Abstract
Entamoeba histolytica is a diarrheal pathogen with the ability to cause profound host tissue damage. This organism possesses contact-dependent cell killing activity, which is likely to be a major contributor to tissue damage. E. histolytica trophozoites were recently shown to ingest fragments of living human cells. It was demonstrated that this process, termed amoebic trogocytosis, contributes to cell killing. Recent advances in ex vivo and 3-D cell culture approaches have shed light on mechanisms for tissue destruction by E. histolytica, allowing amoebic trogocytosis to be placed in the context of additional host and pathogen mediators of tissue damage. In addition to its relevance to pathogenesis of amoebiasis, an appreciation is emerging that intercellular nibbling occurs in many organisms, from protozoa to mammals.
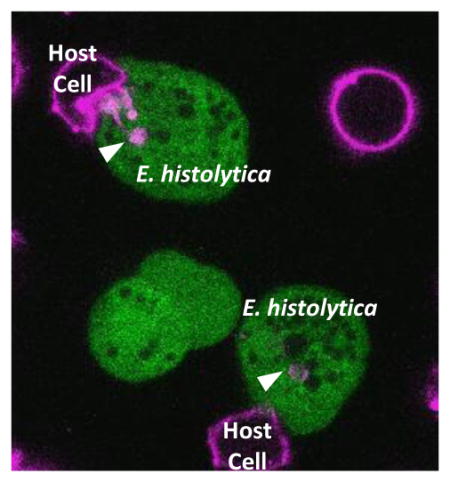
Graphical Abstract
Introduction
Entamoeba histolytica was named for its ability to damage the large intestine and other tissues (histo-: tissue; lytic-: dissolving), which is likely driven by its potent cell killing activity. It was recently shown that E. histolytica trophozoites kill human cells by biting off and ingesting distinct fragments of host cellular material, which was termed amoebic trogocytosis (trogo-: nibble). Amoebic trogocytosis occurred when E. histolytica trophozoites interacted with ex vivo mouse intestine, suggesting it plays a role in tissue damage. In addition to its apparent relevance to pathogenesis of amoebiasis, the discovery of trogocytosis in E. histolytica fits with an emerging appreciation that many organisms, from protozoa to mammals, undergo intercellular nibbling. This review discusses the mechanism and biology of cell nibbling, including its relevance to the tissue-dissolving properties of E. histolytica.
Amoebiasis
E. histolytica is the agent of amoebiasis in humans and is responsible for an estimated 50,000,000 diarrheal infections and 100,000 deaths per year. This infection is remarkably common in children in developing nations (1, 2). Following ingestion of the cyst form that contaminates water sources, excystation occurs and the motile, actively dividing trophozoite form colonizes the large intestine. Trophozoites are extracellular throughout infection, and can encyst to produce new cysts that are passed in the feces. Trophozoites can remain luminal, resulting in asymptomatic colonization or diarrhea. They can also invade the intestine resulting in colitis with profound ulceration. Trophozoites that have invaded the intestine can spread to other tissues, most commonly the liver, where they result in potentially fatal abscesses.
Amoebic Trogocytosis in E. histolytica
E. histolytica trophozoites possess potent, contact-dependent cell killing activity (3–5). This is likely to be a driver of host tissue damage, but the mechanism for cell killing has been unclear (6). It was recently shown that amoebic trophozoites rapidly ingested “bites” of host material after host cell contact (Figure 1A) and preceding host cell death (7). The ingestion of bites of host cell material was similar to phagocytosis, but portions of host cells were ingested, rather than entire cells. This process was termed “amoebic trogocytosis,” in recognition of potential similarity to cell nibbling processes that had been described in other organisms (see below). Interestingly, “stretching” or “suction” of host cell material into the amoeba had been previously described during E. histolytica ingestion (8–10). Host cell material can be seen “stretched” into the amoeba during amoebic trogocytosis (e.g., Figure 1A, top panel and Figure 1B, second panel from left), suggesting that ingested bites may potentially fragment off of stretched host cell material.
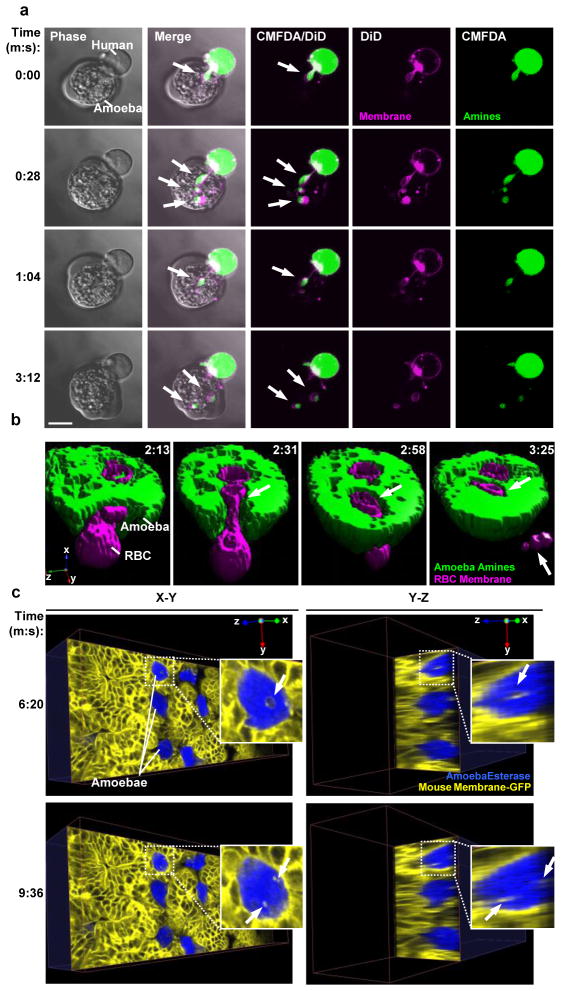
Figure 1. Amoebic trogocytosis occurs with cells and tissues relevant to invasive amoebiasis.
