Abstract
Recently developed calcitonin gene-related peptide (CGRP) receptor antagonistic molecules have shown promising results in clinical trials for acute treatment of migraine attacks. Drugs from the gepant class of CGRP receptor antagonists are effective and do not cause vasoconstriction, one of the major limitations in the use of triptans. However their use had to be discontinued because of risk of liver toxicity after continuous exposure. As an alternative approach to block CGRP transmission, fully humanized monoclonal antibodies towards CGRP and the CGRP receptor have been developed for treatment of chronic migraine (attacks >15 days/month). Initial results from phase I and II clinical trials have revealed promising results with minimal side effects and significant relief from chronic migraine as compared with placebo.
The effectiveness of these various molecules raises the question of where is the target site(s) for antimigraine action. The gepants are small molecules that can partially pass the blood–brain barrier (BBB) and therefore, might have effects in the CNS. However, antibodies are large molecules and have limited possibility to pass the BBB, thus effectively excluding them from having a major site of action within the CNS. It is suggested that the antimigraine site should reside in areas not limited by the BBB such as intra- and extracranial vessels, dural mast cells and the trigeminal system. In order to clarify this topic and surrounding questions, it is important to understand the localization of CGRP and the CGRP receptor components in these possible sites of migraine-related regions and their relation to the BBB.
Keywords: BBB, CGRP, CGRP receptor, CLR, gepants, monoclonal antibodies
Introduction
Migraine is a common neurological disorder that affects up to 16 % of the adult population in Western countries 1. It is characterized by episodic, often disabling headache, associated with sensory (aura), autonomic (nausea, vomiting), phonophobia and photophobia, and cognitive symptoms. Although still debated, the general view is that migraine is a disorder in which central nervous system (CNS) dysfunction plays a pivotal role while various parts of the trigeminal system are necessary for the expression of peripheral symptoms and aspects of pain 2. In support, a recent study reported brain activation already during the premonitory phase of glycerol trinitrate-induced migraine attacks 3.
Although the triptan group of drugs provides effective relief from acute migraine attacks for many patients, a substantial number (up to 40% in the case of oral triptans) of affected individuals are unresponsive 4. Subcutaneous sumatriptan provides about 81% headache relief at 2 h 5 while the efficacy of oral triptans is lower. Ferrari et al. reported sumatriptan 100 mg oral had a response rate of 58% improvement at 2 h (therapeutic gain was 33%) while the pain-free response was 35% (therapeutic gain was 26%) 4. In addition, such therapy can lead to cardiovascular symptoms in 10% of the subjects 6. The gepants represent a new class of antimigraine drugs that act as calcitonin gene-related peptide (CGRP) receptor blockers. They have proven efficacy in clinical trials 7 and act at several sites in the trigeminal system and in the CNS resulting in pain relief 8. The gepants do not cause vasoconstriction per se, either in cranial or in coronary arteries 9–11, which avoids one of the major limitations of using triptans 6. In comparisons with triptans in head-to-head clinical trials on acute treatment of migraine attacks, it has been revealed that the clinical efficiency of gepants is comparable with that of triptans and superior to placebo 7. Recently, telcagepant was reported to have a prophylactic effect 12. However, this group of molecules was terminated for further development because of liver toxicity during repeated exposures. This effect was attributed to the molecular structure of the compound.
In a subgroup of migraine patients (1–2%) the frequency of migraine may increase over time to multiple monthly attacks. These patients are extremely difficult to treat. Furthermore, their attacks may become chronic (attacks > 15 days per month) which is often associated with medication overuse 13. The development of monoclonal antibodies to CGRP or to its receptor has reopened the development of therapeutics for this group of patients. The first published reports indicate that this novel antibody strategy is effective in such patients 14,15. It is suggested that these molecules act by binding to CGRP that is released from the trigeminovascular system or attached to CGRP receptors during the migraine attack. The antibodies, however, act in various parts of the body and are not limited to cranial structures only 16.
