antigens.
To exclude that such results were due to crossreactivity between the donor and third-party strains, we examined the regulatory function of CD4+CD25+ T cells from chimeric mice where B10-reactive T cells have been inactivated. We confirmed that alloreactive CD4+CD25- T cells were indeed inactivated. Although we cannot exclude that some antigen-specific CD4+CD25+ regulatory T cells survived deletion, we demonstrate that these mice did not exhibit linked suppression. As a consequence, we established that any regulatory T cells persisting in such mice were not able to exert this form of dominant regulation. Despite this outcome, CD4+CD25+ T cells from chimeric mice remained competent to prevent rejection of both BALB/c and B10 skin grafts, in what seems to be a form of regulation independent of donor antigen specificity.
Given the recent evidence that CD4+CD25+ T cell regulation may operate by interference with lymphopenia-driven proliferation (28, 29), we cannot exclude the possibility that the lack of observed donor antigen specificity of CD4+CD25+ T cells represents an artifact of lymphopenic conditions. It is possible that CD4+CD25+, as well as CD4+CD25-, T cells might behave differently in T cell-replete mice. The issue of whether CD4+CD25- T cells can contribute to the specificity of dominant tolerance remains unresolved. Contrary to what we have observed in tolerance induced to minor transplantation antigens, CD4+CD25- T cells isolated from mice tolerized across MHC barriers were not able to prevent graft rejection. Indeed, they retained the capacity to reject grafts (n = 5, MST = 12.5 days). However, CD4+CD25-CD45RBlow T cells from the same animals were as efficient as CD4+CD25+ T cells in preventing grafts rejection in lymphopenic conditions (data not shown). A hypothesis consistent with much of the published data is that CD4+CD25+ T cells are indeed involved as regulators in dominant transplantation, but that specificity is dependent on interaction with other regulatory T cells, such as the CD45RBlow subset within the CD4+CD25- T cell population (8).
We have confirmed that CD4+CD25+ T cells are powerful regulators of immune responses, being able to prevent transplant rejection, albeit without demonstrating specificity for donor antigens.
Supplementary Material
Acknowledgments
We thank N. Rust for cell sorting, S. Humm for mAb production, and the staff of the animal facility for expert assistance. This work was supported by the Medical Research Council, U.K., and by the Fonds National de la Recherche Scientifique and Fondation Wiener-Anspach (A.L.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: B10, C57BL/10; BM, bone marrow; BMT, BM transplantation; MST, median survival time.
References
- 1.Sakaguchi, S. (2000) Cell 101, 455-458. [DOI] [PubMed] [Google Scholar]
- 2.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389-400. [DOI] [PubMed] [Google Scholar]
- 3.Maloy, K. J. & Powrie, F. (2001) Nat. Immunol. 2, 816-822. [DOI] [PubMed] [Google Scholar]
- 4.Wood, K. J. & Sakaguchi, S. (2003) Nat. Rev. Immunol. 3, 199-210. [DOI] [PubMed] [Google Scholar]
- 5.Honey, K., Cobbold, S. P. & Waldmann, H. (1999) J. Immunol. 163, 4805-4810. [PubMed] [Google Scholar]
- 6.Cobbold, S. P., Martin, G. & Waldmann, H. (1990) Eur. J. Immunol. 20, 2747-2755. [DOI] [PubMed] [Google Scholar]
