Abstract
Aims
The aim of the study was to determine the effect of renal impairment and prior platinum-based chemotherapy on the toxicity and pharmacokinetics of oral topotecan and to identify recommended doses for patients with renal impairment or prior platinum-based (PB) chemotherapy.
Methods
A multicentre phase I toxicity and pharmacokinetic study of oral topotecan was conducted in patients with advanced solid tumours. Patients were grouped by normal renal function with limited or prior PB chemotherapy or impaired renal function (mild [creatinine clearance (CLcr) = 50–79 ml min−1], moderate [CLcr = 30–49 ml min−1], severe [CLcr <30 ml min−1]).
Results
Fifty-nine patients were evaluable. Topotecan lactone and total topotecan area under the concentration–time curve (AUC) was significantly increased in patients with moderate and severe renal impairment (109% and 174%, respectively, topotecan lactone and 148% and 298%, respectively, total topotecan). Asian patients (23 in total) had higher AUCs than non-Asian patients with the same degree of renal impairment. Thirteen dose-limiting toxicities (DLTs) were observed, which were mostly haematological. The maximum tolerated dose (MTD) was 2.3 mg m−2 day−1, given on days 1 to 5 in a 21 day cycle, for patients with prior PB chemotherapy or mild renal impairment, and 1.2 mg m−2 day−1 for patients with moderate renal impairment (suggested dose 1.9 mg m−2 day−1 for non-Asians). Due to incomplete enrolment of patients with severe renal impairment, the MTD was determined as ≥ 0.6 mg m−2 day−1 in this cohort.
Conclusions
Oral topotecan dose adjustments are not required in patients with prior PB chemotherapy or mildly impaired renal function, but reduced doses are required for patients with moderate or severe renal impairment.
Keywords: oral topotecan, pharmacodynamics, pharmacokinetics, renal impairment, toxicity
What is Already Known About this Subject
Topotecan is a specific inhibitor of topoisomerase-I and is approved for treatment of patients with cancer.
Topotecan exposure is increased in patients with moderate and severe renal impairment after intravenous administration.
The effect of renal impairment on the toxicities and pharmacokinetics of oral topotecan in patients with advanced solid tumours is unknown.
What this Study Adds
Dose-limiting toxicities were mostly haematological.
Topotecan lactone and total topotecan area under the concentration–time curve (AUC) was significantly increased in patients with moderate and severe renal impairment.
Asian patients had higher AUCs than non-Asian patients with the same degree of renal impairment.
Introduction
Topotecan (SK&F-104864; Hycamtin; GlaxoSmithKline) is a semi-synthetic analogue of the alkaloid camptothecin and is a specific inhibitor of topoisomerase-I 1. Inhibition of this enzyme results in lethal deoxyribonucleic acid (DNA) damage during the course of DNA replication 2. Intravenous (i.v.) topotecan monotherapy is approved for treatment of patients with metastatic ovarian carcinoma as second line therapy and for patients with relapsed small cell lung cancer (SCLC) for whom re-treatment with the first line regimen is not considered appropriate. Intravenous topotecan in combination with cisplatin is indicated for patients with cervical cancer with recurrence after radiotherapy and for patients with stage IV-B disease. Safety and efficacy of oral topotecan alone 3–7 and in combination with other chemotherapeutic and anti-angiogenic agents 8–16 has been explored in various phase I and II trials. In phase II and III studies in SCLC patients, intravenous (1.5 mg m−2 day−1) and oral (2.3 mg m−2 day−1) topotecan regimens demonstrateD similar response rates 17,18. Oral topotecan has shown improved benefit for survival and quality of life compared with supportive care alone in a phase III trial in SCLC patients 19. Oral topotecan is approved for the treatment of patients with relapsed SCLC. The maximum tolerated dose (MTD) for oral topotecan in a regimen of 5 days every 21 days is 2.3 mg m−2 day−1 in patients with normal renal function 20.
Excretion of oral topotecan was studied in a human mass-balance study and the principal routes of excretion were renal and faecal. Approximately 20% was recovered as parent (total topotecan) drug in urine and 33% of the oral dose was found to be unchanged (total topotecan) in faeces 21. The contribution of metabolism to topotecan total body clearance is limited (<10%). Topotecan undergoes reversible pH-dependent hydrolysis, yielding topotecan carboxylate. It is thought that the lactone form of the drug, which is the predominant form in an acidic environment (pH ≤ 4) is pharmacologically more active 22,23. Topotecan has relatively low plasma protein binding of approximately 30% in humans 24,25 and the mean absolute bioavailability of oral topotecan is 30 – 40% [6, 7, 21]6,7,21. Topotecan is a substrate for ABCB1 (P-glycoprotein, P-gp) and ABCG2 (breast cancer resistant protein, BCRP) transporter proteins and inhibition of these transporters affects the oral bioavailability of oral topotecan 26.
The disposition and toxicological profile of intravenous topotecan was studied by O’Reilly et al. in patients with varying degrees of renal dysfunction. This study showed marked reduction in plasma clearance of topotecan in patients with moderate renal impairment (defined as measured 24 h creatinine clearance (CLcr) 20–39 ml min−1) 27. (Please note CLcr for moderate renal impairment in the present study with oral topotecan is 30–49 ml min−1). The current labelling for i.v. topotecan recommends a dose reduction of 50% for patients with moderate renal impairment (CLcr=20–39 ml min−1). It is unknown whether the dose adjustments for i.v. topotecan in patients with renal dysfunction can be directly applied to oral topotecan administration. Another issue is whether prior platinum-based (PB)-chemotherapy would alter the safety and pharmacokinetics of topotecan 28. Evidence indicates that cisplatin has a direct toxic effect on the renal proximal tubule 29 that could change tubular secretion of topotecan and could increase systemic exposure to topotecan without affecting CLcr. As a result, prior PB based chemotherapy may affect the toxicodynamics and pharmacokinetics of oral topotecan.
The objectives of this study were to determine the effect of renal impairment and prior PB chemotherapy on the toxicodynamics and pharmacokinetics of oral topotecan and to identify appropriate dose adjustments for patients with mild, moderate or severe renal impairment and patients with normal renal function who have received prior PB chemotherapy. Secondary objectives were to evaluate the pharmacokinetics of oral topotecan and to explore the relationship between pharmacokinetic parameters and the degree of renal impairment.
Methods
Patient selection
Patients with histologically or cytologically confirmed advanced solid tumours, for whom no standard treatment was available or for whom single agent topotecan therapy was considered suitable, were eligible. Other inclusion criteria were ability to provide written informed consent, ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) 30 performance status ≤ 2 and stable renal function, defined as < 10% change in estimated CLcr for more than 4 weeks prior to start. In addition to standard exclusion criteria, the following were included current dialysis, participation in another clinical study within 30 days or five elimination half-lives of the drug under investigation, uncontrolled emesis, bilirubin > 1.5 times the upper limit of normal (ULN) alanine aminotransferase, aspartate aminotransferase or alkaline phosphatase > two times ULN, in case of liver metastases < five times ULN, haemoglobin < 5.6 mmol l−1 or < 9 g dl−1, white blood cell count < 3.5 × 109 l−1, absolute neutrophil count < 1.5 × 109 l−1 or platelets < 100 × 109 l−1, active infection, less than 4 weeks since last chemo-, radio-biologic-therapy or surgical procedure, failure to recover from any prior chemotherapy toxicity at baseline with the exception of grade 1 neuropathy or any grade alopecia, impaired gastro-intestinal absorption or motility, concurrent ciclosporin A treatment, concurrent severe medical problems unrelated to the malignancy significantly limiting compliance with protocol/study events, history of allergic reactions to topotecan or chemically related compounds. Patients were not allowed to receive drugs known to inhibit or induce human breast cancer resistance protein (BCRP, ABCG2), P-glycoprotein (P-gp, ABCB1) and multidrug resistance protein (MRP1, ABCC1) during pharmacokinetic sampling. The study protocol was approved by the Medical Ethics committee and all patients had to give written informed consent prior to entry into the study. The study was conducted in accordance with the ICH guidelines of good clinical practice (GCP).
Study design, study procedures and treatment administration
This was a multicentre, phase I, multiple dose, open label, safety and pharmacokinetic study of oral topotecan in patients with advanced solid tumours (NCT00483860, www.clinicaltrials.gov) 31. In total, six centres participated in the study. The first patient visit occurred on 20 June 2007 and the last visit occurred on 6 February 2012.