However, the site action of CGRP and CGRP receptor interacting agents in migraine therapy is still debated. The gepants pass poorly through the BBB 17. For telcagepant the CSF : plasma ratio in primates was found to be about 1.4% which suggests the potential for a small amount of brain penetration 18. On the other hand, the antibodies represent a different class of molecules that are considerably larger in size with even less possibility to cross the BBB. It is often argued that triptans, gepants or antibodies may pass the BBB to some degree and that this is enough to deliver antimigraine efficiency. However, it should be kept in mind that the agonist–antagonist behaviour at a receptor site follows operational criteria for pharmacological interactions as outlined by Black & Leff 19. It seems unlikely, given their poor penetration of the BBB, that these molecules will achieve the CNS concentrations necessary to provide reasonable antagonism at the receptor sites. The neurotransmitter is released at a synapse at a very high concentration (the site of interaction where the antagonist competes). In addition to poor penetration into the brain, telcagepant is also a substrate for P-glycoprotein transport out of the brain. Based on the above considerations, there is a need to examine the localization of CGRP receptors in relation to the brain, the trigeminal ganglion and the meninges in order to understand where these molecules may act in migraine therapy.
Role of CGRP
A significant role of CGRP in migraine pathophysiology was established by the demonstration that CGRP concentrations are increased in the cranial circulation during genuine migraine attacks 20. CGRP in saliva has been shown to be elevated in acute migraine 21, and its concentrations correlate with pain and abortion of the attack by triptan administration 22. Technical issues related to peptide analysis, such as specificity of the detection antibodies and concerns regarding breakdown of CGRP and other peptides in the circulation, are important to keep in mind when evaluating positive as well as negative results 23,24. Further support for the role of CGRP is established by the antimigraine effect of CGRP receptor blockade. This was initially demonstrated using intravenous olcegepant 25 and subsequently with several other gepants given orally 7.
The trigeminal origin of perivascular CGRP-containing nerve fibres was first revealed using immunohistochemistry and quantitative radioimmunoassay combined with specific denervation 26. Subsequent neuronal tracing studies in rat using True Blue in temporal 27, middle meningeal 28 and cerebral arteries 29 localized the tracer in a subpopulation of CGRP-containing trigeminal neurons. Later studies using the sensory transganglionic tracers wheat germ agglutinin-conjugated horseradish peroxidase (WGA-HRP) and cholera toxin subunit b (CTb) revealed a specific somatotopic localization in brainstem projection areas, the trigeminal nucleus caudalis, and the spinal C1–2 levels of projections of both the thin nociceptive (unmyelinated C-fibres and small myelinated Aδ - fibers) and the thick low threshold mechanoreceptive myelinated A- fibres. These projections are connected to the middle meningeal 30, superior sagittal 31, temporal 32 and cerebral arteries 33.
Over many years, our research group has carefully mapped out available messenger molecules in the trigeminovascular system and revealed the central role of the trigeminal sensory system in migraine. Of the various neuronal messenger molecules found in this system, CGRP stands out as the most prevalent molecule with a particular relation to primary headache disorders 34. CGRP is expressed throughout the central and peripheral nervous systems, consistent with modulation of vasodilatation, nociception, motor function, secretion and olfaction 8. Two forms of CGRP are expressed, αCGRP is prominently localized in primary spinal afferent C and Aδ fibres of sensory ganglia, while ßCGRP is the main isoform in the enteric nervous system 7,31. There is a rich plexus of CGRP containing perivascular nerves in intracranial blood vessels, both in cerebral and meningeal arteries and in the dura mater 7,34. Tracing and denervation studies have revealed their origin in the first division of the trigeminal ganglion 34–36. The peripheral projections of the trigeminal system are involved in neurogenic vasodilatation, sensitization and inflammation, while central release may induce hyperalgesia. Trigeminal nerve activation results in antidromic release of CGRP 36 which acts via a CGRP receptor complex that consists of calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) 37. This receptor complex is linked to a G-protein that is coupled to the ‘receptor component protein (RCP) - adenylate cyclase’ 38. This pathway underlies non-endothelium mediated and cAMP-associated vasodilatation in cerebral and meningeal arteries 39,40. At synapses in the trigeminal nucleus caudalis/C1–2 levels of the brain stem, CGRP, with or without a co-transmitter, acts on other neurons to transmit nociceptive signals centrally via the brainstem and midbrain to the thalamus and higher cortical regions 35. CGRP binding sites are widely expressed throughout the brain 41. CLR and RAMP1 have been localized to the cytoplasm of trigeminal neurons, at peripheral sites on the intracranial vasculature (in the smooth muscle cells), in the dura mater and in the brainstem 42–44. Most CGRP containing neurons in the trigeminal ganglion, however, do not have the receptor elements. Instead CLR and RAMP1 are co-expressed on non-CGRP neurons and on glial cells, suggestive of intraganglionic interaction 45.
