Abstract
The trans-fluoro-2-butenyl group has been previously utilized as an N-substituent on several nortropanes for imaging the dopamine transporter with positron emission tomography. We report here a simplified and shorter synthesis of trans-1-tosyloxy-4-substituted-2-butenes using Ag(I) salts. This methodology was also applied to the synthesis of the cis-isomers. Furthermore, these procedures allow for the recovery of the majority of the Ag(I) ions.
Keywords: Fluorobutenyl-tosylate, N-fluorobutenyl-nortropane, Fluorine-18, Positron emission tomography
Graphical Abstract

Several N-(E)-fluoro-2-butenyl-nortropanes (Scheme 1) have been previously reported as high affinity ligands of the dopamine transporter (DAT) and which can be radiolabeled with fluorine-18 for imaging the DAT with positron emission tomography (PET).1–4 trans-1,4-Ditosyloxy-2-butene (1) was initially prepared by multi-step syntheses and then reacted with tetrabutylammonium fluoride to give trans-4-fluoro-1-tosyloxy-2-butene (2) which was then reacted with the various nortropanes to provide the N-(E)-fluoro-2-butenyl-nortropanes (Scheme 1).1–4 Radiolabeling was performed by reacting 1 with K18F/K2.2.2 to give [18F]2, which was then reacted with the various nortropanes to give the 18F PET tracers.1, 3, 5 Subsequently, trans-4-chloro-1-tosyloxy-2-butene (3) was prepared and reacted with the tolyl-nortropane to provide the N-(E)-chloro-2-butenyl-nortropane that could then be used as a one-step 18F-radiolabeling precursor.6 The preparation of 1, 2, and 3 all require multiple synthetic steps.2–4, 6 Herein, we report a simplified synthesis of 1, 2, and trans-4-bromo-1-tosyloxy-2-butene (4), which can serve as a replacement for 3, all of which can be obtained in 1 or 2 steps from commercially available trans-1,4-dibromo-2-butene (5) using Ag(I) salts.7–9 Additionally, we have applied this methodology to the synthesis of the cis-isomers using commercially available cis-1,4-dichloro-2-butene (6). Furthermore, these procedures allow for the recovery of the majority of the Ag(I) ions which would reduce the cost if these compounds were to be prepared commercially.
Scheme 1.
As shown in Scheme 2, reaction of 5 with AgOTs (2.5 equivalents) in refluxing CH3CN for 3 hours afforded 1 in 95% yield after silica gel purification (see Supplementary Data for experimental details). This reaction was significantly faster (3 hours) than a similar reaction with 1,4-dibromobutane which took 17 hours to afford 1,4-ditosyloxybutane in 91% yield.10 The proposed mechanism8, 9 of the reaction of silver salts with alkyl iodides involves an ion-pair intermediate in the transition state. Presumably, the carbocation formed from 5 is stabilized by being in an allylic position which accelerates the reaction relative to the non-stabilized carbocation that is formed from 1,4-dibromobutane. When 5 was reacted with AgOTs (1 equivalent) in refluxing CH3CN for 2 hours (Scheme 2), compound 4 was obtained in 56% yield and compound 1 was obtained in 20% yield. Thus, the product distribution of 1 and 4 can be controlled by the ratio of AgOTs employed and the reaction time. When compound 4 was reacted with AgF (1.1 equivalents) in refluxing CH3CN for 2 hours, compound 2 was obtained in 51% yield and compound 1 was obtained in 15% yield. The fact that 1 was obtained from this reaction suggests that AgOTs was formed in situ and then reacted with 4. AgOTs could be formed in situ if fluoride ion displaced the tosylate group from 4 or 2 by an SN2 mechanism. The nucleophilicity of AgF was tested by reacting 1 with AgF (1.2 equivalents) in refluxing CH3CN for 2 hours which afforded 2 in 27% yield (unreacted 1 was recovered in 59% yield). Thus, the fluoride ion of AgF is capable of nucleophilic displacement of a tosylate group. This was repeated by reacting 1 with KF/18-crown-6 in refluxing CHCl3 for 16 hours which produced 2 in 38% yield (unreacted 1 was recovered in 33% yield). Thus, it is not clear if AgF reacts with alkyl bromides through an SN2 mechanism, through the previously proposed ion-pair mechanism,8, 9 or a combination of both. For example, in the reaction of 4 with AgF, fluoride ion could displace the tosylate group of 4 to give 1-bromo-4-fluoro-2-butene which would then react with in situ-generated AgOTs to give 2. Alternatively, Ag ion could abstract bromide from 4 to give the ion pair8, 9 which would then react with fluoride to give 2.