Estimated creatinine clearance (CLcr) was calculated using the Cockcroft–Gault formula 32. Prior PB chemotherapy was defined as ≥ three courses of cisplatin or carboplatin chemotherapy documented with normal estimated CLcr at the time of screening or < three courses of cisplatin or carboplatin chemotherapy associated with a significant change in CLcr (documented >20% decrease from baseline CLcr) but documented with normal CLcr at the time of screening or documented decreased CLcr, resulting in cisplatin or carboplatin chemotherapy being withheld, but documented with normal CLcr at the time of screening. The definition of limited prior PB chemotherapy was applicable in patients in whom the aforementioned definition of ‘prior PB chemotherapy’ was not met but who had received prior platinum based therapy. Patients were assigned to one of five groups (Table1) according to measured CLcr at baseline based on the amount of creatinine excreted in a 24 h urine collection and prior platinum treatments: CLcr > 80 ml min−1 and limited prior PB chemotherapy (group A), CLcr > 80 ml min−1 and prior PB chemotherapy (group B), mild renal impairment (CLcr = 50–79 ml min−1, group C), moderate renal impairment (CLcr = 30–49 ml min−1, group D) and severe renal impairment (CLcr <30 ml min−1, group E).
Patient demographics and characteristics of the PK evaluable population
| Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | A | B | C | C | D | D | D | E | E | E | Total |
| Dose (mg m–2 day–1) | 2.3 | 2.3 | 1.9 | 2.3 | 1.2 | 1.5 | 1.8 | 0.6 | 0.8 | 1.2 | |
| Number (n) | 6 | 12 | 6 | 13 | 5 | 6 | 3 | 5 | 1 | 2 | 59 |
| Age (years) | |||||||||||
| Median (range) | 63 (54–74) | 53 (38–67) | 62 (36–69) | 63 (39–77) | 59 (53–68) | 64 (52–77) | 54 (43–63) | 62 (46–75) | 68 (NA) | 56 (37–75) | 62 (36–77) |
| Weight (kg) | |||||||||||
| Median (range) | 76 (64–91) | 81 (43–92) | 64 (55–74) | 75 (41–115) | 64 (53–99) | 73 (55–97) | 75 (72–117) | 62 (55–100) | 60 (NA) | 62 (56–68) | 73 (41–117) |
| Height (cm) | |||||||||||
| Median (range) | 171 (151–186) | 169 (149–182) | 159 (146–167) | 168 (154–178) | 166 (150–183) | 166 (161–180) | 172 (170–179) | 167 (155–176) | 160 (NA) | 165 (160–170) | 167 (146–186) |
| BSA (m2) | |||||||||||
| Median (range) | 1.88 (1.71–2.17) | 1.92 (1.40–2.12) | 1.70 (1.49–1.74) | 1.90 (1.37-2.30) | 1.71 (1.50–2.21) | 1.80 (1.58–2.17) | 1.94 (1.84–2.28) | 1.68 (1.60–2.08) | 1.60 (NA) | 1.68 (1.64–1.71) | 1.81 (1.37–2.30) |
| CLcr * (ml min–1) | |||||||||||
| Median (range) | 103 (90–119) | 92 (83–155) | 69 (63–79) | 63 (54–80) | 42 (36–47) | 36 (33–48) | 38 (32–41) | 20 (10–26) | 23 | 29 (29–29) | NA |
| Gender | |||||||||||
| Female (%) | 1 (17) | 3 (25) | 3 (50) | 5 (38) | 3 (60) | 4 (67) | 1 (33) | 3 (60) | 1 (100) | 2 (100) | 26 (44) |
| Male (%) | 5 (83) | 9 (75) | 3 (50) | 8 (62) | 2 (40) | 2 (33) | 2 (67) | 2 (40) | N.A. | N.A. | 33 (56) |
| Ethnicity, n (%) | |||||||||||
| African | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (50) | 1 (2) |
| Asian† | 1 (17) | 6 (50) | 5 (83) | 2 (15) | 3 (60) | 2 (33) | 1 (33) | 3 (60) | 0 | 0 | 23 (39) |
| Caucasian | 5 (83) | 6 (50) | 1 (17) | 11 (85) | 2 (40) | 4 (67) | 2 (67) | 2 (40) | 1 (100) | 1 (50) | 35 (59) |
| Primary tumour site, n (%) | |||||||||||
| Bile duct | 0 | 2 (17) | 0 | 0 | 0 | 0 | 1 (33) | 0 | 0 | 0 | 3 (5) |
| Breast | 0 | 0 | 1 (17) | 1 (8) | 0 | 0 | 0 | 1 (20) | 1 (100) | 0 | 4 (7) |
| Cervix | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) | 2 (40) | 0 | 0 | 3 (5) |
| Colorectal | 1 (17) | 0 | 0 | 1 (8) | 0 | 1 (17) | 0 | 1 (20) | 0 | 0 | 4 (7) |
| NSCLC | 0 | 3 (25) | 1 (17) | 0 | 1 (20) | 0 | 0 | 1 (20) | 0 | 0 | 6 (10) |
| Ovary | 0 | 2 (17) | 1 (17) | 2 (15) | 0 | 2 (33) | 0 | 0 | 0 | 0 | 7 (12) |
| Prostate | 3 (50) | 0 | 0 | 3 (23) | 0 | 1 (17) | 0 | 0 | 0 | 0 | 7 (12) |
| Renal | 0 | 0 | 0 | 2 (15) | 1 (20) | 0 | 0 | 0 | 0 | 0 | 3 (5) |
| Other | 2 (33) | 5 (42) | 3 (50) | 4 (30) | 3 (60) | 2 (33) | 1 (33) | 0 | 0 | 2 (100) | 22 (37) |
Both group A and B have CLcr > 80 ml min–1 (according calculation from 24 h urine collection at baseline), but group A had received limited platinum-based therapy, defined as < three courses of cisplatin or carboplatin chemotherapy without significant change in CLcr.
All Asian patients were of East Asian ethnicity, except one who was of South-East Asian ethnicity. Abbreviations: BSA, body surface area; CLcr, creatinine clearance; NA, not applicable; NSCLC, non-small cell lung cancer.
Study procedures
Complete physical examinations, ECG (including QT interval) and clinical laboratory testing for standard haematologic and metabolic profiles were performed at screening and at regular intervals during treatment and at study termination. Patients remained in the study until occurrence of unacceptable toxicities, disease progression, withdrawal of consent, investigator discretion or treatment delay for more than 2 months.
Dosing
Oral topotecan was provided as capsules containing topotecan HCl, equivalent to 0.25 mg or 1 mg of the anhydrous free base. Topotecan was administered on days 1–5 of a 21 day cycle. On day 1 of course 1, topotecan was administered with water after an overnight fast and a light meal was scheduled 2 h after dosing.
The maximum tolerated dose (MTD) and recommended dose for patients without renal impairment has been established as 2.3 mg m−2 day−1 on days 1 to 5 of a 21 day cycle 20. The initial starting doses were: 2.3 mg m−2 day−1 (groups A–C), 1.2 mg m−2 day−1 (group D) and 0.6 mg m−2 day−1 (group E). In the control, normal renal function, group (group A) six patients were enrolled and in the four test groups (groups B–E) patients were to be enrolled in cohorts of three. These study cohorts were expanded to six patients if a DLT occurred in one of the three subjects initially enrolled. The MTD was exceeded if two or more patients out of six in a test cohort experienced a dose limiting toxicity (DLT). Topotecan dose reductions were allowed in all groups. Dose escalations were only allowed in groups D and E.
A DLT was defined as any grade 3 or 4 non-haematological toxicity (except alopecia, liver toxicity based on tumour progression or grade ≥ 3 nausea, vomiting or diarrhoea without adequate supportive therapy), grade ≥ 4 neutropenia of any duration associated with fever or infection or ≥ 7 days without associated fever or infection, dose delay due to grade ≥ 3 neutropenia, grade 4 thrombocytopenia (prior to an amendment dated 3 May 2008, grade 3 thrombocytopenia was also considered a DLT), dose delay of > 28 days or dose reduction of > 75%; grade ≥ 2 toxicity for > 28 days or selected grade ≥ 2 (if agreed to by the sponsor and investigator).
Toxicity assessments
Adverse events (AE) and their relation to the study drug were assessed throughout the study. The incidence and severity of AEs were evaluated and graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events (CTCAE) version 3.0 33.
Evaluability
Patients were considered evaluable it they received one dose of oral topotecan and provided adequate pharmacokinetic samples through the 24 h post-dosing collection period.
Pharmacokinetic sampling and analysis
Venous blood samples for the determination of total topotecan and topotecan lactone plasma concentrations were obtained on day 1 at baseline, and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 h following oral topotecan administration. Urine was collected pre-dose, 0 to 6, 6 to 12 and 12 to 24 h following oral topotecan administration on day 1. Blood and urine sample processing is described in Supplementary Information 1.
Plasma concentrations of topotecan lactone and total topotecan (both carboxylate and lactone form) and urine concentrations of total topotecan were quantified using a validated and previously described method based on protein precipitation followed by high performance liquid chromatography with fluorescence detection (HPLC-FL) 34 at a central laboratory. The lower limit of quantitation (LOQ) was 0.1 ng ml−1 and 25 ng ml−1 for topotecan in human plasma and human urine, respectively. The coefficient of variation (CV) was always less than 10%. Detailed information on pharmacokinetic analysis is included in Supplementary Information 1.