Localization of CGRP receptors
Clinical studies with imaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have demonstrated that certain brainstem areas are activated during migraine attacks 46. These include the midbrain, pons, substantia nigra, red nucleus, periaqueductal grey (PAG), nucleus raphe magnus (NRM) and the locus coeruleus (LC) 47. Recently, investigation of the premonitory phase of migraine (without headache) using functional neuroimaging revealed that the right ventral midbrain and the right PAG in the dorsal pons were activated by systemic administration of glyceryl trinitrate in episodic migraine subjects 3. However, it is not known what drives this early activation of the brainstem, but these data support the involvement of diencephalic and brainstem mechanisms in the pathophysiology of migraine 2.
In the CNS there is a wide distribution of CGRP with the highest concentrations in the striatum, amygdalae, colliculi and cerebellum 8,41. The first CGRP ligand binding study was carried out by Sexton and revealed a number of binding sites in the CNS of rat 48. Recent studies have verified many of these findings in the rhesus monkey brainstem with the use of novel methodologies and specific antibodies towards CLR and RAMP1 49. These studies in primate brain also show CLR and RAMP1 mRNA and protein expression in the pineal gland (related to light and darkness registration and circadian rhythm control), the medial mammillary nucleus, PAG, area postrema, the raphe nucleus, the gracile nucleus and the spinal trigeminal nucleus 49.These data indicate multiple localizations of CGRP receptor components and suggests several putative functions of CGRP within the CNS.
The cerebellum is important in modulating many cortical motor and sensory inputs. The cerebellum exerts an inhibitory control in the cerebral cortex and, thus, may play an important role in filtering of sensory inputs 50. Purkinje cell bodies store large amounts of vesicular CGRP and the CLR/RAMP1 complex is expressed both on the cell body and in axons and dendrites 51,52. Early studies of acute migraine attacks by PET revealed activation of cerebellar regions 46,53. However, the studies did not provide any deeper explanation for the activation that was observed.
Based on the view that migraine starts in the CNS 2,3 and the fact that triptans and gepants can modify acute attacks, it is logical to search for sites in the CNS that are targeted by these drugs. Hence, Merck (West Point, USA) developed a CGRP receptor related PET ligand that passed the BBB and could be used to test the hypothesis in a small group of patients. They first administered a brain penetrant PET tracer that binds to the CNS. Amazingly, the main binding was observed in the cerebellum of the patients 54. Then the group administered telcagepant in two doses, one in a clinically effective dose and one nearly 10 times higher 54. Surprisingly, the brain PET signal was not modified by telcagepant when it was given in the clinically effective dose that aborts the migraine headache 7. However, when telcagepant was given in the higher dose, they observed a competition with the PET signal, demonstrating interaction at CNS binding sites with this dose. However, there was no further clinical efficiency reported in this study. These findings are compatible with the view that the principle site of antimigraine action of telcagepant lies outside the CNS.
Is the trigeminal ganglion located outside the BBB?
