Abstract
Orosomucoid (ORM, composed of two isoforms, ORM1 and ORM2) has been described as an inducer of M2 macrophages, which are cells that decrease host antibacterial innate immunities. However, it is unknown which phenotypes of M2 macrophages are induced by ORM. In this study, healthy donor monocytes stimulated with ORM (ORM-monocytes) were characterized phenotypically and biologically. CCL1 (a biomarker of M2b macrophages) and IL-10 were detected in monocyte cultures supplemented with ORM1; however, CCL17 (a biomarker of M2a macrophages) and CXCL13 (a biomarker of M2c macrophages) were not produced in these cultures. All of these soluble factors were not detected in the culture fluids of monocytes stimulated with ORM2. Monocytes stimulated with ORM1 were characterized as CD64−CD209−CD163+CCL1+ cells. MRSA and Enterococcus faecalis infections were accelerated in chimeras (NOD/scid IL-2Rγnull mice reconstituted with white blood cells) after inoculation with monocytes stimulated with ORM1 or treatment with ORM1; however, the infections were greatly mitigated in both chimeras inoculated with ORM1-stimulated monocytes and treated with ORM1, after an additional treatment with an inhibitor of M2b macrophages (CCL1 antisense ODN). These results indicate that ORM1 stimulates quiescent monocytes to polarize to M2b monocytes. The regulation of M2b macrophages may be beneficial in controlling opportunistic infections in patients with a large amount of plasma ORM1.
Keywords: CCL1, monocytes, orosomucoid
1. Introduction
Human alpha-1 acid glycoprotein (AGP, a 44 kD immunosuppressive glycoprotein) has been identified as orosomucoid (ORM), which is composed of the ORM1 and ORM2 isoforms [1-6]. As compared to healthy individuals, the plasma levels of ORM in cancer patients [7, 8] and thermally injured patients [9-13] are greatly increased, and these patients are shown to be susceptible to various opportunistic infections [14-16]. ORM has been described as an inducer of M2 macrophages [17-23], which play a major role in the increased susceptibility of these patients to opportunistic infections.
The importance of macrophages in the host's antibacterial innate immunities against opportunistic infections has been established [24, 25]. Healthy individuals are usually resistant against opportunistic infections; however, immunocompromised hosts are susceptible to various opportunistic pathogens [26-28]. Macrophages are one of the major barriers of host antibacterial defense, and their plasticity and diversity are well-described [29, 30]. The generation of bactericidal M1 macrophages (IL-12+IL-10−CD80+CCR7+CD68+ cells) was demonstrated in healthy donors exposed to antigens [31-33], while M2 macrophages (immunosuppressive IL-12−IL-10+CD80−CCR7− CD68+ cells) were generated in response to various type 2 cytokines in cancer patients and thermally injured patients [33-37]. Type 2 cytokines are predominated in these patients [31, 34]. M2 macrophages inhibit the pathogen-stimulated macrophage conversion to M1 macrophages [33, 38]. Therefore, M1 macrophages are not generated in patients with a high level of ORM [28, 33, 37].
So far, three different subsets of M2 macrophages have been described: M2a, M2b, and M2c macrophages [31, 32, 34, 35]. All of the subsets of M2 macrophages are equally immunosuppressive, but can be distinguished from one another by their surface antigens and cytokine/chemokine-producing profiles. In humans, CD163−CD209 (DC-SIGN)+ macrophages that produce CCL17 and IL-10 are identified as M2a, CD163+CD209− macrophages that produce CCL1 and IL-10 are classified as M2b, and CD163+CD209+ macrophages that produce CXCL13 and IL-10 are recognized as M2c [34, 35]. In our previous murine studies [39], M2a and M2c macrophages appeared together in mice 1 day after burn injury and disappeared within 7 days of burn injury. Both macrophage subsets are responsible for the increased susceptibility of mice to opportunistic infections early after burn injury [39]. In contrast, M2b macrophages were not isolated from mice within the first week of burn injury [40], but rather 10 to 30 days after burn injury. Therefore, the elimination of M2b macrophages caused the decreased severities of opportunistic infections in sub-acutely burned mice [40].
Although many papers have described that ORM is an inducer of M2 macrophages [17-23], it is unknown which phenotypes of M2 macrophages are induced by ORM. In the present study, we confirmed that monocytes treated with ORM1 were M2 monocytes. Then, these cells were further characterized phenotypically and biologically. In the results, ORM1 was shown to be active in the monocyte polarization to the M2b phenotype. ORM2 was inactive in monocyte polarization. M2a and M2c phenotypes were not demonstrated in the cultures of quiescent monocytes stimulated with ORM1.
2. Materials and methods
2.1. Orosomucoids
Human rORM1 (>95% of purity by SDS-PAGE) was purchased from Abcam (Cambridge, MA). Human rORM2 (>90% of purity by SDS-PAGE) was obtained from Sino Biological (Beijing, China). These compounds were dissolved in macrophage-serum free medium (Mϕ-SFM) at 10 mg/ml and stored at 4°C. The stock was diluted by Mϕ-SFM to the appropriate concentrations when used in the experiments.
2.2. Reagents and bacteria
Human CpG DNA, human IgG, dexamethasone, and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO). MRSA (BAA-44 strain) and E. faecalis (29212 strain) were purchased from the American Type Culture Collection (Manassas, VA). Before being used in the experiments, bacteria were cultured in tryptic soy broth for 16 h at 37°C in aerobic conditions. Human rIL-1β, rIL-4, rIL-10, rIL-12, rIL-13, rCCL1, rCCL17, and rCXCL13 were obtained from PeproTech (Rocky Hill, NJ). Magnetic beads coated with anti-CD14 mAb and mAbs for human IL-10, IL-12, CCL1, CCL17, and CXCL13 were purchased from R&D Systems (Minneapolis, MN). IMag buffer and Cytofix/Cytoperm were obtained from BD Biosciences (San Jose, CA). FITC-labeled anti-human CD64, APC-labeled anti-human CD163, and PE-labeled anti-human CD209 mAbs were obtained from BioLegend (San Diego, CA). Alexa Fluor 488-labeled anti-human CCL17, Alexa Fluor 488-labeled anti-human CCL1, and Alexa Fluor 488-labeled anti-human CXCL13 mAbs were purchased from Bioss (Woburn, MA). Mϕ-SFM was obtained from GIBCO (Grand Island, NY). Single-stranded nucleic acid that inhibits the production of CCL1 (CCL1 antisense ODN; 5’-GAAGCCCGAGAACATCAT-3’) was synthesized by Sigma-Proligo (Woodlands, TX). To protect antisense ODN from nucleolytic degradation in mice, CCL1 antisense ODN with phosphorothioate modification was utilized. As a control reagent, phosphorothioated scrambled ODN (5’-CATCACAAATGCGACAGG-3’) was utilized.