Topotecan lactone and total topotecan concentrations and actual sample collection times were used to perform non-compartmental analysis using WinNonLin (v 6.2). AUC(0,∞) and AUC(0,t) calculation methods in this software were the linear trapezoidal rule for increasing values and the log trapezoidal rule for decreasing values (uniform weighting for λz calculations).The following pharmacokinetic parameters were determined from the plasma concentration–time data: area under the concentration–time curve extrapolated to infinity and to 12 h (AUC(0,∞), AUC(0,12 h), respectively) and terminal half-life (t1/2).The maximum observed plasma concentration (Cmax) and time to maximum observed plasma concentration (tmax) were directly obtained from the plasma concentration vs. time data.
The following total topotecan parameters were determined from the urine concentration–time data: fraction dose excreted/% dose recovered (fe), urinary excretion rate and renal clearance (CLr).
In addition to Cockcroft–Gault methods for estimation of renal function, Modification of Diet in Renal Disease (MDRD) was used to calculate eGFR and the resulting values were used to determine the relationship between glomerular filtration rate and dose-normalized AUC.
The study sample size was based on feasibility. However, based on an estimate of within-subject variability of 26% for AUC and 53% for Cmax of topotecan and topotecan lactone (GlaxoSmithKline Document Number CM2003/00071/02), a sample size of 60 subjects (12 per group) was expected to provide approximately 19.1% precision for AUC and 33.5% precision for Cmax, where precision is expressed as the half-width of the 90% confidence interval (CI). Calculations were based on a two-tailed procedure on the log-scale and a type I error rate of 0.1. No adjustments were made for pre-planned multiple comparisons.
Pharmacogenetic sampling and analysis
A peripheral blood sample was obtained following specific informed consent and DNA was extracted using standard methods. Single nucleotide polymorphisms (SNPs) of genes of interest, based on involvement in topotecan disposition and metabolism, were selected for exploratory analysis and and the analysis was performed using the TaqMan assay (Applied Biosystems, Foster City, CA, USA). These included ABCB1 (rs1128503, rs2032582, rs1045642), ABCG2 (rs2231137, rs2231142) and SLC22A8 (rs1156842). Detailed information regarding the primers used is available in Supplementary Information 2.
Statistical analysis
Statistical analyses were performed using the SAS/STAT module of the SAS System, Version 8.2 or higher (SAS Institute Inc., Cary, NC, USA). Pharmacokinetic parameters were summarized by group and dose. Geometric means and 90% CIs for the ratios of B: A, C: A, D: A and E: A for dose-normalized AUC(0,∞) and Cmax of total topotecan and topotecan lactone were calculated to provide a range of plausible values for the comparisons of interest. Following loge transformation, dose-normalized AUC and Cmax of topotecan and topotecan lactone were separately analyzed by analysis of variance ( anova). The estimated means were back-transformed to obtain an estimate for the ratio. During the study it was determined that race may have an effect on the AUC (exposure) of topotecan lactone and total topotecan. Therefore, a similar analysis was conducted to explore differences in AUC for non-Asian patients vs. Asian patients. tmax and tlag were compared between groups using the Wilcoxon Rank-Sum test. Correlations between pharmacokinetic parameters and oral clearance were explored graphically.
Results
Patient inclusion and demographics
In total, 59 patients were included in the study, of whom 56 completed cycle 1. Two patients withdrew due to lack of efficacy (groups C and E) and one patient withdrew informed consent (group E). Detailed patient demographics and characteristics are summarized in Table1. The median age was 62 (range 36–77) years. The majority of patients were male (56%) and Caucasian (59%). Twenty-three patients (39%) had Asian ethnicity. The study population consisted most frequently of patients with primary tumours of the ovary, the prostate (both 12%) and non-small lung (NSCLC, 10%). The majority of patients had an ECOG performance score at baseline of 0 or 1 (85%). Nearly all patients (93%) had received at least one prior chemotherapy regimen.
Toxicity
All 59 enrolled patients were evaluable for topotecan related toxicity: six in group A, 12 in group B, 19 in group C, 14 in group D and eight in group E. The topotecan doses that were administered are described in Table1. The most frequently reported grade 3 or 4 AEs were haematological and included neutropenia (36%), thrombocytopenia (31%), anaemia (19%) and leukopenia (15%). The most frequently reported grade 3 or 4 non-haematological AEs were fatigue (8%), hypokalaemia (8%), nausea (7%), vomiting (5%) and increased γ-glutamyltransferase (5%). Generally, a higher frequency of AEs, including the AEs of interest, diarrhoea and neutropenia, and AEs grade ≥ 3 occurred in the cohorts with renal impairment or previous platinum therapy (group B), groups (B–E) compared with the cohort with normal renal function (group A).
The most frequently reported AEs related to oral topotecan are described in detail in Table2. These consisted of nausea (51%), anaemia and thrombocytopenia (both 49%), neutropenia (42%), fatigue (39%), diarrhoea (37%), vomiting (36%) and decreased appetite (29%). AEs that led to dose reductions or delays occurred in 11 (11/59, 19%) and 19 (19/59, 32%) patients, respectively. The leading cause for dose reduction or delay was haematological toxicity.
Table 2.
Most frequently reported drug-related adverse events (AE, occurring in ≥ 10% of patients)
| Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | A | B | C | C | D | D | D | E | E | E | Total |
| Dose (mg m–2 day–1) | 2.3 | 2.3 | 1.9 | 2.3 | 1.2 | 1.5 | 1.8 | 0.6 | 0.8 | 1.2 | |
| Number (n, %) | 6 | 12 | 6 | 13 | 5 | 6 | 3 | 5 | 1 | 2 | 59 |
| Any drug-related AE | 6 (100) | 11 (92) | 6 (100) | 12 (92) | 5 (100) | 6 (100) | 3 (100) | 5 (100) | 1 (100) | 2 (100) | 57 (97) |
| Nausea | 3 (50) | 5 (42) | 3 950) | 6 (46) | 4 (80) | 3 (50) | 1 (33) | 3 (60) | 1 (100) | 1 (50) | 30 (51) |
| Anaemia | 1 917) | 9 (75) | 1 (17) | 89 (62) | 2 (40) | 3 (50) | 2 (67) | 0 | 1 (100) | 2 (100) | 29 (49) |
| Thrombocytopenia | 3 (50) | 7 (58) | 0 | 9 (69) | 2 (40) | 2 (33) | 2 (67) | 1 (20) | 1 (100) | 2 (100) | 29 (49) |
| Neutropenia | 2 (33) | 5 (42) | 1 (17) | 9 (69) | 0 | 3 (50) | 2 (67) | 2 (40) | 0 | 1 (50) | 25 (42) |
| Fatigue | 5 (83) | 3 925) | 0 | 6 (46) | 3 (60) | 3 (50) | 2 (67) | 0 | 0 | 1 (50) | 23 (39) |
| Diarrhoea | 0 | 7 (58) | 1 (17) | 6 (46) | 1 (20) | 2 (33) | 3 (100) | 1 (20) | 0 | 1 (50) | 22 (37) |
| Vomiting | 1 (17) | 4 (33) | 1 (17) | 6 (46) | 2 (40) | 2 (33) | 1 (33) | 1 (20) | 1 (100) | 2 (100) | 21 (36) |
| Decreased appetite | 1 (17) | 2 (17) | 4 (67) | 5 (38) | 2 (40) | 2 (33) | 1 (33) | 0 | 0 | 0 | 17 (29) |
| Leukopenia | 3 (50) | 3 (25) | 0 | 6 (46) | 0 | 1 (17) | 1 (33) | 1 (20) | 0 | 0 | 15 (25) |
| Alopecia | 1 (17) | 3 (25) | 0 | 2 (15) | 0 | 1 (17) | 1 (33) | 0 | 0 | 1 (50) | 9 (15) |
| Asthenia | 0 | 2 (17) | 0 | 0 | 1 (20) | 2 (33) | 1 (33) | 0 | 0 | 0 | 6 (10) |
| Constipation | 2 (33) | 0 | 2 (33) | 0 | 0 | 2 (33) | 0 | 0 | 0 | 0 | 6 (10) |
SAEs were reported in 21 (36%) patients. The most frequently reported SAEs were thrombocytopenia (5%), vomiting (5%) and dyspnoea (3%). There were two patients with grade 5 SAEs. One patient in group C (2.3 mg m−2 day−1) died from neutropenic sepsis that was considered related to the study drug. One patient in group D (1.5 mg m−2 day−1) died of a sudden cardiac death not considered to be related to the study drug.