- 7.Qin, S. X., Wise, M., Cobbold, S. P., Leong, L., Kong, Y. C., Parnes, J. R. & Waldmann, H. (1990) Eur. J. Immunol. 20, 2737-2745. [DOI] [PubMed] [Google Scholar]
- 8.Graca, L., Thompson, S., Lin, C.-Y., Adams, E., Cobbold, S. P. & Waldmann, H. (2002) J. Immunol. 168, 5558-5567. [DOI] [PubMed] [Google Scholar]
- 9.Davies, J. D., Leong, L. Y., Mellor, A., Cobbold, S. P. & Waldmann, H. (1996) J. Immunol. 156, 3602-3607. [PubMed] [Google Scholar]
- 10.Chen, Z. K., Cobbold, S. P., Waldmann, H. & Metcalfe, S. (1996) Transplantation 62, 1200-1206. [DOI] [PubMed] [Google Scholar]
- 11.Wong, W., Morris, P. J. & Wood, K. J. (1997) Transplantation 63, 1490-1494. [DOI] [PubMed] [Google Scholar]
- 12.Wise, M. P., Bemelman, F., Cobbold, S. P. & Waldmann, H. (1998) J. Immunol. 161, 5813-5816. [PubMed] [Google Scholar]
- 13.Wekerle, T., Kurtz, J., Ito, H., Ronquillo, J. V., Dong, V., Zhao, G., Shaffer, J., Sayegh, M. H. & Sykes, M. (2000) Nat. Med. 6, 464-469. [DOI] [PubMed] [Google Scholar]
- 14.Durham, M. M., Bingaman, A. W., Adams, A. B., Ha, J., Waitze, S. Y., Pearson, T. C. & Larsen, C. P. (2000) J. Immunol. 165, 1-4. [DOI] [PubMed] [Google Scholar]
- 15.Seung, E., Mordes, J. P., Rossini, A. A. & Greiner, D. L. (2003) J. Clin. Invest. 112, 795-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bemelman, F., Honey, K., Adams, E., Cobbold, S. & Waldmann, H. (1998) J. Immunol. 160, 2645-2648. [PubMed] [Google Scholar]
- 17.Micklem, H. S., Clarke, C. M., Evans, E. P. & Ford, C. E. (1968) Transplantation 6, 299-302. [PubMed] [Google Scholar]
- 18.Kolb, H., Thierfelder, S., Baumann, P. & Balk, O. (1971) Rev. Eur. Etudes Clin. Biol. 16, 810-814. [PubMed] [Google Scholar]
- 19.Tutschka, P. J. & Santos, G. W. (1975) Transplantation 20, 101-106. [DOI] [PubMed] [Google Scholar]
- 20.Wekerle, T., Kurtz, J., Bigenzahn, S., Takeuchi, Y. & Sykes, M. (2002) Curr. Opin. Immunol. 14, 592-600. [DOI] [PubMed] [Google Scholar]
- 21.Graca, L., Moine, A. L., Cobbold, S. P. & Waldmann, H. (2003) Curr. Opin. Immunol. 15, 499-506. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Fueyo, A., Tian, J., Picarella, D., Domenig, C., Zheng, X. X., Sabatos, C. A., Manlongat, N., Bender, O., Kamradt, T., Kuchroo, V. K., et al. (2003) Nat. Immunol. 4, 1093-1101. [DOI] [PubMed] [Google Scholar]
- 23.Kingsley, C. I., Karim, M., Bushell, A. R. & Wood, K. J. (2002) J. Immunol. 168, 1080-1086. [DOI] [PubMed] [Google Scholar]
- 24.van Maurik, A., Herber, M., Wood, K. J. & Jones, N. D. (2002) J. Immunol. 169, 5401-5404. [DOI] [PubMed] [Google Scholar]
- 25.Taylor, P. A., Lees, C. J. & Blazar, B. R. (2002) Blood 99, 3493-3499. [DOI] [PubMed] [Google Scholar]
- 26.Cohen, J. L., Trenado, A., Vasey, D., Klatzmann, D. & Salomon, B. L. (2002) J. Exp. Med. 196, 401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann, P., Ermann, J., Edinger, M., Fathman, C. G. & Strober, S. (2002) J. Exp. Med. 196, 389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annacker, O., Pimenta-Araujo, R., Burlen-Defranoux, O., Barbosa, T. C., Cumano, A. & Bandeira, A. (2001) J. Immunol. 166, 3008-3018. [DOI] [PubMed] [Google Scholar]
- 29.Barthlott, T., Kassiotis, G. & Stockinger, B. (2003) J. Exp. Med. 197, 451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.