Abstract
Aims
Ketamine analgesia is limited by low intrinsic efficacy compounded by large interindividual variability in drug responses, possibly due to the heterogeneity in drug concentration. The CYP2B6*6 allele is associated with substantially reduced ketamine metabolism in vitro and, therefore, may affect ketamine clearance. Our aims were to examine the impact of the CYP2B6*6 allele on ketamine plasma clearance and on adverse effects in chronic pain patients.
Methods
CYP2B6 genotypes were identified in 49 chronic pain patients who received 24 h continuous subcutaneous infusions of ketamine. Steady-state plasma concentrations of ketamine (Css,k) and norketamine (Css,nk) were determined using HPLC.
Results
The median plasma clearance of ketamine after 100 mg 24 h–1 dose was significantly lower in patients with the CYP2B6*6/*6 (21.6 l h–1) and CYP2B6*1/*6 (40.6 l h–1) genotypes compared with patients with the CYP2B6*1/*1 genotype (68.1 l h–1, P < 0.001). The ketamine : norketamine plasma metabolic ratio was significantly higher in patients with the CYP2B6*6/*6 genotype than in those with the CYP2B6*1/*6 and the CYP2B6*1/*1 genotypes (P < 0.001). Patients who experienced adverse effects had lower plasma clearance (45.6 l h-1) than those who did not (52.6 l h-1, P = 0.04). The CYP2B6*6 genotype and age, and their combined impact explained 40%, 30% and 60% of the variation in Css,k, respectively. Similar results were observed after higher doses.
Conclusions
The CYP2B6*6 allele is associated with a substantial decrease in steady-state ketamine plasma clearance in chronic pain patients. The decreased clearance and resultant higher plasma concentrations may be associated with a higher incidence of ketamine adverse effects.
Keywords: adverse effects, age factors, cytochrome P-450 CYP2B6, ketamine, metabolic clearance rate, polymorphism
What is Already Known about this Subject
Ketamine analgesia is limited by large interindividual variability in response and adverse effects, possibly due to drug concentration factors.
Ketamine is mainly metabolized to norketamine by hepatic CYP2B6 and CYP3A4.
CYP2B6*6 allele is associated with reduced ketamine metabolism in vitro and, hence, may affect ketamine clearance.
What this Study Adds
The CYP2B6*6 allele substantially decreased ketamine steady-state plasma clearance and metabolic ratios to norketamine in chronic pain patients.
The decreased clearance may be associated with a higher incidence of ketamine adverse effects.
Introduction
Ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, has been used as an anaesthetic for over 40 years. The drug has also been used at subanaesthetic doses in the management of acute post-operative pain and chronic refractory pain, either solely or in combination with opioids 1–3. Although subanaesthetic ketamine is said to produce some degree of analgesic and opioid-sparing effects in chronic pain patients, the analgesia, both when it is given alone or combined with an opioid, is limited by low efficacy and large interindividual variability in analgesic responses and by adverse effects warranting drug cessation 2–6.
One potential causal factor of this variability is the interindividual differences in ketamine pharmacokinetics (PK). A previous clinical trial reported an up to four-fold variation in plasma concentrations between neuropathic pain patients receiving a single 40 min intravenous infusion of 0.4 mg kg-1 ketamine 4. Ketamine is a high hepatic clearance drug with an estimated high hepatic extraction ratio (0.9) indicating that its clearance is sensitive to changes in hepatic blood flow 7. Additional variability in hepatic clearance may arise from its metabolism which is primarily to norketamine by two cytochrome P450 (CYP) enzymes, CYP2B6 and CYP3A4, whose expression and catalytic activities show large variability in humans 8,9. CYP3A5, if it is expressed, may also be involved in ketamine metabolism, since it has a catalytic specificity similar to CYP3A4 10. Moreover, a previous randomized placebo-controlled trial reported a negative contribution of (S)-norketamine to the analgesic effects of (S)-ketamine on heat pain in healthy volunteers, indicating another potential pharmacokinetic influence of ketamine on analgesia 11. Although large interindividual differences in the clearance of subanaesthetic ketamine were not observed in previous PK studies 12–14, these studies were conducted in young healthy male volunteers, which may not accurately represent clinical populations whose liver function, blood flow and CYP enzyme activities show large variability due the differences in age, disease states and medication use 8. In addition, the population size of these PK studies 12–14 was too small (n ≤ 10) to detect systematically any impact of genetic variations, particularly the CYP2B6*6 allele, the most prevalent loss-of-function allelic variant of the CYP2B6 gene, on ketamine metabolism and potentially on response 15. Our recent in vitro study identified that the intrinsic clearance of ketamine was decreased by up to 89% in human liver microsomes and 55% in cDNA-expressed CYP2B6 protein by the presence of the CYP2B6*6 allele 16. However, the clinical impact of the CYP2B6*6 allele on in vivo ketamine clearance and adverse effects is yet to be determined.
The primary aim of the present study was to examine the impact of the CYP2B6*6 allelic variant on the steady-state plasma clearance of racemic ketamine and plasma concentrations of ketamine and norketamine in patients with chronic opioid-refractory pain. The secondary aim was to investigate the association between adverse effects and the plasma clearance of ketamine.
Methods
Subjects
Two study populations with a total number of 49 patients were included in this study. Characteristics of the 49 patients were as follows: 1) 23 of 49 were male, 2) median age was 64 (range 35–87) years and 3) all were Caucasians except two patients with Sri Lankan ethnic origin. Blood samples were obtained from all patients and clinical profiles (including, when available, information about disease states, pain types, comorbidities, opioid consumption and other concurrent non-opioid medications) were obtained from 45 patients. Medications that may induce or inhibit the function of CYP2B6 or CYP3A4 were identified according to a cytochrome P450 drug interaction table 17.
