Abstract
Objectives
Farmers may be at increased risk for adverse respiratory outcomes compared with the general population due to their regular exposures to dusts, animals and chemicals. However, early life farm exposures to microbial agents may result in reduced risk. Understanding respiratory disease risk among farmers and identifying differences between farmers and other populations may lead to better understanding of the contribution of environmental exposures to respiratory disease risk in the general population.
Methods
We compared the prevalence of self-reported respiratory outcomes in 43548 participants from the Agricultural Health Study (AHS), a prospective cohort of farmers and their spouses from Iowa and North Carolina, with data from adult participants in the National Health and Nutrition Examination Survey (NHANES) over the same period (2005–2010).
Results
AHS participants had lower prevalences of respiratory diseases (asthma, adult-onset asthma, chronic bronchitis and emphysema), but higher prevalences of current respiratory symptoms (wheeze, cough and phlegm) even after controlling for smoking, body mass index and population characteristics. The overall prevalence of asthma in the AHS (7.2%, 95% CI 6.9 to 7.4) was 52% of that in NHANES (13.8%, 95% CI 13.3 to 14.3), although the prevalence of adult-onset asthma among men did not differ (3.6% for AHS, 3.7% for NHANES). Conversely, many respiratory symptoms were more common in the AHS than NHANES, particularly among men.
Conclusions
These findings suggest that farmers and their spouses have lower risk for adult-onset respiratory diseases compared with the general population, and potentially higher respiratory irritation as evidenced by increased respiratory symptoms.
INTRODUCTION
Farmers have a complex set of occupational and lifestyle exposures that may influence their respiratory health in both positive and negative ways. Since the 1500s, farmers have been identified as an occupational group at higher risk of respiratory disease than many other occupations due to their exposures to hays, grains and animals.1 However, recent evidence suggests that some farmers may have reduced risk of allergy and asthma as a result of early life2 and continued farm exposures.3 Farmers tend to have lower rates of smoking and higher levels of physical activity than the general population,4 tendencies that should also reduce respiratory risk. However, their exposures to inflammatory microbial agents from animals, hays and grains may contribute to chronic obstructive disease later in life.5 Many of the exposures potentially linked to respiratory disease in farmers are also important for the general population. Understanding risks among farmers and how these differ from the population at large may shed light on environmental contributors to respiratory disease in the population as a whole.
Evaluating how farmers compare with the general population is challenging. Few large population-based studies collect complete data on respiratory history. In Europe, respiratory symptoms among crop and animal farmers have been compared with population-based data from the European Community Respiratory Health Survey (ECRHS) for individuals 20–44 years;6,7 similar comparisons have not been done in the USA. The present manuscript compares the respiratory health of a US farming population with that of the general population of the USA. Our data on a farming population come from The Agricultural Health Study (AHS) which is a cohort of farmers and their spouses in Iowa (IA) and North Carolina (NC) who have been followed since 1993. Our data on the general population come from the National Health and Nutrition Examination Survey (NHANES). Lifetime respiratory disease history along with current symptom information was collected in both studies from 2005 to 2010.
METHODS
Population
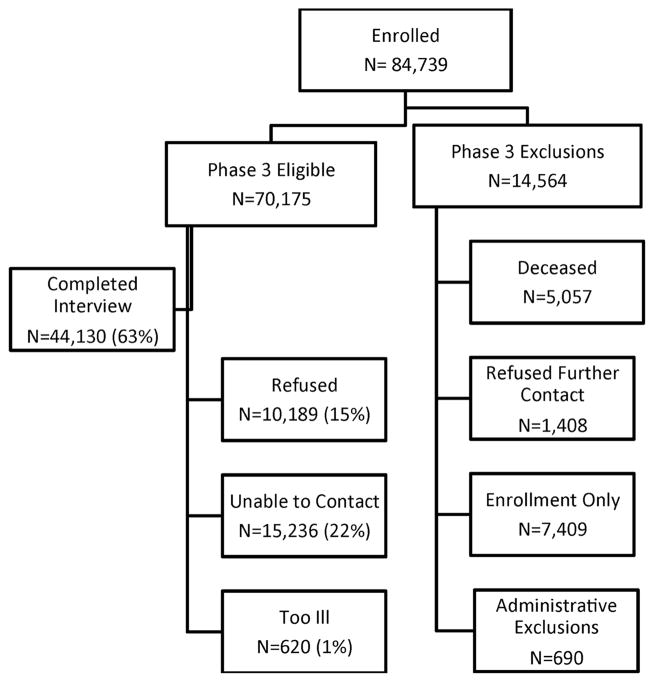
The AHS is a prospective cohort including 52 394 licensed private pesticide applicators (farmers) and 32 345 of their spouses from IA and NC enrolled in 1993–1997 as well as 4916 commercial applicators from IA (phase I). Licensed applicators and those newly seeking licenses were recruited at pesticide licensing sites. Approximately 82% of licensed pesticide applicators in both states enrolled. At enrolment, private applicators were given a second take-home questionnaire for themselves as well as an enrolment questionnaire for their spouse. Approximately 44% of applicators completed the take-home questionnaire and the spouses of 75% of married applicators enrolled. Since enrolment, private applicators and their spouses have completed up to two follow-up telephone interviews, approximately 5 years apart; commercial applicators only received the first follow-up interview. Respiratory disease information was collected during the second follow-up phone interview (phase III). The phase III follow-up interview was conducted from November 2005 to February 2010. Individuals were eligible for the phase III interview if they were a private applicator or a spouse, were alive, had not refused future contact, were able to do a phone interview and, most importantly, had participated in some AHS activity (ie, take-home questionnaire, spouse enrolled, first follow-up interview) in addition to the enrolment questionnaire (figure 1). This decision was made to minimise the expense of trying to contact individuals for phase III who appeared to have little interest in additional AHS participation. After some additional administrative exclusions (eg, people who participated in earlier pilot interviews), there were 70 175 phase III eligible AHS participants. A total of 44 130 individuals (24 171 applicators, 19 959 spouses) completed the phase III interview with an overall response rate of 63% of those eligible. Response rates were slightly higher for spouses (66%) than for applicators (60%). The majority of the non-participants were those not reachable by phone (59%). This analysis focuses on the 43 548 AHS participants who completed the phase III questionnaire and provided information on respiratory outcomes, as they represent the largest sample of the AHS with data on respiratory health.
