Abstract
Background
Breast brachytherapy after lumpectomy is controversial in younger patients, as effectiveness is unclear and selection criteria are debated.
Methods
Using MarketScan® healthcare claims data, we identified 45,884 invasive breast cancer patients (ages 18–64), treated from 2003–2010 with lumpectomy, followed by brachytherapy (n=3,134) or whole breast irradiation (WBI) (n=42,750). We stratified patients into risk groups, based on age (Age<50 vs. Age≥50) and endocrine therapy status (Endocrine− vs. Endocrine+). “Endocrine+” patients filled an endocrine therapy prescription within 1 year after lumpectomy. Pathologic hormone receptor status was not available in this dataset. In brachytherapy vs. WBI patients, utilization trends and 5-year subsequent mastectomy risks were compared. Stratified, adjusted subsequent mastectomy risks were calculated using proportional hazards regression.
Results
Brachytherapy utilization increased from 2003 to 2010: In patients Age<50, from 0.6% to 4.9%; patients Age≥50 from 2.2% to 11.3%; Endocrine− patients, 1.3% to 9.4%; Endocrine+ patients, 1.9% to 9.7%. Age influenced treatment selection more than endocrine status: 17% of brachytherapy patients were Age<50 vs. 32% of WBI patients (P<0.001); while 41% of brachytherapy patients were Endocrine- vs. 44% of WBI patients (P=0.003). Highest absolute 5-year subsequent mastectomy risks occurred in Endocrine−/Age<50 patients (24.4% after brachytherapy vs. 9.0% after WBI (Hazard ratio[HR]=2.18, 1.37–3.47); intermediate risks in Endocrine−/Age≥50 patients (8.6% vs. 4.9%; HR=1.76, 1.26–2.46); and lowest risks in Endocrine+ patients of any age: Endocrine+/Age<50 (5.5% vs. 4.5%; HR=1.18, 0.61–2.31); Endocrine+/Age≥50 (4.2% vs. 2.4%; HR=1.71, 1.16–2.51).
Conclusion
In this younger cohort, endocrine status was a valuable discriminatory factor predicting subsequent mastectomy risk after brachytherapy vs. WBI and therefore may be useful for selecting appropriate younger brachytherapy candidates.
Introduction
Breast brachytherapy is a popular adjuvant radiotherapy modality, intended to decrease local tumor recurrence risks after lumpectomy (1). In recent years, breast brachytherapy has gradually replaced traditional whole breast irradiation (WBI) in a subset of older breast cancer patients, used in lieu of WBI in approximately 16% of eligible patients ages 65 and older. Breast brachytherapy is generally accepted as a standard treatment option in such older patients, especially in those with a low predicted risk of recurrence (2,3).
In younger patients, however, the suitability of breast brachytherapy remains controversial, with conflicting statements from current treatment guidelines. American Society for Radiation Oncology (ASTRO) guidelines identify either age<60 or estrogen receptor (ER) negative status alone as sufficient to categorize a patient as “cautionary” or “unsuitable” for breast brachytherapy (4). In contrast, American Brachytherapy Society (ABS), Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO), and American Society of Breast Surgeons (ASBS) guidelines classify patients as young as 45 to 50 years old as “acceptable” for brachytherapy. Furthermore, none of these guidelines deems ER negative status alone sufficient to disqualify patients from brachytherapy (5–7).
Existing data are insufficient for reconciling these divergent recommendations. While it is clear that younger age and negative ER status are risk factors for in-breast tumor recurrence (8–12), it remains unclear whether, in patients with these risk factors, recurrences after brachytherapy occur more frequently than after WBI (5). Thus not surprisingly, there is considerable heterogeneity in breast brachytherapy utilization patterns associated with the conflicting and insufficient data (3). Approximately 60–70% of current patients in the US treated with breast brachytherapy are classified as ASTRO “cautionary” or “unsuitable”, suggesting that these guidelines may be viewed as overly restrictive (1,13).