Migraine is considered a neurovascular disorder that originates in the brain, involving the hypothalamus and thalamus, as well as certain brainstem regions 2. The attack is often preceded by prodromal symptoms, which again suggests the CNS as a likely point of origin 3. The pain during a migraine attack is associated with the release of CGRP which appears to have a key role in migraine pathophysiology 8,20–22,25. Basic studies have revealed that CGRP does not pass the cerebral vessel walls 55. Hence it is suggested that the elevated concentrations of CGRP found in the jugular venous blood during migraine attacks derives from various parts of the trigeminovascular system during its activation 56. Systemic intravenous infusion of CGRP has been found to trigger migraine-like headache in migraine patients 57. With magnetic resonance angiography imaging, the authors observed that CGRP-induced migraine was associated with dilatation of extracranial branches of the middle meningeal artery. The headache was located in association with the dilatation and hence it was proposed that vasodilatation was causative for the attack because there was at the same time no dilatation of the middle cerebral artery 57. However, a more recent MRI angiography study showed that pain in spontaneous migraine attacks was not accompanied by extracranial meningeal artery dilatation but with a small intracranial artery dilatation 58. This shows a difference in intra- and extracranial arterial diameter alterations in drug induced migraine-like attacks vs. genuine migraine attacks 58. The interpretation of these results remains to be related to other neuroimaging findings 47.
Antibodies towards CGRP and the CGRP receptor, as well as ‘Spiegelmer’ molecules with CGRP-binding RNA 59,60 provide new tools that may assist in explaining the site of action of antimigraine drugs 16. These molecules are relatively large and therefore appear to have little chance to pass the BBB in effective doses. However, some studies have shown that IgG molecules can pass the BBB via transcytosis 61, via transfer mechanisms 62 or via facilitated transport 63,64. In addition, some regions of the CNS are more penetrable than others which also offers possibilities for systemic drugs to interact with the CNS. Since gepants as well as antibodies to CGRP and CGRP receptors are therapeutically effective in migraine, it could be suggested that they share a site of action. However, it is my view that it is unlikely that these drugs act in the CNS in clinically effective doses.
It has been well known for decades that the dura mater and the middle meningeal artery are devoid of a BBB 65. In order to shed some light on this issue, research has recently been focused on the trigeminal ganglion to determine if CGRP receptor antagonists may act there 66. In addition, it is known that trigeminal CGRP-containing nerve fibres innervate cerebral 26 and dural blood vessels 67 and that release of CGRP mainly causes vasodilatation 36,39. CGRP also was found to degranulate rodent mast cells, which contain CGRP receptor components 42.
The trigeminal ganglion contains CGRP where the peptide is found in small to medium-sized neurons (about 50%) and in nerve fibres that are dispersed within the ganglion and in perivascular CGRP positive nerve fibres. In addition large neurons (37%), satellite glial cells and vascular smooth muscle cells have CGRP receptor elements CLR/RAMP1 45,66 (Figure1). Centrally the trigeminal system projects to parts of the CNS where nociceptive information is processed to higher cortical regions 35,43,44. This pattern of expression is similar in rat, monkey and human trigeminal ganglia.
Figure 1.
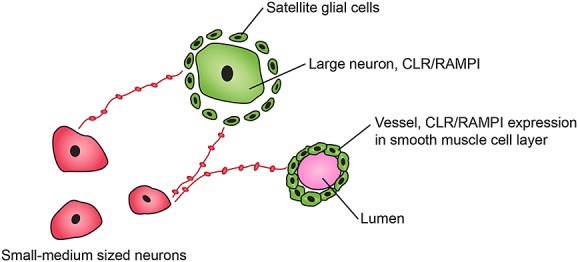
A schematic illustration of the immunofluorescence results 62. CGRP is expressed in small/medium sized cells and in thin ‘pearl’-like fibres among the trigeminal cells (red). The receptor components, CLR and RAMP1, are co-expressed in larger neurons, satellite glial cells and in the vascpular smooth muscle cells located within the trigeminal ganglion (green).  co-expression CLR/RAMPI;
co-expression CLR/RAMPI;  CGRP expression.