Figure 1.
Agricultural Health Study (AHS) sample selection criteria and response rates.
Respiratory outcome information
At enrolment, all participants completed questionnaires on demographic characteristics, smoking history, medical history and agricultural activities. This information was collected over the two instruments (enrolment and take-home questionnaires) for applicators, but only one (the spouse enrolment questionnaire) for spouses. Information on respiratory outcomes was collected from applicators on the take-home questionnaire and from spouses on their enrolment questionnaire. The enrolment questionnaires for applicators included information on current respiratory symptoms as well as respiratory disease history while spouses were asked only about respiratory diseases. The phase III follow-up interview included respiratory health outcomes and all participants were asked for the same information (http://aghealth.nih.gov/background/questionnaires.html).
NHANES
We used data from the NHANES 2005–2010 surveys (http://www.cdc.gov/nchs/nhanes.htm) to estimate the prevalence and incidence of respiratory outcomes in the US population. The NHANES is a population-based survey of the US non-institutionalised population designed to assess the health and nutritional status of adults and children. It collects detailed medical history, including respiratory disease history, from approximately 5000 people each year. For comparison with the AHS cohort, we used the data for adults 20 years and older. The NHANES is aggregated to represent the USA as a whole; it does not provide state-specific data.
Outcome definitions
From the AHS phase III interview, we had detailed information on respiratory symptoms (wheeze, cough, phlegm and shortness of breath) and respiratory diseases (asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD), emphysema and farmer’s lung). We created variables from the AHS data that were similar to the questionnaire information collected in NHANES. In both the AHS and NHANES questionnaires, if participants reported a respiratory disease, they were asked to provide the age at diagnosis. We were unable to assess differences in allergic conditions because questions about allergy differed considerably between AHS and NHANES questionnaires. NHANES did not collect information on COPD or farmer’s lung and did not collect all symptom information (cough, phlegm and shortness of breath) for participants younger than 40. For completeness, we present all data on all respiratory outcomes collected in the AHS.
We defined the respiratory outcomes as follows:
Respiratory diseases:
Asthma=ever diagnosed with asthma
Childhood asthma=asthma diagnosed before age 20
Adult-onset asthma=asthma diagnosed age 20 or older
Farmer’s lung=ever diagnosed with farmer’s lung disease
Emphysema=ever diagnosed with emphysema
Chronic bronchitis=ever diagnosed with chronic bronchitis
COPD=ever diagnosed with COPD.
Symptoms:
Cough=usually cough either at waking or during the rest of the day
Phlegm=usually produce phlegm either at waking or during the rest of the day
Chronic cough and phlegm=cough and phlegm for 2 or more years
Wheeze=any episode of wheeze in the past 12 months
Shortness of breath=shortness of breath when hurrying on level ground, walking up a hill or fiight of stairs.
Statistical methods
We calculated the population prevalence and 95% CI for each respiratory disease and symptom within the AHS and NHANES populations. We also calculated the incidence rate and 95% CI for newly diagnosed respiratory diseases. Using the age at diagnosis information at the most recent interview, we defined incident disease as diagnoses occurring at an age greater than or equal to the age at enrolment for AHS or within the past 12 years for NHANES. We assumed a 12-year follow-up interval for NHANES for incident outcomes, such that diseases diagnosed within 12 years of NHANES participation were considered incident for comparison with the AHS data, as the median time from AHS enrolment to phase III interview was 12.0 years (range 8.1–16.1 years). Age at diagnosis was available for the majority of respiratory disease cases in the AHS with fewer than 5% failing to report an age at diagnosis. Additionally, for the AHS, we used previously reported information on respiratory outcomes (available for 75% of participants; 55% of applicators and 100% of spouses) to correct for misreports of incident disease by excluding those who reported the outcome at enrolment (7% of emphysema, 13% of asthma, 19% of farmer’s lung and 20% of incident chronic bronchitis cases). Data for making such corrections were unavailable for the NHANES sample. All estimates from NHANES were weighted using the supplied survey weights to yield nationally representative data. The weights take into account factors such as the complex survey design, survey non-response and poststratification. To compare the prevalence and incidence of respiratory outcomes between AHS and NHANES, we used a Z-test to compare the two proportions. To account for differences in population characteristics between AHS and NHANES, we adjusted for age (categories), race (white, non-white), gender, smoking (ever, never) and body mass index (BMI; <25, 25–30, >30) via logistic regression. To accommodate the sampling design of NHANES, we fit models with PROC SURVEYLOGISTIC that specified sampling stratum, primary sampling unit and sampling weight. The stratum variable contained the NHANES stratum (SDMVSTRA) for NHANES subjects and state for AHS subjects. The sampling-unit variable contained the NHANES sampling unit (SDMVSPSU) for NHANES subjects and an individual identifier for each AHS participant. For NHANES, we used NHANES sampling weights rescaled to sum to the total number of NHANES participants; for AHS, each participant had a sampling weight of 1. All analyses were conducted using SAS V.9.3.
