Abstract
The properties of γ-aminobutyric acid (GABA) type A receptors (GABAA receptors) microtransplanted from the human epileptic brain to the plasma membrane of Xenopus oocytes were compared with those recorded directly from neurons, or glial cells, in human brains slices. Cell membranes isolated from brain specimens, surgically obtained from six patients afflicted with drug-resistant temporal lobe epilepsy (TLE) were injected into frog oocytes. Within a few hours, these oocytes acquired GABAA receptors that generated GABA currents with an unusual run-down, which was inhibited by orthovanadate and okadaic acid. In contrast, receptors derived from membranes of a nonepileptic hippocampal uncus, membranes from mouse brain, or recombinant rat α1β2γ2-GABA receptors exhibited a much less pronounced GABA-current run-down. Moreover, the GABAA receptors of pyramidal neurons in temporal neocortex slices from the same six epileptic patients exhibited a stronger run-down than the receptors of rat pyramidal neurons. Interestingly, the GABAA receptors of neighboring glial cells remained substantially stable after repetitive activation. Therefore, the excessive GABA-current run-down observed in the membrane-injected oocytes recapitulates essentially what occurs in neurons, rather than in glial cells. Quantitative RT-PCR analyses from the same TLE neocortex specimens revealed that GABAA-receptor β1, β2, β3, and γ2 subunit mRNAs were significantly overexpressed (8- to 33-fold) compared with control autopsy tissues. Our results suggest that an abnormal GABA-receptor subunit transcription in the TLE brain leads to the expression of run-down-enhanced GABAA receptors. Blockage of phosphatases stabilizes the TLE GABAA receptors and strengthens GABAergic inhibition. It may be that this process can be targeted to develop new treatments for intractable epilepsy.
Keywords: temporal lobe epilepsy, microtransplantation into Xenopus oocyte, okadaic acid, γ-aminobutyric acid-current run-down, human tissue slices
Epilepsy, a condition with recurrent seizures, is one of the most common neurological disorders. Although some forms of epilepsy are successfully treated, many patients develop resistance to the anticonvulsant drug treatment in chronic temporal lobe (TL) epilepsy (TLE). Although many attempts have been made to determine the mechanisms underlying this drug resistance, surgical treatment is the final option, yielding beneficial outcomes to patients afflicted with intractable epilepsy.
By using promising molecular and electrophysiological approaches, TLE is being extensively studied through the analyses of brain tissues surgically resected from medically refractory TLE patients. Whereas some forms of epilepsy have been associated with mutations in genes coding for ion channels and γ-aminobutyric acid (GABA) receptors (1, 2); there is no clear genetic background in the refractory TLE patients. Nevertheless, there are clear associations to changes in metabolite concentrations (3), modifications in the properties of receptor and transporter systems of excitatory and inhibitory amino acid neurotransmitters (4–6), changes in signaling proteins of postsynaptic densities (7), altered expression of neurotransmitter receptors (6, 8, 9), and altered GABA activity (10, 11). Thus, epileptogenesis, extensively investigated in both animal models and humans, may be related to many events, including GABA type A receptor (GABAA-receptor) dysfunction (12). To address this issue in human TLE, we recently introduced the “microtransplantation” of plasma membranes from the human brain to Xenopus oocytes. Essentially, this method consists in injecting the oocytes with cell membranes prepared from foreign tissues. The oocyte plasma membrane incorporates the foreign membranes and efficiently acquires functional human neurotransmitter receptors and voltage-operated channels, which retain the properties they have in their native tissues (13–15).
In this study we used a multipronged approach that included microtransplantion of brain membranes, whole-cell patch clamp of nerve and glial cells of TLE neocortex slices, and real-time quantitative RT-PCR (qRT-PCR). We found a marked GABAA-receptor run-down and alterations in the amount of GABAA subunit transcripts in samples of the brains of TLE patients.