Thirteen dose-limiting toxicities (DLTs) occurred which are summarized in Supplementary Table 1. The most frequently observed DLTs were grade 3 or 4 neutropenia and thrombocytopenia. Only two (15%) DLTs were non-haematological, being grade 3 fatigue. In summary, for all groups, dosing occurred on days 1–5 of a 21 day cycle and the MTD was determined as 2.3 mg m−2 day−1 for groups B and C. In group D, two patients in each of the 1.8 mg m−2 day−1 and 1.5 mg m−2 day−1 groups experienced DLTs. Three of four DLTs occurred in Asian patients. However, in the 1.2 mg m−2 day−1 group, none of the five patients experienced a DLT. Therefore, the MTD was determined as 1.2 mg m−2 day−1 for group D. In group E, no DLTs were observed at the 0.6 mg m−2 day−1 dose level and in the 0.8 mg m−2 day−1 enrolment was incomplete. In the 1.2 mg m−2 day−1 group, two patients had erroneously been assigned to group D and later re-assigned to group E. One DLT occurred in this latter group. Due to incomplete enrolment in group E, the MTD was defined as ≥ 0.6 mg m−2 day−1. However, the precise dosing level could not be determined.
Pharmacokinetic analysis
The pharmacokinetic population included the 59 patients enrolled in the study as all had received at least one dose of oral topotecan. For one patient in group C, AUC(0,∞) could not be calculated accurately due to an incomplete pharmacokinetic sample collection following the 3 h post-dosing time point. Mean extrapolated AUCs were < 20% for all groups, except for topotecan lactone group E (0.6 mg m−2 day−1). However, individual values > 20% were included as estimated in AUC and t1/2 summary statistics since group E had limited patient numbers.
Mean (SD) plasma concentration–time curves are presented in Figure1 (topotecan lactone) and Figure2 (total topotecan). The curves show higher mean plasma concentrations for group D (dosed at 1.8 mg m−2 day−1) for both topotecan lactone and total topotecan due to three-fold and two-fold higher AUCs, respectively, in one out of the three patients. For total topotecan in group E, the shape of the plasma concentration–time curves appeared to be different compared with the reference group (A), with a less steep rise in plasma concentration and a slower decline after tmax up to 24 h.
Figure 1.
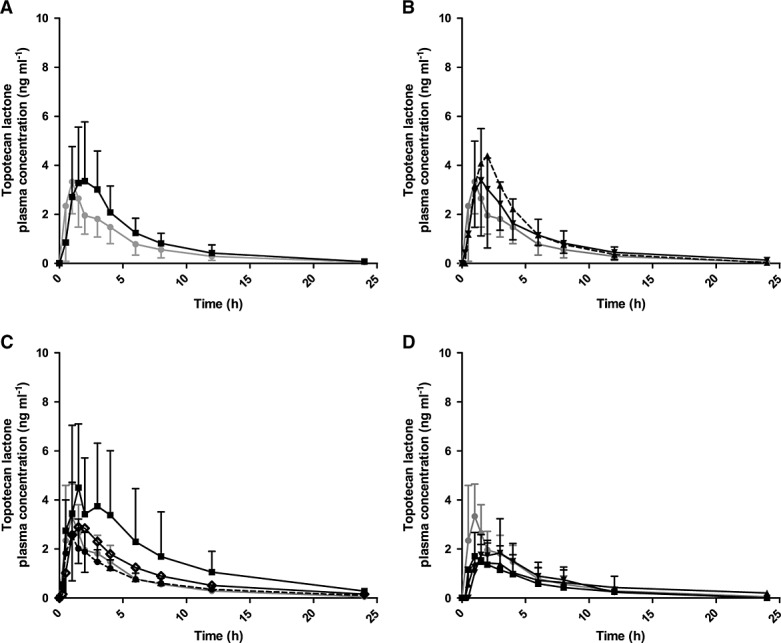
Mean (± SD) plasma concentration (ng ml–1)–time curves of topotecan lactone following oral administration. Groups A–E represent different groups with normal renal function, with limited previous platinum-based chemotherapy (group A) and with platinum-based chemotherapy (group B, Figure1A), or impaired renal function (group C: mild, group D: moderate and group E: severe, Figure1 B–D). Group A is depicted in all graphs as reference (normal renal function). Oral topotecan doses per group are indicated in mg m–2 day–1. n, number of patients. (A)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group B dose 2.3, n = 12, (B)
Group B dose 2.3, n = 12, (B)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group C dose 1.9, n = 6;
Group C dose 1.9, n = 6;  Group C dose 2.3, n = 13; (C)
Group C dose 2.3, n = 13; (C)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group D dose 1.2, n = 5;
Group D dose 1.2, n = 5;  Group D dose 1.5, n = 6;
Group D dose 1.5, n = 6;  Group D dose 1.8, n = 3; (D)
Group D dose 1.8, n = 3; (D)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group E dose 0.6, n = 5;
Group E dose 0.6, n = 5;  Group E dose 0.8, n = 1;
Group E dose 0.8, n = 1;  Group E dose 1.2, n = 2.
Group E dose 1.2, n = 2.
Figure 2.
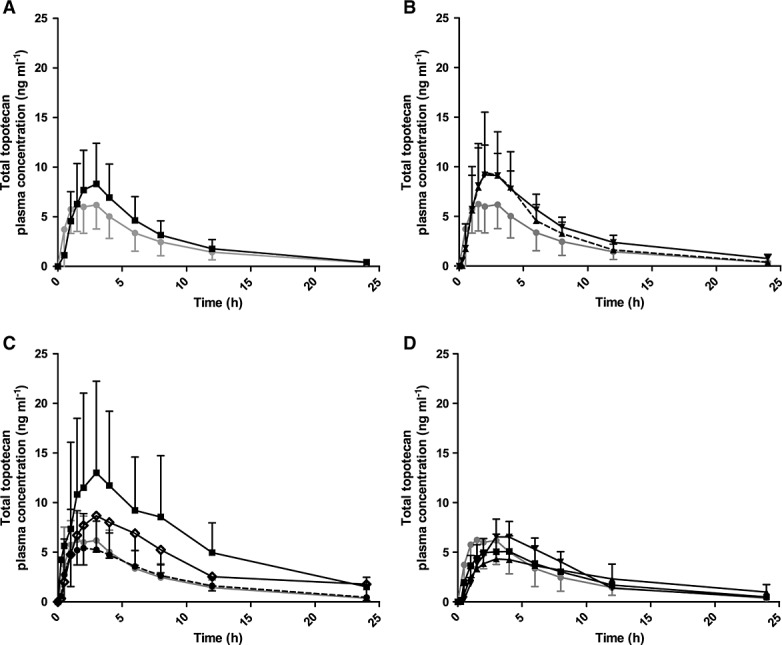
Mean (± SD) plasma concentration (ng ml–1)–time curves of total topotecan following oral administration. Groups A–E represent different groups with normal renal function, with limited previous platinum-based chemotherapy (group A) and with platinum-based chemotherapy (group B, Figure1A), or impaired renal function (group C: mild, group D: moderate and group E: severe, Figure1 B–D). Group A is depicted in all graphs as reference (normal renal function). Oral topotecan doses per group are indicated in mg m−2 day–1. n, number of patients. (A)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group B dose 2.3, n = 12, (B)
Group B dose 2.3, n = 12, (B)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group C dose 1.9, n = 6;
Group C dose 1.9, n = 6;  Group C dose 2.3, n = 13; (C)
Group C dose 2.3, n = 13; (C)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group D dose 1.2, n = 5;
Group D dose 1.2, n = 5;  Group D dose 1.5, n = 6;
Group D dose 1.5, n = 6;  Group D dose 1.8, n = 3; (D)
Group D dose 1.8, n = 3; (D)  Group A dose 2.3, n = 6;
Group A dose 2.3, n = 6;  Group E dose 0.6, n = 5;
Group E dose 0.6, n = 5;  Group E dose 0.8, n = 1;
Group E dose 0.8, n = 1;  Group E dose 1.2, n = 2.
Group E dose 1.2, n = 2.
Detailed pharmacokinetic data are summarized in Tables3 and 4. Generally, mean topotecan lactone and total topotecan exposure increased as renal function decreased. There was large inter-subject variability (CV ranged from ranged from 43% to 111% (Cmax) and 31% to 121% (AUC(0,∞)). Prior PB based chemotherapy did not appear to have an effect on topotecan lactone or total topotecan pharmacokinetic parameters.
Table 3.