a, Live confocal microscopy demonstrating amoebic trogocytosis of human T cell material. Human Jurkat T cells were pre-labeled with DiD (pink) and CMFDA (green). H, human cell; A, amoeba. Arrows, ingested “bites.” Time is indicated in minutes:seconds. Bar, 10 μm. b, Live 4-D confocal microscopy (shown as a surface-rendered 3-D reconstruction) demonstrating amoebic trogocytosis of a human red blood cell. Human red blood cells were pre-labeled with DiD (pink) and amoebae were pre-labeled with CMFDA (green). Internalization of a bite (arrow) of the red blood cell can be seen over the time course, and a fragment of the red blood cell that remains extracellular and not internalized is also seen (arrow at T= 3:25). c, Live 4-D multiphoton microscopy demonstrating amoebic trogocytosis of mouse intestinal cells. ex vivo mouse cecal tissue was from a mouse expressing membrane-targeted enhanced green fluorescent protein (EGFP) (yellow false color) and amoebae were pre-labeled with calcein violet (blue). Shown are X-Y and Y–Z planes from a 3-D reconstruction with arrows indicating internalized enterocyte bites. All panels are reprinted with permission from (7).
Bites of host cell material ingested by E. histolytica trophozoites contained host membrane and cytoplasm (Figure 1A), and sometimes contained mitochondria (7). Amoebic trogocytosis occurred with Jurkat T lymphocytes, Caco-2 intestinal epithelial cells, human red blood cells (Figure 1B), and during interaction with ex vivo mouse intestinal tissue (Figure 1C) (7). Amoebic trogocytosis was inhibited by incubation on ice, treatment with cytochalasin D, anti-Gal/GalNAc lectin blocking antibodies, an EhC2PK dominant negative mutant, and treatment with wortmannin (7). Hence, this process requires physiological temperature, actin rearrangements, Gal/GalNAc lectin, EhC2PK and PI3K signaling (Figure 2) (7). In all cases when amoebic trogocytosis was inhibited, host cell killing was also quantitatively reduced (7). Host cell calcium intracellular calcium became elevated during amoebic trogocytosis and prior to host cell death (Figure 2). Host cells eventually were killed, as evidenced by loss of membrane integrity and loss of mitochondrial potential. This process was specific to live human cells since trophozoites ceased ingestion once human cells had been killed (Figure 2).
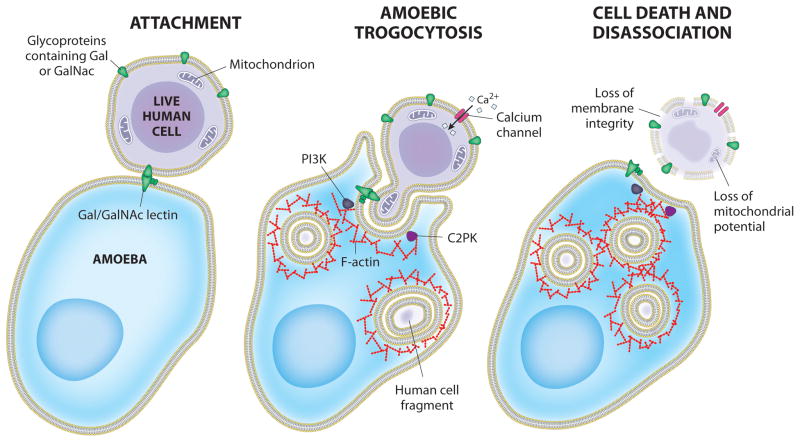
Figure 2. Model for host cell killing via amoebic trogocytosis.
Attachment to host glycoproteins containing D-galactose (Gal) or N-acetyl-D-galactosamine (GalNAc) is mediated by the amoeba surface (Gal/GalNAc)-specific lectin. Following attachment, amoebic trogocytosis is initiated. Signal transduction in the initiation of amoebic trogocytosis includes PI3K and EhC2PK, both of which influence actin polymerization. Host cell intracellular calcium becomes elevated through the activation of calcium channels. Tehe ingestion of fragments of host cell material eventually leads to host cell death. Host cell death can be assessed by the loss of membrane integrity and mitochondrial potential. Following cell killing, amoebae dissociate from the killed cells
Mechanism and Biology of Trogocytosis
It is not yet clear whether the underlying mechanisms are shared, but it has become clear that intercellular nibbling occurs in a variety of protozoa as well as multicellular organisms, and it plays many biological roles (Figure 3).
