Abstract
Little is known about the role of Abs in determining the outcome of hepatitis C virus (HCV) infection. By using infectious retroviral pseudotypes bearing HCV glycoproteins, we measured neutralizing Ab (nAb) responses during acute and chronic HCV infection. In seven acutely infected health care workers, only two developed a nAb response that failed to associate with viral clearance. In contrast, the majority of chronically infected patients had nAbs. To determine the kinetics of strain-specific and crossreactive nAb emergence, we studied patient H, the source of the prototype genotype 1a H77 HCV strain. An early weak nAb response, specific for the autologous virus, was detected at seroconversion. However, neutralization of heterologous viruses was detected only between 33 and 111 weeks of infection. We also examined the development of nAbs in 10 chimpanzees infected with H77 clonal virus. No nAb responses were detected in three animals that cleared virus, whereas strain-specific nAbs were detected in six of the seven chronically infected animals after ≈50 weeks of infection. The delayed appearance of high titer crossreactive nAbs in chronically infected patients suggests that selective mechanism(s) may operate to prevent the appearance of these Abs during acute infection. The long-term persistence of these nAbs in chronically infected patients may regulate viral replication.
Hepatitis C virus (HCV) is an enveloped positive-stranded RNA virus classified in the Flaviviridae family. An estimated 170 million individuals are infected with HCV worldwide. The acute phase of infection is often subclinical, and ≈70% of individuals develop a chronic infection that may result in progressive liver disease. The high frequency of chronic infection suggests that an effective antiviral immune response is not initiated or maintained and that virus-mediated immune escape strategies may be operating. Although the mechanisms leading to clearance versus viral persistence are not clearly defined, there is growing evidence from studies in humans and chimpanzees that an early and strong intrahepatic CD4+ and CD8+ cell response is associated with viral clearance (1, 2).
Neutralizing Ab (nAb) responses after natural infection or vaccination comprise a major component of protection from virus infection (3). However, the role of nAbs in HCV infection and disease progression are unclear, largely because of the lack of assays to measure and quantify their activity. A hypervariable region (HVR) in the E2 envelope glycoprotein (gp) has been proposed to be a target for nAbs (4, 5), and studies on the rate of HVR evolution suggest that variation is a function of the immune pressure exerted by the Ab response (6, 7). Previous experiments showed that serum from a chronically infected patient could neutralize HCV infectivity in a chimpanzee model, suggesting the presence of nAbs (4). In the absence of a cell-culture system capable of generating infectious HCV particles, truncated soluble version(s) of the viral encoded gps have been used to study virus–cell interactions (8). Rosa et al. (9) reported that the presence of Abs that could inhibit soluble E2 gp binding to cells associated with viral clearance in immunized animals; however, the relevance of such blocking Abs to neutralization is unknown.
The recent development of infectious retroviral HCV pseudotypes, comprising HIV capsids bearing HCV envelope gps, have allowed the study of nAbs during HCV infection (10, 11). Bartosch et al. (12) recently validated this system, reporting an association between samples able to neutralize HCV pseudotypes and those able to inhibit HCV infection of chimpanzees. nAbs can be classified as strain-specific, showing a restricted neutralization of autologous virus, or crossreactive, being able to neutralize both autologous and heterologous viruses. In this study, we demonstrate that the majority of chronically infected patients have high-titer, crossreactive nAb responses. In contrast, crossreactive nAbs were detected in only two of seven acutely infected patients, and their presence failed to associate with viral clearance. We studied the response in chronically infected patient H, from whom the prototype genotype 1a H77 HCV strain was cloned. Interestingly, this patient developed a strain-specific nAb response at seroconversion that only broadened to neutralize other viral strains between 33 and 111 weeks after infection. Because the chimpanzee is the only animal model currently available for HCV vaccine studies, we compared the nAb response in animals infected with clonal H77 virus. The majority of infected chimpanzees developed a low-titer, strain-specific nAb response late in disease, which failed to associate with viral clearance. In most viral infections, nAbs are generally considered to “blunt” viral replication, allowing CD4 and CD8 T cell responses to clear virus-infected cells (3). It may be a critical mechanism of HCV persistence that crossreactive nAb responses are delayed until a time when the cellular immune response is dysfunctional and unable to clear infected cells. If so, strategies to induce such a nAb response during the immunocompetent acute phase of infection may have beneficial effects in controlling viral replication.
Materials and Methods
Cells and Preparation of Plasma. 293T and Hep3B cells were propagated in DMEM with 10% FBS. IgG depletion was performed by using the Aurum serum protein kit (Bio-Rad), designed for the simultaneous removal of albumin and IgG from plasma. IgG was purified from human plasma by using a HiTrap Affinity Protein G column (Amersham Pharmacia) and was quantified by using a human IgG ELISA kit (Bethyl Laboratories, Montgomery, TX).