Heterogeneous practice patterns, discrepancies in expert opinions, and high prevalence of care discordant with current guidelines all point to the pressing need to compare utilization and efficacy outcomes in younger, higher-risk patients treated with brachytherapy versus WBI. Such a comparison could inform evolving selection criteria for breast brachytherapy. Accordingly, in a national, contemporary cohort of women with incident breast cancer ages 64 and younger, we directly compared: 1) radiation treatment utilization patterns; 2) risks of subsequent mastectomy; and 3) costs of radiation treatment in patients treated with brachytherapy vs. WBI.
Methods
Dataset
MarketScan® Commercial Claims & Encounters database (Truven, Ann Arbor, MI) is an employment-based, healthcare claims database including employees and beneficiaries. The data include claims from approximately 45 large, self-insured employers who contract with Truven Health to manage the cost and design of their healthcare plans, and also include employees and dependents who receive their healthcare coverage from small- and medium-sized firms (14,15).
Cohort selection
Based on an algorithmic claims approach similarly utilized in prior studies (15,16), we searched diagnosis and procedure claims to initially identify 59,956 women ages 18 to 64 with breast cancer, treated between 2003 and 2010 using lumpectomy followed by radiation. To ensure a homogeneous population treated with definitive intent, we excluded patients with metastatic cancer, as well as patients who underwent mastectomy before radiation treatment, those who failed to receive an entire radiation course (as indicated by no simulation or delivery codes), and those receiving a combination of WBI plus brachytherapy; our final sample yielded 45,884 patients (eTable 1).
Radiation treatment
Radiation treatment delivered within 12 months of lumpectomy was determined using Common Procedural Terminology (CPT) and International Classification of Diseases (ICD, version 9) procedure codes, and classified as follows: WBI (including external beam radiation treatment with or without intensity modulated radiation [IMRT]); or brachytherapy, including specific indicators of single- versus multi-channel applicator (eTable 2).
Subsequent mastectomy and other covariates
In accordance with published methods, subsequent mastectomy was defined as a procedure code for mastectomy occurring at any time between 1 year after lumpectomy and date of last follow up (17). Locoregional tumor recurrences are not available in this data set. The MarketScan® (administrative) enrollment file provided covariate data including age (which was available as a continuous variable, but also classified dichotomously a priori into Age<50 or Age≥50, based on brachytherapy treatment guidelines) (4,5), as well as Census Bureau geographic region (18). Inpatient and outpatient claims files were used to define axillary lymph node surgery, axillary lymph node involvement, and use of systemic chemotherapy (including a specific indicator for trastuzumab treatment). The variable “systemic chemotherapy (yes/no)” does not include endocrine therapy. Separately, the National Drug Code (NDC) pharmacy file was used to determine endocrine therapy use and to supplement definitions of chemotherapy and trastuzumab (eTable 3). Patients were considered “Endocrine+” if they filled a prescription for tamoxifen, anastrozole, letrozole, or exemestane within −3 to +12 months of surgery. Of note, pathologic hormone receptor status and long-term endocrine therapy compliance were not available in this dataset. Modified Charlson comorbidity score was derived from claims during the 3 months preceding lumpectomy, and specific claims for diagnoses of cardiovascular disease and diabetes were also identified (19). Area-level socioeconomic variables were derived using linkage with the Area Health Resource File, according to patients’ county and health service area (HSA) of residence in the year of their treatment. HSA-level surgeon and radiation oncologist density was calculated using HSA population density.
Risk subgroups by age and endocrine therapy status
We prospectively stratified patients into risk groups, based on age (Age<50 vs. Age≥50) and endocrine therapy status (Endocrine− vs. Endocrine+), including the following combinations defined a priori: Endocrine−/Age<50; Endocrine−/Age≥50; Endocrine+/Age<50; and Endocrine+/Age≥50.
Costs of care
Radiation treatment-related costs were calculated based on the total amount reimbursed by the insurer to providers for radiation-related claims occurring within 1 year of lumpectomy, including claims related to radiation treatment planning and delivery and placement of a brachytherapy catheter. Costs were adjusted for inflation and normalized to the year 2013 using the Medicare Economic Index (20).