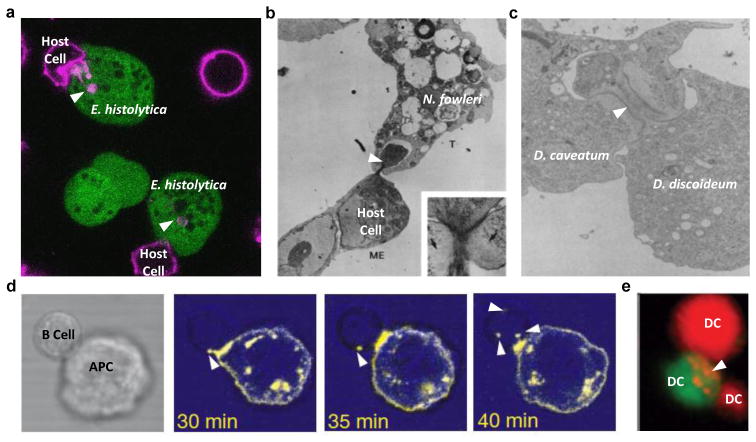
Figure 3. Trogocytosis in amoebae and multicellular organisms.
a, Amoebic trogocytosis between E. histolytica and mammalian cells. Live confocal microscopy with human Jurkat cells pre-labeled with DiD (pink) and amoebae pre-labeled with CMFDA (green); arrowheads indicate internalized bites. b, Trogocytosis between N. fowleri and mammalian cells. Transmission electron micrograph demonstrating ingestion of a bite (arrowhead) of a mouse embryo cell by an N. fowleri trophozoite. Reprinted with permission from (13). c, Trogocytosis between Dictyostelium species. Transmission electron micrograph demonstrating ingestion of a bite (arrowhead) of a D. discoideum cell by a D. caveatum cell. Reprinted with permission from (16). d, Trogocytosis between B cells and antigen presenting cells. Live fluorescence microscopy demonstrating acquisition of fragments (arrowheads) by a HEL-specific B cell interacting with an mHEL-GFP (yellow false color) target cell. Reprinted with permission from (18). e, Trogocytosis between dendritic cells. Live microscopy with dendritic cells pre-labeled with DiI (red) or CMFDA (green). Ingestion of fragments (arrowheads) can be seen. Reprinted with permission from (59).
Amoebae
Amoebae do not comprise a taxonomic group, thus it is notable that nibbling has thus far been observed in amoebae from two distinct eukaryotic supergroups, the Amoebozoa (e.g., Entamoeba, Hartmanella and Dictyostelium) and Excavates (e.g., Naegleria) (Figure 3A–C). There were initially reports of “nibbling, piecemeal” ingestion of red blood cells by Naegleria fowleri and Hartmanella (11, 12). These amoebae also ingested nucleated cells in a nibbling fashion, but did not ingest the nuclei (11, 12). The term trogocytosis was later coined in more detailed studies of the process in N. fowleri (Figure 3B) (13). After co-incubation with target cells, it was shown that N. fowleri trophozoites contained distinct “bites” of target cells, and in ultrastructural studies, target cell mitochondria could be identified within the ingested bites (13). During N. fowleri trogocytosis and prior to target cell death, targeted cells appeared morphologically normal and their cell membranes remained intact. Treatment with the actin filament polymerization inhibitor cytochalasin B inhibited both the nibbling process and the cytopathic effect (13, 14). A process termed “nibbling” has also been described in Dictyostelium caveatum during predation of other Dictyostelium species (Figure 3C) (15, 16).
Multicellular organisms
In addition to the occurrence of nibbling processes in amoebae, a similar process that is also termed trogocytosis occurs in mammals (17). This was first seen at the immunological synapse, where lymphocytes obtained fragments from target cells (Figure 3D) (18–21). Trogocytosis is now recognized to occur with T cells, B cells, natural killer (NK) cells, and antigen-presenting cells (Figure 3E). The ingested material is thought to primarily consist of membrane, based on some studies that have been unable to detect transfer of cytosolic dyes (e.g., (22)). Transfer can be uni-directional (one cell obtains bites from the other) or bi-directional (both cells obtain bites from each other) and typically involves specific ligand-receptor engagement. This leads to the direct transfer of engaged molecules, together with the indirect transfer of other molecules in the transferred fragment (22).
Expanding the occurrence of trogocytosis beyond mammalian immune cells to other organisms and cell types, retinal pigment epithelium cells appear to extract bites containing the outer segment distal tips of rod photoreceptor cells in Xenopus laevis (23). Potentially representing trogocytosis, transfer of membrane proteins has been seen in the Drosophila melanogaster eye (24), in mouse fibroblasts (25), in HeLa cells (26), and between oocytes and fertilizing spermatozoa in mice (27). Transfer of membrane proteins, possibly together with cytoplasm, has been detected between Plasmodium falciparum-infected red bloods cells and endothelial cells (28). Trogocytosis has also been reported to occur between mammalian cell lines and Trypansoma cruzi (29).
Molecular mechanism
The molecular mechanisms underlying trogocytosis are not well understood in any organism, and it is not known if the mechanistic underpinnings are shared. It is possible that processes that have been termed trogocytosis and/or nibbling are similar in name only. It is also not yet clear whether the mechanisms underlying phagocytosis (whole cell ingestion) and trogocytosis are distinct. The mechanism for phagocytosis is the best characterized by the paradigm of Fc receptor-mediated phagocytosis. In the initiation of Fc receptor-mediated phagocytosis, clustering of Fc receptor proteins leads to phosphorylation of the intracellular domain of the receptors by the membrane-associated kinase Src. The phosphorylated receptor in turn recruits the kinase Syk, which activates many downstream signals, including lipid-modifying enzymes (e.g., PI3K and phospholipase C), kinases (e.g., PKC), and small GTPases (e.g., Rac and Cdc42), resulting in actin reorganization and pseudopod extension (30). Initiation of phagocytosis in E. histolytica has been experimentally demonstrated to similarly involve numerous receptors, PI3K, transmembrane kinases and small GTPases, as well as an expanded family of calcium-binding proteins (31).