2.3. Preparation of monocytes
Peripheral blood specimens were obtained from randomly selected healthy donors, under protocols approved by the UTMB Institutional Review Board. Peripheral blood mononuclear cells were isolated from the heparinized blood by Ficoll-Hypaque density gradient centrifugation [33]. To isolate monocytes, mononuclear cells (5 × 106 cells/ml) suspended in IMag buffer were incubated with magnetic beads coated with anti-CD14 mAb (30 min at 4°C). According to the manufacturer's instruction, 50 μl of the magnetic particles were added to every 107 mononuclear cells. Then, CD14+ cells were magnetically harvested. Magnetic beads that were not coated with anti-CD14 mAb were used as a control, and CD14+ cells were not recovered by these beads. The purity of monocytes isolated by this procedure was routinely >97% [33]. Magnetic beads coated with anti-CD14 mAb did not cause any cytotoxic or stimulatory effects on isolated CD14+ cells. Because peripheral blood monocytes are predisposed toward a M2 phenotype during cultivation with M-CSF [41], Mϕ-SFM (Invitrogen, Carlsbad, CA) was utilized for the cultivation of monocytes to avoid the possible influence of M-CSF that is slightly contained in FBS. Therefore, in our assay system, the influence of FBS on the monocyte conversion to M2 monocytes is minimal.
2.4. Preparation of M2a, M2b, and M2c monocytes
M2a monocytes were generated from healthy donor peripheral blood monocytes in 2-day cultures supplemented with a mixture of IL-4 (20 ng/ml) and IL-13 (20 ng/ml) [42]. By flow cytometric analysis, approximately 80% of cells in this M2a monocyte preparation expressed CD209 surface antigen, but not CD64 and CD163. M2b monocytes were generated from healthy donor monocytes in 2-day cultures supplemented with immobilized human IgG (100 μg/ml) and IL-1β (20 ng/ml) [41]. This M2b monocyte preparation expressed intracellular CCL1 (but not CCL17 and CXCL13) and more than 85% of total cells expressed CD163 surface antigen (but not CD64 and CD209). M2c monocytes were generated from healthy donor peripheral blood monocytes in 2-day cultures supplemented with a mixture of IL-10 (100 ng/ml) and dexamethasone (100 nM) [43]. More than 90% of cells in the M2c monocyte preparation expressed CD163 surface antigen (but not CD64 and CD209). Healthy donor peripheral blood monocytes 2 days after cultivation in Mϕ-SFM were utilized as untreated control monocytes [44, 45].
2.5. Cytokine/chemokine-producing abilities of ORM-monocytes
Monocytes (1 × 106 cells/ml) were cultured with 0.04 to 7.0 mg/ml of ORM1 for 12 to 48 h (ORM1-monocytes). Monocytes were also cultured with 0.02 to 4.0 mg/ml of ORM2 in the same fashion. Concentrations of ORM1 and ORM2 tested in this study were determined in response to the previous studies [5, 9, 10]. Natural levels of ORM1 in the plasma of healthy donors are shown to be 0.2 to 0.6 mg/ml [5, 6]. The plasma level of ORM1 significantly increases in cancer patients, while the plasma level of ORM2 shows only a slight increase [5, 6]. Culture fluids harvested were assayed for IL-10, IL-12, CCL17, CCL1, and CXCL13 by ELISA. The minimum detection limits for the above cytokines and chemokines were 4-13 pg/ml in our assay system. In other experiments, monocytes previously cultured with ORM1 (24 h, 1 mg/ml) were washed 2 times with culture media, then treated with or without CpG DNA (24 h, 0.5 μg/ml). Culture fluids harvested from CpG DNA-treated cells were assayed for IL-12. Culture fluids harvested from CpG-DNA-untreated cells were assayed for IL-10.
2.6. Bactericidal activity of monocytes
ORM-monocytes were tested for their abilities to inhibit bactericidal activities of monocytes stimulated with CpG DNA. Thus, healthy donor monocytes (2 × 106 cells/ml, lower chamber) and ORM1-monocytes (1 × 106 cells/ml, upper chamber) were cultured with 0.5 μg/ml of CpG DNA in transwells (0.4 μm pore size, Costar, Corning, NY). Twenty-four h after cultivation, cells in the lower chamber were washed and suspended in the antibiotic-free media, then infected with 105 CFU/well of MRSA. The bacteria suspended in antibiotic-free media were cultured alone in the same fashion and utilized as controls. Three h after incubation, cells were lysed in 0.1% Triton X-100. Serial 10-fold dilutions of these fluids were plated on tryptic soy agar. The number of colonies were counted 24 h after incubation at 37°C. The following formula was used to determine the bactericidal activity of monocytes: bactericidal activity (%) = [(1 – test group CFU/control group CFU) × 100] [33].
2.7. Flow cytometric analysis of monocytes
Phenotypic analysis of ORM1-monocytes was performed by flow cytometry. Cells were first washed 2 times with calcium and magnesium free PBS supplemented with 2% FBS. About 1 × 105 cells were stained for 30 min at 4°C with 1 μg/ml isotype control or specific mAbs for CD64 (FITC), CD163 (APC), and CD209 (PE). Fluorescence was measured by SE500 flow cytometer (Stratedigm, San Jose, CA) and analyzed by FlowJo 7.5.5 software (Tree Star, Inc., Ashland, OR). For staining of the intracellular chemokines, monocyte preparations were washed twice with PBS supplemented with 2% FBS, incubated with Cytofix/Cytoperm solution at 4°C for 20 min, and washed twice with Perm/Wash solution. The cells were incubated with 1 μg/ml isotype control or specific mAb for CCL17 (Alexa Fluor 488), CCL1 (Alexa Fluor 488) and CXCL13 (Alexa Fluor 488), at 4°C for 30 min. Then, the cells were analyzed for intracellular CCL17, CCL1, CXCL13, and surface CD163 expression by flow cytometry.