Statistical analysis
To address our first objective, we calculated percent use of brachytherapy by year, standardized by geographic region to account for fluctuations in the geographic composition of the MarketScan® cohort over time (21). Brachytherapy use rates by state were calculated to demonstrate variations in local practice patterns.
Temporal trends in radiation treatment utilization were tested using the Cochran-Armitage trend test. Univariate predictors of radiation treatment selection were evaluated using Pearson’s Chi-Square. Multivariate logistic models identified independent predictors of selection for brachytherapy vs. WBI. Goodness of fit was assessed using the method of Hosmer and Lemeshow.
To address our second objective, we calculated cumulative incidence of subsequent mastectomy using the Kaplan-Meier method, with patients censored at date of last follow-up. Unadjusted risk of subsequent mastectomy for patients treated with brachytherapy versus WBI was compared using the log-rank test. Adjusted subsequent mastectomy risks were determined using multivariate Cox proportional hazards models. Covariates for multivariate models were selected a priori, based on clinical relevance and/or univariate significance (P<0.25). Proportionality assumption was verified using visual inspection of the log(-log) plot and proportionality test. Models tested for prespecified interactions between radiation treatment type and age, endocrine therapy status, and combined age/endocrine therapy risk group. Stratum-specific hazard ratios (HR) were generated using interaction terms. A subgroup analysis further divided brachytherapy treatment to specifically compare outcomes in patients treated with single- versus multi-channel applicators.
To address our third objective, we compared differences in radiation-related costs for patients treated with WBI +/− IMRT versus brachytherapy with single- vs. multi-catheters using the Wilcoxon two-sample test. All analyses assumed two-tailed alpha=0.05 and were conducted using SAS version 9.3. Our study was approved by the Institutional Review Board.
Results
Patient sample
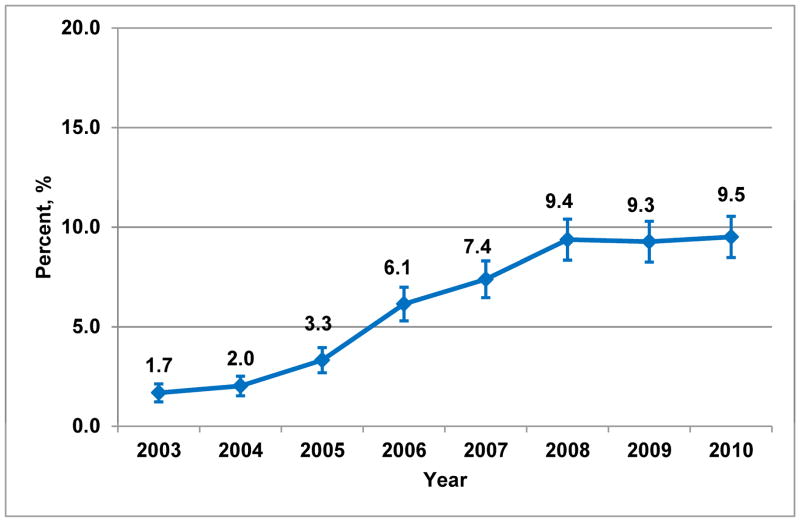
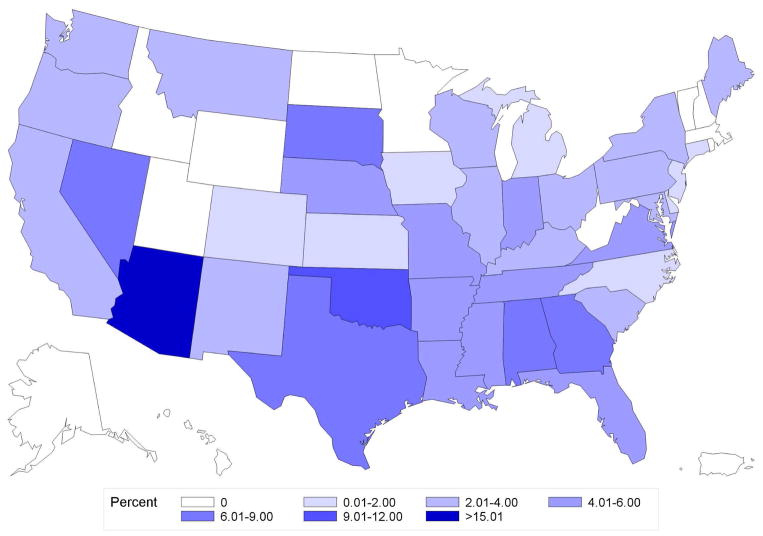
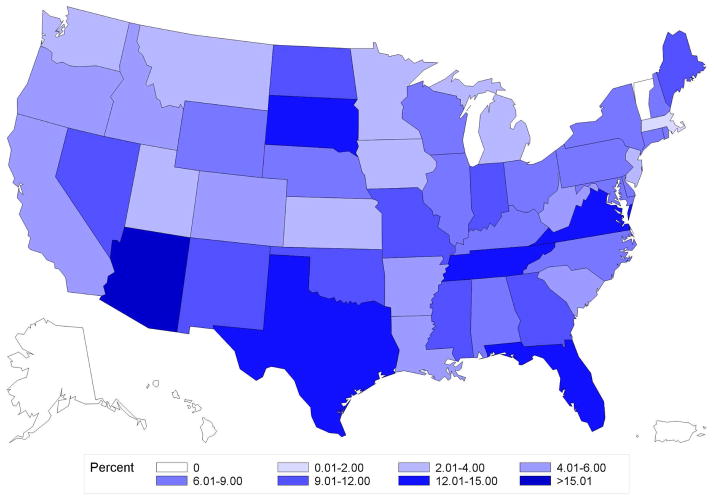
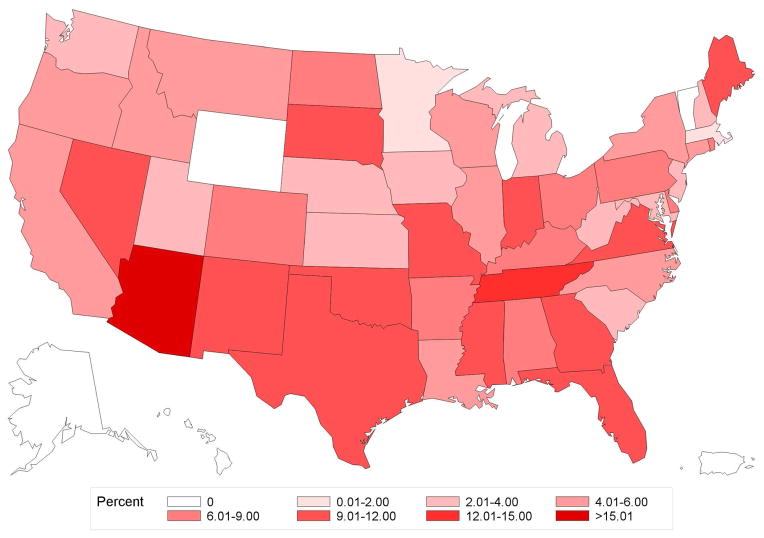
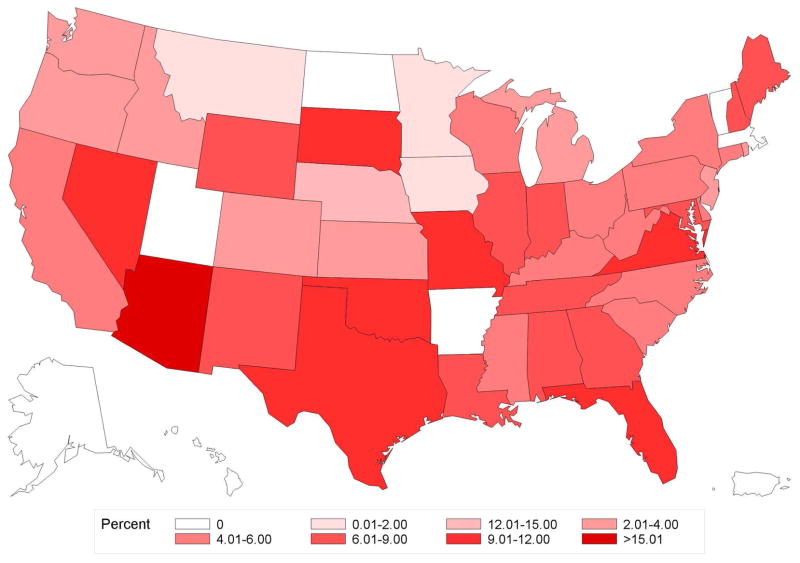
Of 45,884 women, median follow-up was 2.4 years (interquartile range [IQR] 1.6–3.8) and median age was 54 years (IQR 48–59). Overall frequency of brachytherapy use was 6.8% (n=3,134 of 45,884), but significantly increased over the study period, from 1.7% in 2003 to 9.5% in 2010 (Ptrend<0.001) (Figure 1a). State-level practice patterns varied widely (P<0.001), with the frequency of brachytherapy ranging from a low of 0% in Alaska (0/57), Hawaii (0/9), and Vermont (0/51) to a high of 23.3% in Arizona (105/451). State-level variation persisted even in patients Age<50 (P<0.001) or Endocrine− (P<0.001) (Figure 2a–b).