Trogocytosis has been shown to require actin rearrangements in E. histolytica, N. fowleri, and T cells and NK cells (7, 13, 32, 33). In both E. histolytica and T cell trogocytosis, PI3K signaling plays a role (7, 32), but it does not appear as critical in NK cell trogocytosis (33). The shared requirements for actin and PI3K in both phagocytosis and trogocytosis suggest mechanistic overlap between phagocytosis and trogocytosis. E. histolytica trogocytosis also involves the amoebic C2-kinase EhC2PK, which initiates phagocytosis in this organism (34). In T cell trogocytosis, two small GTPases have been identified that are involved, TC21 and RhoG (35). RhoG has an established role in phagocytosis, again suggesting overlap with phagocytosis (35). Trogocytosis by CD4+ T cells also involves Src and Syk signaling (32, 35). Trogocytosis by NK cells also involves Src signaling, and is enhanced by drugs that increase intracellular calcium or PKC activity (33).
Potentially hinting at differences between phagocytosis and trogocytosis, E. histolytica clearly has receptors that bind to live cell targets (e.g., the Gal/GalNAc lectin), and different receptors that bind to dead cell targets (e.g., calreticulin and the serine-rich E. histolytica protein SREHP) (31). Since ameobic trogocytosis is specific to live cells, and phagocytosis occurs with pre-killed cells (7), this suggests that different receptors may activate different amoebic trogocytosis and phagocytosis. Given the expanded families of calcium-binding proteins with roles in E. histolytica ingestion, and the expanded families of genes involved in vesicle trafficking (31), it seems possible that distinct intracellular machinery might be engaged for each process.
Biology of trogocytosis
Thus far, reports of trogocytosis by unicellular organisms are tied to killing of other species. In contrast, trogocytosis has not yet been tied to cell death in multicellular organisms. The reason for this distinction is not yet apparent, but this may be because the described examples of trogocytosis in multicellular organisms appear to involve the ingestion of fewer bites, and these bites are thought to be primarily fragments of cell membrane. In contrast, bites of host cells ingested by E. histolytica and N. fowleri can contain cytoplasm and mitochondria in addition to membrane, potentially resulting in greater damage to the host cell (7, 13).
Trogocytosis can also impact cell signaling. For example, receptors that are transferred can be functional, leading to activation of downstream signaling pathways in the recipient cell (36). Conversely, the loss of a receptor protein via trogocytosis may dampen signal transduction (35). The acquisition of proteins not normally found in a particular cell type may endow it with uncharacteristic phenotypes, or influence its ability to signal to other cells (37). In immune cells, trogocytosis in different contexts could potentially stimulate or downregulate immune responses. However, the impact of trogocytosis on immune responses in whole animals is not yet clear. In microbes, trogocytosis could potentially serve a cell-signaling role by allowing cells to sense their environment and respond appropriately to a particular niche.
Additionally, it is possible that trogocytosis could also serve a nutritional role. Transfer of membrane appears to be a common feature of trogocytosis, and it has been suggested that acquisition of lipids by the recipient cell could be beneficial since lipids are energetically costly to synthesize (17). However, a nutritional function for trogocytosis has not yet been directly demonstrated.
Amoebic Trogocytosis and Tissue Damage in Amoebiasis
Amoebic trogocytosis when E. histolytica trophozoites interacted with ex vivo mouse intestine, suggesting relevance to tissue damage. As outlined below, recent approaches employing ex vivo tissue and 3-D culture have shed light on mechanisms for tissue destruction, allowing amoebic trogocytosis to be placed in the context of additional host and pathogen mediators of damage (Figure 4).
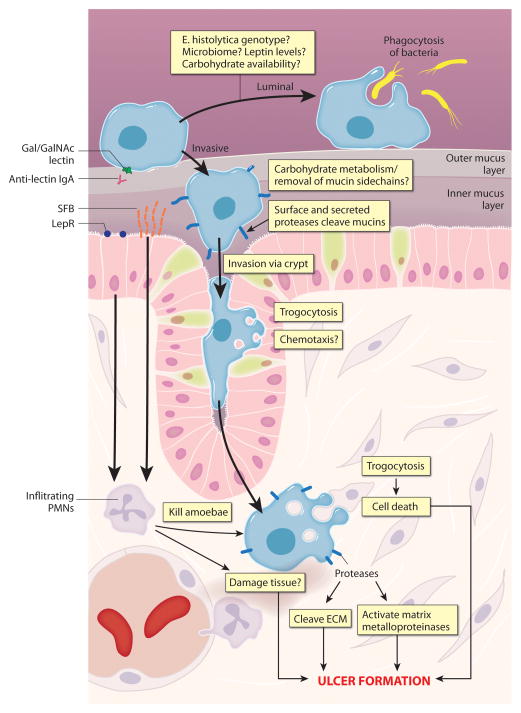
Figure 4. Model for tissue destruction in amoebiasis.
Model for host and pathogen factors contributing to intestinal damage. Amoebic trophozoites can attach to intestinal mucus via the Gal/GalNAc lectin. Host IgA directed to the carbohydrate recognition domain of the Gal/GalNAc lectin is associated with protection, and is likely to directly interfere with amoebic attachment to host substrates. Trophozoites can remain in the intestinal lumen and can undergo phagocytosis of bacteria. Infection can become invasive for unknown reasons. Whether an infection becomes invasive or not is likely to be impacted by such factors as host leptin levels, carbohydrate availability, the composition of the gut microbiome, and the E. histolytica genotype. Amoebic carbohydrate metabolism may play a role in removal of mucin side chains. Amoebic cysteine proteases, including CP-A5, contribute to invasion, most likely by acting on mucins and extracellular matrix proteins. Trophozoites invade via the intestinal crypts, which may involve chemotaxis. Amoebic trogocytosis occurs during this time. Once in the submucosa, trophozoites are likely to continue to undergo amoebic trogocytosis, which may drive tissue damage. Cysteine proteases acting on collagen and other ECM components may further disrupt tissue, and also activate host matrix metalloproteinases. Host responses, particularly involving neutrophils, are critical for protection from E. histolytica. Differential polymorphonuclear neutrophil (PMN) recruitment to the lamina propria may underlie differential susceptibility to amoebiasis associated with leptin receptor polymorphisms. PMN recruitment is also associated with protection from E. histolytica in segmented filamentous bacteria (SFB)-colonized mice. SFB colonize the small intestine of mice but appear to have systemic effects. Hence, infiltrating PMN are correlated with protection. However, it is possible that in some cases, PMN and associated inflammatory reactions could exacerbate tissue damage.