RESULTS
A total of 44 130 AHS participants completed the phase III interview, and 43 548 provided information on respiratory outcomes. At that time, ages ranged from 27 to 97 years (mean=59.4, SD=11.6). The AHS is primarily white (98%) and 66% from IA. The pesticide applicators are mainly men, while the spouses are primarily women. A majority of AHS participants grew up on farms, 62% of women and 92% of men. At the time of the 2005–2010 follow-up interview, 73% of men and 30% of women were still engaged in farming (table 1). Farmers in the AHS engage in a wide range of agricultural activities involving both crops and animals (see online supplementary table). The NHANES 2005–2010 sample consisted of 17 132 adult Americans with results weighted to represent the US adult, non-institutionalised population. The average age was 46.7 years, ranging from 20 to 85 years. In contrast to the AHS, the NHANES population is 30% non-white. Compared with NHANES, a greater proportion of AHS participants were lifetime never smokers (68% never smokers in AHS vs 53% in NHANES) and are less likely to currently smoke (7% vs 22%, table 1). In both groups, 85% reported visiting a doctor within the past year, though the frequency was lower among NHANES men (78%) than NHANES women (91%). A greater proportion of individuals in the AHS reported excellent health status (19% vs 10% for NHANES), but the per cent of individuals reporting poor health was similar in both groups (3% for AHS, 4% for NHANES).
Table 1.
Demographic characteristics of the AHS participants and adult NHANES participants, 2005–2010
| Variable | AHS phase III follow-up participants
|
NHANES 2005–2010
|
||||
|---|---|---|---|---|---|---|
| Total N=43 548 % |
Females N=19 818 % |
Males N=23 730 % |
Total N=17 132 % |
Females N=8829 % |
Males N=8303 % |
|
| Type of participant | ||||||
| Applicator | 54 | 3 | 100 | |||
| Spouse | 46 | 97 | <1 | |||
| Still farming | 53 | 30 | 73 | |||
| Grew up on farm* | 74 | 62 | 92 | |||
| Current age group | ||||||
| 20–29 years old | <1 | <1 | <1 | 19 | 18 | 20 |
| 30–39 years old | 3 | 3 | 4 | 18 | 18 | 19 |
| 40–49 years old | 19 | 20 | 18 | 21 | 20 | 21 |
| 50–59 years old | 30 | 30 | 30 | 18 | 18 | 18 |
| 60–69 years old | 26 | 27 | 25 | 12 | 12 | 12 |
| 70–79 years old | 18 | 17 | 19 | 7 | 8 | 7 |
| 80+ years old | 4 | 3 | 5 | 4 | 5 | 3 |
| Race | ||||||
| White | 98 | 98 | 98 | 70 | 69 | 70 |
| Non-white | 2 | 2 | 2 | 30 | 31 | 30 |
| Smoking status | ||||||
| Never smoked | 68 | 78 | 58 | 53 | 60 | 46 |
| Past smoker | 25 | 16 | 34 | 24 | 21 | 28 |
| Current smoker | 7 | 6 | 8 | 22 | 19 | 25 |
| Cigarettes/day | ||||||
| <10 | 29 | 44 | 22 | 49 | 56 | 43 |
| 11–20 | 48 | 44 | 50 | 33 | 31 | 34 |
| 21–40 | 19 | 11 | 23 | 15 | 11 | 19 |
| >40 | 4 | 1 | 5 | 3 | 2 | 4 |
| Years smoked | ||||||
| 1–10 years | 21 | 25 | 20 | 25 | 26 | 25 |
| 11–20 years | 22 | 21 | 22 | 24 | 23 | 25 |
| 21–30 years | 23 | 22 | 23 | 21 | 22 | 20 |
| 31–40 years | 20 | 19 | 20 | 17 | 16 | 18 |
| >40 years | 14 | 13 | 15 | 13 | 13 | 12 |
| Lifetime pack years | ||||||
| <3 | 12 | 18 | 10 | 24 | 26 | 22 |
| 3–10 | 18 | 22 | 17 | 24 | 26 | 23 |
| 10.25–27 | 31 | 31 | 30 | 26 | 27 | 26 |
| >27 | 39 | 29 | 43 | 26 | 21 | 29 |
| Current alcohol | 57 | 53 | 60 | 81 | 80 | 83 |
| BMI category | ||||||
| <25 | 27 | 38 | 19 | 32 | 36 | 27 |
| 25–30 | 49 | 42 | 55 | 38 | 32 | 46 |
| >30 | 24 | 21 | 26 | 30 | 32 | 28 |
| Last doctor visit | ||||||
| <1 year | 85 | 85 | 85 | 85 | 91 | 78 |
| 1–3 years | 11 | 11 | 11 | 9 | 6 | 12 |
| >3 years | 4 | 4 | 4 | 6 | 3 | 9 |
| Self-reported health status | ||||||
| Excellent | 19 | 20 | 19 | 10 | 10 | 9 |
| Very good | 39 | 39 | 40 | 27 | 27 | 27 |
| Good | 30 | 30 | 30 | 39 | 39 | 39 |
| Fair | 9 | 8 | 9 | 20 | 20 | 20 |
| Poor | 3 | 3 | 3 | 4 | 4 | 4 |
All other variables reported in the most recent interview.
Available for 75% of participants (those who completed the take-home or spouse at enrolment).
AHS, Agricultural Health Study; BMI, body mass index; NHANES, National Health and Nutrition Examination Survey.
Asthma was the most common respiratory disease and emphysema was the least common in both populations (table 2). All respiratory diseases had significantly lower prevalence in the AHS cohort than in NHANES; however, symptom prevalence was higher in the AHS. The largest difference in disease prevalence was for asthma, with an estimated prevalence of 7.2% (95% CI 6.9 to 7.4) among AHS participants, approximately half the prevalence observed among NHANES participants (13.8%, 95% CI 13.3 to 14.3). While still lower in the AHS, the difference in the prevalence of adult-onset asthma was not as large (adjusted prevalence ratio (PR)=0.65 (95% CI 0.57 to 0.74)). Some current respiratory symptoms (wheeze, cough and phlegm) were more common in AHS than NHANES participants while respiratory symptoms potentially more indicative of poorer health (eg, shortness of breath and chronic cough and phlegm) were higher among NHANES participants. The prevalence of wheeze in the AHS (20.1%, 95% CI 19.7 to 20.5) was higher than that for NHANES (14.1%, 95% CI 13.5 to 14.6). The PR for wheeze was 1.66 (95% CI 1.51 to 1.82) when adjusted for differences between NHANES and AHS. For those 40 years and older, reports of cough and phlegm were 14%–16% higher in the AHS than in NHANES.