Scheme 2.
Compound 2 can also be prepared directly from 5 in one step by the following three methods (performed side-by-side on the same day and with the same molar scale of 5; see experimental details in the Supplementary Data): 1) Compound 4 was prepared in situ by refluxing 5 and AgOTs (1 equivalent) for 1 hour in CH3CN, and then converted to 2 by addition of AgF (1.25 equivalents) and refluxing for 1 hour. After purification, 2 was obtained in 40% yield and 1 was obtained in 27% yield. 2) 1-Bromo-4-fluoro-2-butene was prepared in situ by refluxing 5 and AgF (1.3 equivalents) for 1 hour in CH3CN, and then converted to 2 by addition of AgOTs (1 equivalent) and refluxing for 1 hour. After purification, 2 was obtained in 27% yield and 1 was obtained in 16% yield. This method was repeated on a separate day and on a slightly larger scale and afforded 2 in 40% yield and 1 in 10% yield. 3) Compound 5, AgOTs (1 equivalent), and AgF (1.3 equivalents) were stirred for 2 hours in refluxing CH3CN. After purification, 2 was obtained in 24% yield and 1 was obtained in 21% yield. Thus, various procedures have been developed that allow 2 to be prepared from 1, 4, or 5. In all cases the sample of 2 had minor baseline impurities in the 1H NMR spectrum (see Figures S6 – S10, Supplementary Data) with the least amount of impurities when 2 was prepared from 5 by Methods 2 and 3, and more impurities when 2 was prepared from 4. The amount of impurities did not increase with storage as 2 was shown to be stable for >1 year when stored in a freezer (Figure S6, Supplementary Data). Furthermore, the impurities did not interfere with the ability to use 2 as an N-alkylating agent.
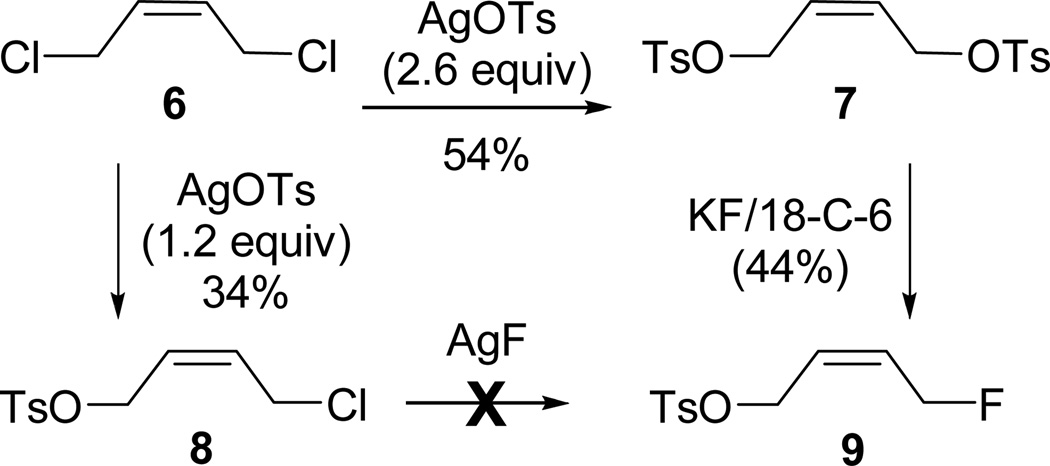
These procedures were then applied to the preparation of the cis-isomers (Scheme 3). Reaction of 6 with AgOTs (2.6 equivalents) in refluxing CH3CN for 15 hours afforded 7 in 54% yield after purification (a mixture of 7 and 8 was also isolated). The lower yield of 7 and longer reaction time required relative to the preparation of 1 may be the result of lower reactivity of chlorides relative to bromides during formation of the ion-pair intermediate.8, 9 Compound 8 was obtained in 34% yield after reacting 6 with AgOTs (1.2 equivalents) in refluxing CH3CN for 75 minutes. Conversion of 8 to 9 using AgF was not successful, and attempted preparation of 9 directly from 6 was also not successful after several attempts, except for one instance where 9 was obtained in 2% yield. Therefore, 9 was prepared from 7 using KF/18-crown-6 in CHCl3 and was obtained in 44% yield after purification (with 40% recovery of unreacted 7).
Scheme 3.