Determinants of tissue damage
Tissue damage does not always occur, and what leads trophozoites to invade and damage tissue is not fully apparent. Some strains of E. histolytica are associated with invasive infection (38). Host genetics play a role, since a polymorphism in the leptin receptor is associated with resistance to E. histolytica infection (39). Age and gender are determinants of spreading to other tissues, since liver abscesses occur 10 times more often in men than women and are uncommon in children (40). By affecting both the host and E. histolytica, it is also possible that the intestinal microbiome has an impact (41).
Carbohydrate metabolism
A gene expression study using ex vivo human intestine found that genes involved in carbohydrate metabolism were highly expressed in trophozoites incubated with intestinal tissue (42). dsRNA silencing of a family of amoebic β–amylase genes led to a decrease in mucus degradation and invasion depth. This potentially suggests that degradation of carbohydrate side chains could expose intestinal mucins for cleavage by amoebic proteases (Figure 4) (42). Fitting with a potential role for carbohydrate metabolism in tissue damage, glucose starvation enhances the ability of E. histolytica to attach to and kill host cells in vitro (43).
Cysteine proteases
Studies using ex vivo human intestine showed that cysteine proteases are involved in tissue invasion and damage. Trophozoites epigenetically silenced for expression of the pore-forming protein amoebapore A and the major cysteine protease CP-A5 appeared less capable of invasion (44). Subsequent studies showed that amoebic cysteine proteases alter the host collagen network (45). More recently, it has been suggested that CP-A5 activates human matrix metalloproteinases (46). Together these studies point to a role for amoebic cysteine proteases in degradation of mucus, and both amoebic and host proteases in degradation of extracellular matrix (Figure 4). Amoebic cysteine proteases are also implicated in degradation of antimicrobial peptides, complement, and antibodies (47, 48).
Amoebic trogocytosis
In addition to cleavage of extracellular matrix by proteases, direct killing of host cells is likely to drive tissue damage. Killing of intestinal epithelial cells may allow trophozoites to breach the barrier of the intestine. Consistent with this idea, amoebic trogocytosis can be detected during invasion of ex vivo mouse intestine and pharmacological inhibition of amoebic trogocytosis reduced invasion depth (7). Once trophozoites have breached the barrier of the intestine, cell killing via amoebic trogocytosis is likely to directly promote tissue damage and ulcer formation. Additionally, since amoebic trophozoites do not ingest cells that they have killed (7), discarded dead cell corpses could exacerbate inflammation. Thus amoebic trogocytosis appears to contribute to initial invasion of tissue, and is likely to both directly and indirectly drive tissue damage once invasion has occurred.
Liver models
Less is known about factors that drive spreading of amoebic trophozoites to other tissues, and what drives abscess formation in these sites. Slices of hamster liver have been used to model interactions occurring in liver abscess (49, 50). A 3-D culture approach was also recently developed, consisting of liver sinusoidal endothelial cells (LSEC), hepatocytes, and layers of collagen (51). These studies identified a role for the host immune regulators galectin-1 and galectin-3 in trophozoite adhesion to LSEC and in stimulating cytokine release (51). Trophozoites invaded the LSEC layer, and fragments of LSEC were seen inside of trophozoites, potentially reflecting the occurrence of amoebic trogocytosis (51). This suggests that amoebic trogocytosis may also contribute to invasion and damage of the liver.
Inflammatory response
Given that after amoebic trogocytosis, trophozoites do not ingest cells that they have killed (7), discarded dead cell corpses could potentially promote inflammation. Recent studies on the host response point to the importance of neutrophil recruitment in the lamina propria (41, 52) (Figure 4). However, virulent E. histolytica trophozoites are capable of killing neutrophils (53), and neutrophils are recognized to contribute to tissue damage in other diseases by releasing proteases and toxic metabolites (54). Thus, neutrophils, and potentially other pro-inflammatory responses could play a role in tissue damage. Consistent with this idea, in a mouse model of liver abscess, kupffer cells and Ly6C(high) monocytes drive the formation of larger abscesses, (55).
Recent studies have also revealed that contact with amoebic trophozoites directly activates inflammation. In intestinal epithelial cells, CP-A5 was shown to trigger an inflammatory response by binding αvβ3 integrin (56). In macrophages, the NLRP3 inflammasome is activated in a mechanism that requires attachment mediated by the Gal/GalNAc lectin and the activity of CP-A5, leading to the recruitment of α5β1 integrin and NLRP3 to the macrophage-amoeba junction (57, 58). Activation of the NLRP3 inflammasome results in the activation and release of the pro-inflammatory cytokine 1L-1β (58). Thus, CP-A5 directly promotes inflammation by engaging with host integrins, in addition to its roles in degradation of host substrates.