Table 2.
Self-reported respiratory symptom and disease prevalence in adults for the AHS and NHANES, 2005–2010
| Respiratory outcome | AHS
|
NHANES
|
Prevalence ratio
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence | 95% CI | Prevalence | 95% CI | PR | 95% CI | ||||
| All adult participants (%) | Adj for age, race, gender, BMI, Smk | ||||||||
| Diseases | |||||||||
| Asthma ever | 7.2 | 6.9 | 7.4 | 13.8 | 13.3 | 14.3 | 0.54 | 0.49 | 0.59 |
| Asthma—adult-onset | 4.3 | 4.1 | 4.5 | 6.0 | 5.6 | 6.4 | 0.65 | 0.57 | 0.74 |
| Chronic bronchitis | 3.5 | 3.3 | 3.7 | 5.7 | 5.4 | 6.1 | 0.53 | 0.45 | 0.62 |
| Emphysema | 1.4 | 1.3 | 1.5 | 1.8 | 1.6 | 2.0 | 0.55 | 0.45 | 0.67 |
| COPD | 1.3 | 1.2 | 1.4 | NA | |||||
| Any obstructive disease* | 5.1 | 4.9 | 5.3 | NA | |||||
| Farmer’s lung | 1.2 | 1.1 | 1.3 | NA | |||||
| Symptoms | |||||||||
| Wheeze | 20.1 | 19.7 | 20.5 | 14.1 | 13.5 | 14.6 | 1.66 | 1.51 | 1.82 |
| Cough | 12.7 | 12.4 | 13.0 | NA | |||||
| Phlegm | 10.4 | 10.1 | 10.7 | NA | |||||
| Shortness of breath | 24.2 | 23.8 | 24.6 | NA | |||||
| Chronic cough and phlegm† | 4.0 | 3.8 | 4.2 | NA | |||||
| Participants 40 and older (%) | |||||||||
| Diseases | |||||||||
| Asthma ever | 7.2 | 7.0 | 7.5 | 12.7 | 12.0 | 13.3 | 0.57 | 0.51 | 0.64 |
| Asthma—adult-onset | 4.4 | 4.2 | 4.6 | 7.4 | 6.9 | 7.9 | 0.65 | 0.57 | 0.75 |
| Chronic bronchitis | 3.6 | 3.4 | 3.8 | 7.0 | 6.6 | 7.5 | 0.54 | 0.45 | 0.63 |
| Emphysema | 1.4 | 1.3 | 1.5 | 2.7 | 2.4 | 3.1 | 0.55 | 0.45 | 0.67 |
| COPD | 1.4 | 1.3 | 1.5 | NA | |||||
| Any obstructive disease* | 5.2 | 5.0 | 5.4 | NA | |||||
| Farmer’s lung | 1.2 | 1.1 | 1.3 | NA | |||||
| Symptoms | |||||||||
| Wheeze | 20.0 | 19.6 | 20.4 | 14.9 | 14.2 | 15.5 | 1.60 | 1.44 | 1.77 |
| Cough | 12.8 | 12.5 | 13.1 | 11.5 | 10.9 | 12.1 | 1.16 | 1.04 | 1.31 |
| Phlegm | 10.5 | 10.2 | 10.8 | 9.5 | 9.0 | 10.1 | 1.14 | 1.03 | 1.26 |
| Shortness of breath | 24.6 | 24.2 | 25.1 | 32.3 | 31.5 | 33.2 | 0.71 | 0.65 | 0.77 |
| Chronic cough and phlegm | 4.0 | 3.8 | 4.2 | 5.1 | 4.7 | 5.5 | 0.79 | 0.70 | 0.90 |
NA, not collected in NHANES (farmer’s lung and COPD).
Cough, phlegm and shortness of breath only collected for those 40 and older.
Includes chronic bronchitis, emphysema and COPD.
Chronic cough and phlegm defined as cough and phlegm for at least 2 years.
AHS, Agricultural Health Study; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NHANES, National Health and Nutrition Examination Survey; PR, prevalence ratio.
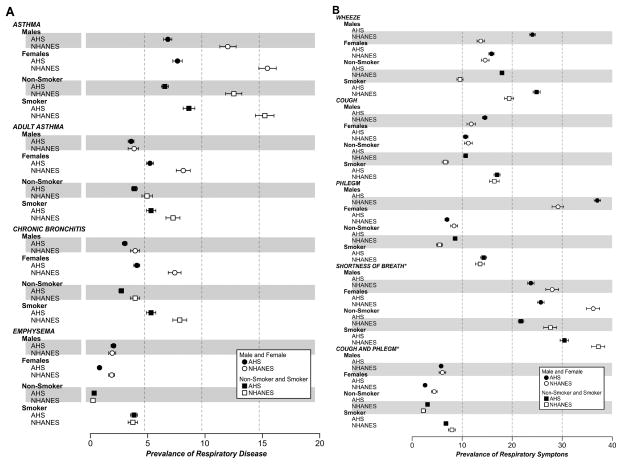
Differences in disease and symptoms varied by gender and smoking. Among men, the prevalence of adult-onset asthma was no longer statistically different between the AHS and NHANES (figure 2A). In general, women were more likely to report asthma and chronic bronchitis than men, but women in the AHS had lower prevalences of all respiratory diseases than women in NHANES. For respiratory symptoms among participants 40 and older (figure 2B), wheeze was more common among men in the AHS (24%, 95% CI 23% to 24%) than among men in NHANES (15%, 95% CI 14% to 16%); this relationship was seen in both smokers and non-smokers. Cough and phlegm were each more common among men in the AHS than men in NHANES; yet women in the AHS had similar (cough) or lower prevalence (phlegm) compared with women in NHANES. Among non-smokers, cough and phlegm were more prevalent in the AHS than in NHANES; but, among smokers, cough and phlegm each showed similar prevalence between the two populations.
Figure 2.
