Abstract
Early-life experiences can dramatically affect adult traits. However, the evolutionary origins of such early-life effects are debated. The predictive adaptive response hypothesis argues that adverse early environments prompt adaptive phenotypic adjustments that prepare animals for similar challenges in adulthood. In contrast, the developmental constraints hypothesis argues that early adversity is generally costly. To differentiate between these hypotheses, we studied two sets of wild female baboons: those born during low-rainfall, low-quality years and those born during normal-rainfall, high-quality years. For each female, we measured fertility-related fitness components during years in adulthood that matched and mismatched her early conditions. We found support for the developmental constraints hypothesis: females born in low-quality environments showed greater decreases in fertility during drought years than females born in high-quality environments, even though drought years matched the early conditions of females born in low-quality environments. Additionally, we found that females born in low-quality years to high-status mothers did not experience reduced fertility during drought years. These results indicate that early ecological adversity did not prepare individuals to cope with ecological challenges in later life. Instead, individuals that experienced at least one high-quality early environment—either ecological or social—were more resilient to ecological stress in later life. Together, these data suggest that early adversity carries lifelong costs, which is consistent with the developmental constraints hypothesis.
Keywords: early-life effects, developmental plasticity, predictive adaptive response, developmental constraints, social buffering, Amboseli baboons
Introduction
Early-life environments can dramatically shape a range of adult phenotypes, from molecular control of gene expression and stress reactivity (Weaver et al. 2004) to immune function (Chen et al. 2011) and disease risk (Roseboom et al. 2006). For example, humans subjected to famine in utero exhibit higher rates of obesity, heart disease, and schizophrenia in adulthood than siblings conceived under non-famine conditions (Jones 1994; Roseboom et al. 2006). In addition, resource competition in early life predicts faster reproductive senescence in wild red deer (Nussey et al. 2007) and explains 35%–55% of the variation in composite measures of fitness in adult sheep, deer, and goats (Hamel et al. 2009). However, while a relationship between early environments and fitness-related traits is well documented across species, the selective import of early environment– induced plasticity remains unclear.
Two influential hypotheses have been proposed to explain the evolution of plasticity in response to the early environment. The predictive adaptive response (PAR) model hypothesizes that animals have evolved to adjust their phenotype during development in anticipation of predicted adult conditions (Gluckman et al. 2005a, 2005b). Individuals that encounter adult environments that are similar to their early conditions are predicted to gain a selective advantage, whereas animals that experience a mismatch between early-life and adult environments should experience a fitness cost (Gluckman et al. 2005a, 2005b; Monaghan 2008). The PAR model is well known as an explanation for the long-term effects of in utero nutrition on later metabolic disease (e.g., Bateson et al. 2004). However, PARs can also include a much broader set of associations between early-life conditions and later-life traits, including cases that extend beyond gestation to later developmental periods (e.g., stress reactivity in rats in response to post-natal maternal behavior, as reported in Meaney 2001; see Gluckman et al. 2005a and Spencer et al. 2006 for additional examples).
Perhaps the most widely known examples of putative PARs come from studies of human cohorts that experienced scarcity in early life but resource-rich environments in adulthood. These cohorts often exhibited elevated rates of cardiovascular disease and metabolic syndrome in adulthood, which is a pattern consistent with PAR-related mis- matches (Barker et al. 1993; Hales and Barker 2001; Gluckman et al. 2005a; Wells 2007). However, in these cases, we usually do not know whether cohorts that experienced scarcity in early life would have been advantaged if resources were limited in adulthood, which is a test required to distinguish PAR from the alternative developmental constraints model (also known as the silver spoon hypothesis; Grafen 1988; Monaghan 2008). This alternative model predicts a simple relationship between early environmental quality and adult fitness: individuals born in high-quality environments experience a fitness advantage regardless of the adult environment.
Because developmental constraints and PAR predict identical outcomes when conditions in adulthood are favorable—that individuals from high-quality early environments will perform better—the same data sets have been invoked as support for both hypotheses (Barker et al. 1993; Gluckman et al. 2005a; Wells 2007). In addition, associations between early-life conditions and adult physiology or health are often interpreted as evidence for PARs (Gluckman and Hanson 2004b; Bol et al. 2010; Kemp et al. 2012; see discussion in Hayward et al. 2013). However, the PAR and developmental constraints models can only be distinguished by comparing fitness-related traits in individuals from high- and low-quality early environments, when each of these sets of individuals experience both high- and low-quality adult conditions.
Indeed, many authors have argued that this is the necessary empirical test to distinguish between the PAR and developmental constraints models (Rickard and Lummaa 2007; Monaghan 2008; Wells 2012; Uller et al. 2013), and a number of such tests have now been conducted in laboratory settings. A recent meta-analysis of studies in plants, arthropods, fish, and reptiles concluded that evidence for the PAR hypothesis is generally weak (Uller et al. 2013). However, because the PAR model is frequently invoked in the context of human health and evolution (Gluckman and Hanson 2004a, 2004b; Gluckman et al. 2005a), research on mammalian species—particularly natural populations— is needed to further assess its generality. To date, only one study has investigated the fitness effects of matched and mismatched early-life and adult environments in wild mammals. In this study, Douhard and colleagues analyzed data from two populations of wild roe deer, one living in a resource-rich environment and one living in a resource-poor environment (Douhard et al. 2014). In the resource-rich environment, the authors found no evidence in support of PAR. However, in the resource-poor environment, deer born in low-quality years sometimes outperformed those born in high-quality years, which is a pattern consistent with PAR but also with viability selection. Specifically, viability selection may eliminate all but the most robust off-spring during low-quality years, leading to high adult sur vival rates for individuals born in poor conditions (Douhard et al. 2014).
Here, we take advantage of data from a long-term study of wild baboons (Papio cynocephalus) in Kenya's Amboseli ecosystem to further evaluate the support for the PAR versus developmental constraints hypotheses. Animals in this population experience considerable environmental variability, particularly in annual rainfall levels, and this variation has known consequences for fertility-related traits in females (Alberts and Altmann 2003; Beehner et al. 2006). Importantly, individuals in this population are observed from birth throughout their lives, and measures of fertility are therefore available for the same females across ecologically variable years. Using these longitudinal data, we were thus able to examine fertility outcomes for the same female when her adult conditions both matched and mismatched her early-life environment.