2.8. Opportunistic infections in chimeras
Nine- to 12-week-old pathogen-free male NOD-SCID IL-2Rγnull mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice), purchased from The Jackson Laboratory (Bar Harbor, ME), were used to create humanized mice (chimeras). According to the recent information obtained from the Jackson Laboratory, NOD-SCID IL-2Rγnull mice are deficient in both innate and adoptive immunities; they are without functional T cells, B cells, and NK cells. Also, these mice are carriers of defective functions (phagocytosis, digestion, antigen presentation, and activation) of macrophages [46-49]. Before being used in our experiments, these mice were exposed to whole body γ-irradiation (4 Gy) in order to deplete neutrophils. These mice were then inoculated with healthy donor WBC (5 × 106 cells) and utilized as chimeras in all animal experiments. The inoculated human WBCs spread throughout the body of NOD-SCID IL-2Rγnull mice within 2 days of the cell inoculation [50]. The chimeras were inoculated with 5 × 106 cells/chimera of ORM1-monocytes (syngeneic CD14+ cells to WBCs) or treated with ORM1 (6 mg/chimera). The chimeras inoculated with healthy donor monocytes, or treated with saline, served as control groups. In some experiments, the chimeras inoculated with ORM1-monocytes or treated with ORM1 were additionally treated with CCL1 antisense ODN (10 μg/mouse, s.c.) twice a day for 2 days beginning 2 h after WBC inoculation. As controls, a group of chimeras were treated with scrambled ODN in the same fashion.
MRSA infection
One day after WBC inoculation, the chimeras shown above were infected i.v. with 2 × 105 CFU/chimera of MRSA. The severities of the infection in these chimeras were evaluated by bacterial growth in spleen, liver, and kidneys as compared to control chimeras. Bacteria in the organs of the tested and control chimeras were quantified by a standard colony counting method, as previously described [40].
E. faecalis oral infection
Decontamination of γ-irradiated NOD-SCID IL-2Rγnull mice was performed by treatment with drinking water containing 4 mg/ml of penicillin, streptomycin, and bacitracin for 4 d [39, 51, 52]. In decontaminated chimeras, significant numbers of any kind of bacteria were not demonstrated in gastrointestinal tracts [52]. On the day of the final antibiotic treatment, these mice were treated orally with lansoprazole (a proton-pump inhibitor, 0.5 mg/ml). It has been described that the acid barrier is of fundamental importance in the inactivation of invading bacteria and for fighting off infection. In the solution below pH 2.0, Enterococcus faecalis and other enteric bacteria are not survived. Also, these bacteria showed strong sensitivity to proteolysis by pepsin (digestive enzyme) at pH 2.0 [53]. Treatment with a proton-pump inhibitor, a gastric acid suppressant [54], enhances bacterial survival in the acid barrier of the stomach. Therefore, in these infection experiments, mice were treated with lansoprazole prior to oral infection with E. faecalis. One day after lansoprazole treatment, the chimeras were created as shown above. One day after WBC inoculation, the chimeras were infected orally with 3 × 105 CFU/mouse of E. faecalis. The severity of infectious complications caused by E. faecalis oral infection was evaluated by the bacterial growth in typical bacteria translocation site organs (mesenteric lymph nodes and liver), and by the mortality rates of the test groups in comparison with the control groups. Bacterial growth was quantified as previously described [39, 52]. To determine the percentage of survival, the chimeras will be monitored twice a day for 10 days after infection.
2.9. Statistical analysis
Data shown in each figure are derived from 3 to 6 independent experiments. Data are presented as mean ± SEM. The results of the test group were compared to those of the control group using a student t-test. Survival curves were analyzed using the Kaplan-Meier test. The result was considered significant if the probability value was lower than 0.05.
3. Results
3.1. Cytokine production by monocytes cultured with ORM1
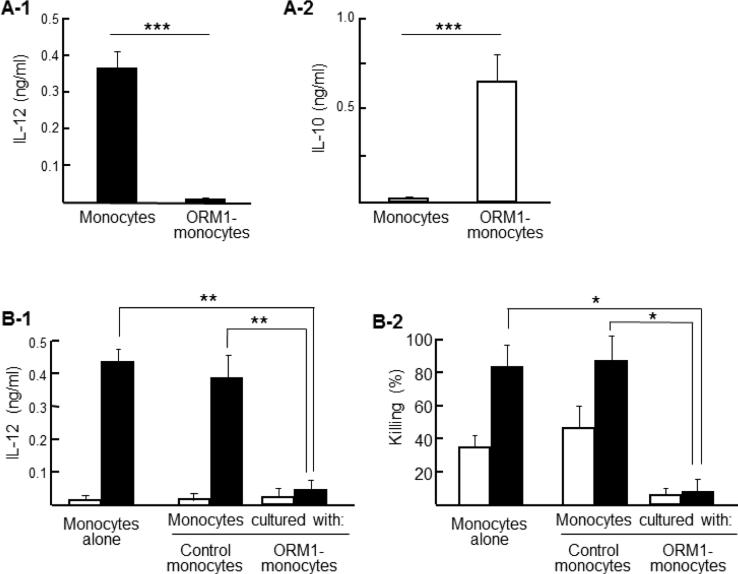
In the first experiments, ORM1 was used to induce M2 monocytes from healthy donor peripheral blood monocytes. Monocytes (1 × 106 cells/ml) were cultured with 1 mg/ml of ORM1 for 24 h (ORM1-monocytes). Then, these cells were further cultured for 24 h with 0.5 μg/ml of CpG DNA. Culture fluids harvested were assayed for IL-12 by ELISA. IL-12 was produced by monocytes cultured with CpG DNA alone; however, IL-12 was not induced by CpG DNA in cultures of ORM1-monocytes (Fig. 1A-1). IL-10 was detected in culture fluids of ORM1-monocytes, while this cytokine was not produced by untreated control monocytes (Fig. 1A-2). Next, ORM-monocytes (upper chamber) and untreated monocytes (lower chamber) were cultured with 0.5 μg/ml of CpG DNA in transwells. Twenty-four h after cultivation, monocytes were harvested from the lower chamber and assayed for their abilities to produce IL-12 and to kill MRSA. IL-12 was not induced by CpG DNA from untreated monocytes cultured with ORM1-monocytes in transwells (Fig. 1B-1). In addition, untreated monocytes cultured with CpG DNA killed 81% of the pathogen, while the significant bactericidal activity was not shown by the same monocytes cultured with ORM1-monocytes in transwells (Fig. 1B-2). These results indicated that M2 monocytes were induced by ORM1.
Fig. 1.
