Abstract
MscL is a mechanosensitive channel of large conductance that functions as an “emergency release valve,” allowing bacteria to survive acute hypoosmotic stress. Although Escherichia coli MscL is the best-studied mechanosensitive channel, structural rearrangements occurring during gating remain disputed. Introduction of a charged residue into the pore of MscL was shown to result in a reduced-viability phenotype. Here, we probe for residues in the transmembrane domains that are exposed to the aqueous environment in the presence and absence of hypoosmotic shock by reacting a charged sulfhydryl reagent with substituted cysteines. Subsequent analysis of cell viability allows for an assessment of residues exposed in the closed and opening states in vivo. The results suggest that the crystal structure of MscL derived from the Mycobacterium tuberculosis orthologue may reflect a nearly closed rather than fully closed state and support a clockwise rotation of the pore-forming first transmembrane domain on gating.
The ability to detect mechanical force is crucial for essentially all life. One paradigm, which has been well studied at the molecular and structural levels, is the ability of microbes to detect membrane stretch invoked by osmotic environment. Escherichia coli contains two mechanosensitive channels that have been shown to be involved in osmotic regulation, MscL and MscS (1). Homologues of these molecules are found in virtually all microbes, including the Archea (2). The genes appear to encode proteins that serve a redundant function in osmotic adaptation because deletion of both mscS and mscL is required to observe a phenotype (1). The resulting double-null strain is osmotically fragile, showing reduced viability on acute hypotonic shock (osmotic downshock).
To date, MscL is the best-studied mechanosensitive channel. Many mutations that effect a gain-of-function (GOF) phenotype, in which the cells show slowed growth or decreased viability, have been isolated and studied (3–6). The pivotal point came when a crystal structure of an orthologous channel from Mycobacterium tuberculosis was resolved to 3.5 Å (7). The channel was found to be a homopentamer. However, the study could not determine whether the solved structure reflects a closed or “nearly closed” state of the channel (the channel constricted to a 4-Å-diameter pore), nor could it accurately predict the structural changes that occur on channel gating. What was clear from the crystal structure was that the first transmembrane domain (TM1) lines the lumen and that to obtain the predicted open-pore size of >30 Å in diameter (8), large structural changes must occur. Subsequent studies have predicted features of the channels' open structure; one suggested model is based on modeling, crosslinking, and disulfide-trapping experiments (9, 10), and the other is based on EPR results (11). Although agreement exists on general aspects of gating, such as tilting of the transmembrane domains, several fundamental differences exist, including the identity of specific residues likely to line the open pore.
One approach that has been often used for identifying residues within a channel pore is the substituted cysteine accessibility method (SCAM) (12). In the SCAM, cysteine substitutions are generated within the protein and sulfhydryl reagents are allowed to react on gating. Hydrophilic, often charged, reagents are typically used to assure that only residues that are accessible to the aqueous environment are modified. If the residue is buried within the closed channel but exposed on gating, then the reagent will react with the cysteine only on channel opening. For channels with small pore sizes, a decrease in conductance is often observed on this modification.
Recently, Batiza et al. (13), studying E. coli MscL, adapted the SCAM to test for accessibility in vivo. This group exploited previous observations demonstrating that hydrophilic or charged substitutions within TM1 often lead to decreased viability (4, 6). This appears to be due to channel misgating because patchclamp analysis demonstrated that the channels gate at lowerthan-normal membrane tensions. In addition, reaction of charged sulfhydryl reagents with a cysteine mutant, G22C, demonstrated that these changes could be effected in situ, as assayed by patch clamp (14). Batiza et al. (13) studied the accessibility of a mutant with a single substitution, L19C, and found a decreased viability on treatment of the charged sulfhydryl reagent, [2-(trimethylammonium) ethyl]methanethiosulfonate bromide (MTSET), with intact cells. This decrease in viability was observed only when the channel was gated by an acute osmotic downshock, suggesting that the residue was only exposed on gating.