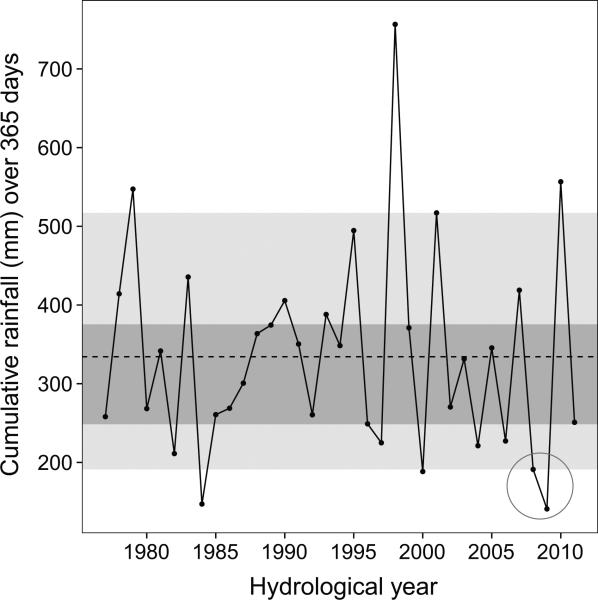
We focused our primary analysis on an extreme climatic event that occurred in 2009, when the Amboseli ecosystem experienced the worst drought recorded in over 4 decades (fig. 1; see also fig. A1; figs. A1–A5 available online). We treated this period as a natural experiment that allowed us to ask whether early-life conditions explained variation in females’ reproductive responses to a severe ecological challenge. Specifically, we asked how a given female's fertility changed during the 2009 drought relative to her fertility during a high-quality year. To do so, we analyzed each female's ability to (i) resume cycling after post-partum amenorrhea or (ii) conceive an offspring during the 2009 drought and during at least one high-quality year (i.e., a year with average rainfall). We then explicitly tested whether a female's fertility was highest when her adult conditions matched her early-life conditions (note that we define early life as the 365 days following birth, and hence we adopt the definition of the PAR model described in Gluckman et al. 2005a). Under the PAR hypothesis, females that experienced high-quality early-life conditions should perform better during high-quality adult years, and females born during dry, low-quality years should perform better during low-quality drought years. In contrast, under the developmental constraints hypothesis, females born during low-quality years should be disadvantaged relative to females born in high-quality years, regardless of the adult environment.
Figure 1.
Consecutive low-rainfall years magnified the severity of the 2009 drought. Cumulative rainfall by hydrological year (November 1 to October 31) is plotted for all years recorded by the Amboseli Baboon Research Project (1977–2011; note that our study focused on females born between 1985 and 2004 and examined fertility measures for these females in the years 1992–2011, when they were multiparous adults). The middle 40% and 90% of the cumulative rainfall distribution are highlighted in dark gray and light gray, respectively; 2009 was the driest hydrological year on record and was preceded by another extremely dry year (sequence circled in gray).
Finally, we also tested whether high social status in early life could mitigate the effects of ecological adversity in early life. This analysis was motivated by observations that high maternal social status predicts faster offspring growth and earlier offspring maturation in the Amboseli baboons (Alt-mann and Alberts 2005; Charpentier et al. 2008). We therefore predicted that high maternal dominance rank might offset the costs associated with early ecological adversity, illustrating the highly multidimensional environment experienced by individuals in natural populations.
Material and Methods
Study Subjects, Fertility Data, and Social Status Data
Study subjects were 50 adult female baboons from a long-term study population in Kenya's Amboseli ecosystem (Alberts and Altmann 2012). Subjects were born between 1985 and 2004 and were individually recognized and monitored from birth. Subjects were observed on a near-daily basis, and reproductive status (pregnant, lactating, cycling, and, if cycling, stage/day of the estrous cycle) was scored retrospectively for each female in adulthood on the basis of observations of the sexual skin and the paracallosal skin (Beehner et al. 2006; Gesquiere et al. 2007). Resumption of cycling was determined through observations of swelling of the sexual skin following a period of postpartum amenorrhea. Conception was documented through failure of both menstruation and sexual swelling after the luteal phase as well as through a subsequent diagnostic change of the paracallosal skin from black to pink. The first day of swelling deturgescence (size decrease) in the previous estrous cycle was taken as the conception date (Beehner et al. 2006). Female dominance hierarchies were constructed monthly for every social group in the study population on the basis of the outcomes of dyadic aggressive encounters; ordinal dominance ranks were assigned to every adult female on the basis of these hierarchies (Hausfater 1974).
Rainfall Data and the 2009 Drought
Precipitation was measured in millimeters, using a rain gauge that was read daily, across all hydrological years beginning in 1977. Hydrological years in Amboseli begin in November, at the end of an annual 5-month dry season (June–October), and continue until the end of the following October. During the 2009 hydrological year (November 2008–October 2009), we observed the lowest cumulative rainfall in the history of the long-term study. In addition, the 2008 hydrological year had the fourth-lowest cumulative rainfall in our records, heightening the effects of low rainfall during 2009 (fig. 1; see also fig. A1). The 2009 drought became the focus of our analysis of low-quality environments in adulthood (see below).
Defining Low-Quality Environments in Early Life and Adulthood
To identify low-quality environments in early life, we calculated cumulative rainfall over the first year of life for every female that reached reproductive maturity in our population (N = 289 females), including the 50 subjects of this study. Females in our data set experienced a wide range of cumulative rainfall values over the first year of life (mean rainfall = 334.29 mm; range = 151.4–767.0 mm; for comparison, an arid desert is defined by <250 mm annual rainfall; Noy-Meir 1973). We defined years with cumulative rainfall values within the bottom 30% of this distribution as low-quality early-life environments. We focused primarily on the first year of life as our early environment of interest, because nutrition during this time period has well-described effects on adult fitness in Amboseli (Altmann 1991). However, because environmental conditions in utero can also be important, we performed parallel analyses that considered rainfall during the gestational and perinatal period (i.e., where the early-life environment was defined by rainfall over the 365 days following conception, which includes gestation and the first 6 months of life).
We defined the 10-month dry period from January 1 to October 31, 2009, as the 2009 drought and focused on this period as the primary low-quality environment of interest for adult females. We focused on this 10-month period because of its extreme properties: during these 10 months, only 72.7 mm of rain fell, compared with a mean ± SD of 224.28 ± 104.03 mm during these months in 34 other years. We excluded 2 months of the 2009 hydrological year (November and December 2008) from our analyses because 68.2 mm of rain fell during this period (i.e., almost as much rain as fell during the entire 10-month drought period). This level of rainfall is low, but it is within 1 standard deviation of the mean rainfall for November–December periods in Amboseli (120.30 ± 66.48 mm), in contrast to the much more extreme drought conditions in the subsequent 10 months of 2009.
Defining High-Quality Environments in Early Life and Adulthood
We initially defined high-quality environments as years with rainfall in the middle 40% of the hydrological year distribution (across all years for which rainfall data were available, 1977–2011; fig. 1). Importantly, the distribution of rainfall values for high-quality early-life and high-quality adult years defined in the manner described here were statistically indistinguishable (Kolmogorov-Smirnov test, D = 0.073, P = .996; fig. A2).
Defining high-quality environments as those with rainfall in the middle 40% of the distribution excluded years with very high rainfall from the definition of high-quality early-life and adult environments. Although low precipitation has well-documented negative effects on baboon behavior (Alberts et al. 2005), life history (Charpentier et al. 2008), and fertility (Beehner et al. 2006), the effects of very high rainfall on baboons in Amboseli are unknown and may include negative outcomes (e.g., flooding and thermo-regulatory stress). We tested this a priori hypothesis by exploring how our results varied depending on the threshold we used to define high-quality conditions (see “Sensitivity to Low and High Rainfall in Early Life and Adulthood”).