Materials and Methods
Patients. Surgical brain specimens were obtained from six patients with cryptogenic drug-resistant TLE (see Table 1, which is published as supporting information on the PNAS web site) operated at the Neuromed Neurosurgery Center for Epilepsy (Venafro, Italy). Informed consent was obtained from all patients for using part of the biopsy material, and the Ethics Committees of Neuromed and of the University of Rome “La Sapienza” approved the patient selection process and surgical procedures. The histopathology of all specimens showed the typical neuropathological features of Ammon's horn sclerosis and did not show sclerosis in the TL. For electrophysiological controls we used specimens of hippocampal uncus resected from a 27-year-old patient (female) afflicted with left TL oligoden-droglioma, who did not suffer epileptic episodes (nonepileptic TL glioma, grade II). These were part of the nontumoral tissue obtained from the tumor margins during surgical resection, and their removal was clinically indicated. In the qRT-PCR analysis, for comparative purpose, we used total RNA extracted from the TL of two 18-year-old healthy men (Ctrl1 and Ctrl2) who died of accidents and underwent necropsy at the Servicio Médico Forense, Procuraduría de Justicia, Querétaro, México. All tissues were frozen in liquid nitrogen and were subsequently processed for membrane preparation or RNA extraction.
Membrane Preparation and Injection Procedures. Membranes from human nervous tissue (TL or hippocampal uncus), or from mouse TL, were prepared and injected into oocytes by using procedures described in detail in refs. 13 and 14 and Supporting Methods, which is published as supporting information on the PNAS web site. Intranuclear injections and neurotransmitter receptor expression were performed as described in ref. 16. Murine α1β2γ2 subunit cDNAs (plasmid PGW1) were a gift of Enrico Cherubini (Scuola Internazionale Superiore di Studi Avanzati, Trieste, Italy).
Electrophysiological Recordings from Oocytes. From 12 to 48 h after injection, membrane currents were recorded from voltage-clamped oocytes by using two microelectrodes filled with 3 M KCl (17). The oocytes were placed in a recording chamber (volume, 0.1 ml) and perfused continuously, 10–11 ml/min, with oocyte Ringer's solution at room temperature (20–22°C). GABA sensitivity was tested by applying GABA (1 mM) every 7–8 min. GABA-current run-down, during repetitive neurotransmitter applications, was evaluated as the impairment (in %) of the peak current amplitude after six GABA applications (1 mM, 10-s duration, at 40-s intervals). Okadaic acid, dissolved in DMSO and stored at -20°C as 0.1 mM stock solution, was applied by cytoplasmic injection at a final concentration of 20 nM (DMSO final dilution, 1:5,000); orthovanadate was dissolved in H2O and kept as 200 mM stock solution at -20°C. The drugs were injected into the oocytes 8–10 min before recording, and GABA-current desensitization was estimated as the time taken for the current to decay from its peak to half-peak value (T0.5).
Electrophysiological Recordings from TL Slices. Neocortical slices were prepared (11, 18) from an ≈1-cm3 block of surgically resected T1 TL. Pyramidal neurons could be recognized easily by visual inspection, and also because they had spontaneous activity, large transient inward currents with voltage steps positive to -40 mV, membrane resting potentials of -67.1 ± 1.8 mV, and membrane resistances of 111 ± 33 MΩ (n = 13). No signs of spontaneous interictal bursts were observed during our recordings. On the other hand, glial cells could be recognized because they had hyperpolarized resting potentials (-87.8 ± 2.9 mV), lower resistance (33 ± 16 mΩ; n = 5; see refs. 19 and 20), and were unable to elicit full action potentials in current-clamp mode (21). The current run-down protocol adopted was the following: after current amplitude stabilization with repetitive applications every 120 s, a sequence of 10 GABA applications every 15 s was delivered. At the end of the run-down protocol the test pulse was resumed at control rate (every 120 s) to monitor the recovery of the GABA current. The reduction in the peak current amplitude was expressed as the ratio of current at the end of the run-down protocol vs. control (Itenth/Ifirst). Okadaic acid (50 nM; DMSO final dilution, 1:2,000) was applied by bath perfusion for 30 min.
RNA Extraction. About 30 mg of resected human TL tissues from TLE patients and from autopsy controls, stored in liquid nitrogen for 1–7 days, was successively homogenized with an Ultra Turrax T8 (IKA Labortechnik; Staufen, Germany) homogenizer, and total RNA was extracted by using an Absolutely RNA RT-PCR miniprep kit (Stratagene Europe; Amsterdam), including DNase I digestion to remove any genomic DNA contamination. Integrity of the RNA was determined by gel electrophoresis, and nucleic acid concentrations were measured with a spectrophotometer (Amersham Pharmacia Biotech).
qRT-PCR Analysis. Expression levels of human GABAA-receptor subunit mRNAs (β1, β2S, β2L, β3, and γ2 subunits) were determined from resected human TL tissues of five TLE patients and from the TL tissues of two healthy autopsy controls. qRT-PCR experiments were conducted as described in Supporting Methods.