Herein, we have described improvements in the preparation of 1,4-substituted-2-butenes. Previously, compound 1 was obtained by ditosylation of trans-1,4-dihydroxy-2-butene1–5, 11, 12 which was obtained by reduction of 1,4-dihydroxy-2-butyne,1, 4, 13, 14 reduction of dimethylfumarate,2, 5 hydrolysis of trans-1,4-diacetoxy-2-butene,3, 11, 15, 16 or cross-metathesis of allyl alcohol.12 The previous methods all required multiple steps whereas our new method allows 1 to be obtained in high yield after a single step and mild conditions from commercially available 5. This new methodology also provides a simplified preparation of 7 compared to the previously reported procedure.17 Furthermore, we have developed a simple method of preparing unsymmetrical 1,4-substituted-2-butenes which only relies on adjusting the ratio of Ag(I) salt to starting material and using shorter reaction times. Compound 2 can now easily be obtained in a single step with a 2-hour reaction time followed by a simple purification. This methodology also affords easy access to 4 which otherwise would have to be prepared from trans-1,4-dihydroxy-2-butene6 or by reduction of methyl 4-bromocrotonate to give trans-1-bromo-4-hydroxy-2-butene18 followed by tosylation of the alcohol. Additionally, these procedures allow for the simple preparation of 7, 8 and 9 which now provides easier access to cis-halo-2-butenylamines.19, 20
In summary, we have developed a simplified and shorter procedure for the preparation of 1, 2, and 4 which allows these compounds to be prepared in a single step from commercially available 5 and Ag(I) salts. These procedures also allow for the recovery of the Ag(I) halides and any unreacted AgOTs. Furthermore, these procedures can be used to prepare 7 and 8 from commercially available 6, and compound 9 can be prepared from 7 using KF/18-crown-6. In all cases of our new methodology stereochemical integrity is maintained.
Supplementary Material
Acknowledgement
This research was sponsored by NIH/NIMH (1-R21-MH-66622-01 and 2U19 MH069056).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Complete experimental details, 1H and 13C NMR data, and 1H spectra. This information is available for free online at
References
- 1.Chen P, Kilts CD, Camp VM, Ely TD, Keil R, Malveaux E, Votaw J, Hoffman JM, Goodman MM. Synthesis, characterization and in vivo evaluation of (N-(E)-4-[18F]fluorobut-2-en-1-yl)-2β-carbomethoxy-3β-(4-substituted-phenyl)nortropanes for imaging DAT by PET. J. Labelled Cpd. Radiopharm. 1999;42(Supplement 1):S400. [Google Scholar]
- 2.Dollé F, Emond P, Mavel S, Demphel S, Hinnen F, Mincheva Z, Saba W, Valette H, Chalon S, Halldin C, Helfenbein J, Legaillard J, Madelmont J-C, Deloye J-B, Bottlaender M, Guilloteau D. Synthesis, radiosynthesis and in vivo preliminary evaluation of [11C]LBT-999, a selective radioligand for the visualisation of the dopamine transporter with PET. Bioorg. Med. Chem. 2006;14:1115–1125. doi: 10.1016/j.bmc.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Stehouwer JS, Daniel LM, Chen P, Voll RJ, Williams L, Plott SJ, Votaw JR, Owens MJ, Howell L, Goodman MM. Synthesis, Fluorine-18 Radiolabeling, and Biological Evaluation of N-((E)-4-Fluorobut-2-en-1-yl)-2β-carbomethoxy-3β-(4'-halophenyl)nortropanes: Candidate Radioligands for In Vivo Imaging of the Brain Dopamine Transporter with Positron Emission Tomography. J. Med. Chem. 2010;53:5549–5557. doi: 10.1021/jm100269c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman MM, Chen P. WO 2000064490 A1. Fluoroalkenyl Nortropanes. 2000 Nov 2;
- 5.Dollé F, Hinnen F, Emond P, Mavel S, Mincheva Z, Saba W, Schöllhorn-Peyronneau M-A, Valette H, Garreau L, Chalon S, Halldin C, Helfenbein J, Legaillard J, Madelmont J-C, Deloye J-B, Bottlaender M, Guilloteau D. Radiosynthesis of [18F]LBT-999, A Selective Radioligand for the Visualization of the Dopamine Transporter with PET. J. Label. Compd. Radiopharm. 2006;49:687–698. [Google Scholar]