Dose-normalized pharmacokinetic parameters of total topotecan and topotecan lactone, after oral administration of topotecan (groups A–E)
| Treatment group | |||||
|---|---|---|---|---|---|
| Group | A | B | C | D | E |
| n | 6 | 12 | 19 | 14 | 8 |
| AUC(0,∞) (ng ml–1 m–2 per unit of dose) | |||||
| Topotecan lactone | 6.1 (3.8, 9.9) | 7.5 (5.0, 11.4) | 9.1 (7.7, 10.8)† | 13.9 (10.7, 18.1) | 17.4 (9.1, 32.9) |
| Total topotecan | 21.4 (12.1, 38.1) | 26.4 (19.5, 35.8) | 36.7 (32.7, 41.2)† | 56.6 (41.7, 76.6) | 87.7 (56.0, 137) |
| AUC(0,12 h) (ng ml–1 h m–2 per unit of dose) | |||||
| Topotecan lactone | 5.2 (3.2, 8.5) | 6.5 (4.5, 9.6) | 7.4 (6.2, 9.0) | 10.1 (7.5, 13.4) | 11.7 (7.0, 19.3) |
| Total topotecan | 16.5 (9.5, 28.6) | 20.5 (15.1, 27.7) | 27.4 (24.4, 30.7) | 38.7 (28.6, 52.3) | 51.6 (35.8, 74.6) |
| Cmax (ng ml–1 m–2 per unit of dose) | |||||
| Topotecan lactone | 1.5 (0.9, 2.5) | 1.6 (1.1, 2.3) | 1.9 (1.5, 2.4) | 2.3 (1.7, 3.2) | 2.2 (1.4, 3.5) |
| Total topotecan | 2.7 (1.7, 4.3) | 3.4 (2.5, 4.6) | 4.5 (3.8, 5.4) | 5.2 (3.9, 7.1) | 6.4 (4.5, 9.1) |
All data are shown as geometric mean (95% confidence interval). Doses are normalized to 1 mg m–2 day–1, actual values were not normalized for different doses. † one patient was excluded because of invalid estimation of AUC(0,∞). Abbreviations: AUC(0,∞), area under the concentration–time curve from zero (pre-dose) extrapolated to infinity; AUC(0,12 h), area under the concentration–time curve from zero (pre-dose) to 12 h; Cmax, maximum observed plasma concentration.
Pharmacokinetic parameters of total topotecan and topotecan lactone after oral administration of topotecan (per dose level within each group; actual values)
| Treatment group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | A | B | C | C | D | D | D | E | E | E |
| Dose (mg m–2 day–1) | 2.3 | 2.3 | 1.9 | 2.3 | 1.2 | 1.5 | 1.8 | 0.6 | 0.8 | 1.2 |
| n | 6 | 12 | 6 | 13 | 5 | 6 | 3 | 5 | 1 | 2†† |
| AUC(0,∞) (ng ml–1 h)) | ||||||||||
| Topotecan lactone | 14.1 (8.7, 22.7) | 17.3 (11.4, 26.1) | 19.3 (12.1, 30.6) | 19.9 (16.4, 24.1)* | 14.8 (9.8, 22.3) | 20.4 (12.9, 32.5) | 32.0 (5.2, 196) | 12.4 (3.8, 40.5) | 10.4 | (12.8, 18.7) |
| Total topotecan | 49.3 (27.8, 87.5) | 60.7 (44.8, 82.3) | 66.9 (47.3, 95.0) | 86.1 (76.7, 96.7)* | 50.8 (27.2, 94.7) | 92.9 (56.8, 152) | 137 (29.8, 630) | 60.8 (26.9, 138) | 57.9 | (77.6, 83.7) |
| AUC(0,12 h) (ng ml–1 h) | ||||||||||
| Topotecan lactone | 11.9 (7.3, 19.5) | 15.0 (10.3, 22.0) | 17.4 (11.0, 27.5) | 15.5 (12.7, 18.8) | 10.95 (6.8, 17.5) | 15.1 (9.5, 23.9) | 21.6 (2.4, 196) | 7.9 (3.3, 19.1) | 8.6 | (7.4, 15.7) |
| Total topotecan | 37.9 (21.9, 65.8) | 47.0 (34.8, 63.6) | 54.1 (37.9, 77.2) | 61.8 (55.5, 68.8) | 36.4 (18.8, 70.3) | 62.6 (38.8, 101) | 89.5 (17.4, 462) | 33.6 (17.1, 66.2) | 41.4 | (43.6, 58.6) |
| Cmax (ng ml–1) | ||||||||||
| Topotecan lactone | 3.4 (2.0, 5.7) | 3.6 (2.5, 5.3) | 4.5 (2.4, 8.6) | 3.8 (3.0, 4.9) | 2.6 (1.2, 5.5) | 3.6 (2.3, 5.8) | 4.2 (0.5, 38.9) | 1.5 (0.7, 3.4) | 1.7 | (1.5, 2.8) |
| Total topotecan | 6.1 (3.8, 9.8) | 7.9 (5.8, 10.7) | 10.0 (6.2, 16.0) | 9.7 (8.1, 11.6) | 5.2 (2.5, 10.7) | 8.3 (5.2, 13.5) | 11.3 (2.2, 57.6) | 4.1 (2.1, 7.8) | 5.1 | (5.4, 7.8) |
| tmax (h) | ||||||||||
| Topotecan lactone | 0.99 (0.55–1.55) | 1.51 (1.00–3.00) | 1.76 (1.00–3.00) | 1.02 (0.25–3.02) | 1.00 (0.98–4.00) | 1.25 (0.52–2.00) | 1.50 (0.57–1.50) | 1.03 (1.00–3.12) | 1.00 | (1.48–2.97) |
| Total topotecan | 2.45 (1.00–3.05) | 3.00 (1.50–3.03) | 2.51 (1.05–3.00) | 2.97 (1.48–4.00) | 1.50 (0.98–6.07) | 2.98 (0.52–3.00) | 3.00 (1.50–3.00) | 3.00 (1.50–3.97) | 4.02 | (2.97–3.93) |
| t1/2 (h) | ||||||||||
| Topotecan lactone | 4.8 (3.1, 7.3) | 4.0 (3.1, 5.1) | 3.6 (2.8, 4.7) | 5.5 (3.9, 7.8)* | 6.5 (3.5, 12.0) | 6.7 (4.4, 10.2) | 7.3 (1.5, 34.8) | 8.2 (4.3, 15.8) | 4.9 | (3.8, 8.3) |
| Total topotecan | 6.0 (5.1, 7.0) | 5.6 (5.0, 6.3) | 5.2 (4.2, 6.4) | 6.6 (5.4, 7.9)* | 6.1 (4.6, 8.2) | 6.7 (4.9, 9.2) | 6.6 (2.4, 18.0) | 9.1 (6.4, 12.9) | 6.2 | (5.9, 9.0) |
| Ae | ||||||||||
| Total topotecan | 0.511 (0.326, 0.802) | 0.341 (0.218, 0.533) | 0.379 (0.192, 0.750) | 0.442 (0.327, 0.596) | 0.243 (0.211, 0.280) | 0.346 (0.160, 0.746) | 0.456 (0.046-4.52) | 0.094 (0.035, 0.247 | 0.165 | 0.134 |
| % excreted | ||||||||||
| Total topotecan | 22.2 (14.2, 34.9) | 14.8 (9.5, 25.1) | 20.0 (10.1, 39.5) | 19.2 (14.2, 25.9) | 20.2 (17.5, 23.4) | 23.0 (20.7, 49.8) | 17.1 (6.35, 46.0) | 15.6 (5.9, 41.1) | 20.6 | 11.1 |
| CLr | ||||||||||
| Total topotecan | 184 (123, 276) | 99.1 (66.1, 149) | 98.9 (46.6, 210) | 94.8 (69.9, 129) | 86.7 (48.0, 157) | 69.3 (45.6, 105) | 62.6 (29.9, 131) | 15.6 (5.9, 41.1) | 51.7 | 34.5 |
All data are shown as geometric mean (95% confidence interval), except tmax which is median (range).
one patient was excluded because of invalid estimation of AUC(0,∞).
(range). Abbreviations: AUC(0,∞), area under the concentration–time curve from zero (pre-dose) extrapolated to infinity; AUC(0,12 h), area under the concentration–time curve from zero (pre-dose) to 12 h; Cmax, maximum observed plasma concentration; tmax, time to maximum observed plasma concentration; t1/2, terminal half-life; Ae, amount excreted; % excreted, fraction excreted; CLr, renal clearance.
Mean dose-normalized AUC(0,∞), AUC(0,12 h) and Cmax increased with increasing renal impairment (from group B through E) compared with reference (Table3). This effect was larger for total topotecan than for topotecan lactone. Mean actual AUC(0,∞), AUC(0,12 h) and Cmax values (Table4) showed large differences which can partly be attributed to the different doses used. Dose escalation within groups C–E appeared to increase actual mean AUC(0,∞), AUC(0,12 h) and Cmax for both topotecan lactone and total topotecan accordingly. Group D (dosed at 1.8 mg m−2 day−1) exhibited by far the highest values for these parameters due to the fact that one patient had topotecan lactone and total topotecan AUC(0,∞) values three- to two-fold higher, respectively, than AUC(0,∞) values obtained in the other two patients in this group whose AUC(0,∞) values were within range seen in group A. Median tmax for topotecan lactone ranged from 1.0–1.8 h. Median tmax ranged from 1.5–4.0 h for total topotecan, being slightly later than topotecan lactone, and was similar across all groups except for the only patient in group E (dosed at 0.8 mg m−2 day−1). Mean t1/2 ranged from 3.6–8.2 h for topotecan lactone and from 5.2–9.1 h for total topotecan. Mean topotecan lactone t1/2 values appeared to be increased in the moderately and severely impaired renal function groups (D and E) by approximately 50% compared with the groups with normal or mildly impaired renal function groups and the group with prior PB chemotherapy (A through C).