Figure 1. Temporal trends in brachytherapy use between 2003 and 2010.
A. Entire sample (Ptrend<0.001)
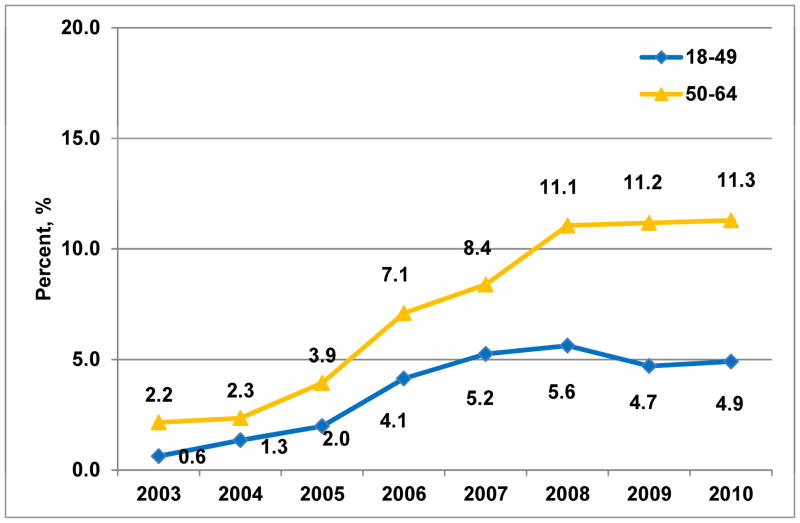
B. Stratified by age group (Ptrend<0.001)
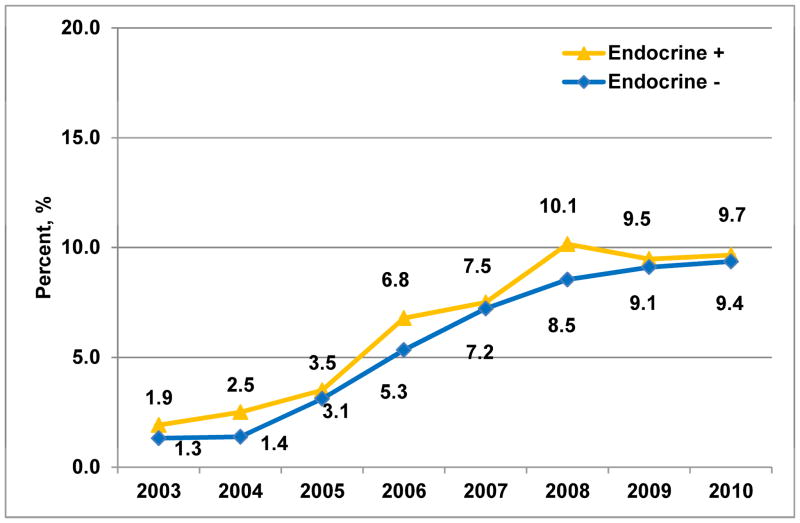
C. Stratified by endocrine therapy status (Ptrend<0.001)
Percentages of brachytherapy use are standardized by region to account for variation in the geographic distribution of the MarketScan® cohort over time. Error bars in panel A indicate 95% confidence intervals around point estimate.