Conclusions
Trogocytosis is now developing as a theme in a variety of cytotoxic amoebae. In multicellular organisms, the list of cell types that undergo trogocytosis continues to grow. Intercellular nibbling is likely to be more common than presently appreciated. One challenge will be to better understand the underlying mechanism(s). Presently it is not clear if cell nibbling in amoebae and in multicellular organisms is similar in name only, or whether the hints of similarities reflect commonalities. An additional important challenge is to ascribe in vivo relevance to this form of intercellular exchange. In immune cell trogocytosis, it has not always been clear whether the in vitro occurrence of trogocytosis translates to the immune response in vivo.
In E. histolytica, the detection of amoebic trogocytosis during interactions with intestinal tissue explants suggests that it contributes to pathogenesis. While a complete picture of mediators of tissue damage in amoebiasis is still emerging, the recent development of ex vivo and 3-D culture approaches nicely complements available whole animal models, and will likely lead to a more holistic view of the host and pathogen factors that initiate and exacerbate tissue destruction. Further studies of amoebic trogocytosis may both improve understanding of the tissue-dissolving properties of this organism, and uncover shared mechanisms for intercellular exchange.
Highlights.
E. histolytica trophozoites ingest bites of host cell material
This contributes to host cell killing and likely contributes to tissue damage
Advances in ex vivo approaches have improved understanding of other aspects of tissue damage
Shared features of cell nibbling in other organisms are discussed
Acknowledgments
I am grateful to colleagues for thoughtful comments on the manuscript, and apologize to those whose work could not be covered owing to space limitations. I thank Anita Impagliazzo for preparing the artwork in Figures 2 and 4. Work in my laboratory is supported by an NIH NIAID Career Transition Award (1K22AI108814).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as: *of special interest, **of outstanding interest
- 1.WHO/PAHO/UNESCO report. Epidemiol Bull; A consultation with experts on amoebiasis; Mexico City, Mexico. 28–29 January, 1997; 1997. pp. 13–4. [PubMed] [Google Scholar]
- 2.Korpe PS, Liu Y, Siddique A, Kabir M, Ralston K, Ma JZ, Haque R, Petri WA., Jr Breast milk parasite-specific antibodies and protection from amebiasis and cryptosporidiosis in Bangladeshi infants: a prospective cohort study. Clin Infect Dis. 2013;56(7):988–92. doi: 10.1093/cid/cis1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralston KS, Petri WA., Jr Tissue destruction and invasion by Entamoeba histolytica. Trends Parasitol. 2011;27(6):254–63. doi: 10.1016/j.pt.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravdin JI, Croft BY, Guerrant RL. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980;152(2):377–90. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravdin JI, Guerrant RL. Studies on the cytopathogenicity of Entamoeba histolytica. Arch Invest Med (Mex) 1980;11(1 Suppl):123–8. [PubMed] [Google Scholar]
- 6.Ralston KS. Chew on this: Amoebic trogocytosis and host cell killing by Entamoeba histolytica. Trends Parasitol. 2015 doi: 10.1016/j.pt.2015.05.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata, Bhattacharya A, Petri WA., Jr Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature. 2014;508(7497):526–30. doi: 10.1038/nature13242. Demonstration that E. histolytica trophozoites undergo trogocytosis of human cells, and that this contributes to human cell killing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lejeune A, Gicquaud C. Evidence for two mechanisms of human erythrocyte endocytosis by Entamoeba histolytica-like amoebae (Laredo strain) Biol Cell. 1987;59(3):239–45. doi: 10.1111/j.1768-322x.1987.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakada-Tsukui K, Okada H, Mitra BN, Nozaki T. Phosphatidylinositol-phosphates mediate cytoskeletal reorganization during phagocytosis via a unique modular protein consisting of RhoGEF/DH and FYVE domains in the parasitic protozoon Entamoeba histolytica. Cell Microbiol. 2009;11(10):1471–91. doi: 10.1111/j.1462-5822.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Palomo A, Gonzalez-Robles A, Chavez B, Orozco E, Fernandez-Castelo S, Cervantes A. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J Protozool. 1985;32(1):166–75. doi: 10.1111/j.1550-7408.1985.tb03033.x. [DOI] [PubMed] [Google Scholar]
- 11.Chi L, Vogel JE, Shelokov A. Selective phagocytosis of nucleated erythrocytes by cytotoxic amebae in cell culture. Science. 1959;130(3391):1763. doi: 10.1126/science.130.3391.1763. [DOI] [PubMed] [Google Scholar]
- 12.Culbertson CG. The pathogenicity of soil amebas. Annu Rev Microbiol. 1971;25:231–54. doi: 10.1146/annurev.mi.25.100171.001311. [DOI] [PubMed] [Google Scholar]
- 13.Brown T. Observations by immunofluorescence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med Microbiol. 1979;12(3):363–71. doi: 10.1099/00222615-12-3-363. [DOI] [PubMed] [Google Scholar]
- 14.Brown T. Inhibition by amoeba-specific antiserum and by cytochalasin B of the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J Med Microbiol. 1979;12(3):355–62. doi: 10.1099/00222615-12-3-355. [DOI] [PubMed] [Google Scholar]
- 15.Nizak C, Fitzhenry RJ, Kessin RH. Exploitation of other social amoebae by Dictyostelium caveatum. PLoS One. 2007;2(2):e212. doi: 10.1371/journal.pone.0000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waddell DR, Vogel G. Phagocytic behavior of the predatory slime mold, Dictyostelium caveatum. Cell nibbling. Exp Cell Res. 1985;159(2):323–34. doi: 10.1016/s0014-4827(85)80006-9. [DOI] [PubMed] [Google Scholar]