- 6.Dollé F, Helfenbein J, Hinnen F, Mavel S, Mincheva Z, Saba W, Schöllhorn-Peyronneau M-A, Valette H, Garreau L, Chalon S, Halldin C, Madelmont J-C, Deloye J-B, Bottlaender M, Le Gailliard J, Guilloteau D, Emond P. One-Step Radiosynthesis of [18F]LBT-999: A Selective Radioligand for the Visualization of the Dopamine Transporter with PET. J. Label. Compd. Radiopharm. 2007;50:716–723. [Google Scholar]
- 7.Emmons WD, Ferris AF. Metathetical Reactions of Silver Salts in Solution. II. The Synthesis of Alkyl Sulfonates. J. Am. Chem. Soc. 1953;75:2257. [Google Scholar]
- 8.Hoffmann HMR. The Preparation of Unstable Toluene-p-Sulphonates. J. Chem. Soc. 1965:6748–6753. [Google Scholar]
- 9.Hammond GS, Hawthorne MF, Waters JH, Graybill BM. Ion-Pair Formation in the Reactions of Alkyl Iodides with Silver Salts. J. Am. Chem. Soc. 1960;82:704–711. [Google Scholar]
- 10.Stehouwer JS, Birnbaum MS, Voll RJ, Owens MJ, Plott SJ, Bourke CH, Wassef MA, Kilts CD, Goodman MM. Synthesis, Structure-Activity Relationships, F-18 Radiolabeling, and MicroPET Evaluation of 3-(2,4-Dichlorophenyl)-N-alkyl-N-fluoroalkyl-2,5-dimethylpyrazolo[1,5-a]pyrimidin-7-amines as Ligands of the Corticotropin-Releasing Factor Type-1 (CRF1) Receptor. 2015 doi: 10.1016/j.bmc.2015.06.036. Submitted for review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asuma Y, Suzuki T, Hidaka T, Asada K, Miyake R, Dekishima Y, Kawabata H. US 2013/0096339 A1; PCT/JP2011/053285. Method For Producing 1-Amino-1-alkoxycarbonyl-2-vinylcyclopropane. 2013
- 12.Edwards GA, Culp PA, Chalker JM. Allyl Sulphides in Olefin Metathesis: Catalyst Considerations and Traceless Promotion of Ring-Closing Metathesis. Chem. Commun. 2015;51:515–518. doi: 10.1039/c4cc07932a. [DOI] [PubMed] [Google Scholar]
- 13.Miller AEG, Biss JW, Schwartzman LH. Reductions with Dialkylaluminum Hydrides. J. Org. Chem. 1959;24:627–630. [Google Scholar]
- 14.Organ MG, Cooper JT, Rogers LR, Soleymanzadeh F, Paul T. Synthesis of Stereodefined Polysubstituted Olefins. 1. Sequential Intermolecular Reactions Involving Selective, Stepwise Insertion of Pd(0) into Allylic and Vinylic Halide Bonds. The Stereoselective Synthesis of Disubstituted Olefins. J. Org. Chem. 2000;65:7959–7970. doi: 10.1021/jo001045l. [DOI] [PubMed] [Google Scholar]
- 15.Raphael RA. Synthesis of Carbohydrates by Use of Acetylenic Precursors. Part II. Addition Reactions of cis- and trans-But-2-ene-1,4-diol Diacetates. Synthesis of DL-Erythrulose. J. Chem. Soc. 1952:401–405. [Google Scholar]
- 16.Bouquillon S, Muzart J. Palladium(0)-Catalyzed Isomerization of (Z)-1,4-Diacetoxy-2-butene - Dependence of η1 – η3-Allylpalladium as a Key Intermediate on the Solvent Polarity. Eur. J. Org. Chem. 2001:3301–3305. [Google Scholar]
- 17.Malanga C, Pagliai L, Menicagli R. Stereocontrolled Preparation of (Z)-1,4-But-2-endiol Esters in Phase-Transfer Conditions. Synth. Commun. 1990;20:2821–2826. [Google Scholar]
- 18.Kinoshita M, Takami H, Taniguchi M, Tamai T. An Enantiospecific Synthesis of the C-21 – C-37 Segment of the Aglycon of Amphotericin B. Bull. Chem. Soc. Jpn. 1987;60:2151–2161. [Google Scholar]
- 19.Casara P, Marchal P, Wagner J, Danzin C. 5'-{[(Z)-4-Amino-2-butenyl]methylamino}-5'-deoxy-adenosine: A Potent Enzyme-Activated Irreversible Inhibitor of S-Adenosyl-L-methionine Decarboxylase from Escherichia coli. J. Am. Chem. Soc. 1989;111:9111–9113. [Google Scholar]
- 20.Hsin L-W, Webster EL, Chrousos GP, Gold PW, Eckelman WC, Contoreggi C, Rice KC. Synthesis of [3H](4-Fluorobutyl)propyl[2,5,6-trimethyl-7-(2,4,6-trimethylphenyl)pyrrolo[2,3-d]pyrimidin-4-yl]amine: A Potent Radioligand for Corticotropin-Releasing Hormone Type 1 Receptor. J. Label. Compd. Radiopharm. 2000;43:899–908. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.