In order to allow better comparison of dose-normalized AUC(0,∞) and Cmax across cohorts with different degrees of renal impairment, ratios of geometric means (90% CI) were calculated for topotecan lactone and total topotecan and are shown in Table5. Test groups were compared with reference (normal renal function, group A) in anova analysis. Topotecan lactone and total topotecan AUCs were significantly increased in patients with moderate and severe renal impairment (109% and 174%, respectively, topotecan lactone and 148% and 298%, respectively, total topotecan, Table5).
Statistical analysis of the primary pharmacokinetic parameters
| Geometric mean | |||||
|---|---|---|---|---|---|
| Parameter | Impaired | Normal renal function | Comparison (Group names) | Ratio of means (impaired: normal function) | 90% confidence interval |
| AUC(0,∞) (ng ml−1 mg−1 m−2); per unit of dose)* | |||||
| Topotecan lactone | 6.33 | 5.90 | B: A | 1.07 | (0.70−1.64) |
| Total topotecan | 24.30 | 21.55 | B: A | 1.13 | (0.78−1.64) |
| Topotecan lactone | 8.03 | 5.90 | C: A | 1.36 | (0.92−2.02) |
| Total topotecan | 34.77 | 21.55 | C: A | 1.61 | (1.14−2.28) |
| Topotecan lactone | 12.33 | 5.90 | D: A | 2.09 | (1.38−3.16) |
| Total topotecan | 53.37 | 21.55 | D: A | 2.48 | (1.72−3.56) |
| Topotecan lactone | 16.13 | 5.90 | E: A | 2.74 | (1.72−4.36) |
| Total topotecan | 85.86 | 21.55 | E: A | 3.98 | (2.64−6.01) |
| Cmax (ng ml−1 mg−1 m−2; per unit of dose)* | |||||
| Topotecan lactone | 1.56 | 1.59 | B: A | 0.98 | (0.63−1.53) |
| Total topotecan | 3.54 | 2.99 | B: A | 1.18 | (0.82−1.70) |
| Topotecan lactone | 1.91 | 1.59 | C: A | 1.20 | (0.79−1.81) |
| Total topotecan | 4.83 | 2.99 | C: A | 1.61 | (1.15−2.26) |
| Topotecan lactone | 2.23 | 1.59 | D: A | 1.41 | (0.91−2.17) |
| Total topotecan | 5.34 | 2.99 | D: A | 1.79 | (1.25−2.55) |
| Topotecan lactone | 2.46 | 1.59 | E: A | 1.55 | (0.95−2.53) |
| Total topotecan | 7.06 | 2.99 | E: A | 2.36 | (1.58−3.53) |
Doses are normalized to 1 mg m−2 day−1. Abbreviations: AUC(0,∞), Area under the concentration-time curve from zero (pre-dose) extrapolated to infinity; Cmax, maximum observed plasma concentration.
A review of the data suggested a difference may exist between Asian and non-Asian patients. To evaluate this, an additional post hoc analysis was performed to compare the geometric means (90% CI) for dose-normalized AUC(0,∞) and Cmax between Asians (test) and non-Asians (reference) in each group, except group A which only had one Asian patient (Table6). Increased dose-normalized AUC(0,∞) ratios greater than or equal to 60% were seen in Asian patients in groups D and E for both topotecan lactone and total topotecan.
Statistical analysis of the primary pharmacokinetic parameters by non-Asian and Asian ethnicity
| Geometric mean | |||||
|---|---|---|---|---|---|
| Parameter | Impaired | Normal renal function | Comparison (Group names) | Ratio of means (impaired: normal function) | 90% confidence interval |
| AUC(0,∞) (ng ml–1 mg–1 m–2 per unit of dose)* | |||||
| Topotecan lactone | 8.69 | 6.49 | B nA: A | 1.34 | (0.67, 2.68) |
| Total topotecan | 30.45 | 22.90 | B nA: A | 1.33 | (0.81, 2.19) |
| Topotecan lactone | 11.03 | 8.07 | C nA: A | 1.37 | (1.04, 1.79) |
| Total topotecan | 36.86 | 36.59 | C nA: A | 1.01 | (0.82, 1.24) |
| Topotecan lactone | 18.17 | 11.40 | D nA: A | 1.59 | (1.08, 2.68) |
| Total topotecan | 73.86 | 46.25 | D nA: A | 1.60 | (1.00, 2.55) |
| Topotecan lactone | 28.56 | 12.87 | E nA: A | 2.22 | (0.83, 5.96) |
| Total topotecan | 124.72 | 70.96 | E nA: A | 1.76 | (0.88, 3.51) |
| Cmax (ng ml–1 mg–1 m–2 per unit of dose)* | |||||
| Topotecan lactone | 1.68 | 1.48 | B nA: A | 1.14 | (0.60, 2.18) |
| Total topotecan | 3.77 | 3.11 | B nA: A | 1.21 | (0.72, 2.03) |
| Topotecan lactone | 2.41 | 1.60 | C nA: A | 1.50 | (1.02, 2.20) |
| Total topotecan | 5.40 | 4.06 | C nA: A | 1.33 | (1.00, 1.76) |
| Topotecan lactone | 3.05 | 1.87 | D nA: A | 1.63 | (0.98, 2.71) |
| Total topotecan | 6.85 | 4.27 | D nA: A | 1.61 | (1.04, 2.57) |
| Topotecan lactone | 2.77 | 1.98 | E nA: A | 1.40 | (0.63, 3.10) |
| Total topotecan | 7.85 | 5.64 | E nA: A | 1.39 | (0.78, 2.50) |
Doses are normalized to 1 mg m–2 day–1. Abbreviations: AUC(0,∞), area under the concentration–time curve from zero (pre-dose) extrapolated to infinity; nA: A, non-Asian vs. Asian; Cmax, maximum observed plasma concentration.
Pharmacokinetic data for total topotecan in urine were available from 57 patients. In group E dose levels 0.8 or 1.2 mg m−2 day−1, urine data were only available for a single patient. Mean measured CLr (95% CI) ranged from 29.5 (12.9, 67.3) in group E to 184 (123, 276) ml min−1 in group A. The topotecan fe (%) and the urinary excretion rate (mg h−1) were calculated. Mean fe (95% CI) was comparable between all groups ranging from 14.8 (9.5, 25.1) to 25.3 (2.6, 251), with the exception of 11.1 in the single patient in group E (1.2 mg m−2 day−1). Mean urinary excretion rate (95% CI) ranged between 65.1 (24.8, 171) and 355 (226, 557) ng min−1 across treatment groups and doses, having the lowest in group E (dose 0.6 mg m−2 day−1) and highest in group A.
In an exploratory analysis, relationships between plasma pharmacokinetics of topotecan and clearance were explored. The strongest linear relationship was between estimated glomerular filtration rate (eGFR) and log-transformed dose-normalized AUC for total topotecan (r2 = 0.46, P <0.0001). For topotecan lactone, the relationship was r2 = 0.21, P <0.0001. The relationship between topotecan lactone CL/F and eGFR was r2 = 0.16, P = 0.046.
Additionally, the relationship between DLTs and topotecan lactone exposure was explored. There was no consistent relationship. In general, dose-normalized Cmax and AUC(0,∞) values were near to or less than the mean value for the renal function group.
Pharmacogenetic analysis
In total, 53 patients provided specific informed consent from which 50 DNA samples were obtained. One sample failed genotyping due to low DNA quality. The remaining 49 patients were analyzed as the pharmacogenetic population. No statistically significant association was seen with a functionally modifying variant in ABCG2 Q141K 35. In addition, haplotype analyses in ABCB1 and ABCG2 did not identify any significant associations.
Discussion
In this study, the effect of renal impairment and prior PB chemotherapy was determined on the toxicity profile and pharmacokinetics of oral topotecan in patients with advanced solid tumours. The most frequently occurring AEs and DLTs were neutropenia and thrombocytopenia, which were also observed as the most common DLTs in patients with impaired renal function treated with i.v. topotecan 27. These are commonly known side effects of i.v. or oral formulations of topotecan as observed in phase I–III trials [3, 17–20]3,17–20. The number of patients reporting AEs including AEs grade ≥ 3 tended to occur more frequently in the patients who received previous PB chemotherapy (group B) and patient cohorts with renal impairment (groups C–E) compared with the study cohort with normal renal function (group A). In total, 11 out of 13 (85%) DLTs were haematological toxicities and the importance of this type of toxicity was underscored by one death as a result of neutropenic sepsis. The only non-haematological DLT was grade 3 fatigue, which occurred once in a patient with prior PB chemotherapy and once in a patient with mild renal impairment. The most frequent non-haematologic toxicities were fatigue, nausea and vomiting, common side effects that are well known from experience in previous phase I–III trials of oral topotecan [3, 17–20]3,17–20.