The first study population consisted of 14 Caucasian subjects (seven males and seven females, median age of 68 [range 45–86] years) who were part of a group of ketamine recipients in a large multicentre Australian-wide double-blinded randomized placebo-controlled trial investigating the clinical benefits of co-analgesic ketamine in cancer pain 6. These 14 subjects agreed to have blood taken for DNA analysis. The eligible criteria and the dosing procedures were previously described 6. The study was registered on the Australian and New Zealand Clinical Trials Registry (ANZCTR with trial ID: ACTRN12607000501448). Ethical approval was obtained from ethics committees at all sites (Human Research Ethics Committees (HREC) including Repatriation General Hospital HREC [reference no. 69/07], Calvary Health Care Sydney HREC [approval no. 2008.12.04], South Western Sydney Area Health Service HREC [reference no. 07/RPAH/193], Mater Health Services HREC [reference no. 1171A], Peter MacCallum Cancer Centre Ethics Committee [reference no. 07/56], and St Vincent’s Hospital HREC [reference no. 07/RPAH/193]). All participants provided written informed consent.
The second study population consisted of 35 chronic opioid-refractory pain patients from an open label study (sixteen males and nineteen females) with a median age of 64 (range 35–87) years. Patients with poor venous access, those who previously had received ketamine and those in whom significant hypertension or tachycardia would be potentially dangerous were excluded. Patients were recruited at the Supportive and Palliative Care Unit and the Pain Unit at Monash Medical Centre, Victoria, Australia. The study was registered on the ANZCTR (ACTRN12613000327785). Ethical approval was obtained from the Southern Health HREC, Victoria (reference no. 12159 L) and the University of Adelaide HREC (reference no. H-2013-002). All participants provided written informed consent.
Ketamine dosing protocol
In both populations, racemic ketamine hydrochloride (Ketalar®) was administered via 24 h continuous subcutaneous infusion (CSCI) with a three level dose-escalation method that has been previously described 18. Briefly, patients received ketamine at an initial dose level of 100 mg 24 h–1. Pain scores (assessed using numerical rating scales) and adverse effects of ketamine (assessed using the Clinician-Administered Dissociative States Scale in the first study and the National Cancer Institute Common toxicity Criteria in both studies) were assessed before, daily (for the first study population) or every 4 h (for the second study population) during the infusion, and at the end of the infusion. The dose was then increased to 300 mg 24 h–1 and finally 500 mg 24 h–1, if the reduction in pain score was < 2 from baseline on the 0 to 10 pain scale with no intolerable adverse effects (requiring cessation of ketamine). Venous whole blood samples were collected at the end of the 24 h infusion. Blood samples (1 to 2 ml) were immediately stored at –20 °C and used for DNA extraction. Plasma samples were separated from the rest of the whole blood samples by centrifugation at 2000 g at room temperature and then stored at –20 °C until the quantification of drug.
DNA extraction and genotyping
Genomic DNA was isolated from whole blood using the Maxwell® 16 instrument with the Maxwell® 16 Blood DNA purification kit (Promega Co. Sydney, NSW, Australia) according to the manufacturer’s protocol. SNPs related to the CYP2B6*4 (c.785A > G, rs2279343), CYP2B6*5 (c.1459C > T, rs3211371), CYP2B6*6 (c.516G > T, rs3745274 and c.785A > G), CYP2B6*7 (c.516G > T, c.785A > G and c.1459C > T), CYP2B6*8 (c.415A > G, rs12721655), CYP2B6*13 (c.415A > G, c.516G > T and c.785A > G) allele and the SNP related to the most common defective allele of CYP3A5, CYP3A5*3 (g.6986A > G, rs776746) were screened by previously described PCR-restriction fragment length polymorphism assays 16,19.
Quantification of ketamine and norketamine plasma concentrations
Plasma concentrations of racemic ketamine and norketamine were quantified by a high performance liquid chromatography assay with u.v. detection. Plasma concentration of each enantiomer of ketamine and norketamine was not examined, since no substantial enantioselective difference in pharmacokinetic parameters (>10%) was reported by relatively recent clinical pharmacokinetic studies 14,20. Quantification of ketamine and norketamine as the free base was performed with standard curves consisting of eight standards that ranged from 20–800 ng ml-1. Ketamine (Sigma-Aldrich, Castle Hill, NSW, Australia) and norketamine (Toronto Research Chemicals, distributed by Sapphire Bioscience Pty. Ltd., Waterloo NSW 2017, Australia) standards were prepared by diluting aqueous stocks (10 µg ml-1) with drug-free human plasma. Plasma sample and standards at a volume of 1 ml were spiked with 150 ng of internal standard (ephedrine, purchased from Faulding Co. Ltd. Torrensville, SA, Australia), and then alkalinized with 500 µl of saturated sodium carbonate before being extracted into 6 ml of 70 : 30 v/v hexane : ether (Chem-supply Pty Ltd., Gillman, SA, Australia). Drugs were then back-extracted into 60 µl of 0.1 m hydrochloric acid after centrifugation at 2000 g at room temperature for 10 min. Aliquots (50 µl) were injected into the HPLC system and analyzed using a previously described method 16. Inter- (n = 6) and intra-assay (n = 6) variabilities for both ketamine and norketamine were determined by the analysis of duplicates of quality control samples (QC) at low (50 ng ml-1), medium (150 ng ml-1) and high (350 ng ml-1) concentrations. The inter- and intra-assay imprecision and inaccuracy of all QC samples were less than 10%. Extraction efficiency was determined by comparing the peak areas of each QC sample and the internal standard from extracted samples with those from non-extracted aqueous solutions. The median (range) extraction efficiency for ketamine, norketamine and the internal standard were 90% (88 to 93%), 88% (86 to 91%) and 81% (79 to 83%), respectively. The lower limit of quantification for both compounds was 10 ng ml-1.