Figure 2. Geographic variation in percentage use of brachytherapy by state.
A. Age<50
B. Age≥50
C. Endocrine+
D. Endocrine−
Variation in use of brachytherapy by state for patients Age≥ 50 (panel A), Age<50 (panel B), Endocrine+ (panel C), or Endocrine− (panel D). The range from 12.01% and 15.00% was not present for any of the states in this data, and hence is not included in the color legend for these maps.
Brachytherapy utilization
Frequency of brachytherapy utilization by year increased within each age and endocrine therapy risk subgroup: in patients Age<50 (n=14,087), from 0.6% to 4.9% (P<0.001); in patients Age≥50 (n=31,797), from 2.2% to 11.3% (P<0.001); in Endocrine− patients (n=20,092), 1.3% to 9.4% (P<0.001); in Endocrine+ patients (n=25,792), 1.9% to 9.7% (P<0.001) (Figure 1b&c). However, age influenced treatment selection more than endocrine therapy status, as demonstrated by absolute differences in the frequency of selection for brachytherapy vs. WBI. Specifically, 17% of brachytherapy patients vs. 32% of WBI patients were Age<50 (P<0.001); while 41% of brachytherapy patients vs. 44% of WBI patients were Endocrine− (P=0.003) (Table 1).
Table 1.
Univariate predictors of selection for brachytherapy vs. WBI
| Characteristic | Brachytherapy (N = 3134)
|
WBI (N = 42750)
|
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| TREATMENT | |||||
|
| |||||
| Age group | <0.001 | ||||
| 18–44 | 127 | 4 | 6023 | 14 | |
| 45–49 | 410 | 13 | 7527 | 18 | |
| 50–54 | 719 | 23 | 9451 | 22 | |
| 55–59 | 944 | 30 | 10860 | 25 | |
| 60–64 | 934 | 30 | 8889 | 21 | |
|
| |||||
| Endocrine therapy | 0.003 | ||||
| Endocrine− | 1294 | 41 | 18798 | 44 | |
| Endocrine+ | 1840 | 59 | 23952 | 56 | |
|
| |||||
| Risk group | <0.001 | ||||
| High(Age<50, E−) | 225 | 7 | 6004 | 14 | |
| Intermediate(Age>=50, E−) | 1069 | 34 | 12794 | 30 | |
| Low(Age<50, E+) | 312 | 10 | 7546 | 18 | |
| Low(Age>=50, E+) | 1528 | 49 | 16406 | 38 | |
|
| |||||
| IMRT | |||||
| No | - | - | 37659 | 88 | - |
| Yes | - | - | 5091 | 12 | |
| Brachytherapy | |||||
| Single-channel | 1869 | 60 | - | - | |
| Multi-channel | 1237 | 39 | - | - | |
|
| |||||
| Systemic chemotherapy | <0.001 | ||||
| No | 2479 | 79 | 24954 | 58 | |
| Yes | 655 | 21 | 17796 | 42 | |
|
| |||||
| Trastuzumab | |||||
| No | 3018 | 96 | 39943 | 93 | <0.001 |