It was hypothesized that prior cisplatin or carboplatin therapy could lead to permanent proximal renal tubular dysfunction without affecting CLcr and that this could compromise the renal secretion of topotecan. This, in turn, could lead to increased systemic drug exposure and increased toxicity. O’Reilly et al. had observed that DLTs were more frequently reported in extensively pre-treated patients when treated with i.v. topotecan and that further dose reductions were required 27. In the present study, no increase in topotecan lactone and total topotecan AUC(0,∞) and Cmax was observed in the ratios of geometric means for PB chemotherapy patients with normal renal function comparing group B: group A (Table5). Although the incidence of AEs was higher in patients who had received prior PB chemotherapy compared with patients who had received limited PB chemotherapy (Table2), the MTD of oral topotecan was the same in both groups (2.3 mg m−2 day−1, days 1–5 of a 21 day cycle). These results showed that there was no clinically relevant difference in systemic topotecan exposure (AUC) that required a reduced dose for patients with prior exposure to PB chemotherapy.
There was a poor correlation between oral topotecan clearance and eGFR. This finding was probably due to low and variable bioavailability of oral topotecan compared with that of the intravenous formulation [6, 26]6,26. Cross study analysis in patients receiving oral topotecan (n= 217, of whom 99 (46%) with CLcr < 80 ml min−1) revealed that <20% of variability in topotecan exposure was due to CLcr. Other factors affecting inter-individual oral bioavailability may include variations in the rate of topotecan passage through the gastrointestinal epithelium and pre-systemic drug metabolism in the gut and liver 36. Other endogenous variables may also affect the exposure of oral topotecan, such as ethnicity and/or diet. Although this study was under-powered to draw definite conclusions, further analysis showed that oral topotecan exposure was higher in Asian patients compared with non-Asian patients and the difference increased as renal function decreased (Table6).
A possible explanation for the observed differences in exposure between ethnicities could have been the presence of an ABCG2 Q141K polymorphism in Asian patients. This polymorphism has been reported to lead to increased oral bioavailability of topotecan compared with patients with wild-type alleles 35.The frequency of this polymorphism was estimated as much higher in Japanese, Koreans and Chinese (26–35%) compared with Caucasians (11%) 37. Unfortunately, only two of the four Asian subjects who were considered outliers for pharmacokinetic parameters had genetic data.
There was an increase in exposure as renal function decreased in this study, but this difference and the magnitude of this difference was confounded by results of group A and ethnicity. Mean AUC(0,∞) for group A (n = 6) was lower in this study than in previous studies (n = 119), 14.1 vs. 23.9 ng ml−1 h for topotecan lactone and 49.3 vs. 67.5 ng ml−1 h for total topotecan 38. Ethnicity also confounded the findings as exposure was higher in Asians vs. non-Asians and this difference increased as renal function decreased.
The MTD for moderate renal impairment patients (group D) was 1.2 mg m−2 day−1, but three of the four patients in whom DLTs occurred were Asians and they had the highest AUCs in this group. Due to this a dose of 1.2 mg m−2 day−1 may result in lower exposures in non-Asian patients and may decrease the patient’s probability of clinical therapeutic benefit. This observation was further investigated in eight other studies with oral topotecan and patients with normal and impaired renal function (n = 276). Indeed, a dose of 1.9 mg m−2 day−1 in non-Asian patients with moderate renal impairment would result in similar exposures to those seen in patients with normal and mild renal impairment given a dose 2.3 mg m−2 day−1. In addition, exposure was approximately 30% less when non-Asians vs. all patients were compared with patients with normal renal function.
Population pharmacokinetic/pharmacodynamic models have been reported that relate pharmacokinetics to probability of experiencing severe toxicity (neutropenia) after i.v. administration of topotecan 39. Inter-patient variability in clearance could be reduced by taking individual characteristics into account, such as performance status and body weight resulting in better prediction of toxicity. Such models have been described for predicting pharmacokinetics following different doses of oral topotecan in individual patients 40. These tools may become valuable in translating the results of the present study into relevant recommendations for the appropriate dose for an individual patient, based on their characteristics such as measured CLcr.
In conclusion, side effects of exposure to oral topotecan were predominantly haematological. AEs appeared to occur more frequently in the renally impaired groups than in the normal renal function group. Prior PB chemotherapy did not appear to have a significant effect on topotecan pharmacokinetics. No dose adjustments of oral topotecan are required in patients with normal renal function and prior PB chemotherapy or mildly impaired renal function (CLcr > 50 ml min−1). While the oral topotecan MTD for moderate renal impairment was 1.2 mg m−2 day−1, this finding was driven by Asian patients with higher systemic topotecan concentrations. A recommended dose of 1.9 mg m−2 day−1 in non-Asian patients would give AUC(0,∞) values similar to those seen in patients with normal renal function and mild renal impairment. Due to incomplete enrolment of patients with severe renal impairment the MTD could not be determined precisely for this patient group.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, D.A.S. and M.M.D. were employed and owned stocks from GSK in the previous 3 years, the institutes of L.D.L. and Y.J.B. received grants from GSK, Y.J.B. received consultancy fees from GSK, H.C.C. received consultancy fees from Lilly, Merck and Taiho and there are no other relationships or activities that could appear to have influenced the submitted work.
This study was funded by the GlaxoSmithKline Group company.
Author Contributions
L.A.D.: Wrote manuscript, performed research, analyzed data; M.M-R: Performed research, P.O.W., D.A.S, L.D.L., D.S.M., Y.J.B., H.C.C., A.D.H, J.H.B, E.E.V, J.H.S, Designed research, wrote manuscript, performed research, analyzed data; M.M.D, Designed research, performed research.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Supporting info item
Supporting info item
Supporting info item
References
- Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–6. [PubMed] [Google Scholar]
- Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- Gerrits CJ, Burris H, Schellens JH, Eckardt JR, Planting AS, van der Burg ME, Rodriguez GI, Loos WJ, van Beurden V, Hudson I, Fields S, Von Hoff DD, Verweij J. Oral topotecan given once or twice daily for ten days: a phase I pharmacology study in adult patients with solid tumors. Clin Cancer Res. 1998;4:1153–8. [PubMed] [Google Scholar]
- Creemers GJ, Gerrits CJ, Eckardt JR, Schellens JH, Burris HA, Planting AS, Rodriguez GI, Loos WJ, Hudson I, Broom C, Verweij J, Von Hoff DD. Phase I and pharmacologic study of oral topotecan administered twice daily for 21 days to adult patients with solid tumors. J Clin Oncol. 1997;15:1087–93. doi: 10.1200/JCO.1997.15.3.1087. [DOI] [PubMed] [Google Scholar]
- Gerrits CJ, Schellens JH, Burris H, Eckardt JR, Planting AS, van der Burg ME, Rodriguez GI, Loos WJ, van Beurden V, Hudson I, Von Hoff DD, Verweij J. A comparison of clinical pharmacodynamics of different administration schedules of oral topotecan (Hycamtin) Clin Cancer Res. 1999;5:69–75. [PubMed] [Google Scholar]