Pseudotype Production and Infection. Pseudotypes were generated by transfection of 293T cells with pNL4-3.Luc.R-E- plasmid containing the env-defective HIV proviral genome and an expression plasmid encoding the HCV gps (strains H, H77, HCJ4, and HCJ6) or murine leukemia virus (MLV) envelope gp, as described in refs. 11 and 13. The virus containing extracellular media was collected 48–72 h after transfection.
Heat-inactivated plasma and virus were mixed at their appropriate dilution in 3% FBS/DMEM plus 4 μg/ml polybrene and incubated at 37°C for 1 h. Virus/plasma mix was transferred to Hep3B cells seeded in 96-well plates (8 × 103 cells per well). Infections with HIV-HCV HCJ4 and HIV-HCV HCJ6 were centrifuged at 400 × g for 1 h and incubated at 37°C for 6 h; unbound virus was removed and incubated for a total of 72 h. Cells were lysed with cell lysis buffer (Promega) and tested for luciferase activity as described in ref. 11. The percentage neutralization was determined by comparing pseudotype infectivity [luciferase relative light units (RLU)] in the presence of a test plasma with infection in the presence of a control HCV-negative plasma at the same dilution. The luciferase signal standard error was ±25%, such that neutralization values >50% were considered significant. The ID50 and ID90 values refer to the dilution of plasma inhibiting pseudotype infectivity by 50% and 90%, respectively.
Measurement of HCV Viral RNA Levels. Total RNA was prepared from 100 μl of chimpanzee plasma by using TRIzol reagent (Life Technologies, Gaithersburg, MD), and HCV RNA levels were quantified by real-time PCR with the PRISM 7700 sequence detection system (PE Applied Biosystems) (detection threshold, 300 RNA copies per ml) as described in refs. 14 and 15.
Measurement of Anti-NS3 and Anti-E1E2 Ab Responses. Enzyme immunoassay plates were coated with purified NS3, E1E2 gp, or a biotinylated peptide (polyprotein amino acids 384–410) representing the HVR and tested for immune reactivity with patient and chimpanzee plasma. Bound immunoglobulins were realized with horseradish peroxidase-conjugated anti-human IgG or IgM as described in ref. 16. Mean optical density (OD) values were expressed as positive/negative (P/N) ratios, calculated by dividing the OD of a test sera by that obtained for a preimmune or irrelevant HCV-negative human serum. The cutoff value was taken as P/N = 2.
Results
HCV-Specific nAb Response. To determine whether nAbs are elicited during infection, plasma samples from uninfected individuals and from those infected with diverse HCV genotypes were screened for their ability to inhibit HCV pseudotype infection. As a specificity control, all plasma samples were tested for neutralization of pseudotypes bearing an MLV envelope gp. Independent of their infecting genotype or viral RNA load, the majority of samples from chronically infected individuals neutralized HCV pseudotypes bearing strains H and H77 gps (genotype 1a) (Fig. 1 shows pseudotype infectivity in the presence of test plasma, and Table 1 shows the percentage neutralization of each plasma for the viruses tested). Plasma from uninfected individuals had no effect on HCV pseudotype infectivity (Fig. 1 and data not shown). All plasma samples had no effect on HIV-MLV pseudotype infectivity, confirming the specificity of the neutralizing response for viruses bearing HCV gps (Fig. 1). Heat treatment of the plasma to inactivate complement had no effect on the ability of plasma to neutralize HCV pseudotype infectivity (data not shown). To study the crossreactive nature of the nAb response, plasma samples were screened for neutralization of pseudotypes bearing HCJ4 and HCJ6 gps of genotypes 1b and 2a, respectively. Both of these pseudotypes appeared less sensitive to neutralization than HIV-HCV H77 (Table 1). However, similar patterns of neutralization were observed for all HCV pseudotypes, with some plasma neutralizing certain pseudotypes more efficiently than others; no genotype-dependent pattern of neutralization was observed (Table 1).
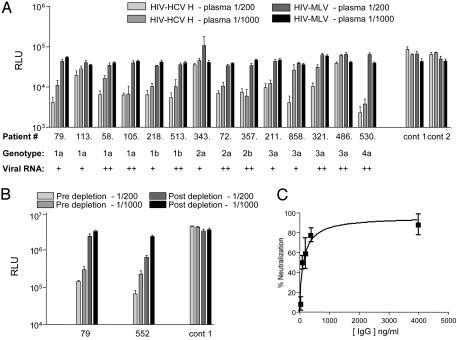
Fig. 1.
