Abstract
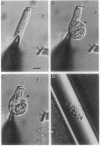
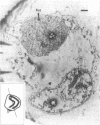
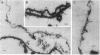
The outer hair cell (OHC) from the organ of Corti is believed to be responsible for the mammal's exquisite sense of hearing. A membrane-based motile response of this cell underlies the initial processing of acoustic energy. The voltage-dependent capacitance of the OHC, possibly reflecting charge movement of the motility voltage sensor, was measured in cells during intracellular dialysis of trypsin under whole cell voltage clamp. Within 10 min after dialysis, light and electron microscopic examination revealed that the subplasmalemmal structures, including the cytoskeletal framework and subsurface cisternae, were disrupted and/or detached from adjacent plasma membrane. Dialysis of heat-inactivated trypsin produced no changes in cell structure. Simultaneous measures of linear and nonlinear membrane capacitance revealed minimal changes, indicating that contributions by subsurface structures to the generation of the nonlinear capacitance are unlikely. This study strongly suggests that voltage-dependent charge movement in the OHC reflects properties of the force generator's voltage sensor and that the sensor/motor resides solely within the lateral plasma membrane.
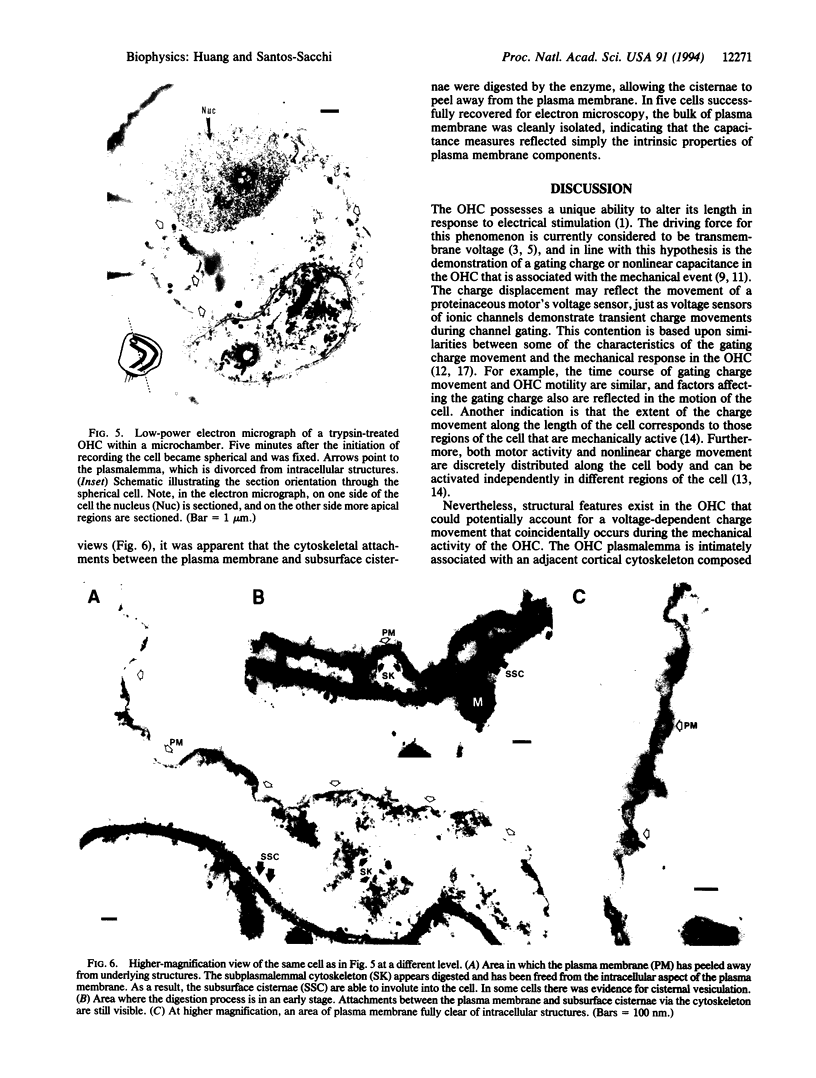
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Tanabe T., Mikami A., Numa S., Beam K. G. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990 Aug 9;346(6284):569–572. doi: 10.1038/346569a0. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Dallos P., Evans B. N., Hallworth R. Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature. 1991 Mar 14;350(6314):155–157. doi: 10.1038/350155a0. [DOI] [PubMed] [Google Scholar]
- Dallos P., Hallworth R., Evans B. N. Theory of electrically driven shape changes of cochlear outer hair cells. J Neurophysiol. 1993 Jul;70(1):299–323. doi: 10.1152/jn.1993.70.1.299. [DOI] [PubMed] [Google Scholar]
- Dieler R., Shehata-Dieler W. E., Brownell W. E. Concomitant salicylate-induced alterations of outer hair cell subsurface cisternae and electromotility. J Neurocytol. 1991 Aug;20(8):637–653. doi: 10.1007/BF01187066. [DOI] [PubMed] [Google Scholar]
- Evans B. N. Fatal contractions: ultrastructural and electromechanical changes in outer hair cells following transmembraneous electrical stimulation. Hear Res. 1990 May;45(3):265–282. doi: 10.1016/0378-5955(90)90126-a. [DOI] [PubMed] [Google Scholar]