Within-Female Analyses: Interaction between Early-Life and Adult Conditions on Female Fertility
We examined fertility for females born in either a low-quality environment or a high-quality environment, restricting our data set to females that experienced both the 2009 drought and at least one high-quality year as a multiparous adult. Nulliparous females exhibit marked differences in fertility characteristics, so we excluded them (Altmann 1980; Gesquiere et al. 2007). For the remaining set of females (N = 50 females, N = 172 female-years), we collated records of resumption of cycling and conception on the basis of long-term records. We also considered other fertility measures, in particular the production of weanlings; females that successfully weaned an infant represented a subset of the females that resumed cycling—specifically, the subset that resumed cycling after weaning a live infant as opposed to the subset that resumed cycling after losing an infant. Thus, analyzing weaning success would involve partitioning the analysis of cycle resumption into its two components: successfully weaning an infant versus losing an infant. Although infants in our data set experienced an overall infant mortality rate of ~22%, only a small number of infant deaths occurred before weaning in any given year. This translated into too little variation in infant survival/weaning success to perform a third, parallel analysis (see figs. A3, A4, for a comparison of the frequency of infant mortality, cycle resumption, and conception in our data set).
We modeled resumption of cycling and conception separately using generalized linear mixed models with a binomial error structure and a logit link function. Models were fit using the function lmer in the R package lme4, and the significance of each fixed effect was estimated from the Wald Z-statistic (R Development Core Team 2012; Bates et al. 2014). In both models, our outcome variable was a binary variable indicating whether a given female resumed cycling or conceived during a 10-month period. Both models also included (i) a binary fixed-effect variable classifying the 10-month period as drought (January 1, 2009, to October 31, 2009) or as high quality (January 1 to October 31 of a high-quality year); (ii) a binary fixed-effect variable classifying the female's early-life environment as low quality or high quality; and (iii) a random effect of female identity. We interpreted a significant main effect of current rainfall as evidence that prevailing conditions influenced reproductive outcomes (either resumption of cycling or conception). We interpreted a significant interaction between early and current rainfall conditions as evidence that females born in low-quality versus high-quality environments were differentially affected by ecological conditions in adulthood.
In both the resumption of cycling models and the conception models, we controlled for three variables known to affect fertility in Amboseli females (Alberts and Alt-mann 2003; Beehner et al. 2006; Altmann et al. 2010). The first variable was the age of the female at the start of the time period, modeled as both a linear and a quadratic effect (after Beehner et al. 2006). All females in our data set were born in the study population, and ages were thus known to within a few days’ error and modeled as a continuous variable. The second variable was total number of individuals in the female's social group at the start of the time period, which influences levels of resource competition and thus potentially influences fertility. The third variable was an ordinal number describing the social status (i.e., dominance rank) of the female at the start of the time period (Hausfater 1974). All three variables were incorporated in our models as fixed effects.
In addition, because a female's ability to conceive during a 10-month time period will depend on her immediate reproductive history, we also controlled for several measures of reproductive readiness. In both models, we controlled for (i) a continuous variable indicating the number of days since the female's last live birth and (ii) a binary variable denoting whether the female had a dependent infant (<1 year of age) that died during the 10-month period (because females that lose a dependent infant tend to quickly cease lactating and resume cycling). In our model for resumption of cycling, we also included a binary variable denoting whether the female had cycled at the start of the 10-month period. This variable controlled for the fact that females who are already cycling are less likely to cease cycling and then resume cycling again during the 10-month period. Similarly, in our conception model, we included a binary variable denoting whether the female was already pregnant at the beginning of the 10-month period.
Between-Condition Analyses: Testing for Early-Life Effects on Adult Fertility in a Given Adult Environment
Our data set was restricted to females born in low-quality or high-quality environments who experienced both drought and high-quality conditions as adults. In the analyses described above, we took advantage of this design to test whether within-individual differences in sensitivity to the adult environment were dependent on early-life ecological circumstances. These within-individual analyses therefore indicate whether females born in low-quality environments showed a larger negative response to adverse environments in adulthood compared with females born in high-quality environments. However, these analyses do not directly compare females born in high-quality environments with females born in low-quality environments, controlling for adult environment. Thus, we also conducted between-condition analyses that directly compared all females born in low-quality environments with all females born in high-quality environments, both in 2009 (N = 51 females) and in randomly selected high-quality years (N = 105 females; see appendix, available online). For these between-condition analyses, we did not constrain the data set to females who had experienced both the 2009 drought and a high-quality year as a multiparous adult.
Sensitivity to Low and High Rainfall in Early Life and Adulthood
To assess the sensitivity of fertility traits to low-rainfall conditions, we explored how the magnitude of the interaction effect between early and current rainfall varied depending on the thresholds we used to define low-quality environments. We set thresholds in increments of 1% between the lowest 10% and the lowest 30% of the distribution of annual rainfall for both early and adult environments. For every threshold value between the lowest 10% and the lowest 30% of the rainfall distribution, we estimated the interaction effect from the generalized linear mixed-effects model for conception probabilities described above. This analysis allowed us to assess whether the interaction between current and early rainfall environments remained significant (i) when our definition of a low-quality adult environment included years that were not as severe as the 2009 drought and (ii) when our definition of low-quality early environments included years of varying severity. For this analysis, we retained the same definition of high-quality years (i.e., the middle 40% of the distribution).
We also tested the effects of very high rainfall on fertility outcomes, which we suspected might be costly to Amboseli females as well. Here, we varied the thresholds we used to define high-quality early and adult environments and estimated the interaction effect from a generalized linear mixed-effects model predicting conception. Specifically, our main analyses defined high-quality environments as years falling between the 30th and 70th percentiles of the early-life or adult rainfall distribution; in our sensitivity analyses, this upper cutoff ranged from the 70th to 100th percentile (in increments of 1%), thus including progressively more extreme high-rainfall years.
Effects of Maternal Dominance Rank on Female Fertility
To assess the effects of early-life social status on fertility during the 2009 drought, we measured maternal dominance rank, defined as the dominance rank of the mother at the offspring's birth. Dominance rank is maternally inherited in baboons (Hausfater 1974; Hausfater et al. 1982), and thus current dominance rank and maternal dominance were collinear in our data set (r2 = 0.67). We therefore tested whether maternal dominance rank accounted for additional variation in fertility after accounting for the effect of an individual's current dominance rank. Specifically, we extracted the residuals from a generalized linear model that predicted resumption of cycling or conception during the 2009 drought (as a function of age, group size, the focal female's own dominance rank, and all reproductive readiness measures; N = 50). We then used a linear model to ask, for females born in low-quality environments (N = 14), whether maternal dominance rank was correlated with residual variation in cycle resumption or conception probabilities during the 2009 drought. We note that the analysis of residuals can produce biased estimates of effect sizes (Darlington and Smulders 2001; Freckleton 2002). In this case, our analyses may underestimate the effect of interest, because true maternal dominance rank effects could be masked by first taking into account the focal individual's current dominance rank; thus, our analyses are likely to be biased toward type II error (false-negative results) rather than type I error. We chose to conduct such an analysis because we were interested in testing for maternal social status effects specifically for females born in low-quality environments during the 2009 drought only (N = 14). As a post hoc contrast, we also repeated this procedure for females born in high-quality environments (N = 36).