We recently generated a mutant library in which every amino acid in the transmembrane domains had been sequentially replaced with cysteine. Because wild-type E. coli MscL contains no cysteines, each substituted cysteine is unique within the subunit. Every mutant in the library was assayed for its ability to confer phenotypic changes, and many were characterized electrophysiologically (5). Although not as severe as reported (3–6), GOF-effecting cysteine substitutions were noted in the middle of TM1, near the proposed channel constriction point and toward the periplasmic and cytoplasmic regions of the second transmembrane domain (TM2). Here, we use this characterized library for a modified in vivo SCAM. The ability to drastically decrease viability on residue modification allows us the unique ability to resolve aspects of the structure of MscL in different conformational states while it resides in a living cell. Although our results are consistent with the overall predictions of the crystal structure, they suggest modifications needed to define the fully closed state of the channel. Our results also provide strong support for one of the contested models for structural changes that occur during channel gating.
Materials and Methods
Strains. The cysteine mutant library was generated as described (5). The wild-type E. coli mscL and cysteine substituted mscL mutants were inserted into pB10b (17), and expression was induced by using isopropyl β-d-thiogalactoside. The E. coli FRAG-1 (18) derivative strain, MJF455 ΔmscL::Cm, ΔmscS (1), was used as host. MTSET was obtained from Toronto Research Chemicals (North York, ON, Canada).
In Vivo Functional Assay. Recently streaked colonies were grown overnight at 37°C in 1 ml of citrate-phosphate-defined medium (per liter: 8.57 g of Na2HPO4, 0.87 g of K2HPO4, 1.34 g of citric acid, 1.0 g of NH4SO4, 0.001 g of thiamine, 0.1 g of Mg2SO4·7H20, 0.002 g of (NH4)2SO4·FeSO4·6H2O) plus 1 mM ampicillin. The fresh overnight culture was diluted 1:20 in 2 ml of this defined medium and grown for l h. The culture was then diluted to an OD600 of 0.05 in 2 ml of the same medium supplemented with 0.5 M NaCl. The cultures were then grown to an OD600 of 0.2–0.25 at which stage expression was induced for 1 h with 1 mM β-d-thiogalactoside. The induced cultures were diluted 1:20 into citrate-phosphate medium containing (i) 0.5 M NaCl as control; (ii) no additives for osmotic downshock; (iii) 0.5 M NaCl and 1 mM MTSET for MTSET alone; or (iv) 1 mM MTSET for MTSET and osmotic downshock. Cells were grown with shaking at 37°C for 15 min, and then six consecutive 1:10 serial dilutions were made in medium containing either no salt (for the osmotic downshock conditions) or 0.5 M NaCl (for the mock-shock conditions). These diluted cultures were plated in duplicate and grown overnight at 37°C on LB-ampicillin agar plates. The colony-forming units were counted and averaged per experiment; no statistically significant differences in colony-forming units for the mock-shock control were observed between wild type and any mutant (all were ≈1.2 × 109 colony-forming units per OD unit). All data are presented as a percentage of the mock-shock condition.
Results
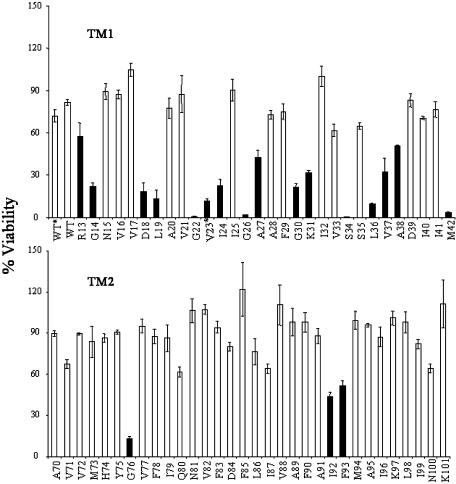
An In Vivo Functional Assay Was Used to Scan the Transmembrane Domains for Residue Positions. The in vivo SCAM was used to identify the amino acids of MscL that are exposed to the aqueous environment on channel gating. We used a transmembranedomain cysteine library to assay all residues within TM1 and TM2 (1). The mutated proteins were expressed in an mscL-, mscS- double-null strain (MJF455). In the primary screen, the library was exposed simultaneously to both the positively charged sulfhydryl reagent MTSET and acute osmotic down-shock. The treated cells were then plated and viability scored. As seen in Fig. 1, several of the cysteine mutants demonstrated a significant decrease in viability on treatment. Mutants exhibiting <60% viability were targeted for further study.