- Flock A., Flock B., Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otorhinolaryngol. 1986;243(2):83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Gulley R. L., Reese T. S. Regional specialization of the hair cell plasmalemma in the organ of corti. Anat Rec. 1977 Sep;189(1):109–123. doi: 10.1002/ar.1091890108. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Holley M. C., Ashmore J. F. Spectrin, actin and the structure of the cortical lattice in mammalian cochlear outer hair cells. J Cell Sci. 1990 Jun;96(Pt 2):283–291. doi: 10.1242/jcs.96.2.283. [DOI] [PubMed] [Google Scholar]
- Huang G., Santos-Sacchi J. Mapping the distribution of the outer hair cell motility voltage sensor by electrical amputation. Biophys J. 1993 Nov;65(5):2228–2236. doi: 10.1016/S0006-3495(93)81248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K. H., Kachar B. Fast in vitro movement of outer hair cells in an external electric field: effect of digitonin, a membrane permeabilizing agent. Hear Res. 1989 Jul;40(3):247–254. doi: 10.1016/0378-5955(89)90165-2. [DOI] [PubMed] [Google Scholar]
- Kachar B., Brownell W. E., Altschuler R., Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986 Jul 24;322(6077):365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kalinec F., Holley M. C., Iwasa K. H., Lim D. J., Kachar B. A membrane-based force generation mechanism in auditory sensory cells. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8671–8675. doi: 10.1073/pnas.89.18.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero M. A. Responses to sound of the basilar membrane of the mammalian cochlea. Curr Opin Neurobiol. 1992 Aug;2(4):449–456. doi: 10.1016/0959-4388(92)90179-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Asymmetry in voltage-dependent movements of isolated outer hair cells from the organ of Corti. J Neurosci. 1989 Aug;9(8):2954–2962. doi: 10.1523/JNEUROSCI.09-08-02954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dilger J. P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988 Sep 15;35(2-3):143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Harmonics of outer hair cell motility. Biophys J. 1993 Nov;65(5):2217–2227. doi: 10.1016/S0006-3495(93)81247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Isolated supporting cells from the organ of Corti: some whole cell electrical characteristics and estimates of gap junctional conductance. Hear Res. 1991 Mar;52(1):89–98. doi: 10.1016/0378-5955(91)90190-k. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. On the frequency limit and phase of outer hair cell motility: effects of the membrane filter. J Neurosci. 1992 May;12(5):1906–1916. doi: 10.1523/JNEUROSCI.12-05-01906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991 Oct;11(10):3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Voltage-dependent ionic conductances of type I spiral ganglion cells from the guinea pig inner ear. J Neurosci. 1993 Aug;13(8):3599–3611. doi: 10.1523/JNEUROSCI.13-08-03599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]