All analyses were conducted in R, version 2.15.0 (R Core Development Team 2012). Data underlying all main analyses are deposited in the Dryad Digital Repository: http://doi.org/10.5061/dryad.5r37g (Lea et al. 2015).
Results
Decreases in Fertility during the 2009 Drought Were Greater for Females Born in Low-Quality Environments Than for Females Born in High-Quality Environments
Overall, females were less likely to resume cycling after postpartum amenorrhea and less likely to conceive during the 2009 drought than during high-quality years (estimates for high-quality years vs. the 2009 drought: β ± SE = 2.58 ± 0.83, P = .002 for resumption of cycling; β ± SE = 3.49 ± 1.34, P = .008 for conception; N = 172 female-years). This estimate translates to a striking 24% decrease in the probability of conception for the average female during the 2009 drought relative to high-quality years (see appendix for an explanation of how model parameters were translated into percentages).
However, our within-female analysis indicated that the cost to fertility associated with the 2009 drought varied across females. Conception rates among females born in high-quality environments were less affected by the 2009 drought than were rates among females born in low-quality environments (interaction between early and adult environments: β ± SE = −3.01 ± 1.49, P = .043). A similar trend was observed for resumption of cycling (β ± SE = −1.70 ± 0.93, P = .069; fig. 2; tables 1, 2). Differences in sensitivity to drought conditions were not explained by genetic relatedness: females born in high-quality environments were not more closely related than females born in low-quality environments (see appendix).
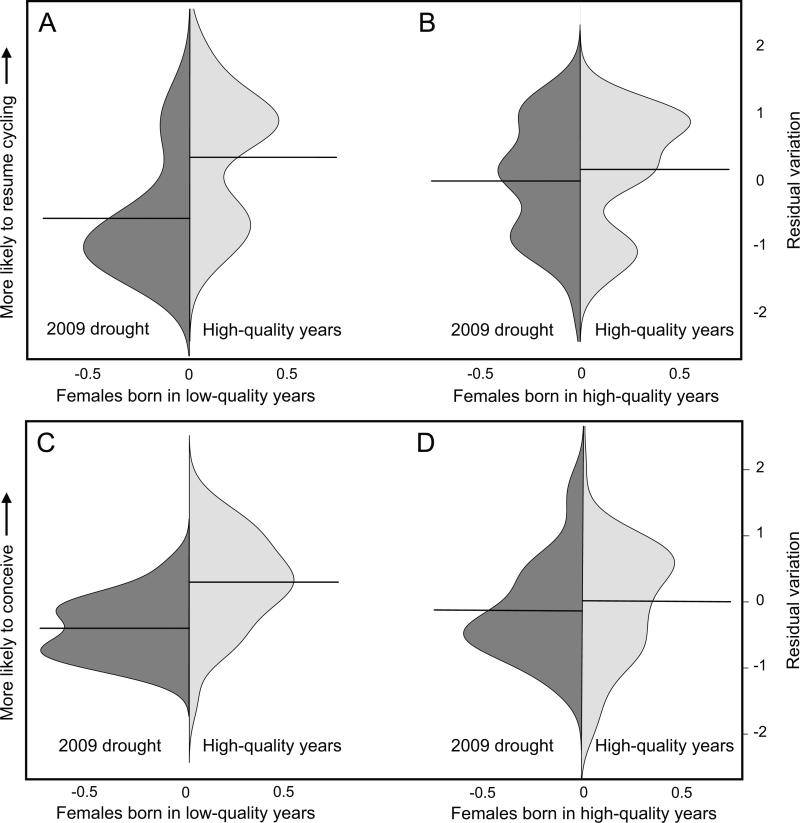
Figure 2.
Females born in low-quality environments were less likely to (A) resume cycling and (C) conceive during the 2009 drought than in high-quality years. Females born in high-quality environments were (B) equally likely to resume cycling and (D) only slightly less likely to conceive during the 2009 drought. Plots are density distributions of residuals from linear mixed-effects models predicting resumption of cycling and conception, respectively, and controlling for individual characteristics, reproductive history, and demographic factors that influence fertility (N = 50). Residual values are shown on the Y-axis, and density is shown on the X-axis. The mean value for each distribution is indicated by a black horizontal line.
Table 1.
Results from a generalized linear mixed-effects model predicting resumption of cycling
| Variable type | Estimate | SE | Z | P | Explanation of effects (P < .10) | |
|---|---|---|---|---|---|---|
| (Intercept) | 1.223 | 1.839 | .665 | .506 | ... | |
| Age | Continuous | –.348 | .252 | –1.380 | .167 | ... |
| Age2 | Continuous | .015 | .010 | 1.486 | .137 | ... |
| Current rainfall environment | Binary (0 = drought/1 = high quality) | 2.578 | .827 | 3.115 | .002 | During the 2009 drought, females were less likely to resume cycling. |
| Early rainfall environment | Binary (0 = low quality/1 = high quality) | .785 | .787 | .997 | .318 | ... |
| Current × early rainfall environment | Interaction between two binary variables | –1.695 | .933 | –1.817 | .069 | Females born in low-quality environments were more likely to resume cycling during high-quality years than during the 2009 drought; females born in high-quality environments were more robust to changes in prevailing ecological conditions |
| Cycling | Binary (N = 0/Y = 1) | –1.619 | .483 | –3.352 | .001 | Females cycling on January 1 were less likely to resume cycling over the following 10 months |
| Time since live birth | Continuous | .003 | .001 | 2.658 | .008 | Females that had recently given birth were less likely to resume cycling |
| Infant death | Binary (N = 0/Y = 1) | .722 | .599 | 1.206 | .227 | ... |
| Dominance rank | Continuous | .019 | .030 | .629 | .529 | ... |
| Group size | Continuous | –.006 | .009 | –.633 | .527 | ... |
Note: All variables described in the table were fitas fixed effects. Female identity was fit as a random effect (random effect variance ± SD = 0.216 ± 0.147).
Table 2.