Data analysis
The deviation of genotype frequencies for each variant allele from Hardy–Weinberg equilibrium was tested by the chi-squared test. Plasma clearance of ketamine at steady-state conditions (CLss) was calculated as K0/Css,k, where K0 was the infusion rate and Css,k was the ketamine steady-state plasma concentration. The differences in Css,k, the steady-state norketamine plasma concentration (Css,nk), CLss and plasma ketamine : norketamine metabolic ratio (KET : NK MR) among patients with different CYP2B6*6 genotypes were examined by Jonckheere-Terpstra tests using SPSS Statistics 20 (IBM, Armonk, NY, USA). The differences in CLss and KET : NK MR between three doses were analyzed using Friedman’s test. The difference in age and CLss at 100 mg 24 h–1 dose between patients who experienced adverse effects and those who did not were analyzed using Mann–Whitney tests. The relative impacts of the CYP2B6*6 allele, gender, age and potential inhibitors/inducers of CYP2B6 or CYP3A4 on the variation in ketamine PK were analyzed using linear regressions in R software with the package ‘relaimpo’ 21,22. Due to a fundamental difference in pain assessment tools between the two studies, the influence of ketamine PK on the ketamine analgesic responses was not analyzed 23. Results were considered statistically significant when P < 0.05. Data are presented as median (range) unless specified.
Results
Genotyping detected the CYP2B6*1 (wild-type), CYP2B6*5, CYP2B6*6 and CYP2B6*7 alleles, with allelic frequencies of 71%, 3%, 24% and 1%, respectively. No CYP2B6*4, CYP2B6*8 or CYP2B6*13 variant alleles was detected in any of the participants. The frequencies of these genotypes were in Hardy–Weinberg equilibrium. The CYP3A5*3 allele was identified in all participants, suggesting CYP3A5 was not expressed in these subjects. Thirty-five patients (72%) received the dose-escalation regimen, 25 of whom received the highest 500 mg 24 h–1 dose. The median values of CLss and KET : NK MR were not substantially different between the three doses (Table1, P = 0.13 and 0.31 for CLss and KET : NK MR, respectively).
Table 1.
Ketamine steady-state plasma concentrations (Css,k), ketamine : norketamine metabolic ratio (KET : NK MR) and ketamine steady-state clearance rates (CLss) in patients with the CYP2B6*1/*5, CYP2B6*5/*6 and CYP2B6*6/*7 genotypes in comparison with median (range) values in patients with the CYP2B6*1/*1, CYP2B6*1/*6, CYP2B6*6/*6 genotypes
| CYP2B6 | Css,k (ng ml-1) | KET : NK MR | CLss (l h-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| genotype | 100 mg 24 h–1 | 300 mg 24 h–1 | 500 mg 24 h–1 | 100 mg 24 h–1 | 300 mg 24 h–1 | 500 mg 24 h–1 | 100 mg 24 h–1 | 300 mg 24 h–1 | 500 mg 24 h–1 |
| CYP2B6*1/*1 | 66 | 159 | 275 | 0.9 | 0.9 | 0.8 | 54.5 | 68.1 | 65.8 |
| (30–158) | (121–317) | (174–483) | (0.5–1.8) | (0.6–2.1) | (0.6–1.6) | (22.8–120.9) | (34.2–89.3) | (37.4–103.8) | |
| (n = 23) | (n = 17) | (n = 9) | (n = 23) | (n = 17) | (n = 9) | (n = 23) | (n = 17) | (n = 9) | |
| CYP2B6*1/*6 | 98 | 267 | 394 | 1.5 | 1.6 | 1.7 | 37.8 | 40.6 | 45.9 |
| (51–136) | (141–366) | (283–687) | (0.7–3.7) | (0.8–4.9) | (0.8–3.1) | (26.5–70.8) | (29.6–76.6) | (26.3–63.9) | |
| (n = 15) | (n = 13) | (n = 12) | (n = 15) | (n = 13) | (n = 12) | (n = 15) | (n = 13) | (n = 12) | |
| CYP2B6*6/*6 | 168 | 395 | 598 | 2.5 | 2.5 | 2.6 | 21.6 | 27.4 | 30.3 |
| (154–181) | (298–407) | (570–626) | (2.1–3.1) | (2.1–3.2) | (2.2–2.9) | (19.9–23.5) | (26.6–36.3) | (28.9–31.7) | |
| (n = 3) | (n = 3) | (n = 2) | (n = 3) | (n = 3) | (n = 2) | (n = 3) | (n = 3) | (n = 2) | |
| CYP2B6*1/*5 | 87 | 2.2 | 41.5 | ||||||
| CYP2B6*1/*5 | 69 | 230 | 466 | 1.6 | 1.4 | 1.7 | 52.6 | 47.1 | 38.8 |
| CYP2B6*5/*6 | 71 | 214 | 365 | 1.7 | 1.8 | 1.9 | 50.9 | 50.6 | 49.5 |
| CYP2B6*6/*7 | 172 | 4.6 | 21.0 | ||||||
| Overall | 76 | 214 | 366 | 1.3 | 1.3 | 1.4 | 47.6 | 50.6 | 49.3 |
| (30–181) | (121–407) | (174–687) | (0.5–4.6) | (0.6–4.9) | (0.6–3.1) | (19.9–120.9) | (26.6–89.3) | (26.3–103.8) | |
| (n = 45) | (n = 35) | (n = 25) | (n = 45) | (n = 35) | (n = 25) | (n = 45) | (n = 35) | (n = 25) | |
The steady-state plasma concentration profiles of ketamine in patients with various CYP2B6 genotypes are listed in Table1. At the end of the 100 mg 24 h–1 CSCI of ketamine, the median values of Css,k and KET:NK MR were significantly higher in patients with the CYP2B6*6/*6 genotype than those with the CYP2B6*1/*6 and the CYP2B6*1/*1 genotypes (P < 0.001, Figure1A and 1B). The estimated CLss in patients with the CYP2B6*6/*6 genotype was approximately 40% and 59% of that in patients with the CYP2B6*1/*1 and the CYP2B6*1/*6 genotypes, respectively (P < 0.001, Figure1C). Similar gene-dose effects were observed after the 300 and 500 mg 24 h–1 CSCIs (Figure2). Linear regression analyses showed that the CYP2B6*6 allele explained 40%, 43% and 41% of the variation in Css,k, 30%, 43% and 52% of the variation in CLss and 42%, 39% and 48% of the variation in KET : NK MR after 24 h CSCI of 100, 300 and 500 mg ketamine, respectively (P < 0.001 for all regressions).