ORM1 is a M2 monocyte inducer. A. Monocytes (1 × 106 cells/ml) were cultured with or without ORM1 (1 mg/ml). Cells harvested 24 h after cultivation were stimulated with (A-1) or without (A-2) CpG DNA (0.5 μg/ml) for 24 h. Culture fluids obtained were assayed for IL-12 (A-1) or IL-10 (A-2). Data are representative of 3 independent experiments. *** P < 0.001 vs control. B. Monocytes (1 × 106 cells/ml, lower chamber) and ORM-monocytes (2 × 106 cells/ml, upper chamber) were cultured with (solid bars) or without (open bars) 0.5 μg/ml of CpG DNA in transwells. As controls, monocytes alone were cultured with CpG DNA in the same fashion. Twenty-four h after cultivation, culture fluids were obtained and assayed for IL-12 (B-1). Cells in the lower chamber were infected with 105 CFU/well of MRSA. Three h after incubation, the remaining bacteria in the wells were counted by a standard colony forming assay (B-2). Data are representative of 3 independent experiments. * P < 0.05 vs control, ** P < 0.01 vs control.
3.2. Characterization of ORM1-monocytes (ORM1-induced M2 monocytes)
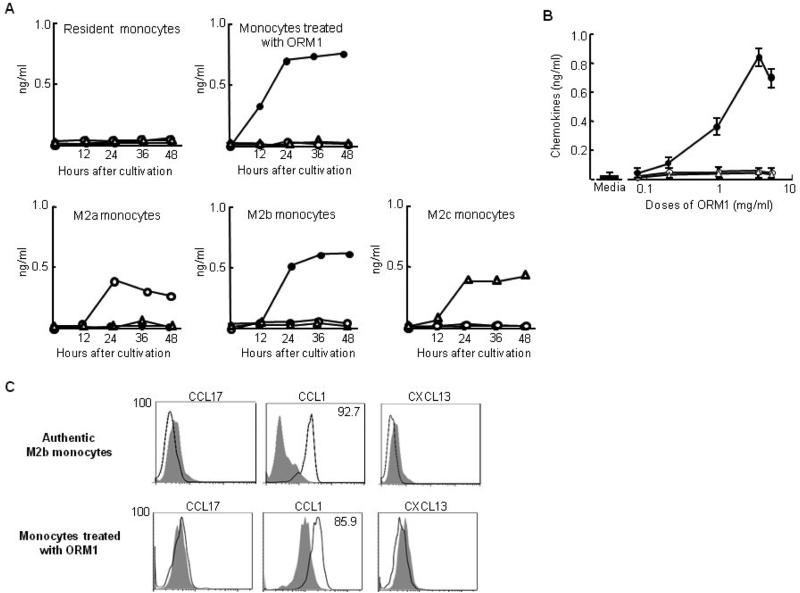
Culture fluids of ORM1-monocytes were assayed for biomarkers (chemokines) of M2a, M2b, and M2c macrophages, and the results were compared to those produced by M2a, M2b, and M2c monocytes induced by IL-4 and IL-13, IgG and IL-1β, and IL-10, respectively. In the results, CCL17 and CXCL13 were not detected in the culture fluids of ORM1-monocytes. ORM1-monocytes produced CCL1 in their culture fluids (Fig. 2A). Dose response effects of ORM1 on CCL1 production by monocytes were shown in Fig. 2B.
Fig. 2.
Production of chemokines by ORM1-monocytes. A. Time course of the production of various chemokines by monocytes after incubation with ORM1 and washing the cells (ORM1-monocytes) or M2a, M2b and M2c monocyte preparations. Various groups of monocytes were cultured in the same fashion. Culture fluids of various groups of monocytes were assayed for CCL17 (○), CCL1 (●) and CXCL13 (Δ) by ELISA. Data are representative of 3 independent experiments. B. The production of chemokines by monocytes stimulated with various doses of ORM1. Culture fluids of monocytes (1 × 106 cells/ml), stimulated with various doses of ORM1 for 24 h, were assayed for CCL17 (○), CCL1 (●) and CXCL13 (Δ) by ELISA. Data are representative of 3 independent experiments. C. Intracellular expression of CCL17, CCL1 and CXCL13 by ORM1-monocytes and M2b monocytes. Cells were stained with Alexa Fluor 488-conjugated anti-human CCL17, CCL1 or CXCL13 antibodies, and analyzed for intracellular chemokine expression by flow cytometry. Figure 2C displays one of the representative results shown in 3 independent experiments.
More than 90% of cells in M2b monocytes stained positive for intracellular CCL1. However, neither intracellular CCL17 nor CXCL13 positive cells were detected in these cell preparations (Fig. 2C). Similarly, around 85% of cells in ORM1-monocytes stained positive for intracellular CCL1, and these cells were not stained with either intracellular CCL17 or CXCL13. These results indicate that ORM1-monocytes and M2b monocytes are IL-12−IL-10+CCL1+CXCL13−CCL17− monocytes.
3.3. Cytokine production by monocytes stimulated with ORM2
Healthy donor peripheral blood monocytes (1 × 106 cells/ml) were stimulated with various doses of ORM2 for 24 h. Culture fluids were harvested and assayed for biomarkers (chemokines) of M2a, M2b, and M2c monocytes. CCL17, CCL1, and CXCL13 were not produced by monocytes 24 h after stimulation with 0.01 to 4.0 mg/ml of ORM2. Also, CCL17, CCL1, and CXCL13 were not demonstrated in culture fluids of monocytes 12 to 48 h after stimulation with 4.0 mg/ml of ORM2. Additionally, IL-10 was not detected in culture fluids of monocytes stimulated with 4.0 mg/ml of ORM2. These results indicate that the monocyte conversion to M2 monocytes is not stimulated by ORM2.