(A) Prevalence of respiratory disease in the Agricultural Health Study (AHS) and NHANES, National Health and Nutrition Examination Survey (NHANES) stratified by gender and by smoking status. (B) Prevalence of respiratory symptoms in the AHS and NHANES stratified by gender and by smoking status for those 40 years and older.
Among non-smokers, AHS participants had lower prevalence of respiratory disease than NHANES participants except for emphysema which had higher prevalence among the AHS participants (0.3%, 95% CI 0.3 to 0.4 vs 0.2%, 95% CI 0.1 to 0.3, p=0.03). In analyses restricted to people over 40, this difference was no longer statistically significant (data not shown). All respiratory symptoms, including chronic cough and phlegm, were more common in AHS non-smokers than NHANES non-smokers. Smokers were more likely to report respiratory disease than non-smokers both in the AHS and NHANES (figure 2A,B); however, among those with respiratory disease, the AHS has a higher proportion of non-smokers (see online supplementary figure).
The estimated 12-year incidence of all respiratory diseases was greater among NHANES than AHS participants (table 3). The incidence rate of asthma in the AHS was 2.1/1000 person-years (PY) compared with 4.0/1000 PY in NHANES. The incidence rate of chronic bronchitis was also lower in the AHS (1.2/1000 vs 2.1/1000 PY). Similarly reduced rates were seen within the smoking and gender strata.
Table 3.
Estimated incident rates for respiratory diseases in the AHS and NHANES using age at diagnosis to estimate person time of follow-up
| Disease | AHS (N=43 548)
|
NHANES
|
||||
|---|---|---|---|---|---|---|
| Incidence rate N/1000 PY | 95% CI | Incidence rate N/1000 PY | 95% CI | |||
| Asthma | 2.1 | 2.0 | 2.2 | 4.0 | 4.0 | 4.0 |
| Chronic bronchitis | 1.2 | 1.1 | 1.3 | 2.1 | 2.1 | 2.1 |
| Emphysema | 0.8 | 0.7 | 0.9 | 1.1 | 1.1 | 1.1 |
| COPD | 0.9 | 0.8 | 1.0 | NR | ||
| Farmer’s lung | 0.3 | 0.3 | 0.4 | NR | ||
| Any obstructive disease* | 2.1 | 2.0 | 2.2 | NR | ||
| Non-smokers | ||||||
| Asthma | 1.8 | 1.7 | 2.0 | 3.4 | 3.4 | 3.4 |
| Chronic bronchitis | 0.8 | 0.8 | 0.9 | 1.3 | 1.3 | 1.3 |
| Emphysema | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 |
| COPD | 0.3 | 0.3 | 0.4 | NR | ||
| Farmer’s lung | 0.3 | 0.2 | 0.3 | NR | ||
| Any obstructive disease* | 1.2 | 1.0 | 1.3 | NR | ||
| Smokers | ||||||
| Asthma | 2.6 | 2.4 | 2.9 | 4.7 | 4.7 | 4.7 |
| Chronic bronchitis | 2.0 | 1.8 | 2.3 | 2.9 | 2.9 | 2.9 |
| Emphysema | 2.1 | 1.9 | 2.4 | 2.3 | 2.3 | 2.3 |
| COPD | 2.0 | 1.8 | 2.2 | NR | ||
| Farmer’s lung | 0.5 | 0.4 | 0.6 | NR | ||
| Any obstructive disease* | 4.2 | 3.9 | 4.5 | NR | ||
| Men | ||||||
| Asthma | 1.7 | 1.5 | 1.9 | 2.6 | 2.6 | 2.6 |
| Chronic bronchitis | 1.1 | 1.0 | 1.2 | 1.3 | 1.3 | 1.3 |
| Emphysema | 1.1 | 1.0 | 1.2 | 1.1 | 1.1 | 1.1 |
| COPD | 1.0 | 0.9 | 1.2 | NR | ||
| Farmer’s lung | 0.6 | 0.5 | 0.7 | NR | ||
| Any obstructive disease* | 2.3 | 2.1 | 2.5 | NR | ||
| Women | ||||||
| Asthma | 2.5 | 2.3 | 2.7 | 5.4 | 5.4 | 5.4 |
| Chronic bronchitis | 1.4 | 1.3 | 1.6 | 2.8 | 2.8 | 2.8 |
| Emphysema | 0.4 | 0.4 | 0.5 | 1.2 | 1.2 | 1.2 |
| COPD | 0.7 | 0.6 | 0.8 | NR | ||
| Farmer’s lung | 0.1 | 0.0 | 0.1 | NR | ||
| Any obstructive disease* | 1.9 | 1.7 | 2.0 | NR | ||
| ≥40 | ||||||
| Asthma | 2.1 | 2.0 | 2.2 | 3.5 | 3.5 | 3.5 |
| Chronic bronchitis | 1.2 | 1.1 | 1.3 | 2.4 | 2.4 | 2.4 |
| Emphysema | 0.8 | 0.7 | 0.9 | 1.7 | 1.7 | 1.7 |
| COPD | 0.9 | 0.8 | 1.0 | NR | ||
| Farmer’s lung | 0.3 | 0.3 | 0.4 | NR | ||
| Any obstructive disease* | 2.2 | 2.0 | 2.3 | NR | ||
NR, not reported in the NHANES.
COPD, emphysema, and chronic bronchitis.
AHS, Agricultural Health Study; COPD, chronic obstructive pulmonary disease; NHANES, National Health and Nutrition Examination Survey; PY, person-years.