Figure 1.
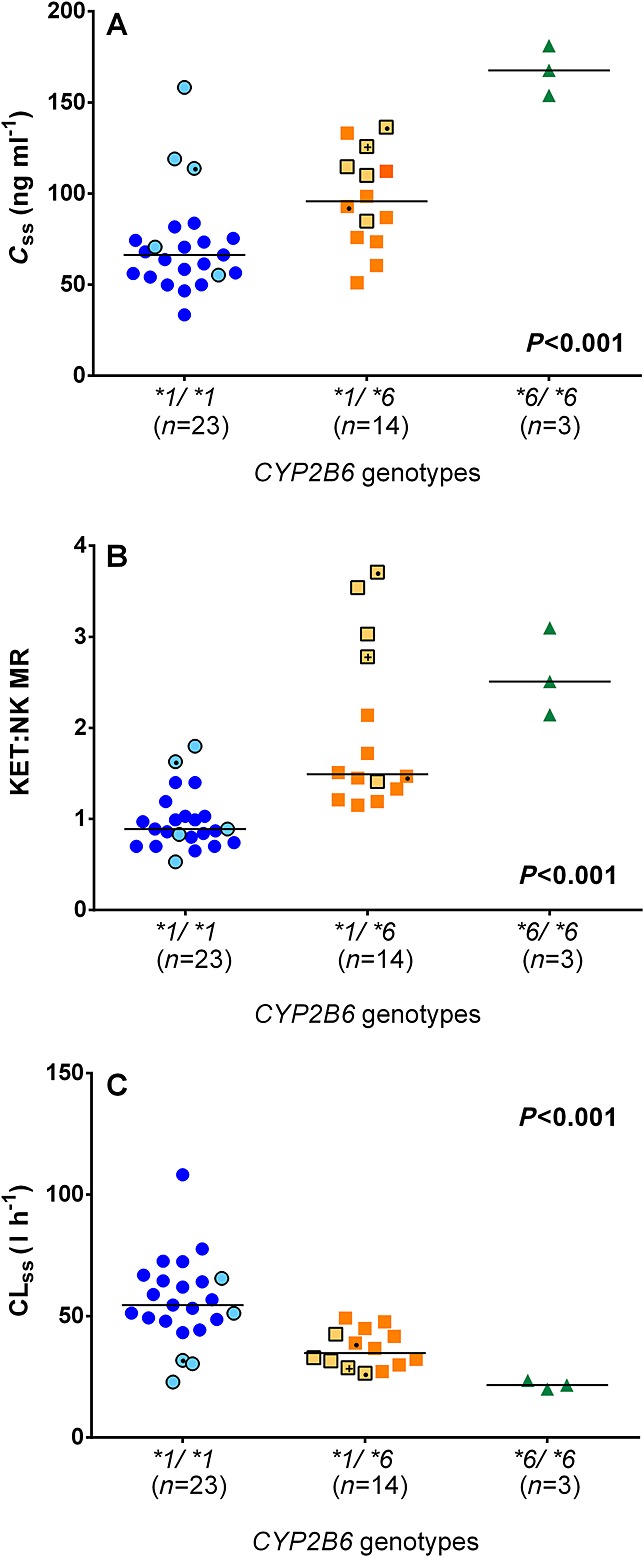
Influence of the CYP2B6*6 allelic variant on A) ketamine steady-state plasma concentration (Css,k), B) ketamine : norketamine metabolic ratio (KET : NK MR) and C) ketamine steady-state plasma clearance (CLss) in chronic pain patients who received a 100 mg 24 h–1 CSCI. Symbols with lighter filled colour and black border represent patients from the first population. Symbols with a dot (●) and plus (+) sign represent patients who received clopidogrel and carbamazepine, respectively. Lines indicate the median values for each genotype group. All P values were obtained from Jonckheere–Terpstra tests
Figure 2.