Results from a generalized linear mixed-effects model predicting conception
| Variable type | Estimate | SE | Z | P | Explanation of effects (P < .10) | |
|---|---|---|---|---|---|---|
| (Intercept) | –5.872 | 2.601 | –2.257 | .024 | ... | |
| Age | Continuous | 1.255 | .382 | 3.281 | .001 | ... |
| Age2 | Continuous | –.054 | .016 | –3.460 | .001 | Very young and very old females were less likely to conceive |
| Current rainfall environment | Binary (0 = drought/1 = high quality) | 3.493 | 1.336 | 2.614 | .009 | During the drought, females were less likely to conceive |
| Early rainfall environment | Binary (0 = low quality/1 = high quality) | 1.562 | 1.357 | 1.151 | .249 | ... |
| Current × early rainfall environment | Interaction between two binary variables | –3.015 | 1.491 | –2.022 | .043 | Females born in low-quality environments were more likely to conceive during high-quality years than during the 2009 drought; females born in high-quality environments were more robust to changes in prevailing ecological conditions |
| Pregnant | Binary (N = 0/Y = 1) | –6.729 | 1.423 | –4.727 | <.001 | Females pregnant on January 1 were less likely to conceive over the following 10 months |
| Time since live birth | Continuous | .005 | .001 | 3.872 | <.001 | Females that had recently given birth were less likely to conceive |
| Infant death | Binary (N = 0/Y = 1) | 6.087 | 1.633 | 3.726 | <.001 | Following a recent infant death, females were more likely to conceive |
| Dominance rank | Continuous | –.040 | .042 | –.951 | .341 | ... |
| Group size | Continuous | –.043 | .013 | –3.208 | .001 | In larger groups, females were less likely to conceive |
Note: All variables described in table 2 were fit as fixed effects. Female identity was fit as a random effect (random effect variance ± SD = 0.843 ± 0.918).
When we shifted our definition of the early environment to include gestation and the first 6 months of life rather than the first year of life, we found less evidence that early rainfall influenced fertility in an adult environment–dependent manner. Specifically, we did not detect evidence of an interaction effect on cycle resumption or conception probabilities (β ± SE = 0.167 ± 0.782, P = .830 for resumption of cycling; β ± SE = 0.195 ± 0.993, P = .844 for conception; see tables A1, A2, available online).
Finally, our between-condition analysis, which examined fertility in the 2009 drought alone, suggests that females born in high-quality environments were more likely to conceive than females born in low-quality environments (t = −1.646, P = .107). The direction of this trend was the same, although weak, for resumption of cycling (t = −1.139, P = .263). However, the two groups were completely indistinguishable for both fertility measures when they were compared in high-quality adult environments (resumption of cycling: P > .10 for 97% of tests; conception: P > .10 for 100% of tests; see appendix). Together, our results suggest that the long-term costs of early ecological adversity become most apparent during periods of ecological challenge later in life.
Sensitivity of Female Fertility to Both Extremely Low and Extremely High Rainfall
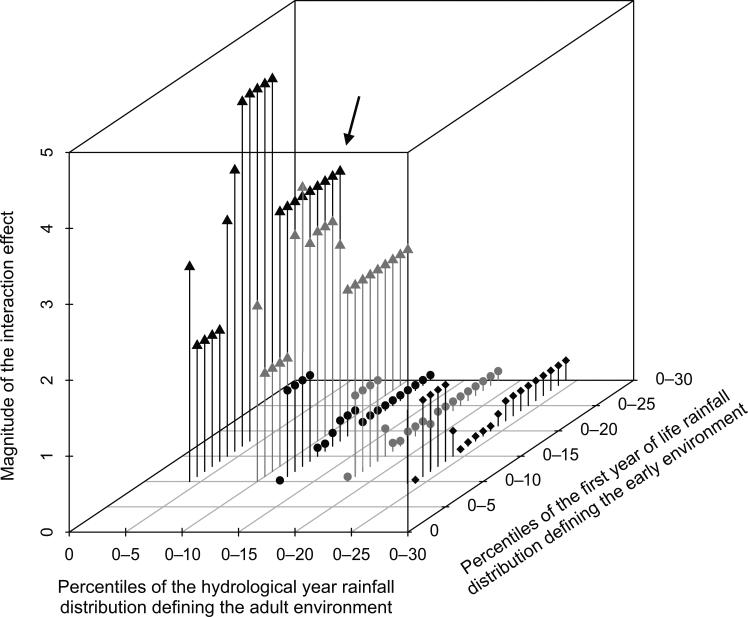
We found that the interaction between early and adult environmental conditions remained significant and was similar in magnitude across a range of thresholds for defining low-quality early environments (lowest 10th to lowest 30th percentile of low-rainfall years; fig. 3). In contrast, only the driest 10% of adult years yielded significant results that were qualitatively similar to our main analysis. These years equate to the driest 3 years experienced by females in our data set, with ≤188.5 mm of cumulative rainfall (hydrological years 2000, 2008, and 2009). Together, our results suggest that relatively modest droughts in early life can impose long-term costs on female fertility but that such costs are strongly expressed only during severe droughts during adulthood.
Figure 3.
Interactions between adult and early-life environments under alternative definitions of low-quality environment. The magnitude (i.e., the absolute value) of the interaction effect between adult and early environments on female conception is plotted on the Z-axis as a function of different definitions of a low-quality environment. Percentiles used as cutoffs for low-quality environments (i.e., where all years below the cutoff are considered low-quality years) are plotted on the X-axis (adult environment) and Y-axis (early environment), respectively. Colors and symbols are used for contrast and to delineate data points associated with a given low-quality adult year. For cases in which model estimates did not change as a result of changes in the definition of the adult environment (because no new data were included in the analysis), points are not plotted. The black triangle points (and all intermediate space between black triangle and gray triangle points) represent the interaction between early environmental conditions and adult environmental conditions when 2009 was the sole low-quality year in adulthood. The gray triangle points represent the interaction between early and adult conditions when low-quality years in adulthood included both 2009 and the next driest year for which data were available (i.e., the next driest year during which females with known early-life environments were present as multiparous adults, the year 2000). The black circle points represent the interaction between early and adult conditions when low-quality years in adulthood included 2009 and the next two driest years. For each definition of low-quality adult years, all points are plotted for definitions of low-quality early years in intervals of 1%. For the two most extreme definitions of low-quality adult years (black triangle and gray triangle points: rainfall in the lowest 10% of hydrological years, 2009 and 2000), the interaction effect was significant across all definitions of a low-quality early year (P <.05), indicating that female fertility is more sensitive to low rainfall in early life than to low rainfall in adult life. The black arrow points to the estimate reported in the main text (i.e., where 2009 is the only low-quality adult year and low-quality early life is defined as the lowest 30% of the distribution for cumulative rainfall in the first year of life).
Similarly, changing our definition of high-quality environments to include years with very high rainfall weakened the evidence for an early life–adult life interaction, which suggests that years with very high rainfall are qualitatively different from years with normal rainfall and may be stressful for female baboons. Specifically, when years with extremely high rainfall (rainfall >88th percentile of years) were included in the definition of high-quality adult environments, fertility during the 2009 drought and during high-quality years became more similar, attenuating the evidence for an interaction effect between low-quality and high-quality environments (fig. A5). Likewise, for any given definition of adult environmental conditions, the magnitude of the interaction effect decreased as more high-rainfall years were included in the definition of high-quality early-life environments. Together, these results suggest that female fertility is highest during moderate rainfall periods in Amboseli, in support of our choice to define high-quality years on the basis of the middle of the overall distribution.