3.4. Surface molecule analysis of ORM1-monocytes
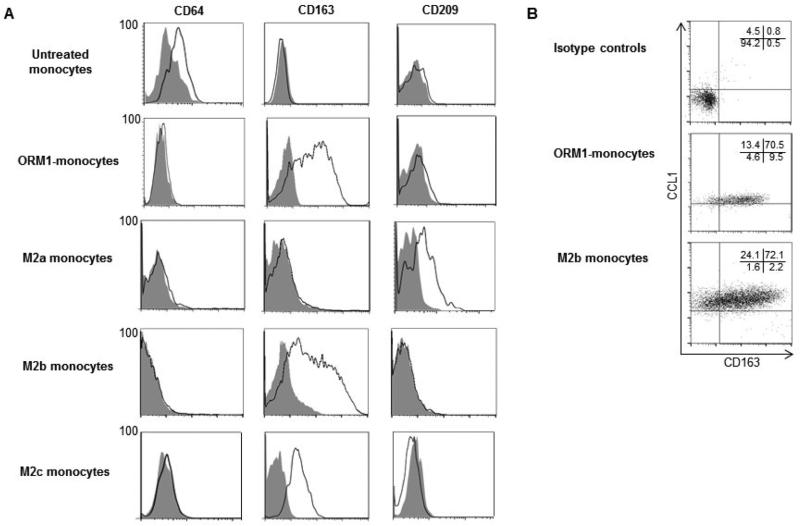
ORM1-monocytes were further analyzed by their surface antigen expressions by flow cytometry (Fig. 3A). Surface expressions of CD64 (FcγR-I), CD163 (a scavenger receptor), and CD209 (DC-SIGN) by ORM1-monocytes were compared to those of M2a, M2b, and M2c monocyte preparations. CD64 is constitutively expressed by untreated control monocytes [55]. CD209 is a well-known marker for M2a monocytes [43, 44]. Expression of CD163 by M2b and M2c monocytes has been reported; however, this antigen is not expressed by M2a monocytes [44, 45]. The results obtained in our experiments are shown in Fig. 4A. Approximately 70% of cells in the M2a monocytes expressed CD209 surface antigen. CD64 and CD163 surface antigens were not expressed by M2a monocytes. More than 90% of cells in the M2c monocytes expressed CD163 surface antigen. CD64 and CD209 surface antigens were not expressed by M2c monocytes. More than 85% of cells in the M2b monocytes expressed CD163 surface antigen. CD64 and CD209 surface antigens were not expressed by M2b monocytes. More than 85% of ORM1-monocytes expressed CD163 surface antigen. CD64 and CD209 surface antigens were not expressed by ORM1-monocytes. These results indicate that M2a monocytes are not present in the ORM1-monocyte preparation. Next, the relationship between surface CD163 expression and intracellular CCL1 expression was examined in M2b monocytes and monocytes stimulated with ORM1. As shown in Fig. 3B, more than 84% of CCL1+ cells in the M2b monocytes and ORM1-monocytes expressed CD163. These results indicate that M2b monocytes and monocytes stimulated with ORM1 are both CCL17−CCL1+CXCL13−CD64−CD163+ CD209− cells.
Fig. 3.
Phenotypic analysis of various preparations of monocytes. (A) M2a monocytes, M2b monocytes, M2c monocytes, ORM1-monocytes and healthy donor monocytes were analyzed for CD64 (FcγR-I), CD163 (a scavenger receptor) and CD209 (DC-SIGN) expression by flow cytometry. (B) M2b monocytes and ORM1-monocytes were double stained with mAbs for anti-CD163 (APC) and anti-CCL1 (Alexa-Fluor 488), and analyzed for their surface expression of CD163 and intracellular expression of CCL1 by flow cytometry. Figs. 3A and 3B display one of the representative results in 5 independent experiments.
Fig. 4.
Effect of ORM1 or ORM1-monocytes on the resistance of the chimeras infected with MRSA or E. faecalis. A. MRSA infection. The chimeras were injected with ORM1-monocytes (i.v., 5 × 106 cells/chimera), ORM1 (i.v., 6 mg/chimera), untreated monocytes (i.v., 5 × 106 cells/chimera, positive control) or saline (0.2 ml/chimera, negative control). Then, all of chimeras were i.v. infected with 2 × 105 CFU/chimera of MRSA. Three days after infection, the growth of bacteria in the liver, spleen and kidneys of these chimeras were determined by a colony-counting method. B. E. faecalis infection. After antibiotic decontamination and proton-pump inhibitor treatment, four groups of the chimeras shown in A were orally infected with 3 × 105 CFU/chimera of E. faecalis. Three days after infection, the growth of bacteria in the liver and mesenteric lymph nodes (MLNs) of these chimeras were determined by a colony-counting method. Data are displayed by the mean ± SEM from 3 or 4 chimeras per group. Data are representative of at least two independent experiments. * P < 0.05, ** P < 0.01.
3.5. Opportunistic infections in chimeras inoculated with ORM1-monocytes or treated with ORM1
The effect of ORM1 or ORM1-monocytes on host antibacterial resistance was determined in the chimeras (γ-irradiated NOD-SCID IL-2Rγnull mice inoculated with healthy donor WBC) infected with MRSA or E. faecalis. The chimeras were inoculated with ORM1-monocytes (syngeneic to the WBC donor) or treated with ORM1 at a dose of 6 mg/chimera. These chimeras were then infected with MRSA (2 × 105 CFU/chimera, i.v.). Three days later, the severity of infections was determined by the bacterial growth in various organs of these chimeras. After MRSA infection, bacterial growth was not shown in the liver, spleen, and kidneys of the chimeras inoculated with healthy donor monocytes or treated with saline, while marked bacterial growth was shown in these organs of the chimeras inoculated with ORM1-monocytes or treated with ORM1 (Fig. 4A). Similar results were shown when E. faecalis was orally infected to these chimeras (Fig. 4B). These results indicated that MRSA infection and E. faecalis translocation were extremely accelerated in the chimeras inoculated with ORM1-monocytes or treated with ORM1.
3.6. Effect of an inhibitor of M2b monocytes on opportunistic infections
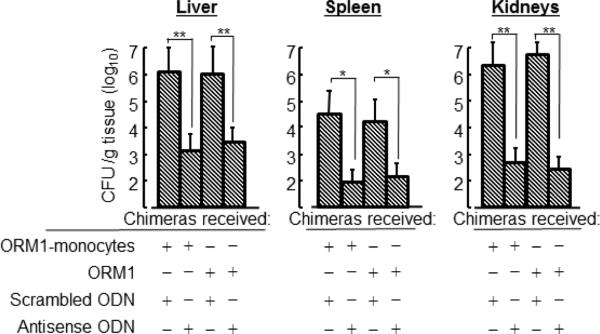
In our previous studies [40], CCL1 has been shown to be an essential chemokine for M2b macrophages to maintain their properties. M2b macrophages reverted to quiescent macrophages during cultivation with an inhibitor of CCL1 (CCL1 antisense ODN). In our preliminary studies, the effect of CCL1 antisense ODN on M1 monocyte generation was tested in the chimeras inoculated with ORM1-stimulated monocytes. Two days after stimulation with 107 heat-killed MRSA (i.v.), human CD14+ cells isolated from the spleens of these chimeras produced IL-12 (a biomarker of M1 macrophages) into their culture fluids. Therefore, we examined the effect of CCL1 antisense ODN on the severities of MRSA infection and E. faecalis translocation in the chimeras inoculated with ORM1-monocytes or treated with ORM1. In the results, the growth of MRSA in various organs of the chimeras inoculated with ORM1-monocytes was greatly inhibited by CCL1 antisense ODN. Similar results were demonstrated when the chimeras treated with ORM1 were given CCL1 antisense ODN (Fig. 5). These results indicate that opportunistic infections with MRSA and E. faecalis are markedly mitigated in the chimeras inoculated with ORM1-monocytes or treated with ORM1 after treatment with an inhibitor of M2b macrophages.