Fig. 1.
Viability of cysteine mutants challenged by osmotic downshock in the presence of MTSET. Cysteine mutants within the first (Upper, TM1) and the second (Lower, TM2) transmembrane domains were tested. Stars indicate channels that were tested at uninduced levels, WT and V23. Mutants showing <60% viability (filled bars) were targeted for further study. All experiments were performed in duplicate with three to eight independent experiments; SEM for each is shown.
All mutants were initially tested after 1 h of β-d-thiogalactoside induction. However, consistent with a previous study (5), at induced expression levels V23C conferred a GOF phenotype that led to a viability too low to measure. Therefore, V23C was assayed in the absence of induced expression. Previous studies had demonstrated that without induction the vector allows the expression of two to six channels per cell (15). As shown in Fig. 1, even at such low expression levels, the viability ratio of treated to untreated V23A-containing cells was quite low for this mutant, supporting it as a candidate for further study.
Mutants Targeted by the Primary Screen Fall into Four Major Categories. At this point we sought to exclude mutants that form a nonfunctional channel, or are loss-of-function (LOF), and thus are not suitable reporters of residue accessibility on gating. Toward this end, we assayed all the mutants indicated by the initial screen for their viability subsequent to the osmotic downshock in the absence of MTSET treatment. The double-null MJF455 strain used in this study, lacking both mscS and mscL, normally shows decreased viability on acute osmotic downshock. Expression of a functional MscL channel rescues this osmoticlysis phenotype, even at uninduced expression levels [see wild type (Fig. 1) and V23C (Table 1)], which we know to be only a few channels per cell (15). We assayed all of the mutants for their viability subsequent to the osmotic downshock in the absence of MTSET treatment. As seen in Table 1, group I, seven of the 19 candidates identified in the primary screen were determined to be LOF by their inability to rescue this osmotic-lysis phenotype.
Table 1. Categorization of mutants as a result of the phenotype exhibited.
| Strain | Osmotic shock | MTSET | MTSET + osmotic shock |
|---|---|---|---|
| Empty vector | 4.6 ± 0.2 | 122 ± 1 | 3.3 ± 0.1 |
| WT MscL | 93 ± 1 | 96 ± 1 | 85 ± 1 |
| Group I | |||
| G14C | 16 ± 1 | 122 ± 3 | 24 ± 2 |
| D18C | 26 ± 9 | 113 ± 10 | 18 ± 6 |
| K31C | 29 ± 3 | 116 ± 9 | 35 ± 1 |
| L36C | 33 ± 4 | 87 ± 4 | 11 ± 1 |
| G76C | 40 ± 2 | 96 ± 8 | 25 ± 5 |
| I92C | 56 ± 4 | 111 ± 5 | 41 ± 2 |
| F93C | 47 ± 2 | 107 ± 4 | 49 ± 3 |
| Group II | |||
| G26C | 13 ± 2 | ≤0.2 | ≤0.2 |
| G30C | 79 ± 4 | ≤0.2 | 25 ± 3 |
| S34C | 89 ± 15 | ≤0.2 | ≤0.2 |
| A38C | 85 ± 3 | 61 ± 4 | 55 ± 1 |
| Group IIIa | |||
| G22C | 80 ± 2 | 48 ± 6 | 0.60 ± 0.08 |
| V23C* | 83 ± 4 | 45 ± 7 | 12 ± 1 |
| A27C | 94 ± 6 | 54 ± 2 | 43 ± 2 |
| V37C | 95 ± 3 | 56 ± 3 | 28 ± 4 |
| Group IIIb | |||
| L19C | 115 ± 19 | 129 ± 13 | 14 ± 6 |
| I24C | 87 ± 2 | 92 ± 2 | 22 ± 4 |
| M42C | 78 ± 8 | 64 ± 9 | 3.3 ± 0.6 |
| Group IV | |||
| R13C | 6 ± 3 | 107 ± 3 | 57 ± 10 |