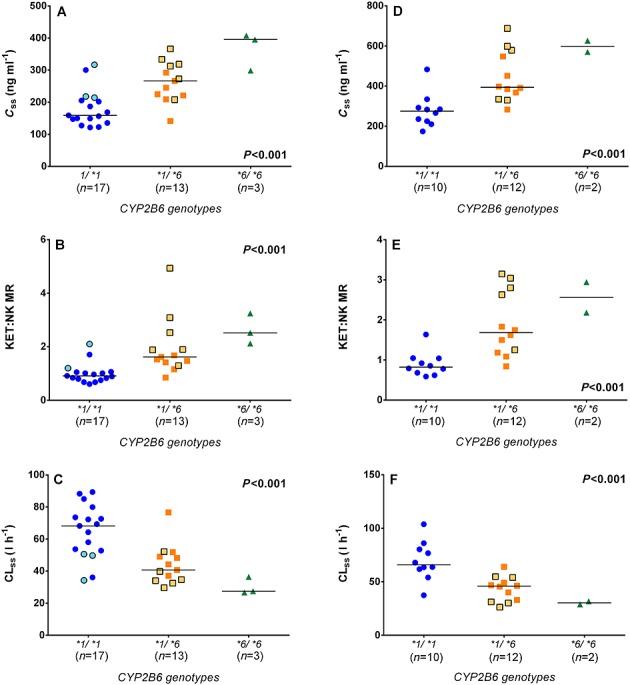
The impact of the CYP2B6*6 allelic variant on A, D) ketamine steady-state plasma concentration (Css,k), B, E) ketamine : norketamine metabolic ratio (KET : NK MR) and C, F) ketamine steady-state plasma clearance (CLss) in chronic pain patients who received a 300 mg 24 h–1 (A, B, C) and 500 mg 24 h–1 (D, E, F) CSCI, respectively. Symbols with lighter filled colour and black border represent patients from the first population. Lines indicate the median values for each genotype group. All P values were obtained from Jonckheere–Terpstra tests
In addition to the CYP2B6*6 genotype, the age of the patients significantly affected ketamine PK at the 100 and 300 mg 24 h–1 doses (Figure3), explaining 28% of Css,k (P = 0.0005), 31% of CLss (P = 0.0002) and 12% of KET : NK MR (P = 0.02) variability at the 100 mg 24 h–1 dose; and 22%, 22% and 11% of Css,k (P = 0.006), CLss (P = 0.006) and KET : NK MR (P = 0.05) variability, respectively, at the 300 mg 24 h–1 dose. Linear two factor regressions showed that together the CYP2B6*6 allele and age explained approximately 62% and 61% of variability in ketamine Css,k after the 100 mg (F(3,37) = 23.9, P < 0.001) and 300 mg 24 h–1 doses (F(3,29) = 54.2, P < 0.001), respectively (Figure4).
Figure 3.
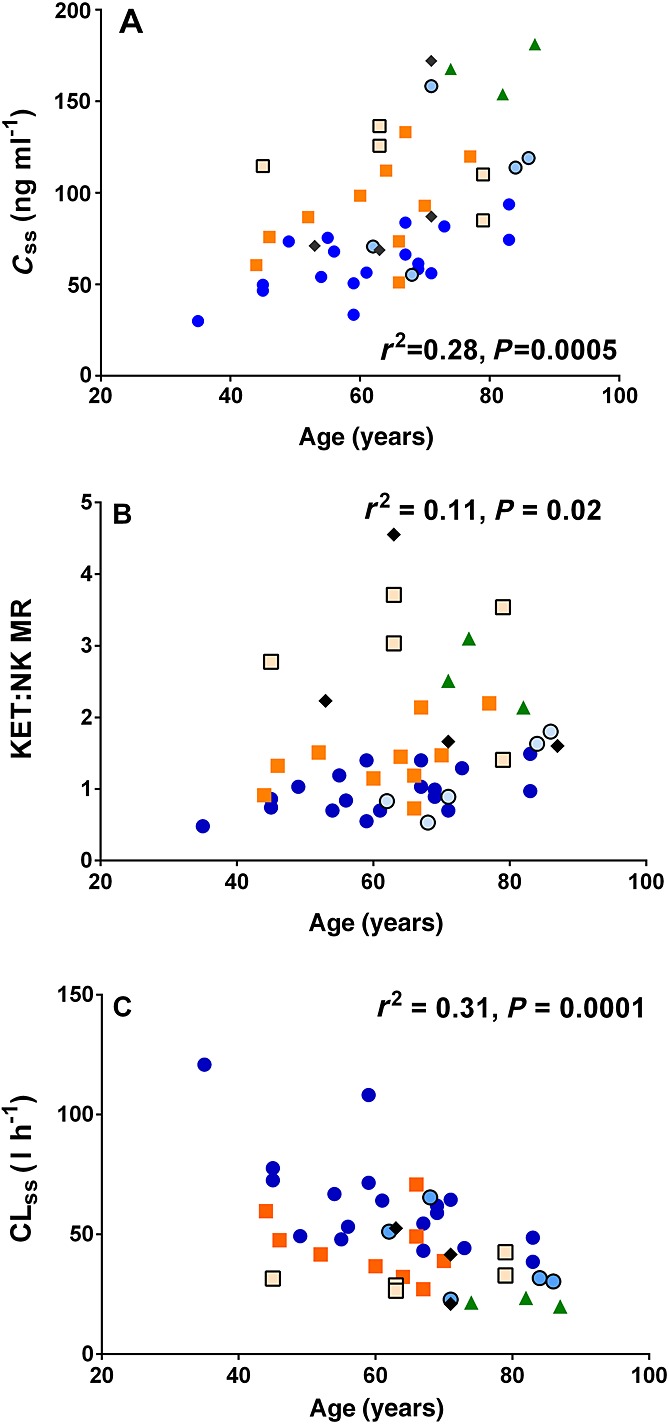
Linear regression between the age of the patients and A) ketamine steady-state plasma concentration (Css,k), B) ketamine : norketamine metabolic ratio (KET : NK MR) and C) ketamine steady-state plasma clearance (CLss) in 45 patients who received a 100 mg 24 h–1 CSCI. Blue circles ( ), orange square (
), orange square ( ), green triangle (
), green triangle ( ) and black rhombus (
) and black rhombus ( ) represent patients with the CYP2B6*1/*1, CYP2B6*1/*6, CYP2B6*6/*6 and other CYP2B6 genotypes, respectively. Symbols with lighter filled colour and black border represent patients from the first population
) represent patients with the CYP2B6*1/*1, CYP2B6*1/*6, CYP2B6*6/*6 and other CYP2B6 genotypes, respectively. Symbols with lighter filled colour and black border represent patients from the first population
Figure 4.