Fig. 5.
Effect of an inhibitor of M2b monocytes on the impaired antibacterial resistance of the chimeras inoculated with ORM1-monocytes or treated with ORM1. The chimeras inoculated with ORM1-monocytes or treated with ORM1 (see Fig. 5 experiments) were i.v. treated with 6 mg/chimera of CCL1 antisense ODN or scrambled ODN (control) twice a day for 2 days beginning 2 h after WBC inoculation. Then, these chimeras were i.v. infected with 2 × 105 CFU/chimera of MRSA. Three days after infection, the growth of bacteria in various organs of these chimeras were determined by a colony-counting method. Data are displayed by the mean ± SEM from 3 or 4 chimeras per group. Data are representative of at least two independent experiments. * P < 0.05, ** P < 0.01.
4. Discussion
Although a certain level of ORM is present in plasma of healthy individuals [5, 6], the amount of the substance markedly elevates in patients with metastatic tumors and in severely burned patients [7-13], who are immensely susceptible to various opportunistic infections [14-16]. Similarly, these patients are known to be carriers of M2 macrophages [33, 37], which inhibit host antibacterial innate immunities [33, 38]. Since ORM is known as a typical inducer of M2 macrophages [17-23], these facts strongly indicate that the increased susceptibility of cancer and burn patients to opportunistic infections may be displayed through the immunosuppressive functions of M2 macrophages that appeared in response to the ORM production.
In the first step of intervention of this increased susceptibility, we characterized M2 monocytes induced by ORM, and determined them as M2b monocytes by their cytokine/chemokine-producing abilities (IL-10 and CCL1), inhibitory effects on monocyte conversion to M1 monocytes, and expressions of surface antigens. CCL17 and CXCL13 were not produced by ORM1-monocytes, indicating that M2a and M2c monocytes were not induced by ORM1 in monocyte cultures. Also, we examined a M2 monocyte-inducing activity of ORM2 in healthy donor monocyte cultures at a dose of 4 mg/ml. Plasma levels of ORM2 in severely burned patients and patients with progressed cancers were less than 0.2 mg/ml [5, 6]. However, significant amounts of IL-10, CCL1, CCL17, or CXCL13 were not produced by ORM2-stimulated monocytes, indicating that any subsets of M2 monocytes were not generated from monocytes stimulated with ORM2.
In the following experiments, these results were flow cytometrically confirmed in comparison with those of M2a, M2b, and M2c monocyte preparations. IL-10 production, intracellular expression of CCL1, and CD163 expression were shown equally by M2b monocytes and ORM1-monocytes. In our preliminary studies, the effect of ORM1 on the induction of M2b monocytes was examined in monocyte cultures supplemented with anti-ORM1 mAb. Both IL-10 and CCL1 were detected in the culture fluids of monocytes treated with 1 mg/ml of ORM-1 for 24 h; however, these cytokines were not produced by monocytes stimulated with ORM-1 in cultures supplemented with 3 μg/ml of anti-ORM1 mAb. Contrarily, IL-12 production and expression of CD64, CCL17, and CXCL13 were not shown by ORM1-monocytes and M2b monocytes. These results indicate that ORM1-monocytes and M2b macrophages are IL-10+IL-12− CD64− CD163+CD209−CCL1+CCL17− CXCL13− cells.
In the next step, M2 monocytes induced by ORM1 were biologically characterized utilizing the chimera mouse system. Thus, the effect of M2b macrophage elimination on the opportunistic infections was examined in the chimeras treated with ORM1 or inoculated with ORM1-monocytes. CCL1 antisense ODN was utilized as a specific inhibitor of M2b monocytes [40, 56]. In our previous studies [40, 56], M2b macrophages reverted to quiescent macrophages after treatment with a CCL1 blocker. M1 macrophages are inducible by a bacterial antigen from M2b macrophages previously treated with CCL1 antisense ODN [40, 56]. The chimeras were shown to be resistant against infections with MRSA and E. faecalis, while severe infections were developed in the chimeras treated with ORM1 or inoculated with ORM1-monocytes. After treatment with CCL1 antisense ODN, however, the chimeras inoculated with ORM1-monocytes or treated with ORM1 were shown to be resistant against MRSA and E. faecalis infections. Also, after treatment with CCL1 antisense ODN and stimulation with a bacterial antigen, human CD14+ cells isolated from the spleens of the chimeras inoculated with ORM1-monocytes were identified as M1 monocytes by their function to produce IL-12. In our previous studies [40], M1 macrophages were induced by a bacterial antigen in severely burned mice treated with CCL1 antisense ODN. Also, severely burned mice treated with CCL1 antisense ODN were shown to be resistant against MRSA systemic infections [40]. Similarly, CCL1 antisense ODN protected mice exposed to 5 Gy of whole body γ-irradiation against sepsis stemming from E. faecalis oral infections [56]. These mice were shown to be carriers of M2b macrophages in the translocation site (mesenteric lymph nodes). The results obtained in these studies suggest that opportunistic infections in patients with ORM1 may be controlled by an inhibitor of M2b monocytes/macrophages.