Chemicals and Solutions. Oocyte Ringer's solution had the following composition: 82.5 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 5 mM Hepes, adjusted to pH 7.4 with NaOH. Except when otherwise indicated, all drugs were purchased from Sigma.
Results
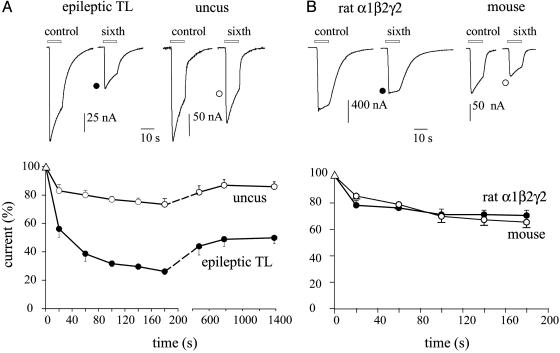
GABA-Current Run-Down in Oocytes. In agreement with analogous experiments (22), applications of GABA (1 mM) to oocytes injected with membranes from the neocortex of six TLE patients elicited bicuculline-sensitive inward currents (bicuculline 100 μM; not shown), of variable amplitudes. Some currents were as large as -400 nA, others as low as -20 nA (depending on both oocytes and donors, i.e., patients or frogs). These currents, generated bona fide by the activation of microtransplanted ionotropic GABAA receptors, exhibited a considerable run-down after repetitive GABA applications (fall to 24 ± 5%, range: 20–33%; 53 oocytes, 20 frogs, 6 patients) not accompanied by a significant change in the current decay (T0.5 = 5.8 ± 0.5 s for control vs. T0.5 = 6.8 ± 0.4 s after six applications, P = 0.21), with a partial recovery after washout (Fig. 1A). As reported previously (22), this current run-down was not related to the amplitude of the initial current elicited in a given oocyte, which in these experiments ranged from -10 to -200 nA (not shown). Despite these variations, it was clear that a much smaller GABA-current run-down occurred in oocytes injected with membranes from the nonepileptic human brain (i.e., hippocampal uncus) resected as margins of a cerebral tumor ganglioglioma (fall to 73 ± 2%, range: 66–86%; 16 oocytes, 4 frogs, 1 patient); or with membranes from a nonepileptic mouse TL (fall to 69 ± 4%, range: 65–77%; 6 oocytes, 3 frogs, 6 mice). Furthermore, cloned GABAA (α1β2γ2) receptors expressed in oocytes also showed weak run-down (fall to 68 ± 7%, range: 60–79%; 19 oocytes, 4 frogs) (Fig. 1B). It is noteworthy that the current run-downs of cloned GABAA receptors and of GABAA receptors transplanted from either mouse TL or human hippocampal uncus, were fully reversible and were not accompanied by changes in T0.5. Thus, the TLE GABA-current run-down is unusual and suggests that an excessive current run-down may represent a GABAA-receptor dysfunction characteristic of TLE.
Fig. 1.
GABA currents recorded from oocytes. (A Upper) Sample currents in response to the first and sixth GABA applications, from oocytes injected with membranes isolated from the indicated brain regions. TL tissue was from patient 4 (see Table 1). (Lower) Time course of GABA-current run-down. Points represent mean current amplitudes (±SEM) from 8–12 oocytes (four frogs; one patient); normalized to Imax: -177 ± 16 nA (○); -107 ± 13 nA (•). (B) Sample currents (Upper) and run-down (Lower) from oocytes injected with rat GABAA-receptor subunit cDNAs (•) or with TL mouse membranes (○). Currents were normalized to Imax = -1.3 ± 0.2 μA (•) and -161 ± 14 nA (○). Number of oocytes, 7–12 (five frogs).