CGRP expression.
In order to determine if the trigeminal ganglion could be one possible site of action for CGRP receptor antagonists, it has recently been shown that Evans blue (which couples with plasma albumin in the circulation to form a large complex) is found in the trigeminal ganglion, among the different cell types and in blood vessel walls within the ganglion (Figure1) 66. The experiments showed that the trigeminal ganglion, like the dura mater 65, lacks a BBB and is freely accessible to circulating compounds 66. Furthermore, a recent study using labelled EDTA, another tracer that allows for quantitative calculation of the transfer constant, showed that the trigeminal ganglion is >100 times more accessible to the passage of this tracer than the cerebral cortex, cerebellum, brainstem and trigeminal nucleus caudalis 68. Thus, it is possible that current antimigraine drugs may reach sites in the dura mater and the trigeminal ganglion, but not in the CNS apart from regions lacking a BBB. However, the suggestion needs more confirmation with functional data and with imaging techniques because it is still often suggested that the BBB is disrupted during migraine attacks 69 and following cortical spreading depression 70. This question has been addressed in a few clinical studies which collectively provide no support for alteration in the BBB during the attacks 71–73.
Conclusion
Clinical trials have demonstrated good efficacy of gepants and triptans in migraine therapy, but they only pass the BBB to a minor degree. The newly developed CGRP and CGRP receptor antibodies are effective prophylactics but they are large molecules that do not readily penetrate the BBB in reasonable doses, suggestive of an action outside the CNS. Therefore, it is suggested that the meninges and, in particular, the trigeminal ganglion are the most logical sites of action for the observed clinical activity. There are neurons and fibres that store CGRP, neurons and satellite glial cells that have CGRP receptors, and the smooth muscle cells of blood vessels have CGRP receptors. All these sites are reachable by molecules present in the circulation.
Competing Interests
The author has completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declares no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by grants from the Swedish Research Council (no 5958) and the Swedish Heart and Lung foundation.
References
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570–84. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activation in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137:232–41. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]
- Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT (1B/1D) agonists in the acute migraine treatment: a metaanalysis of 53 triptan trials. Lancet. 2001;358:1668–75. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P. Optimal balance of efficacy and tolerability of oral triptans and telcagepant: a review and a clinical comment. J Headache Pain. 2011;12:275–80. doi: 10.1007/s10194-011-0309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick DW, Martin VT, Smith T, Silberstein S. Cardiovascular tolerability and safety of triptans: a review of clinical data. Headache. 2004;44(Suppl 1):S20–30. doi: 10.1111/j.1526-4610.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet. 2010;376:645–55. doi: 10.1016/S0140-6736(10)60323-6. [DOI] [PubMed] [Google Scholar]
- Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–82. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Chan KY, Eftekhari S, Nilsson E, de Vries R. Säveland H, Dirven CMF, Danser AHJ, MaassenVanDenBrink A. Effect of the calcitonin gene-related peptide (CGRP) receptor antagonist telcagepant in human cranial arteries. Cephalalgia. 2010;30:1233–40. doi: 10.1177/0333102410362122. [DOI] [PubMed] [Google Scholar]
- Chan KY, Edvinsson L, Eftekhari S, Kimblad PO, Kane SA, Lynch J, Hargreaves RJ, de Vries R, Garrelds IM, van den Bogaerdt AJ, Danser AHJ, MaassenVanDenBrink A. Characterization of the calcitonin gene-related peptide receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J Pharmacol Exp Ther. 2010;334:746–52. doi: 10.1124/jpet.110.165993. [DOI] [PubMed] [Google Scholar]