In the 1980s, Dr. Ishida and his co-workers, including myself, at Tohoku University found an immunosuppressive substance in the sera of cancer patients [17, 23]. This substance was described as IAP (immunosuppressive acid protein). Subsequently, the substance was identified as α1-acid glycoprotein (orosomucoid, ORM). ORM has been shown to be coded by two loci, ORM1 and ORM2 [1-6]. Therefore, the question of which ORMs are immunosuppressive arose. In this study, we compared their immunosuppressive activities using a model of macrophage polarization. The M2 macrophage inducing activity is one of the typical immunosuppressive activities of ORM. As a result, M2b monocytes were specifically induced by ORM1 in cultures of healthy donor monocytes. M2a monocytes and M2c monocytes were not detected in cultures of healthy donor monocytes stimulated with ORM1. In addition, all subsets of M2 monocytes were not isolated from healthy donor monocyte cultures supplemented with ORM2. Further studies will be required to determine the role of ORM2 on the host defense of immunosuppressed patients who are carriers of ORMs. The immune complex [34, 42], TLR agonists [32], and CCL2 [33] are known to be involved in the generation of M2b macrophages. Since ORM1 binds directly to macrophages using CCL5 and Siglect5 [57, 58], ORM1 may directly stimulate the monocyte conversion from healthy donor monocytes to M2b monocytes, but further studies will be needed.
Highlights.
ORM1 was shown to be active in the monocyte polarization to the M2b phenotype.
ORM2 was inactive in monocyte polarization.
Infections were accelerated in humanized mice after treatment with ORM1.
Infections in these mice were mitigated by the elimination of M2b monocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmid K, Kaufmann H, Isemura S, Bauer F, Emura J, Motoyama T, et al. Structure of alpha-1-acid glycoprotein. The complete amino acid sequence, multiple amino acid substitutions, and homology with the immunoglobulins. Biochemistry. 1973;12:2711–24. doi: 10.1021/bi00738a026. [DOI] [PubMed] [Google Scholar]
- 2.Kishino S, Nomura A, Itoh S, Nakagawa T, Takekuma Y, Sugawara M, et al. Age- and gender-related differences in carbohydrate concentrations of α1-acid glycoprotein variants and the effects of glycoforms on their drug-binding capacities. Eur J Clin Pharmacol. 2002;58:621–8. doi: 10.1007/s00228-002-0530-x. [DOI] [PubMed] [Google Scholar]
- 3.Ceciliani F, Pocacqua V. The acute phase protein α1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8:91–108. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- 4.Duché JC, Urien S, Simon N, Malaurie E, Monnet I, Barré J. Expression of the genetic variants of human alpha-1-acid glycoprotein in cancer. Clin Biochem. 2000;33:197–202. doi: 10.1016/s0009-9120(00)00048-5. [DOI] [PubMed] [Google Scholar]
- 5.Budai L, Ozohanics O, Ludányi K, Drahos L, Kremmer T, Krenyacz J, et al. Investigation of genetic variants of alpha-1 acid glycoprotein by ultra-performance liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2009;393:991–8. doi: 10.1007/s00216-008-2518-6. [DOI] [PubMed] [Google Scholar]
- 6.Hanada K, Yamanaka E, Yamamoto N, Minami H, Kawai S, Sasaki Y, et al. Effects of surgery and chronic disease states on the concentrations and phenotype distribution of α1-acid glycoprotein: studies in patients with breast cancer and patients with chronic inflammatory disease. Int J Clin Pharmacol Ther. 2011;49:415–21. doi: 10.5414/cp201539. [DOI] [PubMed] [Google Scholar]
- 7.Kundin WD, Mechali P, Hollinshead AC, Bensimon H, Miller H. Cancer serum index: a useful nonspecific test as a parameter in multimodality screening and assessment of patients with cancer of the prostate. Prostate. 1981;2:207–17. doi: 10.1002/pros.2990020209. [DOI] [PubMed] [Google Scholar]
- 8.Ongay S, Martín-Álvarez PJ, Neusüss C, de Frutos M. Statistical evaluation of CZE-UV and CZE-ESI-MS data of intact α-1-acid glycoprotein isoforms for their use as potential biomarkers in bladder cancer. Electrophoresis. 2010;31:3314–25. doi: 10.1002/elps.201000244. [DOI] [PubMed] [Google Scholar]
- 9.Bloedow DC, Hansbrough JF, Hardin T, Simons M. Postburn serum drug binding and serum protein concentrations. J Clin Pharmacol. 1986;26:147–51. doi: 10.1002/j.1552-4604.1986.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonate PL. Pathophysiology and pharmacokinetics following burn injury. Clin Pharmacokinet. 1990;18:118–30. doi: 10.2165/00003088-199018020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Melezy ska-Matej M, Koziell J, Tolkacz M, Czernik J, Grzybek-Hryncewicz K. The dependence of serum opsonic activity on fibronectin and alpha-1 acid glycoprotein levels in children with burns. Pediatr Pol. 1996;71:511–6. [PubMed] [Google Scholar]
- 12.Wu X, Thomas SJ, Herndon DN, Sanford AP, Wolf SE. Insulin decreases hepatic acute phase protein levels in severely burned children. Surgery. 2004;135:196–202. doi: 10.1016/j.surg.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Xia ZF, Coolbaugh MI, He F, Herndon DN, Papaconstantinou J. The effects of burn injury on the acute phase response. J Trauma. 1992;32:245–50. doi: 10.1097/00005373-199202000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Vostrugina K, Gudaviciene D, Vitkauskiene A. Bacteremias in patients with severe burn trauma. Medicina. 2006;42:576–9. [PubMed] [Google Scholar]
- 15.Sharma BR. Infection in patients with severe burns: causes and prevention thereof. Infect Dis Clin North Am. 2007;21:745–59. doi: 10.1016/j.idc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Kosmidis CI, Chandrasekar PH. Management of gram-positive bacterial infections in patients with cancer. Leuk Lymphoma. 2012;53:8–18. doi: 10.3109/10428194.2011.602770. [DOI] [PubMed] [Google Scholar]
- 17.Shibata Y, Tamura K, Ishida N. In vivo analysis of the suppressive effects of immunosuppressive acidic protein, a type of alpha 1-acid glycoprotein, in connection with its high level in tumor-bearing mice. Cancer Res. 1983;43:2889–96. [PubMed] [Google Scholar]
- 18.Bories PN, Guenounou M, Féger J, Kodari E, Agneray J, Durand G. Human alpha 1-acid glycoprotein-exposed macrophages release interleukin 1 inhibitory activity. Biochem Biophys Res Commun. 1987;147:710–5. doi: 10.1016/0006-291x(87)90988-0. [DOI] [PubMed] [Google Scholar]
- 19.Majima T, Otsuji K, Nagatomi R, Konno T. Polysaccharide (ANK-102) from Polianthes tuberosa cells deteriorates the resistance of mice to Listeria monocytogenes infection. Immunopharmacol Immunotoxicol. 1995;17:59–68. doi: 10.3109/08923979509052720. [DOI] [PubMed] [Google Scholar]