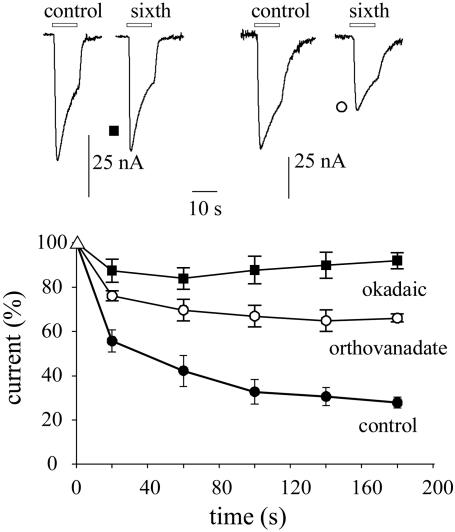
It is well known that phosphorylation influences the activity of GABAA receptors, including receptor run-down (23–27). To investigate whether the run-down of epileptic receptors is modulated by phosphorylation, oocytes injected with TL membranes were exposed to phosphatase inhibitors. Within 8–10 min after injecting the oocytes with the nonselective phosphatase inhibitor okadaic acid (≈20 nM final concentration in the oocyte) the GABA-current run-down was prevented (95.3 ± 5%, range 83–106%; 28 oocytes, 9 frogs, 6 patients). In contrast, after injecting an inhibitor of protein tyrosine phosphatase, orthovanadate (≈200 μM in the oocyte), the current run-down was significantly reduced but was not abolished (e.g., Fig. 2). These results suggest strongly that phosphorylation processes are involved in the increased GABAA-receptor run-down of epileptic tissues.
Fig. 2.
Suppression of GABAA-current run-down by phosphatase inhibitors. (Lower) Time course of current run-down during repetitive applications of GABA to oocytes injected with TL membranes from patient 3 (see Table 1). Points represent means (±SEM) from nine oocytes (three frogs). Symbols denote different treatments, as indicated. (Upper) Sample currents from the same experiments, as indicated by symbols. Currents were normalized to Imax = -55 ± 7 nA (▪), -72 ± 6 nA (○), and -98 ± 4 nA (•). Note current run-down is reduced by orthovanadate and prevented by okadaic acid.
GABA-Current Run-Down in Tissue Slices. Injection of membranes from the brain tissue into an oocyte leads to the incorporation of receptors from glial, neuronal, and other cells into the oocyte membrane. To examine the properties of the epileptic GABAA receptors from neurons versus glial cells, recordings were performed from pyramidal neurons and neighboring glial cells in slices of the same TLE tissues used for the microtransplantation experiments. Furthermore, for comparative purposes, recordings were also carried out from pyramidal neurons of TL slices of nonepileptic rats.
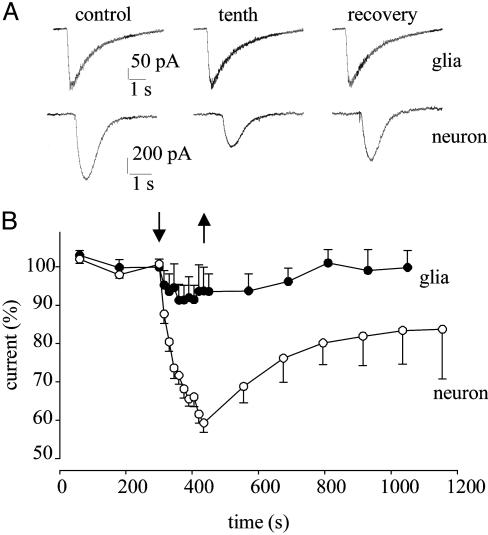
When GABA (100 μM) was applied onto a pyramidal neuron, by means of a “puffer pipette” positioned above the cell in the TLE brain slice, a rapid inward current developed, very likely because of the activation of postsynaptic GABAA receptors. Stable inward currents, ranging from 100 pA to 1 nA, were obtained with low-frequency GABA applications. However, a series of relatively high-frequency GABA jets caused a progressive decrease of the GABA-current peak amplitude. Specifically, in 13 nerve cells examined the current decreased to 59 ± 2% (P < 0.02), without significant change in the current decay (control T0.5 = 450 ± 50 ms vs. T0.5 = 412 ± 44 ms after 10 applications, P = 0.09). This reduction of the current amplitude was also observed after prolonged whole-cell recordings (>40 min). After the run-down, the current amplitude recovered to 83 ± 8% within 6 min; after 15–20 min it recovered fully in only 4 cells. In sharp contrast, when the same run-down protocol was applied to neighboring glial cells, the run-down of the GABA-evoked current was relatively weak (fall to 92 ± 4%; 5 cells, P < 0.05) and disappeared within about 6 min (101 ± 2%, P > 0.05) (see Fig. 3). Pyramidal neurons in the nonepileptic TL rat slices behaved like the human glial cells, i.e., their GABA-current amplitude decreased to 81 ± 3% (n = 6, P < 0.05) during the run-down protocol and again recovered fully within about 6 min (not shown). Thus, only in the human epileptic pyramidal neurons was the function of repetitively activated GABAA receptors not maintained. Therefore, the marked GABA-current run-down observed in oocytes should be attributed to the TLE GABAA receptors transplanted from the human neurons rather than from the glial cells.