DISCUSSION
Participants in the AHS had higher prevalence of self-reported respiratory symptoms than the general population despite lower rates of respiratory disease diagnoses and smoking and presumably more physically demanding work than the general population. Respiratory symptoms may be indicative of respiratory disease or may occur in response to irritants or other stressors. Farmers are exposed to many potential respiratory irritants including allergens, diesel exhaust, pesticides, hays, grains and dusts. How these exposures influence long-term respiratory health is an area of active research.1 Diesel exhaust enhances the allergenicity of allergens;8 thus, coexposures on farms may result in increased airway symptoms. Endotoxins and glucans from bacteria and molds are agents that, in addition to irritating airways, can also contribute to airway inflammation.6,9–12 These inflammatory agents are believed to contribute to COPD in non-smokers in microbial rich environments, such as farms or developing countries where biomass fuels are used and many other environmental and infectious agents are present.12,13 Although specific pesticides have been shown to increase allergic airway responsiveness in animals and humans,14–17 growing up on a farm reduces an individual’s lifetime risk of allergy and asthma.2,3,18,19
Current symptoms of wheeze, cough and phlegm were higher among AHS participants, whereas symptoms of shortness of breath and chronic cough and phlegm were higher in the general population even after controlling for differences in age, race, gender, smoking and BMI. Shortness of breath as well as chronic cough and phlegm are indicative of poorer health and, thus, possibly inversely associated with the ability to farm.20 Growing up on a farm protects against allergy and asthma, and probably results in sensitive individuals removing themselves from farms, such that those with reactive airways may choose not to become or remain farmers.21 This hypothesis is supported by the lower prevalence of ever asthma among those in the AHS, but a more similar prevalence for adult-onset asthma among men in the AHS and NHANES. For other adult-onset respiratory diseases, namely, chronic bronchitis and emphysema, we see evidence of a healthy worker effect as the prevalence and incidence of these diseases was lower among AHS than NHANES participants. In a recent US survey, the prevalence of COPD was much higher among those who were unable to work compared with those who were employed.22 Radon and colleagues have demonstrated a healthy worker selection bias in cohort studies of chronic bronchitis, as those with symptoms tend to remove themselves from exposure.20
Lower smoking rates should reduce risk of obstructive airway disease and symptoms and, yet, in our population, we see somewhat conflicting information. The individual symptoms of current cough and phlegm were higher among AHS participants, even among non-smokers. However, the overall prevalence of chronic cough and phlegm was higher in NHANES than the AHS, but when restricted to lifetime never smokers, individuals in the AHS had higher prevalence of chronic cough and phlegm. Among farmers, working with livestock has been associated with chronic bronchitis in both smokers and non-smokers.10,11 Approximately 15% of COPD is attributable to occupation.23 Among non-smokers, however, occupation probably plays a larger role, particularly in a population such as farmers where exposures may occur over a lifetime. Approximately 31% of COPD was attributable to work among never smokers based on the NHANES 1988–1994 data.24
The AHS represents the largest sample of farm women studied for respiratory conditions. As in other studies, women were at higher risk of adult-onset respiratory diseases than men.25 Like the men in the AHS, women in the AHS had a lower prevalence of respiratory disease than women in NHANES and, with the exception of wheeze, had a lower prevalence of symptoms as well. Only 22% of the women in the AHS had ever smoked, compared with 40% in NHANES. This lower prevalence of smoking coupled with the lower risk of asthma and allergy potentially due to growing up on the farm and performing farm work likely explains the reduced risk among farm women compared with the general population.
The prevalence of respiratory disease and symptoms among farmers has been evaluated around the world in studies large and small using varying outcome definitions. To our knowledge, the AHS with 44 130 participants providing detailed respiratory information is the largest prospective study of respiratory disease incidence and prevalence among farmers and their spouses to date. Wheeze has been reported in a number of other studies of farmers with prevalence ranging from 11.7% for male Swedish farmers26 to 18% for New Zealand farmers3 and New York farmers and farm residents.27 Our overall estimate for wheeze of 20.1% is at the high end of the estimates, but is consistent with the wheeze prevalence at enrolment for private applicators (referred to here as farmers, 19%)28 and commercial pesticide applicators (21%).29 The prevalence of asthma among farmers in previous studies varies from 2.8% among European animal farmers7 to 14.8% for New Zealand farmers;3 our estimated prevalence of 7.2% is consistent with estimates from New York dairy farmers (7.7%)30 and Swedish farmers (8.9%).31 Our estimated prevalence for chronic bronchitis of 3.5% was lower than most other previous studies of farmers, but chronic bronchitis is a more difficult disease to assess through self-report, and differences in diagnostic practices as well as agricultural exposures may contribute to these differences.
Other investigators have compared groups of farmers to population-based estimates of respiratory symptoms and disease. In Iceland, Sigurdarson and colleagues saw no significant differences in respiratory disease and symptoms among 1107 farmers compared with national estimates.32 Similar to our findings, Icelandic farmers had a lower prevalence of lifetime asthma with no difference in current asthma. Among Norwegian farmers, both atopic and non-atopic asthma were less prevalent than in the general population.33 In a larger study focusing on farmers from four European countries (Denmark, Germany, Switzerland and Spain), Radon et al7 and Monso et al6 compared respiratory health data from more than 6000 animal farmers and almost 4800 crop farmers with data from the general population ECRHS conducted in these regions; the same instrument was used to collect respiratory data in all participants. Among animal farmers, the prevalence of the respiratory symptoms (wheeze, shortness of breath and asthma) was lower than the comparison population of ECRHS participants; only phlegm production was more common among farmers and prevalence of this symptom remained elevated when limited to younger farmers (<45 years).7 Among crop farmers, the prevalence of respiratory symptoms was similar to the general European population.6 While the European studies compared respiratory disease experience for different types of farming, animal and crop, we did not analyse our data by specific commodity groups, as many farmers in our cohort produce both crops and animals over the course of their lifetimes. Additionally, we only have data for production at three points in time; thus, it would be difficult to correctly classify an individual with respect to lifetime farming activities.
Ours is the first large study of farmers to report asthma incidence rates. In a review of exposure and respiratory health of farmers, Omland5 noted the lack of longitudinal studies of asthma in farming populations. Previous studies have estimated the incidence rates for asthma among farmers from occupational codes on questionnaires or death certificates, resulting in heterogeneity in classifying farmers.5,34 In a 12-year follow-up study of 418 Swedish farmers, Rask-Andersen31 reported that the prevalence of doctor-diagnosed asthma increased from 2% to 8.9% from 1982 to 1994, suggesting an estimated incident rate of 5.8/1000 PY, much higher than our estimate for the AHS (2.1/1000 PY) or NHANES (4.0/1000PY).