Protective Effects of High Social Status in Early Life
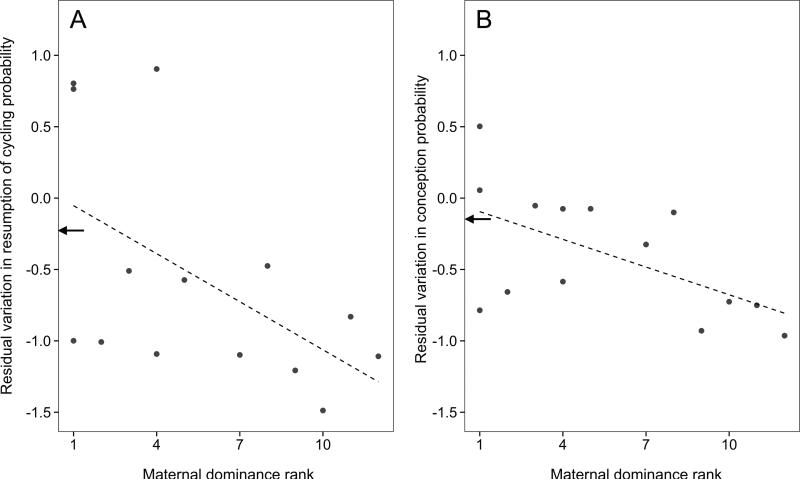
Finally, we hypothesized that another important component of the developmental environment, maternal social status, might buffer the effects of early-life drought on adult fertility. Indeed, for females born in low-quality environments (N = 14), fertility was significantly less depressed by the 2009 drought for females that were born to high-ranking mothers compared to females that were born to low-ranking mothers (resumption of cycling: β ± SE =−0.112 ± 0.050, R2 = 0.296, P = .044; conception: β ± SE = −0.065 ± 0.027, P = .033; fig. 4). In contrast, maternal dominance rank did not significantly affect the probability of conception (β ± SE = 0.031 ± 0.023, P = .178) or cycle resumption (β ± SE = 0.008 ± 0.025, P = .751) for females born in high-quality environments (N = 36). Thus, high mater- nal dominance rank provided important protection for females exposed to early-life ecological adversity but did not affect adult fertility for females born in high-quality ecological conditions.
Figure 4.
Fertility during the 2009 drought. Females born in low-quality ecological conditions were protected by high-quality social environments. Females born to high-ranking mothers in low-quality environments were more likely to resume cycling (A) and conceive (B) during the 2009 drought than females born to low-ranking mothers in low-quality environments. The best-fit lines from a linear regression of maternal dominance rank on the residuals of a model relating resumption of cycling or conception probability to ecological and reproductive readiness variables (N = 14) are plotted with a dashed line. The mean performance for females born during high-quality years during the drought is indicated with arrows.
Discussion
Support for the Developmental Constraints Model in Wild Baboons
Overall, our analyses do not support the PAR hypothesis: individuals born in low-quality environments did not outperform other individuals when they encountered similar low-quality conditions in adulthood. Instead, our within-female analysis revealed that females born in low-quality environments experienced an estimated mean decrease in conception probability of 60.0% during the 2009 drought. In contrast, females born in high-quality environments experienced an estimated mean decrease in conception probability of only 10.2%, an almost sixfold difference in effect sizes (see appendix for an explanation of how model parameters were translated into percentages). Notably, our between-condition analysis (which focuses on differences between females born in different environments, instead of the degree to which individual females are affected by the adult environment) indicated that the two sets of females were statistically indistinguishable in high-quality adult environments, at least with respect to the fertility traits that we investigated here. Taken together, these results indicate that females born in high-quality environments were more resilient to drought conditions than females born in low-quality environments but, during normal years, did not have an advantage over females born in poor conditions. Thus, females born in high-quality environments may experience a long-term advantage over females born in low-quality environments, but only if the population is exposed to adverse conditions. Consequently, early-life effects are most accurately assessed by measuring fitness effects across a wide range of adult conditions (Nussey et al. 2007; Hamel et al. 2009).
We concentrated on fertility-related outcomes in our study, rather than mortality, because annual death rates are low in baboons, as in most primates (Morris et al. 2011). Specifically, the small number of adult female deaths that occurred in the study population in 2009 (N = 13 of 100 adult females) were too few to assess the effects of early-life conditions on survival during the 2009 drought. Thus, although our findings exclude a PAR explanation for fertility-related fitness components in the Amboseli baboons, the PAR model could, in principle, hold for survival-related fitness components, which is a possibility that demands further testing.
Our results are consistent with studies of both wild and laboratory animals that also report consistent advantages to individuals born in high-quality environments (Reid et al. 2006; Nussey et al. 2007; Uller et al. 2013). In addition, they are consistent with tests of the PAR versus developmental constraints models in preindustrial humans and wild roe deer (although these studies did not use within-subjects designs, as presented here; Hayward and Lummaa 2013; Douhard et al. 2014). For example, Douhard and colleagues found that, in a resource-rich habitat, wild female roe deer born in high-quality years experienced a lifelong fitness advantage over individuals born in low-quality years. Similarly, Hayward and Lummaa (2013) demonstrated that, in a preindustrial Finnish population, poor early-life environments were associated with increased mortality in later life. In both cases, these patterns held regardless of the adult environment. Notably, some support for the PAR hypothesis was identified by Douhard and colleagues in a second population of wild roe deer, which inhabited a more marginal environment. Specifically, females born in low-quality years were more likely to survive in harsh adult conditions than females born in high-quality years (Douhard et al. 2014). However, the authors attributed this result to increased viability selection on deer born in poor environments rather than to PAR: under poor early-life conditions, only the most robust roe deer survived to adulthood. Because we focused on within-individual comparisons of performance in both low-quality and high-quality years in adulthood, viability selection could not have influenced our results. This difference in study design may account for the absence of support for PAR in our study.
Factors That Influence the Relationship between Early-Life Ecology and Adult Fertility
Although our findings are largely consistent with the developmental constraints hypothesis, we found that these constraints were contingent on at least two factors. First, we found that the negative consequences of early adversity were highly contingent on the adult environment. Specifically, we found that the long-term consequences of early adversity were only detectable in the worst conditions during adulthood, consistent with evidence from laboratory mice (Giovanoli et al. 2013) and humans (Hayward et al. 2013). For example, in a preindustrial human population, individuals born during periods of low crop yield exhib- ited lower survival and fertility during periods of famine compared with individuals born during periods of high crop yield (Hayward et al. 2013). Thus, the effects of early-life adversity may, in certain cases, remain undetected unless individuals face challenging circumstances later in life, which is a possibility with important ramifications for understanding resilience.