Shown is the percent survival ± SEM under the different conditions assayed. All experiments were performed in duplicate with three to eight independent experiments. The first column indicates the ability of the cells to survive osmotic downshock alone, the second indicates their ability to survive MTSET alone, and the third indicates their ability to survive both MTSET and osmotic downshock. All V23 data, indicated by *, is from uninduced cultures due to low viability when induced. Boldface indicates the condition in which the largest and statistically significant decreases in viability are observed. Differences between osmotic shock and MTSET are statistically significant (P < 0.05 as determined by Student's t test) for groups I, II, IIIa, and IV. Differences between MTSET and MTSET + osmotic shock are statistically significant for groups I, IIIa IIIb, and IV and for G30C. Differences between osmotic shock and MTSET + osmotic shock are statistically significant for groups II, IIIa IIIb, and IV, and for L36C.
The remaining 12 candidates identified in the primary screen showed decreased viability only in the presence of MTSET. To determine whether MTSET was reacting with the channel when in the closed state, we tested viability in the absence of osmotic downshock. Four candidates demonstrated decreased viability on MTSET treatment alone (Table 1, group II). In contrast, seven of the candidates showed decreased viability that depended on both MTSET and osmotic downshock (Table 1, groups IIIa and IIIb). Four of these demonstrated some decrease in viability when treated with MTSET alone (group IIIa). R13C was assigned to a fourth category, group IV, because it showed the interesting property that MTSET actually increased viability on osmotic downshock.
Discussion
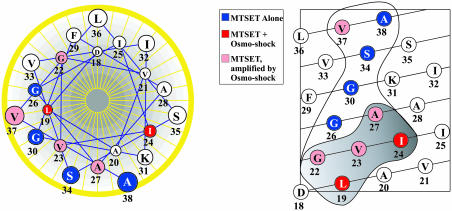
The modified SCAM used here allowed us to identify MscL transmembrane residues exposed in different conformational states while the channel is within its native environment of a living cell. Sulfhydryl reagents, including methanethiosulfonate compounds, have been used in the past to probe the pore of several other channels (12). However, the pore of the MscL channel is much larger than those other channels and is estimated to be ≈30 Å (8). Therefore, it seems unlikely that MTSET would block the passage of ions, as is observed for other channels. Instead, the assay depends on the assumption that the addition of a large hydrophilic group onto the residue will cause the channel to gate more easily. Precedent exists: previous studies found that hydrophilic substitutions within the TM1 domain, which lines the pore (7), leads to channels that gate at lower membrane tension, thus resulting in a GOF phenotype (3, 4, 6). These substitutions appear to result in channels that more easily go through transitions between closed and open states (6, 16). The assumption appears to be valid given the finding that at least one substituted residue per α-helical turn of the poreforming region of TM1 was found to lead to MTSET-dependent decreases in viability (Fig. 2).
Fig. 2.
Relative position of reactive TM1 residues. An idealized helical wheel (Left) and helical net (Right) are shown. Residues have been colored according to the condition under which decreased viability was seen. Blue indicates that the residues respond to MTSET alone; pink indicates that the residues respond to MTSET alone but show an increased response on osmotic downshock; red indicates that the residues require both MTSET and osmotic downshock to show decreased viability. On the right is the helical net representation of TM1. Enclosed residues react with MTSET; those within the shaded region are residues in the gate that show increased accessibility to MTSET when the channel is gated by osmotic downshock.