Like other large surveys, we relied on self-reported respiratory disease and symptom history to assess disease incidence and prevalence. Some diseases, such as asthma, are more accurately reported than other diseases, such as chronic bronchitis, but it is unlikely that the reporting errors of these diseases differ between the NHANES and AHS populations. We do not anticipate diagnostic differences between smokers and non-smokers because the frequency of pulmonary function testing and medication use for COPD did not differ by smoking status in another US population-based sample.22 We used age at diagnosis to estimate disease incidence, a measure that has been used by other researchers;35 in addition, we were able to correct incidence estimates by removing prevalent disease reported at enrolment. The impact of this correction on incidence rates was small, as the actual number of cases affected was low and the number of PY was large; thus, this correction was too small to explain the differences in incidence rates between the AHS and NHANES. Among Finnish adults, the sensitivity of asthma incidence (63%) was less than for asthma prevalence (91%) when disease information was collected 4 years apart.36 With a longer follow-up period and the use of enrolment information to correct misreported information, it is likely that our sensitivity for incident disease is higher.
We compared the respiratory health in the AHS cohort with a nationally representative sample collected over the same calendar period. The advantage in using nationally representative data is that it allows comparison with a standard set of data without restricting it to unique subgroups (eg, older white adults) that no longer represent the whole. While the AHS population was older and less racially diverse than NHANES, these factors did not explain the differences in prevalence observed between the two groups. It is unlikely that access to medical care influenced these findings as 85% of participants in both the AHS and NHANES had visited a doctor within the past year. Another advantage is that the data from the AHS and NHANES were collected over the same calendar years. Asthma rates in the USA have been increasing over time,37 and diagnostic practices change over time, and so use of contemporaneous information is critical for valid comparisons.
The AHS is a cohort of farm owners and operators and their spouses and does not include seasonal, temporary or other agricultural workers. Cohort members engage in a wide range of agricultural activities representative of Midwestern and Southern agriculture in the USA. In this sample, we observed higher prevalence of respiratory symptoms, yet lower prevalence and incidence of respiratory disease compared with a representative US sample. While respiratory disease prevalence was lower than the general population, the proportion of those with respiratory disease who were non-smokers was higher among farmers. Even though farmers have lower rates of disease, understanding the exposures that contribute to respiratory symptoms and disease among non-smoking farmers should be explored in detail.
Supplementary Material
Supplemental Table. Crops Grown and Animals Raised by AHSParticipants at Enrollment and at the Phase 3 Followup Interview.
What this paper adds.
Farmers have different respiratory exposures and different respiratory risks than the general population.
Few studies have been able to compare farmers with general population data.
In the largest study of farmers to date, this study shows that US farmers and their spouses have lower rates of respiratory disease than the US population, but higher rates of respiratory symptoms, perhaps due to occupational exposure to respiratory irritants.
Acknowledgments
We used the following AHS data releases for this analysis: phase I (P1REL201005.01), phase III (P3REL1000.00) and Demographic (AHSREL201103.00).
Funding This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES025041) and National Cancer Institute (Z01-CP010119). JLR was supported by the National Institute of Environmental Health Sciences (award no. T32ES007018).
Footnotes
Contributors: JAH, MCRA, AB, DPS, LEBF, SJL and PKH designed the data collection instruments and collected the data. JAH, DMU and SL conducted the statistical analyses. JAH drafted the manuscript. DMU, SL, JLR, PKH, PMS, DCZ, SJL, MCRA, AB, LEBF and DPS reviewed and provided critical comments on the draft manuscript. All authors reviewed and approved the final manuscript.
Ethics approval: IRB of the National Institutes of Health.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We used data from the Agricultural Health Study. Interested investigators can apply to gain access to the data through the AHS study management site (http://www.aghealthstars.com).
References
- 1.Schenker MB, Christiani D, Cormier Y, et al. Respiratory health hazards in agriculture. Am J Respir Crit Care Med. 1998;158:S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 2.Leynaert B, Neukirch C, Jarvis D, et al. Does living on a farm during childhood protect against asthma, allergic rhinitis, and atopy in adulthood? Am J Respir Crit Care Med. 2001;164:1829–34. doi: 10.1164/ajrccm.164.10.2103137. [DOI] [PubMed] [Google Scholar]
- 3.Douwes J, Travier N, Huang K, et al. Lifelong farm exposure may strongly reduce the risk of asthma in adults. Allergy. 2007;62:1158–65. doi: 10.1111/j.1398-9995.2007.01490.x. [DOI] [PubMed] [Google Scholar]
- 4.Blair A, Malker H, Cantor KP, et al. Cancer among farmers. A review. Scand J Work Environ Health. 1985;11:397–407. doi: 10.5271/sjweh.2208. [DOI] [PubMed] [Google Scholar]
- 5.Omland O. Exposure and respiratory health in farming in temperate zones—a review of the literature. Ann Agric Environ Med. 2002;9:119–36. [PubMed] [Google Scholar]
- 6.Monso E, Magarolas R, Radon K, et al. Respiratory symptoms of obstructive lung disease in European crop farmers. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1246–50. doi: 10.1164/ajrccm.162.4.9912093. [DOI] [PubMed] [Google Scholar]