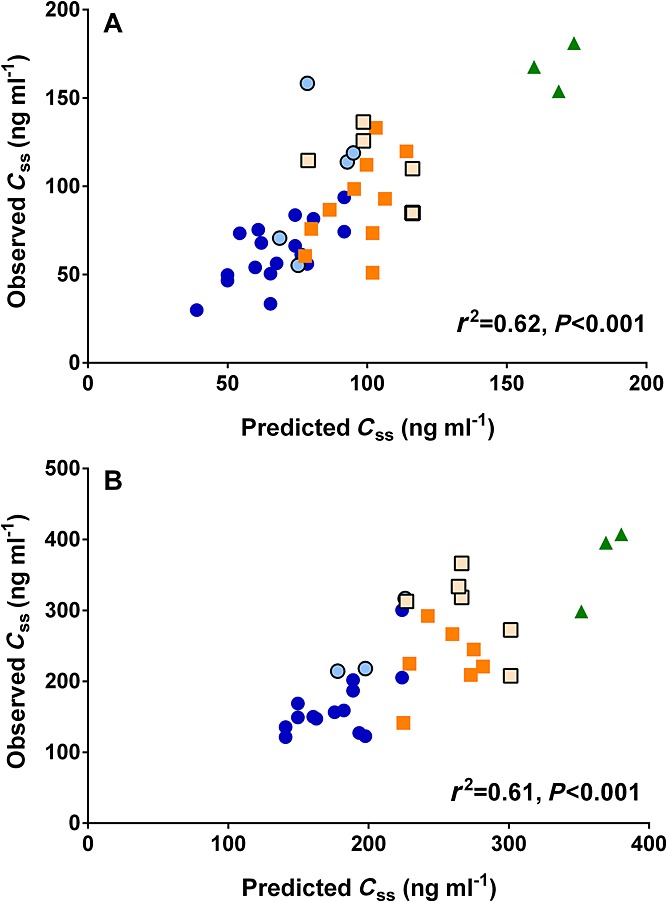
Linear two factor regressions (CYP2B6*6 allele and age) between the model-predicted and observed ketamine steady-state plasma concentrations (Css,k) at A) a 100 mg 24 h–1 and B) 300 mg 24 h–1 doses. Blue circles ( ), orange square (
), orange square ( ) and green triangle (
) and green triangle ( ) represent patients with the CYP2B6*1/*1, CYP2B6*1/*6 and CYP2B6*6/*6 genotypes, respectively. Symbols with lighter filled colour and black border represent patients from the first population
) represent patients with the CYP2B6*1/*1, CYP2B6*1/*6 and CYP2B6*6/*6 genotypes, respectively. Symbols with lighter filled colour and black border represent patients from the first population
No significant gender differences in ketamine plasma concentration were observed (P = 0.16, 0.15 and 0.41 at the 100, 300 and 500 mg 24 h–1 doses, respectively). Three patients received a medication that may inhibit the function of CYP2B6 (clopidogrel) and one patient received an inducer of CYP2B6 and CYP3A4 (carbamazepine). The PK parameters for these patients at the 100 mg 24 h–1 dose are highlighted in Figure1. No patient received any inhibitor of CYP3A4. Neither inclusion of gender nor other drugs or both in the linear two factor (CYP2B6 genotype and age) regressions improved the model in predicting ketamine steady-state plasma concentrations. Exclusion of the data of the two patients of Sri Lankan ethnic origin did not alter the results of data analysis.
Adverse effects of ketamine were reported by 18 patients, including 11 patients with the CYP2B6*1/*1 genotype (which was approximately 42% of all patients with the CYP2B6*1/*1 genotype), three patients with CYP2B6*1/*6 genotype (20% of all CYP2B6*1/*6 carriers), all three patients with the CYP2B6*6/*6 genotype and one patient with the CYP2B6*5/*6 genotype. The adverse response rate was 36% and 42% in the first and second study population, respectively. No significant difference in the median age of subjects between patients who experienced adverse effects (63 [35–83] years) and those who did not (67 [45–87] years) was identified (P = 0.18). Although, when the two study populations were analyzed together, the CLss at the 100 mg 24 h–1 dose in patients who experienced adverse effects of ketamine (45.6 [20.0–77.6] l h–1) was significantly lower than that for those who did not (52.6 [22.8–121] l h–1, P = 0.04), no statistically significant difference in CLss was found in each individual study (CLss in patients who reported adverse effects and those who did not was 30.4 [21.0-32.9] l h–1 and 42.0 [22.8–65.5] l h–1, respectively, for the first population, P = 0.12; and 47.9 [20.0–72.6] and 60.8 [21.6–108.2] l h–1, respectively, for the second population, P = 0.09). Drowsiness was the most common adverse effect, which was reported by approximately 7% of all 45 patients who received 100 mg 24 h–1, 23% of 35 patients who received 300 24 h–1 dose and 12% of 25 patients who received the 500 mg 24 h–1 dose. Hallucination was the second most common adverse effects, which was reported by 13%, 23% and 20% patients who received the 100, 300 and 500 mg 24 h–1 dose, respectively. Other adverse effects, including dizziness and confusion, were reported by less than 10% of patients irrespective of dose.
Discussion
The present study provides the first evidence that the in vivo steady-state metabolic clearance of ketamine to norketamine during CSCI is substantially decreased by the number of CYP2B6*6 variant alleles present and is also affected by increasing age. To the best of our knowledge, this is also the first study reporting the steady-state plasma concentration profiles of ketamine and norketamine following a subcutaneous administration with a dose-escalation regimen.