HCV-specific nAb response. (A) Plasma samples from uninfected individuals (cont 1 and cont 2) and those chronically infected with HCV genotypes 1, 2, 3, and 4, with low (+, <103 copies per ml) and high (++, >103 copies per ml) viral RNA levels, were tested for their ability to neutralize pseudotype viruses bearing H (HIV-HCV H) and MLV (HIV-MLV) gps at plasma dilutions of 1/200 and 1/1,000. The graph depicts infectivity, expressed as luciferase RLU, of HIV-HCV H and HIV-MLV (RLU ×10) in the presence of various plasma levels. Values are the mean of quadruplicate wells with the standard deviation shown. (B) Plasma from two chronically HCV-infected (79 and 552) individuals were tested for neutralization of HIV-HCV H at final dilutions of 1/200 and 1/1,000 before and after depletion of IgG. As a specificity control, virus infection in the presence of plasma from an uninfected individual (cont 1) is shown. Virus infectivity is shown as RLU, and values are the mean of quadruplicate wells with the standard deviation shown. (C) Neutralization of HIV-HCV H by plasma IgG purified from chronically infected patient 552. Data are shown as percentage neutralization, derived from quadruplicate wells with the standard deviation shown.
Table 1. Genotype-independent pattern of neutralization in chronic HCV infection.
| Neutralization of plasma from individuals infected with different HCV genotypes,* %
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infecting genotype
|
||||||||||||||
| 1a
|
1b
|
2a
|
2b
|
3a
|
4a
|
|||||||||
| Patient 79 | Patient 113 | Patient 58 | Patient 105 | Patient 218 | Patient 513 | Patient 343 | Patient 72 | Patient 357 | Patient 211 | Patient 858 | Patient 321 | Patient 486 | Patient 530 | |
| HIV-HCV H (1a) | 95 | 74 | 92 | 92 | 92 | 93 | 53 | 91 | 90 | 87 | 95 | 86 | – | 97 |
| HIV-HCV H77 (1a) | >99 | >99 | 98 | >99 | >99 | >99 | 85 | >99 | >99 | >99 | >99 | >99 | >99 | >99 |
| HIV-HCV HCJ4 (1b) | 92 | 55 | 88 | 91 | 86 | 94 | 85 | 93 | 95 | 89 | 64 | 78 | 63 | 92 |
| HIV-HCV HCJ6 (2a) | 96 | 94 | 85 | – | 84 | 89 | 81 | 72 | 63 | 77 | 63 | 93 | 76 | 59 |
–, percentage neutralization <50%. Viral genotypes are listed in parentheses.
All plasma samples were tested at a dilution of 1/200
To confirm that the inhibitory activity of plasma from chronically infected individuals was mediated by Ig, we analyzed the effect of depleting IgG from plasma on its ability to neutralize HCV pseudotypes and the ability of purified plasma IgG to neutralize HCV pseudotypes. Depletion of IgG from the plasma of two chronically infected individuals significantly reduced the neutralization of HIV-HCV H infection, and this finding associated with the loss of anti-HCV E1E2 reactivity (Fig. 1B and data not shown). Plasma IgG purified from HCV-infected patient 552 demonstrated specific neutralization of HIV-HCV H infection in a dose-dependent relationship, confirming the Ab-dependent nature of the neutralizing activity in this sample (Fig. 1C).
Development of nAbs During Acute Infection. To address whether nAb activity associates with immune control of HCV infection and viral clearance, a number of health care workers infected by needlestick exposure were studied for the presence of nAbs during the acute phase of infection (Table 2) (17). All seven individuals [numbered with respect to an earlier study (17)] were infected by genotype 1 viruses and were screened for the presence of nAbs to pseudotypes bearing genotype 1 HCV gps (H77 and HCJ4) or MLV gp. Patient 1, who spontaneously cleared HCV after 16 weeks of infection, failed to neutralize any of the HCV pseudotypes tested (Table 2). In contrast, patient 5, who cleared HCV after IFN/ribavirin therapy initiated at 36 weeks of infection, had nAb activity for HIV-HCV H77 at 15 weeks (Table 2). Of the remaining five patients who became chronically infected, one (patient 4) had nAb activity detected at 93 weeks after infection for HIV-HCV H77 (Table 2). None of the acute patient plasma samples neutralized HIV-MLV infectivity. All patients seroconverted to HCV nonstructural antigens between 2 and 9 weeks after infection; however, Abs to HCV E1E2 gps were detected only in patients 4 and 5 coincident with the detection of nAbs (data not shown). In conclusion, nAbs were detected in only two of seven acutely infected individuals, and their presence failed to associate with viral clearance. The low frequency of individuals with crossreactive nAbs during the early stages of infection contrasts markedly with that observed during the chronic phase, suggesting that crossreactive nAbs appear late during infection.