- Gupta S, Mehrotra S, Avezaat CJJ, Villalón CM, Saxena PR, MaassenVanDenBrink A. Characterization of CGRP receptors in the human isolated middle meningeal artery. Life Sci. 2006;79:265–71. doi: 10.1016/j.lfs.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Ho TW, Connor KM, Zhang Y, Pearlman E, Koppenhaver J, Fan X, Lines C, Edvinsson L, Goadsby PJ, Michelson D. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958–66. doi: 10.1212/WNL.0000000000000771. [DOI] [PubMed] [Google Scholar]
- Diener HC, Dodick DW, Goadsby PJ, Lipton RB, Olesen J, Silberstein SD. Chronic migraine - classification, characteristics and treatment. Nat Rev Neurol. 2012;8:162–71. doi: 10.1038/nrneurol.2012.13. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Spierings ELH, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13:885–92. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J. for the ALD403 study investigarors. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100–7. doi: 10.1016/S1474-4422(14)70209-1. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S. Monoclonal antibodies for migraine: preventing calcitonin gene-related peptide activity. CNS Drugs. 2014;28:389–99. doi: 10.1007/s40263-014-0156-4. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics. 2010;7:164–75. doi: 10.1016/j.nurt.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Hargreaves R, Bell I, Dancho M, Graham S, Hostetler E, Kane S, Kim J, Michener M, Miller P, O’Malley S, Salvatore C, Selnick H, Staas D, Stump C, Williams D, Wood M, Zeng Z, Cook J. CSF levels and binding pattern of novel CGRP receptor antagonists in rhesus monkey and human central nervous system: toward the development of a PET tracer. Cephalalgia. 2009;29(Suppl 1):136–7. [Google Scholar]
- Black JW, Leff L. Operational models of pharmacological agonism. Proc R Soc Lond. 1983;220:141–62. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. 2009;49:1258–66. doi: 10.1111/j.1526-4610.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekman R, Goadsby PJ. Measurement of vasoactive neuropeptides in biological materials: Problems and pitfalls from 30 years of experience and novel future approaches. Cephalalgia. 2010;30:761–6. doi: 10.1177/0333102409351807. [DOI] [PubMed] [Google Scholar]
- Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–8. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett. 1985;62:131–6. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Hara H. Axonal tracing of autonomic nerve fibers to the superficial temporal artery in the rat. Cell Tissue Res. 1989;256:559–65. doi: 10.1007/BF00225604. [DOI] [PubMed] [Google Scholar]
- Uddman R, Hara H, Edvinsson L. Neuronal pathways to the rat middle meningeal artery revealed by retrograde tracing and immunocytochemistry. J Auton Nerv Syst. 1989;26:69–75. doi: 10.1016/0165-1838(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Hara H, Uddman R. Retrograde tracing of nerve fibers to the rat middle cerebral artery with true blue: colocalization with different peptides. J Cereb Blood Flow Metab. 1989;9:212–8. doi: 10.1038/jcbfm.1989.31. [DOI] [PubMed] [Google Scholar]
- Liu Y, Broman J, Edvinsson L. Central projections of the sensory innervation of the rat middle meningeal artery. Brain Res. 2008;1208:103–10. doi: 10.1016/j.brainres.2008.02.078. [DOI] [PubMed] [Google Scholar]
- Liu Y, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superior sagittal sinus. Neuroscience. 2004;129:431–7. doi: 10.1016/j.neuroscience.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang M, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superficial temporal artery. Brain Res. 2003;966:126–33. doi: 10.1016/s0006-8993(02)04222-1. [DOI] [PubMed] [Google Scholar]