| Yes | 116 | 4 | 2807 | 7 | |
|
| |||||
| Diagnosis code indicating axillary lymph node involvement | <0.001 | ||||
| No | 3050 | 97 | 36618 | 86 | |
| Yes | 84 | 3 | 6132 | 14 | |
|
| |||||
| Axillary lymph node surgery | 0.60 | ||||
| No | 640 | 20 | 8902 | 21 | |
| Yes | 2494 | 80 | 33848 | 79 | |
|
| |||||
| COMORBIDITIES | |||||
|
| |||||
| Modified Charlson comorbidity score | 0.40 | ||||
| 0 | 3038 | 97 | 41552 | 97 | |
| 1+ | 96 | 3 | 1198 | 3 | |
|
| |||||
| Cardiac disease | 0.04 | ||||
| No | 2952 | 94 | 40620 | 95 | |
| Yes | 182 | 6 | 2130 | 5 | |
|
| |||||
| Diabetes | <0.001 | ||||
| No | 2816 | 90 | 39474 | 92 | |
| Yes | 318 | 10 | 3276 | 8 | |
|
| |||||
| SOCIAL DEMOGRAPHIC | |||||
|
| |||||
| Region | <0.001 | ||||
| Northeast | 312 | 10 | 6398 | 15 | |
| Midwest | 683 | 22 | 11466 | 27 | |
| South | 1681 | 54 | 17516 | 41 | |
| West | 456 | 15 | 7274 | 17 | |
|
| |||||
| Median area-level household income in HSA | <0.001 | ||||
| ≤ $42529 | 580 | 19 | 10895 | 26 | |
| $42529 to $50042 | 862 | 28 | 10619 | 25 | |
| $50043 to $59796 | 842 | 27 | 10554 | 25 | |
| >$59796 | 848 | 27 | 10586 | 25 | |
|
| |||||
| Surgeon density per 100,000 population in HSA | <0.001 | ||||
| ≤ 7.9 | 830 | 27 | 10617 | 25 | |
| 8.0 to 11.4 | 879 | 28 | 10567 | 25 | |
| 11.5 to 14.7 | 716 | 23 | 10694 | 25 | |
| > 14.7 | 707 | 23 | 10776 | 25 | |
|
| |||||
| Radiation oncologist density per 1,000,000 population in HSA | <0.001 | ||||
| ≤9.6 | 870 | 28 | 10685 | 25 | |
| 9.7 to 13.9 | 859 | 27 | 10575 | 25 | |
| 14.0 to 19.5 | 656 | 21 | 10910 | 26 | |
| > 19.5 | 749 | 24 | 10580 | 25 | |
35 patients treated with brachytherapy could not be classified as single- or multi-channel
Abbreviations: E (endocrine), HSA (health services area), WBI (whole breast irradiation)
On multivariate analysis, younger patients were less likely than older patients to be selected for brachytherapy (for example, odds ratio [OR]=0.25, 95% Confidence Interval [CI] 0.21–0.30 for age 18–44 years versus referent age group 60–64 years). In contrast, Endocrine− patients had only marginally decreased odds of receiving brachytherapy (OR=0.93; 95% CI 0.86–1.00; P=0.051) (eTable 4). Other factors associated with brachytherapy use included no chemotherapy, no axillary lymph node involvement, history of diabetes, and higher area-level median household income. Geographic and local practice patterns suggested higher brachytherapy use in the South and in areas with lower density of radiation oncologists (Table 1, eTable 4).