Table 2. nAbs during acute HCV infection.
| Neutralization by plasma collected at different times after infection,* %
|
|||
|---|---|---|---|
| Patient | Week | HIV-HCV H77 | HIV-HCV HCJ4 |
| 1 | 2 | – | – |
| 7 | – | – | |
| 12 | – | – | |
| 17 | – | – | |
| 70 | – | – | |
| 112 | – | – | |
| 2 | 4 | – | – |
| 11 | – | – | |
| 23 | – | – | |
| 64 | – | – | |
| 3 | 38 | – | – |
| 81 | – | – | |
| 135 | – | – | |
| 260 | – | – | |
| 395 | – | – | |
| 4 | 6 | – | – |
| 17 | – | – | |
| 59 | – | – | |
| 93 | 91 | – | |
| 5 | 15 | 89 | – |
| 71 | 93 | 50 | |
| 97 | 80 | 50 | |
| 130 | 60 | 54 | |
| 6 | 49 | – | – |
| 80 | – | – | |
| 171 | – | – | |
| 572 | – | – | |
| 7 | 232 | – | – |
| 316 | – | – | |
–, percentage neutralization <50%.
All plasma samples were tested at a dilution of 1/200
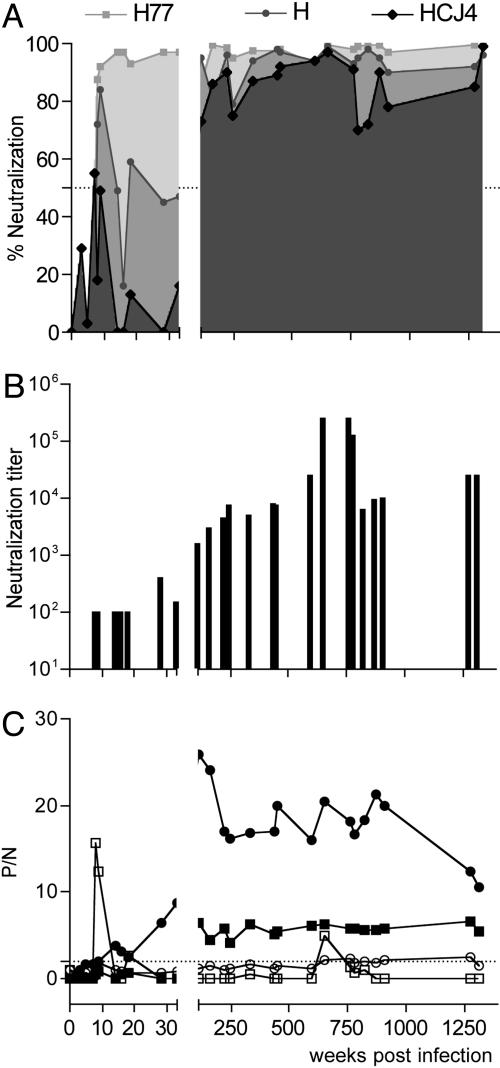
To address whether strain-specific nAbs develop, we studied samples over a 26-year period from patient H who was infected by blood transfusion in 1977 (18). HCV from this patient has been used in multiple studies, including quasispecies measurement, transmission, titration of infectivity in chimpanzees, and the generation of an infectious molecular clone (19, 20). Both strain H and the consensus molecular clone, H77, were obtained from a plasma sample 7 weeks after infection. Sequential plasma samples were tested for their ability to neutralize pseudotypes bearing control MLV gp, autologous strain H and H77 gps, and heterologous HCJ4 and HCJ6 HCV gps. None of the plasma samples had significant neutralizing activity against HIV-MLV (data not shown). nAbs specific for HIV-HCV H and H77 were first detected at 7 weeks postinfection, coincident with acute viremia and seroconversion (Fig. 2A) (18). nAbs capable of inhibiting pseudotypes bearing heterologous HCJ4 and HCJ6 gps were first detected after 111 weeks of infection (Fig. 2 A and data not shown). It should be noted that samples were not available for study between 33 and 111 weeks after infection. The neutralization titer of sequential plasma for HIV-HCV H77 increased over time, with the early, strain-specific response being of low titer (ID90 of 1:100) and the later, more broadly crossreactive response of higher titer (ID90 of 1:1,000–1:10,000) (Fig. 2B). The late appearance of crossreactive nAbs associated with the first detectable IgG response to the E1E2 gps, in contrast to the detection of an anti-NS3 IgG response at 14 weeks after infection (Fig. 2C). A transient anti-E1E2 IgM response was observed during the acute phase of infection that subsequently declined to undetectable levels (Fig. 2C).