Fig. 3.
GABA-current run-down in native cells from human TLE slices. (A) Currents elicited by repetitive applications of GABA to a glial cell (Upper) and to a pyramidal neuron (Lower), before (control), at the end of the run-down protocol (tenth), and at recovery 6 min later. (B) Time course of GABA-current run-down and recovery, from pyramidal neurons (○; n = 13) and glial cells (•; n = 5). Currents were normalized to Imax = -409 ± 48 pA (○) and -144 ± 20 pA (•). Arrows indicate run-down protocol.
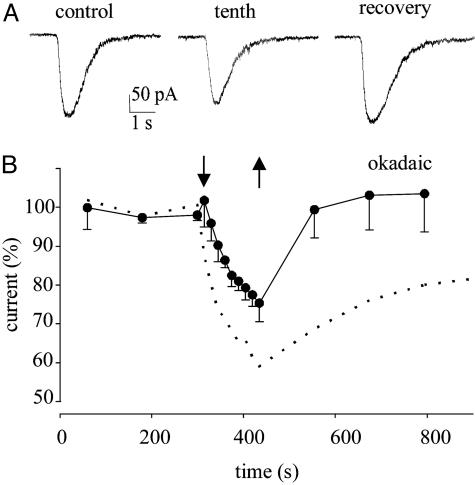
We then returned to human brain slices to see whether protein phosphorylation is involved in the neuronal GABA-current run-down (24, 26, 27). After okadaic acid treatment, GABAA receptors from human TLE slices exhibited a reduced GABA-current run-down and a rapid and full recovery. Specifically, in five neurons the treatment with okadaic acid reduced the GABA-current run-down to 77 ± 6% (P < 0.02), with a full current recovery in all neurons within 6 min (Fig. 4). Consequently, the inhibition of phosphatases stabilizes the epileptic GABAA-receptors and enhances GABA inhibition.
Fig. 4.
Okadaic acid-induced inhibition of GABA-current run-down in pyramidal neurons from human TLE slices. (A) Currents elicited by repetitive applications of GABA to a pyramidal neuron treated with okadaic acid (50 nM, 30 min) before (control), at the end of the run-down protocol (to 81%), and at recovery after 6 min (to 104%). (B) Amplitude of GABA currents elicited by repetitive GABA applications to five pyramidal neurons (patients 2–5; see Table 1), 30 min after okadaic acid treatment, as indicated. Currents were normalized to Imax = -189 ± 23 pA. Superimposed dotted curve is that for pyramidal neurons in Fig. 3B. Arrows are as in Fig. 3.
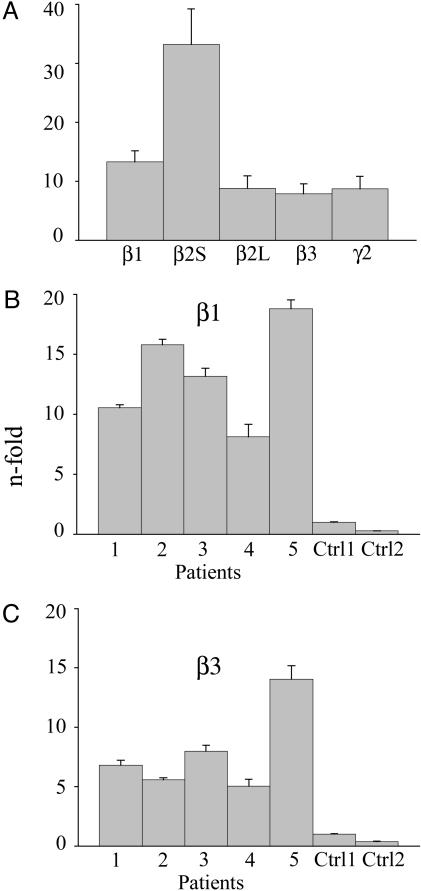
Overexpression of TLE GABAA-Receptor Subunit mRNAs. A number of kinases modulate GABAA receptors through phosphorylation of conserved sites in the intracellular loops of the β1–3 and γ2 subunits (26). Accordingly, we decided to analyze by qRT-PCR the expression of the β1, β2S, β2L, β3, and γ2 subunit mRNAs in the TL of the same TLE patients, using as reference the RNA extracted from the TL of two healthy autopsy controls (Ctrl1 and Ctrl2).