- 17.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4(9):815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 18.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411(6836):489–94. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 19.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 20.Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166(6):3645–9. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- 21.Hudson L, Sprent J, Miller JF, Playfair JH. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature. 1974;251(5470):60–2. doi: 10.1038/251060a0. [DOI] [PubMed] [Google Scholar]
- 22.Vanherberghen B, Andersson K, Carlin LM, Nolte-’t Hoen EN, Williams GS, Hoglund P, Davis DM. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci U S A. 2004;101(48):16873–8. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto B, Defoe DM, Besharse JC. Membrane turnover in rod photoreceptors: ensheathment and phagocytosis of outer segment distal tips by pseudopodia of the retinal pigment epithelium. Proc R Soc Lond B Biol Sci. 1987;230(1260):339–54. doi: 10.1098/rspb.1987.0023. [DOI] [PubMed] [Google Scholar]
- 24.Cagan RL, Kramer H, Hart AC, Zipursky SL. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 1992;69(3):393–9. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- 25.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5(10):879–88. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 26.Anderson SM, Yu G, Giattina M, Miller JL. Intercellular transfer of a glycosylphosphatidylinositol (GPI)-linked protein: release and uptake of CD4-GPI from recombinant adeno-associated virus-transduced HeLa cells. Proc Natl Acad Sci U S A. 1996;93(12):5894–8. doi: 10.1073/pnas.93.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barraud-Lange V, Chalas Boissonnas C, Serres C, Auer J, Schmitt A, Lefevre B, Wolf JP, Ziyyat A. Membrane transfer from oocyte to sperm occurs in two CD9-independent ways that do not supply the fertilising ability of Cd9-deleted oocytes. Reproduction. 2012;144(1):53–66. doi: 10.1530/REP-12-0040. [DOI] [PubMed] [Google Scholar]
- 28.Jambou R, Combes V, Jambou MJ, Weksler BB, Couraud PO, Grau GE. Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions. PLoS Pathog. 2010;6(7):e1001021. doi: 10.1371/journal.ppat.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Mukherjee S, Mukhopadhyay A, Andriani G, Machado FS, Ashton AW, Huang H, Weiss LM, Tanowitz HB. Trypanosoma cruzi invasion is associated with trogocytosis. Microbes Infect. 2015;17(1):62–70. doi: 10.1016/j.micinf.2014.10.009. Potentially expands trogocytosis to ingestion of fragments of protozoa by mammalian cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Garcia E, Rosales C. Signal transduction during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2002;72(6):1092–108. [PubMed] [Google Scholar]
- 31.Ralston KS. Chew on this: amoebic trogocytosis and host cell killing by Entamoeba histolytica. Trends Parasitol. 2015 doi: 10.1016/j.pt.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111(12):5621–8. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabiasco J, Espinosa E, Hudrisier D, Joly E, Fournie JJ, Vercellone A. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur J Immunol. 2002;32(5):1502–8. doi: 10.1002/1521-4141(200205)32:5<1502::AID-IMMU1502>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Somlata, Bhattacharya S, Bhattacharya A. A C2 domain protein kinase initiates phagocytosis in the protozoan parasite Entamoeba histolytica. Nat Commun. 2011;2:230. doi: 10.1038/ncomms1199. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Martin N, Fernandez-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, Irvine DJ, Huang B, Bustelo XR, Shaw A, Alarcon B. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35(2):208–22. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.HoWangYin KY, Caumartin J, Favier B, Daouya M, Yaghi L, Carosella ED, LeMaoult J. Proper regrafting of Ig-like transcript 2 after trogocytosis allows a functional cell-cell transfer of sensitivity. J Immunol. 2011;186(4):2210–8. doi: 10.4049/jimmunol.1000547. [DOI] [PubMed] [Google Scholar]
- 37.Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26(5):1423–33. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali IK, Mondal U, Roy S, Haque R, Petri WA, Jr, Clark CG. Evidence for a link between parasite genotype and outcome of infection with Entamoeba histolytica. J Clin Microbiol. 2007;45(2):285–9. doi: 10.1128/JCM.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC, Jr, Myers MG, Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA., Jr A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. 2011;121(3):1191–8. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petri WA, Haque R. Entamoeba species, including amoebic colitis. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 2014. pp. 3047–3058. [Google Scholar]
- 41**.Burgess SL, Buonomo E, Carey M, Cowardin C, Naylor C, Noor Z, Wills-Karp M, Petri WA., Jr Bone marrow dendritic cells from mice with an altered microbiota provide interleukin 17A-dependent protection against Entamoeba histolytica colitis. M Bio. 2014;5(6):e01817. doi: 10.1128/mBio.01817-14. Demonstration that a component of the mouse microbiome profoundly impacts E. histolytica burden in challenged mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Thibeaux R, Weber C, Hon CC, Dillies MA, Ave P, Coppee JY, Labruyere E, Guillen N. Identification of the virulence landscape essential for Entamoeba histolytica invasion of the human colon. PLoS Pathog. 