- 20.Fu YX, Watson GA, Kasahara M, Lopez DM. The role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. I. Induction of regulatory macrophages in normal mice by the in vivo administration of rGM-CSF. J Immunol. 1991;146:783–9. [PubMed] [Google Scholar]
- 21.Komori H, Watanabe H, Shuto T, Kodama A, Maeda H, Watanabe K, et al. α1-Acid glycoprotein up-regulates CD163 via TLR4/CD14 protein pathway: possible protection against hemolysis-induced oxidative stress. J Biol Chem. 2012;287:30688–700. doi: 10.1074/jbc.M112.353771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi M, Katakura T, Shimoda M, Roberts NJ, Jr, Herndon DN, Suzuki F. α1-Acid glycoprotein (AGP) stimulates resident macrophages to generate alternatively activated macrophages (AAMΦ). Part I. Biological properties of AGP-induced AAMΦ. FASEB J. 2003;17:C160. [Google Scholar]
- 23.Takano S, Sami S, Majima T, Ishida N. Low molecular weight immunosuppressive factors found in elevated amounts in cancer ascitic fluids of mice. 2. 1-Methyladenosine isolated from cancer ascitic fluids enhances Listeria infection in mice. J Immunopharmacol. 1986;8:59–73. doi: 10.3109/08923978609031085. [DOI] [PubMed] [Google Scholar]
- 24.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 25.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J. Innate Immun. 2011;3:550–64. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberger CM, Finlay BB. Phagocyte sabotage: disruption of macrophage signaling by bacterial pathogens. Nat Rev Mol Cell Biol. 2003;4:385–96. doi: 10.1038/nrm1104. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Miyazaki S, Sumiyama Y, Kakiuchi T. Role of macrophages in a mouse model of postoperative MRSA enteritis. J Surg Res. 2004;118:114–21. doi: 10.1016/S0022-4804(03)00355-X. [DOI] [PubMed] [Google Scholar]
- 28.Katakura T, Yoshida T, Kobayashi M, Herndon DN, Suzuki F. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin Exp Immunol. 2005;142:419–25. doi: 10.1111/j.1365-2249.2005.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol. 2009;86:1105–9. doi: 10.1189/jlb.0209073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards JP, Zhang X, Frauwirth KF, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi M, Jeschke MG, Shigematsu K, Asai A, Yoshida S, Herndon DN, et al. M2b monocytes predominated in peripheral blood of severely burned patients. J Immunol. 2010;185:7174–9. doi: 10.4049/jimmunol.0903935. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 37.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumor-associated macrophages. Immunology. 2013;138:93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–13. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 39.Shigematsu K, Asai A, Kobayashi M, Herndon DN, Suzuki F. Enterococcus faecalis translocation in mice with severe burn injury: a pathogenic role of CCL2 and alternatively activated macrophages (M2aMΦ and M2cMΦ). J Leukoc Biol. 2009;86:999–1005. doi: 10.1189/jlb.0409235. [DOI] [PubMed] [Google Scholar]
- 40.Asai A, Nakamura K, Kobayashi M, Herndon DN, Suzuki F. CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. J Leukoc Biol. 2012;92:859–67. doi: 10.1189/jlb.0212107. [DOI] [PubMed] [Google Scholar]
- 41.Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, et al. Differential regulation of chemokine production by Fcγ receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2). J Leukoc Biol. 2006;80:342–9. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 42.Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–27. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 43.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–20. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mellins ED, Macaubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol. 2011;7:416–26. doi: 10.1038/nrrheum.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, et al. Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol. 2009;85:779–87. doi: 10.1189/jlb.0908579. [DOI] [PubMed] [Google Scholar]
- 46.Piganelli JD, Martin T, Haskins K. Splenic macrophages from the NOD mouse are defective in the ability to present antigen. Diabetes. 1998;47:1212–8. doi: 10.2337/diab.47.8.1212. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51:2481–2488. doi: 10.2337/diabetes.51.8.2481. [DOI] [PubMed] [Google Scholar]
- 48.Marée AF, Komba M, Finegood DT, Edelstein-Keshet L. A quantitative comparison of rates of phagocytosis and digestion of apoptotic cells by macrophages from normal (BALB/c) and diabetes-prone (NOD) mice. J Appl Physiol. 2008;104:157–69. doi: 10.1152/japplphysiol.00514.2007. [DOI] [PubMed] [Google Scholar]
- 49.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–23. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 50.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/Rγnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 51.Ohsugi T, Kiuchi Y, Shimoda K, Oguri S, Maejima K. Translocation of bacteria from the gastrointestinal tract in immunodeficient mice. Lab Anim. 1996;30:46–50. doi: 10.1258/002367796780744956. [DOI] [PubMed] [Google Scholar]
- 52.Tsuda Y, Shigematsu K, Kobayashi M, Herndon DN, Suzuki F. Role of polymorphonuclear neutrophils on infectious complications stemming from Enterococcus faecalis oral infection in thermally injured mice. J Immunol. 2008;180:4133–8. doi: 10.4049/jimmunol.180.6.4133. [DOI] [PubMed] [Google Scholar]
- 53.Zhu H, Hart CA, Sales D, Roberts NB. Bacterial killing in gastric juice — effect of pH and pepsin on Escherichia coli and Helicobacter pylori. J Med Microbiol. 2006;55:1265–70. doi: 10.1099/jmm.0.46611-0. [DOI] [PubMed] [Google Scholar]
- 54.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–81. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 55.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi M, Nakamura K, Cornforth M, Suzuki F. Role of M2b macrophages on the acceleration of bacterial translocation and subsequent sepsis in mice exposed to whole body 137Cs γ-irradiation. J Immunol. 2012;189:296–303. doi: 10.4049/jimmunol.1200350. [DOI] [PubMed] [Google Scholar]
- 57.Atemezem A, Mbemba E, Vassy R, Slimani H, Saffar L, Gattegno L. Human alpha1-acid glycoprotein binds to CCR5 expressed on the plasma membrane of human primary macrophages. Biochem J. 2001;356:121–8. doi: 10.1042/0264-6021:3560121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunnarsson P, Levander L, Pahlsson P, Grenegard M. The acute-phase protein alpha 1-acid glycoprotein (AGP) induces rises in cytosolic Ca2+ in neutrophil granulocytes via sialic acid binding immunoglobulin-like lectins (siglecs). FASEB J. 2007;21:4059–69. doi: 10.1096/fj.07-8534com. [DOI] [PubMed] [Google Scholar]