The estimated median steady-state plasma clearance of ketamine was approximately 50 l h-1, which is expected to be similar to the blood clearance rate, as the blood : plasma ratio of ketamine is approximately 1 24. This clearance rate was relatively lower compared with the estimates of a previous study by Goldberg et al. 20, who reported that the mean clearance of ketamine was approximately 60 l h-1 in 16 patients with complex regional pain syndrome who received a 5 day continuous intravenous infusion of racemic ketamine. The discrepancy is possibly due to the older population in our study (mean ± SD age: 67 ± 12 years) compared with that of Goldberg et al. study (mean ± SD age 33 ± 10 years). Linear regression analyses of our study showed that age explained 22 to 31 % of interindividual variation in clearance rates. The reduction in ketamine clearance in elderly subjects is possibly a consequence of substantial age-related decreases in liver volume, hepatic blood flow, and total P450 content, and possibly the enzymatic function of CYP2B6 and CYP3A4 25–28.
Additionally, the results of linear regression analyses suggested that the CYP2B6*6 allele is likely to be the major factor influencing ketamine steady-state plasma concentration, which by itself explained approximately 40% of the over five-fold interindividual variability. Such an effect of the CYP2B6*6 allele on ketamine plasma concentrations is most likely due to its impact on metabolic clearance to norketamine, as the median CLss in patients carrying the CYP2B6*1/*6 and CYP2B6*6/*6 genotypes was 60%–70% and 40%, respectively, of those in patients with the CYP2B6*1/*1 genotype. According to the equation: CLH = QH × fubCLint/(QH + fubCLint), where CLH is hepatic clearance, QH is hepatic blood flow, fub is unbound fraction of drug in blood and CLint is intrinsic clearance 29. Given that ketamine’s fraction unbound was approximately 53% and the average hepatic blood flow in people aged 60–70 years was approximately 72 l h-1, the estimated intrinsic clearance of ketamine was decreased by at least 64% and 86% in patients carrying the CYP2B6*1/*6 and CYP2B6*6/*6 genotypes, respectively 26,30. Such a finding is almost identical to the result of our recent in vitro study, in which the intrinsic clearance of ketamine to norketamine was decreased by at least 62% and 84% in human liver microsomes with the CYP2B6*1/*6 and CYP2B6*6/*6 genotypes, respectively 16. In addition to the most common CYP2B6*6 allele, the CYP2B6*5 and CYP2B6*7 alleles were also identified in our patients. Although the extremely low genotype frequencies prevent a feasible analysis, it is interesting to note that the ketamine clearance in the patient with the CYP2B6*6/*7 genotype was almost identical to that in patients with the CYP2B6*6/*6 genotype. This may reflect a possible functional impairment in carriers of the CYP2B6*7 allele. The CYP2B6*5 allele may also have a minor effect on ketamine clearance, as the ketamine to norketamine metabolic ratios in the two patients with the CYP2B6*1/*5 genotype was at least 1.4-fold higher than that in the carriers of the CYP2B6*1/*1 genotype. However, further in vivo and in vitro studies are required to examine these individual effects of the CYP2B6*5 and CYP2B6*7 alleles on ketamine clearance.
In contrast to the CYP2B6*6 genotype and age, gender and potential inhibitors/inducers of CYP2B6 had no significant effect on ketamine clearance and hence were not included in our model of prediction of steady-state ketamine plasma concentration following CSCI. However, the influence of inhibitors/inducers may need to be taken into consideration when predicting ketamine concentration after oral administration, as previous studies reported large effects of a CYP2B6 inhibitor (ticlopidine) and CYP3A4 inducers (rifampicin and St John’s Wort) on the exposure to oral ketamine in healthy volunteers 31–33.
The variability in plasma clearance and subsequent influence on plasma concentrations may potentially impact on the incidence of ketamine adverse effects, but the plasma clearance of ketamine in patients who experienced adverse effects was only 15% lower than those who did not. Notably, the adverse effects were reported by all three patients with the CYP2B6*6/*6 genotype. The study’s primary aim was to investigate whether CYP2B6 polymorphisms affect ketamine clearance based on the in vitro study 16 and the adverse effect data were a secondary aim and were insufficiently powered (power was 45% and 60% for the first and second population, respectively) to be conclusive. Future studies are needed to inform the potential clinical utility of this finding.
In conclusion, we showed that the CYP2B6*6 allele, the most common genetic variant of the CYP2B6 gene, was associated with substantial decreases in ketamine steady-state plasma clearance in chronic pain patients. In addition, clearance was substantially reduced in the elderly. The combined impact of the CYP2B6*6 allele and age explained approximately 60% of interindividual variation in steady-state ketamine plasma concentrations. This variability in ketamine concentration may contribute to the incidence of adverse effects. However, as this study was limited by the small subgroup sample size, particularly the small number of the CYP2B6*6 carriers, the impact of the CYP2B6*6 allele on ketamine plasma clearance and its consequent influence on ketamine adverse effects requires further assessment with a larger population size.
Competing Interests
There are no competing interests to declare.
We thank all staff at the various hospitals for their valuable contribution to this work and patients for their contribution.
This work was supported by University of Adelaide, School of Medical Sciences funding, Adelaide Graduate Fee Scholarship, the Australian Research Council Australian Research Fellowship (DP110100297) and FTT Fricker Research Fellowship (Medical Endowment Funds, University of Adelaide).