- Arbab MA, Delgado T, Wiklund L, Svendgaard NA. Brain stem terminations of the trigeminal and upper spinal ganglia innervation of the cerebrovascular system: WGA-HRP transganglionic study. J Cereb Blood Flow Metab. 1988;8:54–63. doi: 10.1038/jcbfm.1988.8. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Uddman R. Neurobiology in primary headaches. Brain Res Rev. 2005;48:438–56. doi: 10.1016/j.brainresrev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia. 2011;31:737–47. doi: 10.1177/0333102411398152. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Uddman R, Kingman T, Edvinsson L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A. 1986;83:5731–5. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin–receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem. 2000;275:31438–43. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Fredholm BB, Hamel E, Jansen I, Verrecchia C. Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett. 1995;58:213–7. doi: 10.1016/0304-3940(85)90166-1. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab. 2002;22:620–9. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Sexton PM, McKenzie JS, Mendelsohn FA. Evidence for a new subclass of calcitonin/calcitonin gene-related peptide binding site in rat brain. Neurochem Int. 1988;12:323–35. doi: 10.1016/0197-0186(88)90171-4. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain. 2013;14:1289–303. doi: 10.1016/j.jpain.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Edvinsson L. Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level. BMC Neurosci. 2011;12:112. doi: 10.1186/1471-2202-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–99. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–96. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–60. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Avramut M, Tepper SJ. Structural and functional neuroimaging in migraine: insights from 3 decades of research. Headache. 2013;53:46–66. doi: 10.1111/j.1526-4610.2012.02274.x. [DOI] [PubMed] [Google Scholar]
- Sexton PM. Central nervous system binding sites for calcitonin and calcitonin gene-related peptide. Mol Neurobiol. 1991;5:251–73. doi: 10.1007/BF02935550. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Gaspar RC, Roberts R, Chen T, Zeng Z, Villarreal S, Edvinsson L, Salvatore CA. Localization of CGRP receptor components and receptor binding sites in rhesus monkey brainstem: a detailed study using in situ hybridization, immunofluorescence and autoradiography. J Comp Neurol. 2015;000:00–00. doi: 10.1002/cne.23828. [DOI] [PubMed] [Google Scholar]
- Strata P, Thach WT, Ottersen OP. New insights in cerebellar function. Neuroscience. 2009;162:545–8. doi: 10.1016/j.neuroscience.2009.06.047. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Eftekhari S, Salvatore CA, Warfvinge K. Cerebellar distribution of calcitonin gene-related peptide (CGRP) and its receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) in rat. Mol Cell Neurosci. 2010;46:333–9. doi: 10.1016/j.mcn.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Salvatore CA, Gaspar RC, Roberts R, O’Malley S, Zeng Z, Edvinsson L. Localization of CGRP receptor components, CGRP, and receptor binding sites in human and rhesus cerebellar cortex. Cerebellum. 2013;12:937–49. doi: 10.1007/s12311-013-0509-4. [DOI] [PubMed] [Google Scholar]
- Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–7. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- Hostetler ED, Joshi AD, Sanabria-Bohorquez S, Fan H, Zeng Z, Purcell M, Gantert L, Riffel K, Williams M, O’Malley S, Miller P, Selnick HG, Gallicchio SN, Bell IM, Salvatore C, Kane SA, Li CC, Hargreaves R, de Groot T, Bormans G, Van Hecken A, Derdelinckx I, de Hoon J, Reynders T, Declercq R, De Lepeleire I, Kennedy WD, Blanchard R, Marcantonio EE, Sur C, Cook JJ, van Laere K, Evelhoch JL. In vivo quantification of calcitonin gene-related peptide (CGRP) receptor occupancy by telcagepant in rhesus monkey and human brain usin the positron emission tomography (PET) tracer [11C]MK-4232. J Pharmacol Exp Ther. 2013;347:478–86. doi: 10.1124/jpet.113.206458. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8-37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–40. doi: 10.1038/sj.bjp.0707134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J, Wecker S, Neeb L, Dirnagl U, Reuter U. Primary trigeminal afferents are the main source for stimulus-induced CGRP release into jugular vein blood and CSF. Cephalalgia. 2012;32:659–67. doi: 10.1177/0333102412447701. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, van der Koning P, Larsson HBW, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–45. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Amin FM, Asghar MS, Hougaard A, Hansen AE, Larsen VA, de Koning PJH, Larsson HBW, Olesen J, Ashina M. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12:454–61. doi: 10.1016/S1474-4422(13)70067-X. [DOI] [PubMed] [Google Scholar]