Although the primary screen used here does not distinguish between mutated residues that become exposed on channel gating and those conferring constitutive LOF phenotypes, it is a simple matter to distinguish among these categories on subsequent analysis. In the previous study in which MTSET was used to identify a residue exposed on gating, the authors used several mscL-null host strains (13). Here, we have specifically used an mscL and mscS double-null strain, MJF455 (1). This use has the advantage of avoiding false-negative results; if a channel is functionally compromised and is significantly less sensitive than normal, then the MscS channel cannot shunt the turgor forces induced by the osmotic downshock. However, because the double-null strain is osmotically fragile, this approach will also identify nonfunctional or LOF mutants. A decreased viability could also be due to MTSET binding in the closed state and lead to a nonfunctional channel that induces lysis on osmotic shock. However, group IIIa shows a phenotype independent of shock, and mutations in group IIIb gave consistent results when performed in PB104 (4, 17), a MscS-containing strain (13, and data not shown). The cysteine library used here has been characterized previously; although the vast majority of mutants were shown to be functional, a few conferred LOF phenotypes due to misfunctioning channels, as opposed to heterologous or decreased expression levels (5). Although the osmotic downshock procedure was different between the original study and the present one, a strong agreement exists between the two; most of the mutants we identify as LOF here (group I) are consistent with the previous study. A few mutants, specifically Q80C, F85C, and A89C, were categorized as LOF in the previous study but were not recognized here, presumably because of differing experimental conditions; none of these mutants were shown to react with MTSET. In addition, we categorize G76C and I92C as LOF; these mutants were not assayed in the previous study because of their GOF phenotype. These mutants may not truly be nonfunctional, but the compromised viability in the LOF assay may in fact be due to the combined stresses of the GOF phenotype (misgating or leaking channels) combined with osmotic downshock.
We found that R13C appears to be sensitive to osmotic downshock alone but is saved by the addition of MTSET (Table 1, group IV). The R13C substitution has been shown to confer a GOF phenotype (5). As with G76C and I92C (discussed above), the LOF phenotype may in fact be due to the combined stresses of the GOF phenotype and osmotic downshock. A likely interpretation is that introduction of the positively charged MTSET restores channel function, and thus viability, because the positive charge at this position is reestablished.
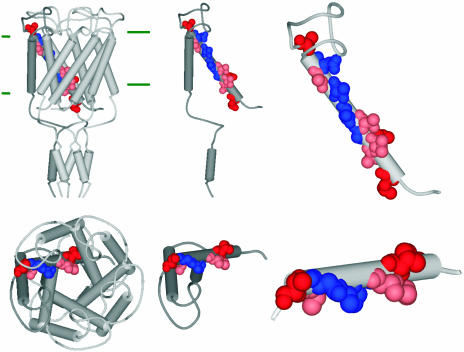
The results presented here give us an image of the vestibule portion of the pore. Several residues reacted with MTSET independent of osmotic downshock and elicited a loss of viability phenotype (group II). These residues reside within the more periplasmic portion of TM1 (Fig. 2). Although we cannot completely rule out the possibility that the cysteine substitution influences the structure for a given mutated MscL, it is impressive that when modeled onto the crystal structure of the M. tuberculosis MscL channel (7), the residues appear to line one face of the helix (Fig. 3). Note, however, that they do not directly face the lumen but are rotated several degrees clockwise as viewed from the periplasm. Although when the MscL channel was originally crystallized, the authors noted that it appeared to be in a closed or “nearly closed” state (7), it has more recently become dogma that the crystal structure reflects the closed conformation. Recently, a proposal was made that the current structural models, including the crystal structure, do indeed reflect a “nearly closed” state and that a slight counterclockwise rotation of TM1 is necessary to achieve the native closed state (5). The evidence underlying this proposal was the formation of a disulfide bridge for a single cysteine mutant, G26C. Here, we find four residues that appear to be exposed in the closed state that support this hypothesis (Fig. 3). In sum, the findings are consistent with the clockwise direction of rotation for TM1 during gating (counterclockwise for closure) suggested by data obtained from site-directed spin-labeling and EPR spectroscopy (11) but not consistent with the countermodel for gating that proposes a slight rotation in the opposite direction (9, 10).