Compared with mRNA of Ctrl1, the mRNAs encoding all of the GABAA subunits analyzed were consistently increased in the five patients examined (Fig. 5A). The n-fold increases were β1 = 13.3 ± 1.9 (range 8–19; see Fig. 5B); β2S = 33.2 ± 7.9 (range 17–62); β2L = 8.8 ± 2.1 (range 6–17); β3 = 7.9 ± 1.6 (range 5–14; see Fig. 5C); and γ2 = 8.70 ± 2.11 (range 5–16). In comparison, as shown for the β1 and β3 subunits, Ctrl2 (Fig. 5 B and C) showed that gene expression was lower than in Ctrl1 (range 0.24- to 0.4-fold). Interestingly, the patient with the most increased expression of the five subunits tested also presented the most severe form of the disease (i.e., patient 5 in Table 1).
Fig. 5.
Quantitative analysis of GABAA receptor subunit mRNAs in TLE patients by using the 2-ΔΔCT method. Columns represent the n-fold change in gene expression normalized to gene expression in the healthy autopsy Ctrl1. (A) Expression of β1–3 and γ2 subunits. Columns show mean values for patients 1–5 (see Table 1). (B and C) Relative β1 and β3 subunit expression from patients 1–5 and Ctrl1 and Ctrl2. Columns show the means of six experiments; bars, standard error. Note the decreased level of expression in Ctrl2 compared with Ctrl1.
Discussion
An increasing variety of experimental approaches are being applied to investigate the pathogenesis of epilepsy. Taking advantage of the nervous tissue resected from six patients afflicted with TLE, we microtransplanted GABAA receptors from the glial and neuronal cells of the epileptic TL to the plasma membrane of Xenopus oocytes. This procedure provided us with a simple and powerful tool to study the GABAA receptors as they exist naturally in the epileptic brain (15). Furthermore, we studied the GABA currents generated by the activation of native GABAA receptors expressed in both pyramidal neurons and glial cells in human TLE slices made from part of the TL specimens resected from the same patients. Finally, we investigated the levels of GABAA-receptor subunit mRNAs in the same tissues to see whether altered subunit expression is related to the abnormal behavior of epileptic GABAA receptors.
We describe here a strong, and partially irreversible, GABA-current run-down of epileptic receptors transplanted to the oocytes. This run-down was much greater than that of the other nonepileptic receptors examined: cloned GABAA receptors, GABAA receptors transplanted from human hippocampal uncus and mouse TL, and those elsewhere examined (24). The GABA-current run-down of transplanted GABAA receptors is similar to the run-down of pyramidal neurons in slices of the same epileptic tissues, and both are removed by phosphatase inhibition. These findings contrast with those from native epileptic glial cells, which exhibit stable GABAA receptors even upon repetitive agonist application. Altogether, our findings indicate that GABAA receptors of pyramidal neurons from the epileptic TL exhibit an unusual use-dependent instability. This behavior is common to both membrane-injected oocytes and native neurons and, whatever the origin of the receptor instability, it was clear that the oocytes recapitulate the properties of TLE neuronal GABA receptors. Thus, membrane injections into oocytes are a convenient alternative to direct electrophysiological recordings from native cells to study the human GABAergic system in TLE.
We speculate that an altered expression of GABAA-receptor subunits shown in both animal models and humans (6, 28) may cause the GABAA-receptor instability. Moreover, it is known that in TLE patients the expression profile of GABAA-receptor subunits is modified after chronic treatment with benzodiazepines (29, 30). Here, qRT-PCR revealed an altered expression of GABAA-receptor subunit mRNAs in the temporal cortex of TLE brains. Particularly interesting is the increase in transcripts of β1 and β2S, subunits that are strongly modulated by phosphorylation (25, 26, 31). Alternatively, the changed mRNA levels in the TLE brain may simply reflect altered tissue composition rather than altered specific subunit transcription. However, that possibility seems unlikely because (i) our tissue specimens were rather homogeneous and lacked obvious gliosis; (ii) the highest expression of GABAA-receptor subunit mRNAs was found in the patient with the most severe TLE; (iii) the putative overexpression in the epileptic temporal cortex of GABAA-receptor subunit mRNAs is in agreement with previous results obtained in an epilepsy animal model (32); and (iv) our data are compatible with the view that chronic seizure activity in humans leads to persistent alterations in gene expression (33). Further work is required to determine precisely the origin of the excessive GABA-current run-down of human epileptic nerve cells.