Fig. 2.
Strain-specific and crossreactive nAb responses in patient H. (A) Sequential plasma samples from chronically infected patient H were monitored for their ability to neutralize pseudotype viruses bearing H77, H, and HCJ4 gps at final dilution of 1/200. Data are shown as percentage neutralization. (B) Neutralization titer of plasma for HIV-HCV H77, defined as the dilution of plasma able to reduce virus infectivity by 90%. (C) Plasma samples (tested at a dilution of 1/100) were tested for anti-NS3 (circle) and anti-E1E2 (square) IgM (open symbols) and IgG (filled symbols) responses. Data are represented as a P/N ratio, calculated by dividing the OD value of a test serum by that obtained with an irrelevant HCV-negative human serum. P/N values >2 were considered positive. All infections were performed in quadruplicate, and the data are representative of two independent experiments.
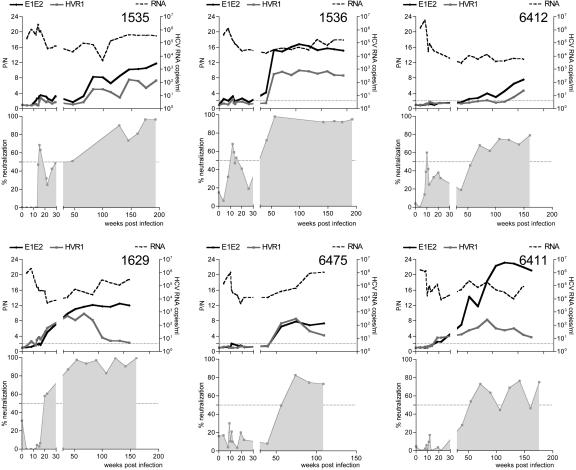
nAb Response in Experimentally Infected Chimpanzees. The chimpanzee is the only available experimental system for HCV vaccine studies. We studied the Ab response in 10 chimpanzees infected with clonal H77 virus for neutralization of pseudotypes bearing autologous (H77) and heterologous (HCJ4) gps. Three of the animals spontaneously cleared virus infection, and samples from these animals at early (20 weeks) and late (100–213 weeks) times after infection failed to neutralize the HCV pseudotypes tested (data not shown). Of the seven persistently infected animals, six demonstrated nAbs detectable at various times after infection (Fig. 3); samples from animal 6394 (55 and 77 weeks after infection) failed to neutralize any of the HCV pseudotypes (data not shown). nAbs were generally detected after the decline of viral RNA (Fig. 3) and alanine transferase (ALT) (data not shown). All of the chimpanzees, with the exception of 1535, failed to neutralize HIV-HCV HCJ4 (data not shown). All plasma samples failed to show any effect on HIV-MLV infectivity (data not shown). In general, there was an association between the detection of a nAb response and the detection of anti-E1E2 and anti-HVR Abs (Fig. 3). Indeed, chimpanzee 6394, which failed to develop a nAb response, had no detectable anti-E1E2 or anti-HVR Abs (data not shown). Sequential plasma from chimpanzee 1629 showed reduced reactivity with the HVR peptide and yet failed to show any significant change in neutralization titer, suggesting that the nAb response may not be specific for the HVR (Fig. 3). The intensity (P/N ratio) of the anti-E1E2 or anti-HVR Ab signal did not appear to associate with neutralization titer, as demonstrated by animal 6412, which had low levels of serologically detectable Ab but similar nAb responses to the other infected animals.
Fig. 3.
The nAb response in experimentally infected chimpanzees. Sequential plasma samples from six chronically H77-virus-infected chimpanzees were monitored for viral RNA levels, nAb for pseudotype virus bearing autologous H77 gp (HIV-HCV H77), and anti-E1E2 and anti-HVR reactivity. All plasma samples were tested at a dilution of 1/100. Data are shown as percentage neutralization. The anti-E1E2 and anti-HVR Ab data are represented as a P/N ratio, calculated by dividing the OD value of the test sera by that obtained with a preimmune serum. P/N values >2 were considered positive. All assays were performed in quadruplicate, and the data are representative of two independent experiments.