Fig. 3.
The location of reactive residues within current models for the closed MscL channel. The structure of the closed or nearly closed M. tuberculosis MscL channel derived from x-ray crystallography is shown. Pictured are profiles of the complex (Left) viewed from the side (Top) and periplasm (Bottom), single subunits (Middle), and a close-up of the TM1 domain (Right). The approximate boundary of the membrane is indicated with green horizontal lines. Residues in TM1 that react with MTSET are shown in CPK format colored according to the condition in which a decreased viability was seen. Blue indicates that the residues respond to MTSET alone; pink indicates that the residues respond to MTSET alone but show an increased response on osmotic downshock; red indicates that residues require both MTSET and osmotic downshock to show decreased viability. Note that in the model the blue-labeled residues are not totally facing the lumen; a slight counterclockwise rotation of TM1 would be required.
Several residues showed changes in MTSET accessibility on osmotic shock. G30C showed an apparent decrease in accessibility on shock (note the difference between MTSET + shock vs. MTSET-alone values), suggesting that the residue is buried on channel opening. Some mutants required both exposure to MTSET and osmotic downshock for the largest decrease in viability (groups IIIa and IIIb), suggesting that these residues are inaccessible in the closed state but become exposed on gating (Fig. 2). In a previous study, V23C and V37C were identified as conferring a strong GOF phenotype when expressed (5). Hence, the small but measurable accessibility of MTSET to these residues in the absence of osmotic downshock may simply be that the reagent can react with channels that are misgating in vivo. Similarly, G26 confers a GOF phenotype and may actually require gating for reactivity. Hence, for members of group IIIa, and even for selected members of group II, we may be under-estimating the requirement of channel gating for MTSET reactivity.
A careful examination of residues categorized within groups IIIa and IIIb can help to predict a profile for the open-pore and transition-state structures. Consistent with the hypothesis that little if any of TM2 lines the pore (10), we found only TM1 mutants within these groups. Two of these TM1 residues reside close to the periplasm, V37 and M42. Presumably these residues are obscured by the periplasmic loop structure and are revealed on gating. Exposure of more cytoplasmic residues may occur in either of two conditions: either the residue is buried within the protein and exposed as the channel opens, or the residue resides within the cytoplasmic compartment and becomes accessible when the channel allows the reagent to flow into the cell. L19C, which is the most cytoplasmic residue identified within these categories, has previously been shown by patch clamp to not be accessible to MTSET when it was placed within the bath of an excised patch (i.e., the cytoplasmic side). A similar electrophysiological study with G22C found that MTSET has some access to this residue when applied to the periplasmic (pipette) but not the cytoplasmic (bath) side of a patch (14). Because V23 and I24 are more periplasmic in location, it seems likely that they would also not be accessible from the cytoplasmic side (Fig. 2). This interpretation is consistent with the crystal structure, which suggests these residues are buried within a tightly packed bundle of TM1 helices surrounded by TM2s.