It is known that the function of GABAA receptors is differentially modulated by phosphorylation of key residues within the intracellular loops of β and γ subunits (26). On the basis of altered expression of GABAA-receptor subunit mRNAs found in TLE patients, we speculate that unusual subunit composition(s) may lead to modifications in the phosphorylation state of GABAA receptors and increase the receptor run-down. Such process may be positively modulated by inhibition of protein phosphatases. For instance, okadaic acid inhibition of protein phosphatases 2A results in hyperphosphorylation of the β3 subunit, yielding an enhanced activity of the GABAA-receptor (26, 27), and orthovanadate inhibition of tyrosine phosphatase reduces GABA-current run-down acting at tyrosine kinase-dependent sites in β subunits (24, 26, 34).
In conclusion, many studies have been aimed at determining how GABA efficacy is altered in human and animal model epileptic tissues (e.g., refs. 6 and 10). The present work contributes to this issue (i) by providing evidence of an increased use-dependent run-down of GABAA receptors, directly in the epileptic neurons as well as in oocytes incorporating membranes from the same epileptic tissue, and (ii) by defining an altered expression pattern of GABAA-receptor subunits in the epileptic tissue. This receptor dysfunction probably results in a diminished inhibitory GABA action and facilitates the development of seizures. The fact that the “epileptic run-down” is corrected by inhibiting phosphatases may pave the way for developing new strategies toward the medical treatment of TLE.
Supplementary Material
Acknowledgments
We are extremely grateful to the epileptic patients C.L.M., P.E., I.A., G.F., S.L., and C.L. and the nonepileptic patient P.C., whose contributions have made this work possible. We thank Drs. Paolo Onorati and Ciriaco Scoppetta for invaluable discussions. This work was supported by grants from the Ministero della Salute (to F.E.), the Ministero Università e Ricerca and the Fondo per gli Investimenti della Ricerca di Base no. RBNE01NR34-003 (to F.E.), the National Science Foundation (to R.M.), the Consejo Nacional de Ciencia y Tecnología 41309Q (to A.M.-T.), and Proyectos de Innovación Tecnológica–Universidad Nacional Autónoma de México IN212702 (to R.M. and A.M.-T.).
Abbreviations: TL, temporal lobe; TLE, TL epilepsy; GABA, γ-aminobutyric acid; GABAA receptors, GABA type A receptors; GABA current, current elicited by GABA; qRT-PCR, quantitative RT-PCR.
References
- 1.Celesia, G. G. (2003) Lancet 361, 1238-1239. [DOI] [PubMed] [Google Scholar]
- 2.Noebels, J. L. (2003) Annu. Rev. Neurosci. 26, 596-625. [DOI] [PubMed] [Google Scholar]
- 3.Mueller, S. G., Suhy, J., Laxer, K. D., Flenniken, D. L., Axelrad, J., Capizzano, A. A. & Weiner, M. W. (2002) Epilepsia 43, 1210-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald, J. W., Garofalo, E. A., Hood, T., Sackellares, J. C., Gilman, S., McKeever, P. E., Troncoso, J. C. & Johnston, M. V. (1991) Ann. Neurol. 29, 529-541. [DOI] [PubMed] [Google Scholar]
- 5.Mathern, G. W., Mendoza, D., Lozada, A., Pretorius, J. K., Dehnes, Y., Danbolt, N. C., Nelson, N., Leite, J. P., Chimelli, L., Born, D. E., et al. (1999) Neurology 52, 453-472. [DOI] [PubMed] [Google Scholar]
- 6.Loup, F., Weiser, H. G., Yonekawa, Y., Aguzzi, A. & Fritschy, J. M. (2000) J. Neurosci. 20, 5401-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyneken, U., Marengo, J. J., Villanueva, S., Soto, D., Sandoval, R., Gundelfinger, E. D. & Orrego, F. (2003) Epilepsia 44, 243-246. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs, J. W., III, Shumate, M. D. & Coulter, D. A. (1997) J. Neurophysiol. 77, 1924-1938. [DOI] [PubMed] [Google Scholar]