2013;9(12):e1003824. doi: 10.1371/journal.ppat.1003824. Identifies genes that are differentially expressed when amoebic trophozoites are incubated with human intestinal tissue. In particular, this uncovered a potential role for carbohydrate metabolism in tissue invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tovy A, Hertz R, Siman-Tov R, Syan S, Faust D, Guillen N, Ankri S. Glucose starvation boosts Entamoeba histolytica virulence. PLoS Negl Trop Dis. 2011;5(8):e1247. doi: 10.1371/journal.pntd.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal D, Ave P, Kerneis S, Frileux P, Boche O, Baglin AC, Dubost G, Leguern AS, Prevost MC, Bracha R, Mirelman D, Guillen N, Labruyere E. An ex-vivo human intestinal model to study Entamoeba histolytica pathogenesis. PLoS Negl Trop Dis. 2009;3(11):e551. doi: 10.1371/journal.pntd.0000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thibeaux R, Dufour A, Roux P, Bernier M, Baglin AC, Frileux P, Olivo-Marin JC, Guillen N, Labruyere E. Newly visualized fibrillar collagen scaffolds dictate Entamoeba histolytica invasion route in the human colon. Cell Microbiol. 2012;14(5):609–21. doi: 10.1111/j.1462-5822.2012.01752.x. [DOI] [PubMed] [Google Scholar]
- 46*.Thibeaux R, Ave P, Bernier M, Morcelet M, Frileux P, Guillen N, Labruyere E. The parasite Entamoeba histolytica exploits the activities of human matrix metalloproteinases to invade colonic tissue. Nat Commun. 2014;5:5142. doi: 10.1038/ncomms6142. Investigation of activation of host melloproteinases by amoebic cysteine protease CP-A5, that uncovers how amoebic trophozoites influence remodeling of the host extracellular matrix. [DOI] [PubMed] [Google Scholar]
- 47.Cobo ER, He C, Hirata K, Hwang G, Tran U, Eckmann L, Gallo RL, Reed SL. Entamoeba histolytica induces intestinal cathelicidins but is resistant to cathelicidin-mediated killing. Infect Immun. 2012;80(1):143–9. doi: 10.1128/IAI.05029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Que X, Reed SL. Cysteine proteinases and the pathogenesis of amebiasis. Clin Microbiol Rev. 2000;13(2):196–206. doi: 10.1128/cmr.13.2.196-206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carranza-Rosales P, Santiago-Mauricio MG, Guzman-Delgado NE, Vargas-Villarreal J, Lozano-Garza G, Ventura-Juarez J, Balderas-Renteria I, Moran-Martinez J, Gandolfi AJ. Precision-cut hamster liver slices as an ex vivo model to study amoebic liver abscess. Exp Parasitol. 2010;126(2):117–25. doi: 10.1016/j.exppara.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Carranza-Rosales P, Santiago-Mauricio MG, Guzman-Delgado NE, Vargas-Villarreal J, Lozano-Garza G, Viveros-Valdez E, Ortiz-Lopez R, Moran-Martinez J, Gandolfi AJ. Induction of virulence factors, apoptosis, and cytokines in precision-cut hamster liver slices infected with Entamoeba histolytica. Exp Parasitol. 2012;132(4):424–33. doi: 10.1016/j.exppara.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 51**.Petropolis DB, Faust DM, Deep Jhingan G, Guillen N. A new human 3D-liver model unravels the role of galectins in liver infection by the parasite Entamoeba histolytica. PLoS Pathog. 2014;10(9):e1004381. doi: 10.1371/journal.ppat.1004381. Development and characterization of a 3-D culture model for host-parasite interactions in the liver. These studies identify a role for host galectins and uncover potential amoebic trogocytosis of liver cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Naylor C, Burgess S, Madan R, Buonomo E, Razzaq K, Ralston K, Petri WA., Jr Leptin receptor mutation results in defective neutrophil recruitment to the colon during Entamoeba histolytica infection. MBio. 2014;5(6) doi: 10.1128/mBio.02046-14. These studies provide an explanation for differential susceptibility to E. histolytica associated with a single amino acid polymorphism in the leptin receptor, by uncovering lower neutrophil recruitment in mice containing the allele associated with susceptibility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guerrant RL, Brush J, Ravdin JI, Sullivan JA, Mandell GL. Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis. 1981;143(1):83–93. doi: 10.1093/infdis/143.1.83. [DOI] [PubMed] [Google Scholar]
- 54.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, Hartl D. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3):e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Helk E, Bernin H, Ernst T, Ittrich H, Jacobs T, Heeren J, Tacke F, Tannich E, Lotter H. TNFalpha-Mediated Liver Destruction by Kupffer Cells and Ly6C(hi) Monocytes during Entamoeba histolytica Infection. PLoS Pathog. 2013;9(1):e1003096. doi: 10.1371/journal.ppat.1003096. in vivo studies demonstrating that Kuppfer cells and monocytes contribute to abscess formation. These studies suggest inflammatory responses can exacerbate tissue damage in amoebiasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou Y, Mortimer L, Chadee K. Entamoeba histolytica Cysteine Proteinase 5 Binds Integrin on Colonic Cells and Stimulates NF{kappa}B-mediated Pro-inflammatory Responses. J Biol Chem. 2010;285(46):35497–504. doi: 10.1074/jbc.M109.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Mortimer L, Moreau F, Cornick S, Chadee K. Gal-lectin-dependent contact activates the inflammasome by invasive Entamoeba histolytica. Mucosal Immunol. 2014;7(4):829–41. doi: 10.1038/mi.2013.100. Demonstration that E. histolytica contact mediated by the Gal/GalNAc lectin activates the macrophage inflammasome. [DOI] [PubMed] [Google Scholar]
- 58**.Mortimer L, Moreau F, Cornick S, Chadee K. The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive Entamoeba histolytica via Activation of alpha5beta1 Integrin at the Macrophage-Amebae Intercellular Junction. PLoS Pathog. 2015;11(5):e1004887. doi: 10.1371/journal.ppat.1004887. Demonstrates engagement of macrophage integrins and NLRP3 at the macrophage-E. histolytica synapse leads to NLRP3 inflammasome activation in a CP-A5 dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471(7340):629–32. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]