Perhaps the most significant finding is that I24 is exposed on gating. This residue is tangential to the constriction point of the closed pore. The crystal structure suggests that the constriction point of the closed pore is V23 (V21 in M. tuberculosis) (7); but, as discussed above, the disulfide bridging of G26C suggested that this residue may be the constriction point in the fully closed state (5). The latter interpretation is more consistent with the data presented here (Figs. 2 and 3). Regardless, exposure of I24 to the pore lumen would require a significant clockwise rotation of TM1. Here again, the data support the clockwise rotation of TM1 on channel gating as proposed (11). In the alternative model proposed by Sukharev et al. (10), F29, not I24, is predicted to line the open pore. Because F29 is on the opposite face of the helix as I24 (Fig. 2), the data presented here strongly suggest that the Sukharev et al. model is incorrect in its predictions of residues lining the open pore. Hence, by using an in vivo SCAM, we have been able to identify residues lining the lumen of the pore in different conformational states of the molecule, support modifications of the closed-state models of the channel, and provide support for a clockwise rotation of the pore-forming first transmembrane domain on gating.
Acknowledgments
We thank Jennifer Trosky for her help in developing this assay and Dr. Paul Moe for a critical reading of the manuscript. This work was supported by National Institutes of Health Grants GM61028 and DK60818, Welch Foundation Grant I-1420, and Air Force Office of Scientific Review Grant F49620-01-1-0503.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GOF, gain-of-function; TM1, first transmembrane domain; TM2, second transmembrane domain; SCAM, substituted cysteine accessibility method; LOF, loss-of-function; MTSET, [2-(trimethylammonium) ethyl]methanethiosulfonate bromide.
References
- 1.Levina, N., Totemeyer, S., Stokes, N. R., Louis, P., Jones, M. A. & Booth, I. R. (1999) EMBO J. 18, 1730-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pivetti, C. D., Yen, M. R., Miller, S., Busch, W., Tseng, Y. H., Booth, I. R. & Saier, M. H. (2003) Microbiol. Mol. Biol. Rev. 67, 66-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurer, J. A. & Dougherty, D. A. (2003) J. Biol. Chem. 278, 21076-21082. [DOI] [PubMed] [Google Scholar]
- 4.Ou, X., Blount, P., Hoffman, R. J. & Kung, C. (1998) Proc. Natl. Acad. Sci. USA 95, 11471-11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin, G. & Blount, P. (2004) Biophys. J. 86, 2862-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshimura, K., Batiza, A., Schroeder, M., Blount, P. & Kung, C. (1999) Biophys. J. 77, 1960-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. (1998) Science 282, 2220-2226. [DOI] [PubMed] [Google Scholar]
- 8.Cruickshank, C. C., Minchin, R. F., Le Dain, A. C. & Martinac, B. (1997) Biophys. J. 73, 1925-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betanzos, M., Chiang, C. S., Guy, H. R. & Sukharev, S. (2002) Nat. Struct. Biol. 9, 704-710. [DOI] [PubMed] [Google Scholar]
- 10.Sukharev, S., Durell, S. & Guy, H. (2001) Biophys. J. 81, 917-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A. & Martinac, B. (2002) Nature 418, 942-948. [DOI] [PubMed] [Google Scholar]
- 12.Akabas, M. H. & Karlin, A. (1999) Methods Enzymol. 294, 123-144. [DOI] [PubMed] [Google Scholar]
- 13.Batiza, A. F., Kuo, M. M., Yoshimura, K. & Kung, C. (2002) Proc. Natl. Acad. Sci. USA 99, 5643-5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura, K., Batiza, A. & Kung, C. (2001) Biophys. J. 80, 2198-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blount, P., Sukharev, S. I., Moe, P. C., Martinac, B. & Kung, C. (1999) Methods Enzymol. 294, 458-482. [DOI] [PubMed] [Google Scholar]
- 16.Blount, P. & Moe, P. (1999) Trends Microbiol. 7, 420-424. [DOI] [PubMed] [Google Scholar]
- 17.Blount, P., Sukharev, S. I., Moe, P. C., Schroeder, M. J., Guy, H. R. & Kung, C. (1996) EMBO J. 15, 4798-4805. [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein, W. & Davies, M. (1970) J. Bacteriol. 101, 836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]