- 9.Schroder, W., Hinterkeuser, S., Seifert, G., Schramm, J., Jabs, R., Wilkin, G. P. & Steinhauser, C. (2000) Epilepsia 41, Suppl. 6, S181-S184. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ari, Y. & Cossart, R. (2000) Trends Neurosci. 23, 580-587. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, I., Navarro, V., Clemenceau, S., Baulac, M. & Miles, R. (2002) Science 298, 1418-1421. [DOI] [PubMed] [Google Scholar]
- 12.Jones-Davis, D. M. & Macdonald, R. (2003) Curr. Opin. Pharmacol. 3, 12-18. [DOI] [PubMed] [Google Scholar]
- 13.Marsal, J., Tigyi, G. & Miledi, R. (1995) Proc. Natl. Acad. Sci. USA 92, 5224-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miledi, R., Eusebi, F., Martinez-Torres, A., Palma, E. & Trettel, F. (2002) Proc. Natl. Acad. Sci. USA 99, 13238-13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palma, E., Trettel, F., Fucile, S., Renzi, M., Miledi, R. & Eusebi, F. (2003) Proc. Natl. Acad. Sci. USA 100, 2896-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palma, E., Mileo, A. M., Eusebi, F. & Miledi, R. (1996) Proc. Natl. Acad. Sci. USA 93, 11231-11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miledi, R. (1982) Proc. R. Soc. London Ser. B 215, 49l-497. [Google Scholar]
- 18.Kohling, R., Lucke, A., Straub, H., Speckmann, E.J., Tuxhorn, I., Wolf, P., Pannek, H. & Oppel, F. (1998) Brain 121, 1073-1087. [DOI] [PubMed] [Google Scholar]
- 19.Walz, W. & MacVicar, B. (1988) Brain Res. 443, 321-324. [DOI] [PubMed] [Google Scholar]
- 20.Hinterkeuser, S., Schroder, W., Hager, G., Seifert, G., Blumcke, I., Elger, C. E., Schramm, J. & Steinhauser, C. (2000) Eur J. Neurosci. 12, 2087-2096. [DOI] [PubMed] [Google Scholar]
- 21.Chvatal, A., Pastor, A., Mauch, M., Sykova, E. & Kettenmann, H. (1995) Eur. J. Neurosci. 7, 129-142. [DOI] [PubMed] [Google Scholar]
- 22.Palma, E., Esposito, V., Mileo, A. M., Di Gennaro, G., Quarato, P., Giangaspero, F., Scoppetta, C., Onorati, P., Trettel, F., Miledi, R., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 15078-15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, Y. F., Angelotti, T. P., Dudek, E. M., Browning, M. D. & MacDonald, R. L. (1996) Mol. Pharmacol. 50, 185-195. [PubMed] [Google Scholar]
- 24.Huang, R. Q. & Dillon, G. H. (1998) J. Pharmacol. 286, 243-255. [PubMed] [Google Scholar]
- 25.Brandon, N. J., Delmas, P., Kittler, J. T., McDonald, B. J., Sieghart, W., Brown, D. A., Smart, T. G. & Moss, S. J. (2000) J. Biol. Chem. 275, 38856-38862. [DOI] [PubMed] [Google Scholar]
- 26.Kittler, J. T. & Moss, S. J. (2003) Curr. Opin. Neurobiol. 13, 341-347. [DOI] [PubMed] [Google Scholar]
- 27.Jovanovic, J. J., Thomas, P., Kittler, J. K., Smart, T. G. & Moss, S. J. (2004) J. Neurosci. 24, 522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks-Kayal, A. R., Shumate, M. D., Jin, H., Rikhter, T. Y. & Coulter, D. A. (1998) Nat. Med. 4, 1166-1172. [DOI] [PubMed] [Google Scholar]
- 29.Zhao, T. J., Chiu, T. H. & Resemberg, H. C. (1994) Mol. Pharmacol. 45, 657-663. [PubMed] [Google Scholar]
- 30.Brown, M. J. & Bristow, D. R. (1996) Br. J. Pharmacol. 118, 1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinley, D. D., Lennon, D. J. & Carter, D. B. (1995) Mol. Brain Res. 28, 175-179. [DOI] [PubMed] [Google Scholar]
- 32.Tsunashima, K., Schwarzer, C., Kirchmair, E., Sieghart, W. & Sperk, G. (1997) Neurosci. 80, 1019-1032. [DOI] [PubMed] [Google Scholar]
- 33.Murray, K. D., Isackson, P. J., Eskin, T. A., King, M. A., Montesinos, S. P., Abraham, L. A. & Roper, S. N. (2000) J. Comp. Neurol. 418, 411-422. [DOI] [PubMed] [Google Scholar]
- 34.Wan, Q., Man, H. Y., Braunton, J., Wang, W., Salter, M. W., Becker, L. & Wang, Y. T. (1997) J. Neurosci. 17, 5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.