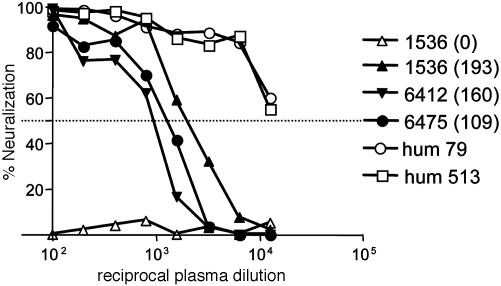
The early nAb response was generally of low titer (ID50 1:250–1:500) and increased over time (ID50 1:1,000–1:4,000) (data not shown). The majority of animals, even at late times after infection, failed to neutralize pseudotype infectivity by 90%. For comparative purposes, the neutralization titer of plasma from a number of chronically infected patients was determined and found to be higher than samples from the infected chimpanzees (Fig. 4; for clarity, data from two patients are shown). In summary, animals that spontaneously cleared virus infection failed to produce Abs capable of neutralizing pseudotypes bearing autologous gps, suggesting that a nAb response is not required for immune clearance of HCV. The nAbs detected in six of the seven persistently infected animals were generally of low titer and strain-specific, in contrast to that observed in patient H at the same time after infection.
Fig. 4.
Comparative neutralization titer of plasma from chronically infected chimpanzees and patients. Plasma from chronically infected chimpanzees 1536, 6412, and 6475 collected at various weeks after infection (shown in parentheses) and two patients, 79 and 513, were tested for their ability to neutralize pseudotype virus bearing H77 gps (HIV-HCV H77). Data are shown as percentage neutralization. All assays were performed in quadruplicate, and the data are representative of two independent experiments.
Discussion
In this study, we show that both strain-specific and crossreactive nAb responses are elicited during HCV infection. The majority of chronically infected patients have high-titer, crossreactive nAb responses that develop late in the chronic phase of infection. To understand whether these nAbs control viral replication, it will be important to determine whether they neutralize pseudotypes bearing autologous gps. Patient H developed nAbs specific for pseudotypes bearing autologous gps after 7 weeks of infection, coincident with acute viremia and seroconversion. In contrast, Abs capable of neutralizing pseudotypes bearing heterologous gps were not detected until after 33 weeks of infection. The appearance of the crossreactive nAb response in patient H coincided with both an increase in neutralization titer and the detection of an anti-E1E2 IgG response by enzyme immunoassay. In contrast, an anti-NS3 IgG response first was detected after 14 weeks, during the acute phase of infection. A similar delay in the appearance of gp-specific IgG responses during acute HCV infection was reported recently (21), suggesting that HCV may selectively delay the production of anti-gp-specific Abs.
The majority of HCV infections are chronic; however, a minority of individuals resolve their infection, suggesting that an effective immune response can be mounted (17). Our study of health care workers during the acute phase of infection suggests that nAbs play a minimal role in viral clearance; however, we were only surveying crossreactive Abs able to neutralize pseudotypes bearing heterologous gps. The early appearance of strain-specific nAbs in patient H suggests that nAbs may contribute to the control viral replication, and further studies are needed to clarify the role of strain-specific nAbs during acute infection. The low frequency (two of seven) of individuals with nAbs at 100 weeks after infection suggests that crossreactive gp-specific nAb responses develop late in the chronic phase of infection. The observation that the majority of chronically infected individuals, presented here and in an independent study of injection drug users (J.A.M. and B. Rehermann, unpublished data), have crossreactive nAbs supports this interpretation.
nAb responses during viral infection generally are thought to develop after the initial control of viremia (22, 23). However, the presence of strain-specific nAb responses during seroconversion in HCV and HIV (24) infection suggests that nAbs may help control viral replication during the acute phase. Several observations support this conclusion. First, immunization of chimpanzees to elicit HCV gp-specific Ab responses failed to induce sterilizing immunity but induced a response that modulated infection and reduced the rate of progression to chronic disease (4, 25, 26). Second, nAb titers are associated with lack of disease progression in long-term survivors during HIV infection (27). Finally, HCV-infected patients with primary Ab deficiencies have been reported to have accelerated rates of disease progression (28, 29). The lymphocytic choriomeningitis virus (LCMV) murine model is often cited as an example of effective cytotoxic T lymphocyte (CTL) control of a virus infection; however, the kinetics of virus elimination do not always correlate with development of a CTL response. Interestingly, when virus-specific CD4+ or CD8+ T cell responses are low or ineffective, LCMV may persist by evading the nAb response (30). It is likely that both CTLs and nAbs play a role in the long-term control of HCV infection, and, ideally, vaccines should elicit both crossreactive nAb and cellular immune responses.