Second, we found that the severity of early-life drought effects was contingent on an individual's early-life social environment, highlighting the multifaceted nature of the early environment. We estimate that, for females born in low-quality environments, each improvement of one maternal rank position would increase resumption of cycling probability and conception probability during severe drought years by 1.8% and 1.3%, respectively (see appendix). Given that 14 rank positions separate the highest- and lowest-ranking females in an average social group, and given that severe drought conditions occur approximately once each decade (fig. 1), differences in female fertility during droughts could create a significant lifetime advantage for the off-spring of high-ranking mothers. This fertility advantage could arise through several mechanisms. For example, off- spring of high-ranking mothers may have increased access to resources, which could protect them from nutritional deficits during dry years (Whitten 1983; Hayward et al. 2013). Alternatively, high-ranking females could experience reduced social stress (Sapolsky 2005), which could have cascading effects on their offspring's physiology (Onyango et al. 2008). Interestingly, similar protective effects of high-quality early-life social environments have also been described in humans. Individuals who grew up in households with low socioeconomic status (SES) but experienced high levels of maternal warmth and affection did not develop the chronic inflammation typically associated with a low-SES childhood (Chen et al. 2011). Social buffering against other sources of adversity may thus have a long evolutionary history in the primate lineage.
Conclusions
Our results join those of other recent studies (Hayward et al. 2013; Uller et al. 2013; Douhard et al. 2014) to motivate a reconsideration of widely accepted PAR explanations for early-life effects on later-life phenotypes, including high disease rates in human cohorts born during famines. The more common situation appears to be that individuals born in low-quality early environments experience a lifelong disadvantage relative to individuals born in high-quality early environments. This disadvantage appears to be most acute when the adult environment deteriorates, which emphasizes the need to study early-life effects under variable environments and across the life course.
We do not believe, however, that the PAR model should be discarded altogether. It has provided a valuable framework for investigating the evolution of early-life effects, and it may still prove to be important under a constrained set of conditions (or for some fitness components but not others). Recent studies have attempted to outline the settings in which PARs are predicted to evolve. Specifically, several authors have argued that PARs should be favored when the environmental cue that triggers alternative phenotypic development is a reliable indicator of the adult environment (Rickard and Lummaa 2007; Wells 2012; Nettle 2013). This condition clearly does not hold for rainfall in Amboseli (fig. 1), and it may rarely be the case for long-lived species. Thus, it is not surprising that the best exam- ples of PARs have been found in short lived species, such as Daphnia (Boersma et al. 1998), locusts (Applebaum and Heifetz 1999), and voles (Lee 1988; Horton and Stetson 1992). In contrast, tests of the PAR model in long-lived species (e.g., wild baboons, deer, and preindustrial humans; Hayward et al. 2013; Hayward and Lummaa 2013; Douhard et al. 2014) have either rejected PAR explanations or, at best, identified weak evidence. If PARs do prove to be more frequent in short-lived species than long-lived species, it will be somewhat ironic that the model was originally proposed to account for early-life effects in humans, a long-lived species that evolved in a highly variable savanna environment. Studies that investigate the relationship between the evidence for PARs, species life history, and environmental variability will be essential for addressing this question. Until it is resolved, however, we argue that developmental constraints, rather than PAR, should be treated as the null model in explaining the evolution of early-life effects, particularly for long-lived organisms.
Supplementary Material
Acknowledgments
We thank the National Science Foundation for support over the past two decades (most recently DGE 1160401, BCS 0323553, DEB 0846286, and IOS 0919200). We are also grateful for support from the National Institute on Aging (R01AG034513 and P01AG031719, as well as P30AG034424 at Duke University and P30AG024361 at Princeton University). We also thank Duke University, Princeton University, and the Max Planck Institute for Demographic Research for important recent support. We thank the Kenya Wildlife Service, Institute of Primate Research of National Museums of Kenya, National Council for Science and Technology, members of the Amboseli-Longido pastoralist communities, Tortillis Camp, Ker and Downey Safaris, Air Kenya, and Safarilink for their cooperation and assistance in Kenya. Thanks also to R. S. Mututua, S. Sayialel, V. Somen, T. Wango, and J. K. Warutere in Kenya and to J. Gordon, N. Learn, L. Maryott, and K. Pinc in the United States. Finally, we thank M. Douhard and a second, anonymous reviewer for valuable comments on a previous submission of this manuscript. This research was approved by the Institutional Animal Care and Use Committees at Princeton University and Duke University and adhered to all the laws and guidelines of Kenya. S.C.A. and J.T. contributed equally to this work.
Acknowledgments
We thank the National Science Foundation for support over the past two decades (most recently DGE 1160401, BCS 0323553, DEB 0846286, and IOS 0919200). We are also grateful for support from the National Institute on Aging (R01AG034513 and P01AG031719, as well as P30AG034424 at Duke University and P30AG024361 at Princeton University). We also thank Duke University, Princeton University, and the Max Planck Institute for Demographic Research for important recent support. We thank the Kenya Wildlife Service, Institute of Primate Research of National Museums of Kenya, National Council for Science and Technology, members of the Amboseli-Longido pastoralist communities, Tortillis Camp, Ker and Downey Safaris, Air Kenya, and Safarilink for their cooperation and assistance in Kenya. Thanks also to R. S. Mututua, S. Sayialel, V. Somen, T. Wango, and J. K. Warutere in Kenya and to J. Gordon, N. Learn, L. Maryott, and K. Pinc in the United States. Finally, we thank M. Douhard and a second, anonymous reviewer for valuable comments on a previous submission of this manuscript. This research was approved by the Institutional Animal Care and Use Committees at Princeton University and Duke University and adhered to all the laws and guidelines of Kenya. S.C.A. and J.T. contributed equally to this work.
Literature Cited
- Alberts SC, Altmann J. Intraspecific variability in fertility and offspring survival in a nonhuman primate: behavioral control of ecological and social sources. In: Wachter KW, Bulatao RA, editors. Offspring: the biodemography of fertility and family behavior. National Academy Press; Washington, DC: 2003. pp. 140–169. [Google Scholar]
- Alberts SC, Altmann J. The Amboseli baboon research project: 40 years of continuity and change. In: Kappeler P, Watts DP, editors. Long-term field studies of primates. Springer; New York: 2012. pp. 261–288. [Google Scholar]
- Alberts SC, Hollister-Smith J, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Seasonality and long term change in a savannah environment. In: Brockman DK, van Schaik CP, editors. Primate seasonality: implications for human evolution. Cambridge University Press; Cambridge: 2005. pp. 157–196. [Google Scholar]
- Altmann J. Baboon mothers and infants. Harvard University Press; Cambridge, MA: 1980. [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behavioral Ecology and Sociobiology. 2005;57:490–501. [Google Scholar]
- Altmann J, Gesquiere L, Galbany J, Onyango PO, Alberts SC. Life history context of reproductive aging in a wild primate model. Annals of the New York Academy of Sciences. 2010;1204:127–138. doi: 10.1111/j.1749-6632.2010.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann S. Diets of yearling female primates (Papio cynocephalus) predict lifetime fitness. Proceedings of the National Academy of Sciences of the USA. 1991;88:420–423. doi: 10.1073/pnas.88.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum S, Heifetz Y. Density-dependent physiological phase in insects. Annual Review of Entomology. 1999;44:317–341. doi: 10.1146/annurev.ento.44.1.317. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Gluckman PD, Godfrey KM, Harding J, Owens J, Robinson J. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:1421–1422. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: linear mixed-effects models using Eigen and S4. 2014 http://cran.r-project.org/web/packages/lme4/index.html.