- Juhl L, Edvinsson L, Olesen J, Jansen-Olesen I. Effect of two novel CGRP-binding compounds in a closed cranial window rat model. Eur J Pharmacol. 2007;567:117–24. doi: 10.1016/j.ejphar.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Hoehlig K, Johnson K, Pryazhnikov E, Maasch C, Clemens-Smith A, Purschke W, Vauléon S, Buchner K, Jarosch F, Khiroug L, Vater A, Klussmann S. A novel CGRP-neutralizing Spiegelmer attenuates neurogenic plasma protein extravasation. Br J Pharmacol. 2015 doi: 10.1111/bph.13110. doi: 10.1111/bph.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero DI, Buciak JB, Yang J, Pardridge WM. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci U S A. 1989;86:4761–5. doi: 10.1073/pnas.86.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BVI, Skundric DS, Segal MB, Lipovac MN, Mackic JB, Davson H. A saturable mechanism for transport of immunoglobulin G across the blood-brain barrier of the guinea pig. Exp Neurol. 1990;107:263–70. doi: 10.1016/0014-4886(90)90144-h. [DOI] [PubMed] [Google Scholar]
- Pardridge WMI, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate. Pharm Res. 1995;12:807–16. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- Bito LZ, Davson H, Hollingsworth JR. Facilitated transport of prostaglandins across the blood-cerebrospinal fluid and blood-brain barrier. J Physiol. 1976;256:273–85. doi: 10.1113/jphysiol.1976.sp011325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, MacKenzie ET, McCulloch J. Cerebral Blood Flow and Metabolism. New York: Raven Press; 1993. pp. 1–683. [Google Scholar]
- Eftekhari S, Salvatore CA, Johansson S, Chen T, Zeng Z. Edvinsson L. Vol. 1600. CGRP receptor, PACAP and glutamate in rhesus monkey trigeminal ganglion. Relation to the blood-brain barrier. Brain Res: Localization of CGRP; 2015. pp. 93–109. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab. 1987;7:720–8. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- Lundberg C, Haanes K, Grände G, Edvinsson L. Microvascular passage and calculation of the transfer constant (Ki) for [51Cr]EDTA and water content following dural application of Freud’s Adjuvant or Inflammatory Soup in trigeminal ganglion and CNS of rat. Cephalalgia. 2015;35(Suppl. 1):00. [Google Scholar]
- Kaube H, Hoskin KL, Goadsby PJ. Inhibition by sumatriptan of central trigeminal neurones only after blood-brain barrier disruption. Br J Pharmacol. 1993;109:788–92. doi: 10.1111/j.1476-5381.1993.tb13643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, Wang X, Rosenberg GA, Lo EH, Moskowitz MA. Cortical spreading depression activates and upregulates MMP-9. J Clin Invest. 2004;113:1447–55. doi: 10.1172/JCI21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrer FM, Sorensen AG, Weisskoff RM, Ostergaard L, Sanchez del Rio M, Lee EJ, Rosen BR, Moskowitz MA. Perfusion-weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25–31. doi: 10.1002/ana.410430108. [DOI] [PubMed] [Google Scholar]
- Jager HR, Giffin NJ, Goadsby PJ. Diffusion- and perfusion-weighted MR imaging in persistent migrainous visual disturbances. Cephalalgia. 2005;25:323–32. doi: 10.1111/j.1468-2982.2004.00858.x. [DOI] [PubMed] [Google Scholar]
- Sanchez del Rio M, Bakker D, Wu O, Aosti R, Mitsikostas DD, Ostergaard L, Wells WA, Rosen BR, Sorensen G, Moskowitz MA, Cutrer FM. Perfusion weighted imaging during migraine: spontaneous visual aura and headache. Cephalalgia. 1999;19:701–7. doi: 10.1046/j.1468-2982.1999.019008701.x. [DOI] [PubMed] [Google Scholar]