The chimpanzee model has been critical for the study of HCV transmission and host immune response(s). nAbs were not detected in any of the animals that resolved their infection, suggesting a minimal role in viral clearance. However, strain-specific nAb responses were observed in the majority of persistently infected animals. One important difference between the infected chimpanzees and humans is that the animals were infected with a clonal source of virus, which may affect the breadth of the Ab response. Future experiments will address this possibility by studying the nAb response in chimpanzees infected with nonclonal virus quasispecies. The patterns of neutralization observed in the chimpanzee are consistent with that seen in patient H and may reflect an early, low-affinity IgM response being replaced by an IgG response of higher affinity and increased neutralization titer. Several reports suggest that HCV gps are poorly immunogenic in infected chimpanzees, with low levels of gp-specific Abs detected (31, 32). The comparison of chimpanzee nAb responses to those observed in patient H supports this conclusion, suggesting that the low-level nAb response observed in the chimpanzees may reflect less immune selection and explain the minimal variation observed in the E1E2 region after clonal virus infection (16).
In summary, this study shows that HCV can induce Abs capable of neutralizing retroviral pseudotypes bearing HCV gps. The delayed appearance of a gp-specific IgG response, coincident with the detection of high-titer, crossreactive nAbs in patient H, suggests that mechanisms may exist to prevent the appearance of these Abs during acute infection. This observation may result from inadequate T cell help, because several reports indicate impairment of HCV-specific T cell function in patients who fail to clear acute infection (reviewed in ref. 2). Although nAb do not appear to be important in the resolution of acute infection, their increasing titer and broadening reactivity during chronic infection raises the possibility that they may contain virus replication and modulate chronic disease.
Acknowledgments
We thank Vicky Kramer for excellent technical help, Shihyun You for providing purified NS3 antigen, and Lynn Dustin for critically reading the manuscript. C.L., A.T., C.M.R., and J.A.M. are supported by the Greenberg Medical Research Institute (Public Health Service Grants CA57973, CA85883, AI40034, and AI60561).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HCV, hepatitis C virus; gp, glycoprotein; nAb, neutralizing Ab; HVR, hypervariable region; MLV, murine leukemia virus; RLU, relative light units; P/N ratio, positive/negative ratio.
References
- 1.Thimme, R., Bukh, J., Spangenberg, H. C., Wieland, S., Pemberton, J., Steiger, C., Govindarajan, S., Purcell, R. H. & Chisari, F. V. (2002) Proc. Natl. Acad. Sci. USA 99, 15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper, S., Erickson, A. L., Adams, E. J., Kansopon, J., Weiner, A. J., Chien, D. Y., Houghton, M., Parham, P. & Walker, C. M. (1999) Immunity 10, 439-449. [DOI] [PubMed] [Google Scholar]
- 3.Burton, D. R. (2002) Nat. Rev. Immunol. 2, 706-713. [DOI] [PubMed] [Google Scholar]
- 4.Farci, P., Shimoda, A., Wong, D., Cabezon, T., De Gioannis, D., Strazzera, A., Shimizu, Y., Shapiro, M., Alter, H. J. & Purcell, R. H. (1996) Proc. Natl. Acad. Sci. USA 93, 15394-15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato, N., Sekiya, H., Ootsuyama, Y., Nakazawa, T., Hijikata, M., Ohkoshi, S. & Shimotohno, K. (1993) J. Virol. 67, 3923-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth, J. C., Kumar, U., Webster, D., Monjardino, J. & Thomas, H. C. (1998) Hepatology 27, 223-227. [DOI] [PubMed] [Google Scholar]
- 7.Ni, Y. H., Chang, M. H., Chen, P. J., Hsu, H. Y., Lu, T. W., Lin, K. H. & Lin, D. T. (1999) J. Med. Virol. 58, 132-138. [DOI] [PubMed] [Google Scholar]
- 8.Pileri, P., Uematsu, Y., Compagnoli, S., Galli, G., Falugi, F., Petracca, R., Weiner, A. J., Houghton, M., Rosa, D., Grandi, G. & Abrignani, S. (1998) Science 282, 938-941. [DOI] [PubMed] [Google Scholar]