- Bateson P, Barker D, Clutton-Brock T, Deb D. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Onderdonk DA, Alberts SC, Altmann J. The ecology of conception and pregnancy failure in wild baboons. Behavioral Ecology. 2006;17:741–750. [Google Scholar]
- Boersma M, Spaak P, De Meester L. Predator-mediated plasticity in morphology, life history, and behavior of Daphnia: the uncoupling of responses. American Naturalist. 1998;152:237–248. doi: 10.1086/286164. [DOI] [PubMed] [Google Scholar]
- Bol V, Desjardins F, Reusens B, Balligand J-L, Remacle C. Does early mismatched nutrition predispose to hypertension and atherosclerosis, in male mice? PLoS One. 2010;5:e12656. doi: 10.1371/journal.pone.0012656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier MJE, Tung J, Altmann J, Alberts SC. Age at maturity in wild baboons: genetic, environmental and demographic influences. Molecular Ecology. 2008;17:2026–2040. doi: 10.1111/j.1365-294X.2008.03724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington RB, Smulders TV. Problems with residual analysis. Animal Behaviour. 2001;62:599–602. [Google Scholar]
- Douhard M, Plard F, Gaillard J-M, Capron G, Delorme D, Klein F, Duncan P, et al. Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20140276. doi: 10.1098/rspb.2014.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freckleton R. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. Journal of Animal Ecology. 2002;71:542–545. [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Hormones and Behavior. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends in Endocrinology and Metabolism. 2004a;15:183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004b;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends in Ecology and Evolution. 2005a;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proceedings of the Royal Society B: Biological Sciences. 2005b;272:671–677. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A. On the uses of data on lifetime reproductive success. In: Clutton-Brock TH, editor. Reproductive success. University of Chicago Press; Chicago: 1988. pp. 454–471. [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. British Medical Bulletin. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Hamel S, Gaillard J-M, Festa-Bianchet M, Côté SD. Individual quality, early-life conditions, and reproductive success in contrasted populations of large herbivores. Ecology. 2009;90:1981–1995. doi: 10.1890/08-0596.1. [DOI] [PubMed] [Google Scholar]
- Hausfater G. Dominance and reproduction in baboons (Papio cynocephalus): a quantitative analysis. Karger; Basel, NY: 1974. [PubMed] [Google Scholar]
- Hausfater G, Altmann J, Altmann S. Long-term consistency of dominance relations among female baboons (Papio cynocephalus). Science. 1982;217:752–755. doi: 10.1126/science.217.4561.752. [DOI] [PubMed] [Google Scholar]
- Hayward AD, Lummaa V. Testing the evolutionary basis of the predictive adaptive response hypothesis in a preindustrial human population. Evolution, Medicine, and Public Health. 2013;2013:106–117. doi: 10.1093/emph/eot007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward AD, Rickard IJ, Lummaa V. Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proceedings of the National Academy of Sciences of the USA. 2013;110:13886–13891. doi: 10.1073/pnas.1301817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton TH, Stetson MH. Maternal transfer of photoperiodic information in rodents. Animal Reproduction Science. 1992;30:29–44. [Google Scholar]
- Jones P. Schizophrenia after prenatal exposure to the Dutch hunger winter of 1944–1945. Archives of General Psychiatry. 1994;51:333–334. doi: 10.1001/archpsyc.1994.03950040077010. [DOI] [PubMed] [Google Scholar]
- Kemp MW, Kallapur SG, Jobe AH, Newnham JP. Obesity and the developmental origins of health and disease. Journal of Paediatrics and Child Health. 2012;48:86–90. doi: 10.1111/j.1440-1754.2010.01940.x. [DOI] [PubMed] [Google Scholar]
- Lea AJ, Altmann J, Alberts SC, Tung J. Data from: Developmental constraints in a wild primate. American Naturalist, Dryad Digital Repository. 2015 doi: 10.1086/681016. http://doi.org/10.5061/dryad.5r37g. [DOI] [PMC free article] [PubMed]
- Lee M. Vole infant development is influenced by maternal photoperiodic history perinatally. American Journal of Physiology. 1988;255:831–838. doi: 10.1152/ajpregu.1988.255.5.R831. [DOI] [PubMed] [Google Scholar]
- Meaney M. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Monaghan P. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris WF, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey AE, Stoinski TS, et al. Low demographic variability in wild primate populations: fitness impacts of variation, covariation, and serial correlation in vital rates. American Naturalist. 2011;177:E14–E28. doi: 10.1086/657443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettle D. The evolution of predictive adaptive responses in human life history. Proceedings of the Royal Society B: Biological Sciences. 2013;280:13–43. doi: 10.1098/rspb.2013.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy-Meir I. Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics. 1973;4:25–51. [Google Scholar]
- Nussey DH, Kruuk LEB, Morris A, Clutton-Brock TH. Environmental conditions in early life influence ageing rates in a wild population of red deer. Current Biology. 2007;17:R1000–R1001. doi: 10.1016/j.cub.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Onyango PO, Gesquiere LR, Wango EO, Alberts SC, Altmann J. Persistence of maternal effects in baboons: mother's dominance rank at son's conception predicts stress hormone levels in subadult males. Hormones and Behavior. 2008;54:319–324. doi: 10.1016/j.yhbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P. Spatial variation in demography and population growth rate: the importance of natal location. Journal of Animal Ecology. 2006;75:1201–1211. doi: 10.1111/j.1365-2656.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- Rickard IJ, Lummaa V. The predictive adaptive response and metabolic syndrome: challenges for the hypothesis. Trends in Endocrinology and Metabolism. 2007;18:94–99. doi: 10.1016/j.tem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Human Development. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Spencer HG, Hanson MA, Gluckman PD. Response to Wells: phenotypic responses to early environmental cues can be adaptive in adults. Trends in Ecology and Evolution. 2006;21:425–426. [Google Scholar]
- Uller T, Nakagawa S, English S. Weak evidence for anticipatory parental effects in plants and animals. Journal of Evolutionary Biology. 2013;26:2161–2170. doi: 10.1111/jeb.12212. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wells JCK. Environmental quality, developmental plasticity and the thrifty phenotype: a review of evolutionary models. Evolutionary Bioinformatics Online. 2007;3:109–120. [PMC free article] [PubMed] [Google Scholar]
- Wells JCK. A critical appraisal of the predictive adaptive response hypothesis. International Journal of Epidemiology. 2012;41:229–235. doi: 10.1093/ije/dyr239. [DOI] [PubMed] [Google Scholar]
- Whitten PL. Diet and dominance among female vervet monkeys. American Journal of Primatology. 1983;5:139–159. doi: 10.1002/ajp.1350050205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.