- Schellens JH, Creemers GJ, Beijnen JH, Rosing H, Boer-Dennert M, McDonald M, Davies B, Verweij J. Bioavailability and pharmacokinetics of oral topotecan: a new topoisomerase I inhibitor. Br J Cancer. 1996;73:1268–71. doi: 10.1038/bjc.1996.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herben VM, Rosing H, Bokkel Huinink WW, van Zomeren DM, Batchelor D, Doyle E, Beusenberg FD, Beijnen JH, Schellens JH. Oral topotecan: bioavailability and effect of food co-administration. Br J Cancer. 1999;80:1380–6. doi: 10.1038/sj.bjc.6690532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PG, Markman M, Bell JG, Fusco NL. Sequential prolonged oral topotecan and prolonged oral etoposide as second-line therapy in ovarian or peritoneal carcinoma: a phase I Gynecologic Oncology Group study. Gynecol Oncol. 2006;102:236–9. doi: 10.1016/j.ygyno.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Bryce AH, Mattar B, Hillman SL, Adjei AA, Kugler JW, Rowland K, Jr, Wender DB, Soori G, Perez EA, Jett JR. Phase II trial of oral topotecan and intravenous carboplatin with G-CSF support in previously untreated patients with extensive stage small cell lung cancer: A North Central Cancer Treatment Group Study. Am J Clin Oncol. 2010;33:353–7. doi: 10.1097/COC.0b013e3181b0c27f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PG, Smrekar M, Haba P, Fusco N, Rodriguez M. A phase I study of oral topotecan and pegylated liposomal doxorubicin (doxil) in platinum-resistant ovarian and peritoneal cancer. Am J Clin Oncol. 2008;31:476–80. doi: 10.1097/COC.0b013e31816a6221. [DOI] [PubMed] [Google Scholar]
- Zarogoulidis K, Mylonaki E, Kakavelas P, Zarogoulidis P, Tsiouda T, Rapti E, Lithoxopoulou H, Zarogoulidou V, Kontakiotis T. Topotecan-carboplatin-etoposide combination as first line treatment in patients with small cell lung cancer. Lung Cancer. 2009;66:226–30. doi: 10.1016/j.lungcan.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Gelderblom AJ, Loos WJ, de Jonge MJ, Sparreboom A, Planting AS, van der Burg ME, Brouwer E, Verheij C, Ouwens L, Hearn S, Verweij J. Phase I and pharmacological study of increased dose oral topotecan in combination with intravenous cisplatin. Ann Oncol. 2000;11:1205–7. doi: 10.1023/a:1008396414915. [DOI] [PubMed] [Google Scholar]
- Gelderblom H, Sparreboom A, de Jonge MJ, Loos WJ, Wilms E, Mantel MA, Hennis B, Camlett I, Verweij J, van der Burg ME. Dose and schedule-finding study of oral topotecan and weekly cisplatin in patients with recurrent ovarian cancer. Br J Cancer. 2001;85:1124–9. doi: 10.1054/bjoc.2001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge MJ, Loos WJ, Gelderblom H, Planting AS, van der Burg ME, Sparreboom A, Brouwer E, van Beurden V, Mantel MA, Doyle E, Hearn S, Ross G, Verweij J. Phase I pharmacologic study of oral topotecan and intravenous cisplatin: sequence-dependent hematologic side effects. J Clin Oncol. 2000;18:2104–15. doi: 10.1200/JCO.2000.18.10.2104. [DOI] [PubMed] [Google Scholar]
- Hanna N, Sweeney C, Fife K, Dropcho S, Seitz DE. Phase I trial of carboplatin and paclitaxel with escalating doses of oral topotecan in patients with solid tumors. Am J Clin Oncol. 2003;26:200–2. doi: 10.1097/00000421-200304000-00021. [DOI] [PubMed] [Google Scholar]
- Spigel DR, Waterhouse DM, Lane S, Legenne P, Bhatt K. Efficacy and safety of oral topotecan and bevacizumab combination as second-line treatment for relapsed small-cell lung cancer: an open-label multicenter single-arm phase II study. Clin Lung Cancer. 2013;14:356–63. doi: 10.1016/j.cllc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- von Pawel J, Gatzemeier U, Pujol JL, Moreau L, Bildat S, Ranson M, Richardson G, Steppert C, Rivière A, Camlett I, Lane S, Ross G. Phase ll comparator study of oral versus intravenous topotecan in patients with chemosensitive small cell lung cancer. J Clin Oncol. 2001;19:1743–9. doi: 10.1200/JCO.2001.19.6.1743. [DOI] [PubMed] [Google Scholar]
- Eckardt JR, von Pawel J, Pujol JL, Papai Z, Quoix E, Ardizzoni A, Poulin R, Preston AJ, Dane G, Ross G. Phase III study of oral compared with intravenous topotecan as second-line therapy in small cell lung cancer. J Clin Oncolt. 2007;25:2086–92. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- O’Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cucevia B, Juhasz G, Thatcher N, Ross GA, Dane GC, Crofts T. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small cell lung cancer. J Clin Oncol. 2006;24:5441–7. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- Gerrits CJ, Burris H, Schellens JH, Planting AS, van den Burg ME, Rodriguez GI, van Beurden V, Loos WJ, Hudson I, Fields S, Verweij J, von Hoff DD. Five days of oral topotecan (Hycamtin), a phase I and pharmacological study in adult patients with solid tumours. Eur J Cancer. 1998;34:1030–5. doi: 10.1016/s0959-8049(97)10173-3. [DOI] [PubMed] [Google Scholar]
- Herben VM, Schoemaker E, Rosing H, van Zomeren DM, Bokkel Huinink WW, Dubbelman R, Hearn S, Schellens JH, Beijnen JH. Urinary and fecal excretion of topotecan in patients with malignant solid tumours. Cancer Chemother Pharmacol. 2002;50:59–64. doi: 10.1007/s00280-002-0454-2. [DOI] [PubMed] [Google Scholar]
- Hertzberg RP, Caranfa MJ, Holden KG, Jakas DR, Gallagher G, Mattern MR, Mong SM, Bartus JO, Johnson RK, Kingsbury WD. Modification of the hydroxy lactone ring of camptothecin: inhibition of mammalian topoisomerase I and biological activity. J Med Chem. 1989;32:715–20. doi: 10.1021/jm00123a038. [DOI] [PubMed] [Google Scholar]
- Underberg WJ, Goossen RM, Smith BR, Beijnen JH. Equilibrium kinetics of the new experimental anti-tumour compound SK&F 104864-A in aqueous solution. J Pharm Biomed Anal. 1990;8:681–3. doi: 10.1016/0731-7085(90)80102-u. [DOI] [PubMed] [Google Scholar]
- Kollmannsberger C, Mrossb K, Jakob A, Kanz L, Bokemeyer C. Topotecan – a novel topoisomerase I inhibitor: pharmacology and clinical experience. Oncology. 1999;56:1–12. doi: 10.1159/000011923. [DOI] [PubMed] [Google Scholar]
- Carmichael J, Ozols RF. Topotecan, an active new antineoplastic agent: review and current status Exp. Opin Invest Drugs. 1997;6:593–608. doi: 10.1517/13543784.6.5.593. [DOI] [PubMed] [Google Scholar]
- Kruijtzer CM, Beijnen JH, Rosing H, Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–50. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- O’Reilly S, Rowinsky EK, Slichenmyer W, Donehower RC, Forastiere AA, Ettinger DS, Chen TL, Sartorius S, Grochow LB. Phase I and pharmacologic study of topotecan in patients with impaired renal function. J Clin Oncol. 1996;14:3062–73. doi: 10.1200/JCO.1996.14.12.3062. [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008;61:903–9. doi: 10.1007/s00280-008-0711-0. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Hanigan MH. Inhibition of gamma-glutamyl transpeptidase or cysteine S-conjugate beta-lyase activity blocks the nephrotoxicity of cisplatin in mice. J Pharmacol Exp Ther. 2002;300:142–8. doi: 10.1124/jpet.300.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- U.S.National Institute of Health. Available at http://www.clinicaltrials.gov (last accessed 3 December 2014)
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program NCI Common Terminology Criteria AE Version 3.0. 2006. (NCI-CTCAE v3.0).
- Rosing H, Doyle E, Davies BE, Beijnen JH. High-performance liquid chromatographic determination of the novel antitumour drug topotecan and topotecan as the total of the lactone plus carboxylate forms, in human plasma. J Chromatogr B Biomed Appl. 1995;668:107–15. doi: 10.1016/0378-4347(95)00054-m. [DOI] [PubMed] [Google Scholar]
- Sparreboom A, Loos WJ, Burger H, Sissung TM, Verweij J, Figg WD, Nooter K, Gelderblom H. Effect of ABCG2 genotype on the oral bioavailability of topotecan. Cancer Biol Ther. 2005;4:650–8. doi: 10.4161/cbt.4.6.1731. [DOI] [PubMed] [Google Scholar]
- Stuurman FE, Nuijen B, Beijnen JH, Schellens JH. Oral anticancer drugs: mechanisms of low bioavailability and strategies for improvement. Clin Pharmacokinet. 2013;52:399–414. doi: 10.1007/s40262-013-0040-2. [DOI] [PubMed] [Google Scholar]
- Kim KA, Joo HJ, Park JY. ABCG2 polymorphisms, 34G>A and 421C>A in a Korean population: analysis and a comprehensive comparison with other populations. J Clin Pharm Ther. 2010;35:705–12. doi: 10.1111/j.1365-2710.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- FDA summary document. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/020981s000_ClinPharmR.pdf (last accessed 3 December 2014)
- Mould DR, Holford NH, Schellens JH, Beijnen JH, Hutson PR, Rosing H, ten Bokkel Huinink WW, Rowinsky EK, Schiller JH, Russo M, Ross G. Population pharmacokinetic and adverse event analysis of topotecan in patients with solid tumors. Clin Pharmacol Ther. 2002;71:334–48. doi: 10.1067/mcp.2002.123553. [DOI] [PubMed] [Google Scholar]
- Leger F, Loos WJ, Fourcade J, Bugat R, Goffinet M, Mathijssen RH, Verweij J, Sparreboom A, Chatelut E. Factors affecting pharmacokinetic variability of oral topotecan: a population analysis. Br J Cancer. 2004;90:343–7. doi: 10.1038/sj.bjc.6601469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item