References
- Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD003351.pub2. CD003351. [DOI] [PubMed] [Google Scholar]
- Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–23. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- Noppers I, Niesters M, Aarts L, Smith T, Sarton E, Dahan A. Ketamine for the treatment of chronic non-cancer pain. Expert Opin Pharmacother. 2010;11:2417–29. doi: 10.1517/14656566.2010.515978. [DOI] [PubMed] [Google Scholar]
- Kvarnström A, Karlsten R, Quiding H, Gordh T. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesth Scand. 2004;48:498–506. doi: 10.1111/j.1399-6576.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- Sörensen J, Bengtsson A, Ahlner J, Henriksson KG, Ekselius L, Bengtsson M. Fibromyalgia - Are there different mechanisms in the processing of pain? A double blind crossover comparison of analgesic drugs. J Rheumatol. 1997;24:1615–21. [PubMed] [Google Scholar]
- Hardy J, Quinn S, Fazekas B, Plummer J, Eckermann S, Agar M, Spruyt O, Rowett D, Currow DC. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol. 2012;30:3611–7. doi: 10.1200/JCO.2012.42.1081. [DOI] [PubMed] [Google Scholar]
- Haas DA, Harper DG. Ketamine: a review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61–8. [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, Iga T. Involvement of CYP2B6 in n-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2001;29:887–90. [PubMed] [Google Scholar]
- Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR. Genetic variability in CYP3A5 and its possible consequences. Pharmacogenomics. 2004;5:243–72. doi: 10.1517/phgs.5.3.243.29833. [DOI] [PubMed] [Google Scholar]
- Olofsen E, Noppers I, Niesters M, Kharasch E, Aarts L, Sarton E, Dahan A. Estimation of the contribution of norketamine to ketamine-induced acute pain relief and neurocognitive impairment in healthy volunteers. Anesthesiology. 2012;117:353–64. doi: 10.1097/ALN.0b013e31825b6c91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JA, Nimmo WS. Pharmacokinetics and analgesic effect of ketamine in man. Br J Anaesth. 1981;53:27–30. doi: 10.1093/bja/53.1.27. [DOI] [PubMed] [Google Scholar]
- White PF, Schuttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers Br J Anaesth. 1985;57:197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- Yanagihara Y, Ohtani M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, Aoyama T, Yamamura Y, Yamada Y, Iga T. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos. 2003;24:37–43. doi: 10.1002/bdd.336. [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Zanger UM. Cytochrome P450 2B6: function, genetics, and clinical relevance. Drug Metabol Drug Interact. 2012;12:1–13. doi: 10.1515/dmdi-2012-0027. [DOI] [PubMed] [Google Scholar]
- Li Y, Coller JK, Hutchinson MR, Klein K, Zanger UM, Stanley NJ, Abell AD, Somogyi AA. The CYP2B6*6 allele significantly alters the N-demethylation of ketamine enantiomers in vitro. Drug Metab Dispos. 2013;41:1264–72. doi: 10.1124/dmd.113.051631. [DOI] [PubMed] [Google Scholar]
- Flockhart DA. 2007. Drug interactions: Cytochrome P450 drug interaction table . Available at http://medicine.iupui.edu/clinpharm/ddis/clinical-table (last accessed August 2014)
- Jackson K, Ashby M, Martin P, Pisasale M, Brumley D, Hayes B. ‘Burst’ketamine for refractory cancer pain: an open-label audit of patients. J Pain Symptom Manage. 2001;22:834–42. doi: 10.1016/s0885-3924(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Pharmacodynamic profiles of ketamine (R)- and (S)- with 5-day inpatient infusion for the treatment of complex regional pain syndrome. Pain Physician. 2010;13:379–87. [PMC free article] [PubMed] [Google Scholar]
- Grömping U. Relative importance for linear regression in R: the package relaimpo. J Stat Softw. 2006;17:1–27. [Google Scholar]
- R Core Team. 2013. R: A language and environment for statistical computing . In, Vienna, Austria R Foundation for Statistical Computing,
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- Hijazi Y, Bolon M, Boulieu R. Stability of ketamine and its metabolites norketamine and dehydronorketamine in human biological samples. Clin Chem. 2001;47:1713–5. [PubMed] [Google Scholar]
- Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61:331–9. doi: 10.1016/S0009-9236(97)90166-1. [DOI] [PubMed] [Google Scholar]
- Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297–301. doi: 10.1002/hep.1840090222. (Baltimore, Md) [DOI] [PubMed] [Google Scholar]
- Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacol Ther. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199:193–209. doi: 10.1016/j.taap.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 4th ed. Philadelphia, United States: Wolters Kluwer Health/Lippincott William & Wilkin; 2011. Editon. [Google Scholar]
- Dayton PG, Stiller RL, Cook DR, Perel JM. The binding of ketamine to plasma proteins: emphasis on human plasma. Eur J Clin Pharmacol. 1983;24:825–31. doi: 10.1007/BF00607095. [DOI] [PubMed] [Google Scholar]
- Noppers I, Olofsen E, Niesters M, Aarts L, Mooren R, Dahan A, Kharasch E, Sarton E. Effect of rifampicin on S-ketamine and S-norketamine plasma concentrations in healthy volunteers after intravenous S-ketamine administration. Anesthesiology. 2011;114:1435–45. doi: 10.1097/ALN.0b013e318218a881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltoniemi MA, Saari TI, Hagelberg NM, Laine K, Neuvonen PJ, Olkkola KT. St John’s wort greatly decreases the plasma concentrations of oral S-ketamine. Fundam Clin Pharmacol. 2012;26:743–50. doi: 10.1111/j.1472-8206.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- Peltoniemi MA, Saari TI, Hagelberg NM, Reponen P, Turpeinen M, Laine K, Neuvonen PJ, Olkkola KT. Exposure to Oral S-ketamine Is Unaffected by Itraconazole but Greatly Increased by Ticlopidine. Clin Pharmacol Ther. 2011;90:296–302. doi: 10.1038/clpt.2011.140. [DOI] [PubMed] [Google Scholar]