- 7.Radon K, Danuser B, Iversen M, et al. Respiratory symptoms in European animal farmers. Eur Respir J. 2001;17:747–54. doi: 10.1183/09031936.01.17407470. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Sanchez D, Penichet-Garcia M, Saxon A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J Allergy Clin Immunol. 2000;106:1140–6. doi: 10.1067/mai.2000.111144. [DOI] [PubMed] [Google Scholar]
- 9.Eduard W, Douwes J, Mehl R, et al. Short term exposure to airborne microbial agents during farm work: exposure-response relations with eye and respiratory symptoms. Occup Environ Med. 2001;58:113–18. doi: 10.1136/oem.58.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest. 2009;136:716–25. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 11.Melbostad E. Chronic bronchitis in farmers. Scand J Work Environ Health. 1997;23:271–80. doi: 10.5271/sjweh.220. [DOI] [PubMed] [Google Scholar]
- 12.Radon K. The two sides of the ‘endotoxin coin’. Occup Environ Med. 2006;63:73–8. 10. doi: 10.1136/oem.2004.017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 14.Cho YS, Oh SY, Zhu Z. Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation. Am J Respir Cell Mol Biol. 2008;39:412–19. doi: 10.1165/rcmb.2007-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppin JA, Umbach DM, London SJ, et al. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. Eur Respir J. 2009;34:1296–303. doi: 10.1183/09031936.00005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proskocil BJ, Bruun DA, Jacoby DB, et al. Macrophage TNF-alpha mediates parathion-induced airway hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol. 2013;304:L519–29. doi: 10.1152/ajplung.00381.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proskocil BJ, Bruun DA, Lorton JK, et al. Antigen sensitization influences organophosphorus pesticide-induced airway hyperreactivity. Environ Health Perspect. 2008;116:381–8. doi: 10.1289/ehp.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heederik D, Sigsgaard T. Respiratory allergy in agricultural workers: recent developments. Curr Opin Allergy Clin Immunol. 2005;5:129–34. doi: 10.1097/01.all.0000162304.66986.7d. [DOI] [PubMed] [Google Scholar]
- 19.von Mutius E. The environmental predictors of allergic disease. J Allergy Clin Immunol. 2000;105(Pt 1):9–19. doi: 10.1016/s0091-6749(00)90171-4. [DOI] [PubMed] [Google Scholar]
- 20.Radon K, Goldberg M, Becklake M. Healthy worker effect in cohort studies on chronic bronchitis. Scand J Work Environ Health. 2002;28:328–32. doi: 10.5271/sjweh.682. [DOI] [PubMed] [Google Scholar]
- 21.Vogelzang PF, van der Gulden JW, Tielen MJ, et al. Health-based selection for asthma, but not for chronic bronchitis, in pig farmers: an evidence-based hypothesis. Eur Respir J. 1999;13:187–9. doi: 10.1034/j.1399-3003.1999.13a34.x. [DOI] [PubMed] [Google Scholar]
- 22.Kosacz N, Punturieri A, Croxton T, et al. Chronic obstructive pulmonary disease among adults—United States, 2011. Morb Mortal Wkly Rep. 2012;61:938–43. [PubMed] [Google Scholar]
- 23.Balmes J, Becklake M, Blanc P, et al. American thoracic society statement: occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167:787–97. doi: 10.1164/rccm.167.5.787. [DOI] [PubMed] [Google Scholar]
- 24.Hnizdo E, Sullivan PA, Bang KM, et al. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156:738–46. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 25.Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176:1179–84. doi: 10.1164/rccm.200704-553CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lembke B, Janson C, Norback D, et al. High risk of adult-onset asthma and work-related wheeze in farmers despite low prevalence of asthma in young farmers. Int J Tuberc Lung Dis. 2004;8:1285–91. [PubMed] [Google Scholar]
- 27.Gomez MI, Hwang SA, Lin S, et al. Prevalence and predictors of respiratory symptoms among New York farmers and farm residents. Am J Ind Med. 2004;46:42–54. doi: 10.1002/ajim.20018. [DOI] [PubMed] [Google Scholar]
- 28.Hoppin JA, Umbach DM, London SJ, et al. Chemical predictors of wheeze among farmer pesticide applicators in the agricultural health study. Am J Resp Crit Care Med. 2002;165:683–9. doi: 10.1164/ajrccm.165.5.2106074. [DOI] [PubMed] [Google Scholar]
- 29.Hoppin JA, Umbach DM, London SJ, et al. Pesticides associated with wheeze among commercial pesticide applicators in the Agricultural Health Study. Am J Epidemiol. 2006;163:1129–37. doi: 10.1093/aje/kwj138. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins PL, Earle-Richardson G, Bell EM, et al. Chronic disease risk in central New York dairy farmers: results from a large health survey 1989–1999. Am J Ind Med. 2005;47:20–6. doi: 10.1002/ajim.20110. [DOI] [PubMed] [Google Scholar]
- 31.Rask-Andersen A. Asthma increase among farmers: a 12-year follow-up. Ups J Med Sci. 2010;116:60–71. doi: 10.3109/03009734.2010.503287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigurdarson ST, Gudmundsson G, Sigurvinsdottir L, et al. Respiratory disorders are not more common in farmers. Results from a study on Icelandic animal farmers. Respir Med. 2008;102:1839–43. doi: 10.1016/j.rmed.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eduard W, Omenaas E, Bakke PS, et al. Atopic and non-atopic asthma in a farming and a general population. Am J Ind Med. 2004;46:396–9. doi: 10.1002/ajim.20088. [DOI] [PubMed] [Google Scholar]
- 34.Greskevitch M, Kullman G, Bang KM, et al. Respiratory disease in agricultural workers: mortality and morbidity statistics. J Agromedicine. 2007;12:5–10. doi: 10.1080/10599240701881482. [DOI] [PubMed] [Google Scholar]
- 35.Winer RA, Qin X, Harrington T, et al. Asthma incidence among children and adults: findings from the Behavioral Risk Factor Surveillance system asthma call-back survey — United States, 2006–2008. J Asthma. 2012;49:16–22. doi: 10.3109/02770903.2011.637594. [DOI] [PubMed] [Google Scholar]
- 36.Oksanen T, Kivimaki M, Pentti J, et al. Self-report as an indicator of incident disease. Ann Epidemiol. 2010;20:547–54. doi: 10.1016/j.annepidem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980–1996. J Asthma. 2007;44:65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Crops Grown and Animals Raised by AHSParticipants at Enrollment and at the Phase 3 Followup Interview.