- 9.Rosa, D., Campagnoli, S., Moretto, C., Guenzi, E., Cousens, L., Chin, M., Dong, C., Weiner, A., Lau, J. Y. N., Choo, Q.-L., et al. (1996) Proc. Natl. Acad. Sci. USA 93, 1759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartosch, B., Dubuisson, J. & Cosset, F. L. (2003) J. Exp. Med. 197, 633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu, M., Zhang, J., Flint, M., Logvinoff, C., Cheng-Mayer, C., Rice, C. M. & McKeating, J. A. (2003) Proc. Natl. Acad. Sci. USA 100, 7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartosch, B., Bukh, J., Meunier, J. C., Granier, C., Engle, R. E., Blackwelder, W. C., Emerson, S. U., Cosset, F. L. & Purcell, R. H. (2003) Proc. Natl. Acad. Sci. USA 100, 14199-14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKeating, J. A., Zhang, L., Logvinoff, C., Flint, M., Zhang, J., Yu, J., Butera, D., Ho, D. D., Dustin, L. B., Rice, C. M. & Balfe, P. (2004) J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 14.Major, M. E., Mihalik, K., Puig, M., Rehermann, B., Nascimbeni, M., Rice, C. M. & Feinstone, S. M. (2002) J. Virol. 76, 6586-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puig, M., Mihalik, K., Yu, M. Y., Feinstone, S. M. & Major, M. E. (2002) J. Virol. Methods 105, 253-263. [DOI] [PubMed] [Google Scholar]
- 16.Major, M. E., Mihalik, K., Fernandez, J., Seidman, J., Kleiner, D., Kolykhalov, A. A., Rice, C. M. & Feinstone, S. M. (1999) J. Virol. 73, 3317-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thimme, R., Oldach, D., Chang, K. M., Steiger, C., Ray, S. C. & Chisari, F. V. (2001) J. Exp. Med. 194, 1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinstone, S. M., Alter, H. J., Dienes, H. P., Shimizu, Y., Popper, H., Blackmore, D., Sly, D., London, W. T. & Purcell, R. H. (1981) J. Infect. Dis. 144, 588-598. [DOI] [PubMed] [Google Scholar]
- 19.Farci, P., Alter, H. J., Wong, D. C., Miller, R. H., Govindarajan, S., Engle, R., Shapiro, M. & Purcell, R. H. (1994) Proc. Natl. Acad. Sci. USA 91, 7792-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolykhalov, A. A., Agapov, E. V., Blight, K. J., Mihalik, K., Feinstone, S. M. & Rice, C. M. (1997) Science 277, 570-574. [DOI] [PubMed] [Google Scholar]
- 21.Chen, M., Sallberg, M., Sonnerborg, A., Weiland, O., Mattsson, L., Jin, L., Birkett, A., Peterson, D. & Milich, D. R. (1999) Gastroenterology 116, 135-143. [DOI] [PubMed] [Google Scholar]
- 22.Battegay, M., Moskophidis, D., Waldner, H., Brundler, M. A., Fung-Leung, W. P., Mak, T. W., Hengartner, H. & Zinkernagel, R. M. (1993) J. Immunol. 151, 5408-5415. [PubMed] [Google Scholar]
- 23.Klenerman, P., Lechner, F., Kantzanou, M., Ciurea, A., Hengartner, H. & Zinkernagel, R. (2000) Science 289, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. (2003) Proc. Natl. Acad. Sci. USA 100, 4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forns, X., Payette, P. J., Ma, X., Satterfield, W., Eder, G., Mushahwar, I. K., Govindarajan, S., Davis, H. L., Emerson, S. U., Purcell, R. H. & Bukh, J. (2000) Hepatology 32, 618-625. [DOI] [PubMed] [Google Scholar]
- 26.Choo, Q. L., Kuo, G., Ralston, R., Weiner, A., Chien, D., Van Nest, G., Han, J., Berger, K., Thudium, K., Kuo, C., et al. (1994) Proc. Natl. Acad. Sci. USA 91, 1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao, Y., Qin, L., Zhang, L., Safrit, J. & Ho, D. D. (1995) N. Engl. J. Med. 332, 201-208. [DOI] [PubMed] [Google Scholar]
- 28.Christie, J. M., Healey, C. J., Watson, J., Wong, V. S., Duddridge, M., Snowden, N., Rosenberg, W. M., Fleming, K. A., Chapel, H. & Chapman, R. W. (1997) Clin. Exp. Immunol. 110, 4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapel, H. M., Christie, J. M., Peach, V. & Chapman, R. W. (2001) Clin. Immunol. 99, 320-324. [DOI] [PubMed] [Google Scholar]
- 30.Ciurea, A., Klenerman, P., Hunziker, L., Horvath, E., Senn, B. M., Ochsenbein, A. F., Hengartner, H. & Zinkernagel, R. M. (2000) Proc. Natl. Acad. Sci. USA 97, 2749-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassett, S. E., Thomas, D. L., Brasky, K. M. & Lanford, R. E. (1999) J. Virol. 73, 1118-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prince, A. M., Brotman, B., Lee, D. H., Ren, L., Moore, B. S. & Scheffel, J. W. (1999) J. Infect. Dis. 180, 987-991. [DOI] [PubMed] [Google Scholar]