Subsequent mastectomy risk
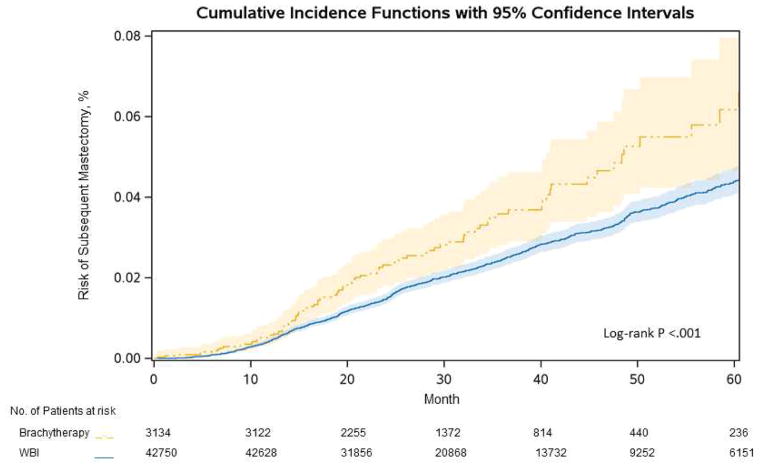
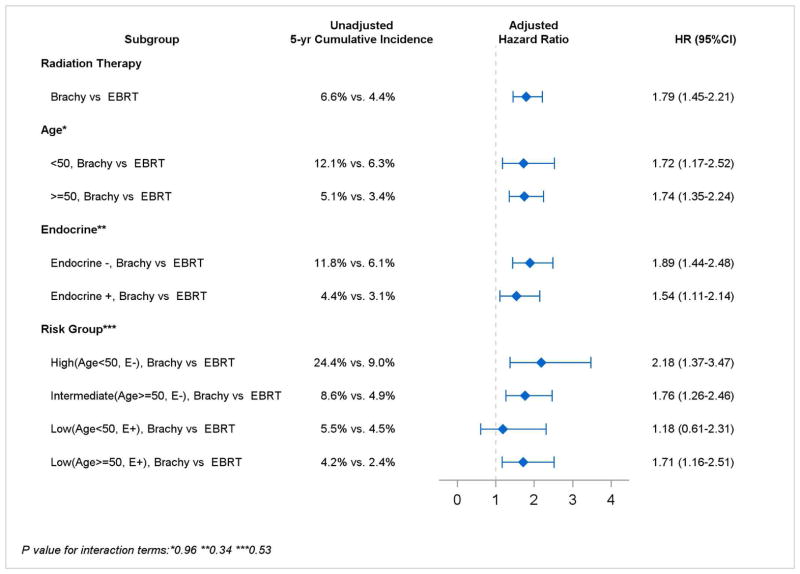
Five-year subsequent mastectomy risk was 6.6% in patients treated with brachytherapy vs. 4.4% in patients treated with WBI (P<0.001) (Figure 3). In multivariable analysis, subsequent mastectomy risk remained elevated in patients treated with brachytherapy (HR=1.79, 95% CI 1.45–2.21; P<0.001) (Table 2). Younger age was an independent predictor of subsequent mastectomy risk (HR=2.86, 95% CI 2.28–3.54; P<0.001 for age 18–44 vs. referent age 60–64), as was Endocrine− status (HR=1.87, 95% CI 1.66–2.11; P<0.001).
Figure 3. Risk of subsequent mastectomy.
A. Overall cumulative incidence of subsequent mastectomy for brachytherapy vs. WBI
B. Unadjusted and adjusted subsequent mastectomy risks stratified by age and Endocrine status
A. Survival curves depict cumulative incidence of subsequent mastectomy by type of radiation therapy. Shaded areas indicate 95% confidence bands. B. Adjusted hazard ratios are derived from the multivariate model in Table 2, but are calculated using the appropriate interaction term to measure effect sizes of type of radiation therapy in specific subgroups, such as Age<50 and Age≥50. Abbreviations: E (endocrine), WBI (whole breast irradiation)
Table 2.
Multivariate proportional hazards model: predictors of subsequent mastectomy
| Factor | HR | 95% CI | P | |
|---|---|---|---|---|
| TREATMENT | ||||
|
| ||||
| Radiation Therapy | ||||
| Brachytherapy (v WBI) | 1.79 | 1.45 | 2.21 | <.001 |
|
| ||||
| Age group | ||||
| 18–44 (v 60–64) | 2.84 | 2.28 | 3.54 | <.001 |
| 45–49 (v 60–64) | 1.68 | 1.34 | 2.10 | <.001 |
| 50–54 (v 60–64) | 1.34 | 1.07 | 1.67 | 0.01 |
| 55–59 (v 60–64) | 1.19 | 0.95 | 1.48 | 0.13 |
|
| ||||
| Endocrine therapy | ||||
| Endocrine − (v Endocrine +) | 1.87 | 1.66 | 2.11 | <.001 |
|
| ||||
| Systemic Chemotherapy | ||||
| Yes (v No) | 0.99 | 0.87 | 1.14 | 0.92 |
|
| ||||
| Trastuzumab | ||||
| Yes (v No) | 1.06 | 0.84 | 1.34 | 0.63 |
|
| ||||
| Axillary lymph node involvement | ||||
| Yes (v No) | 1.38 | 1.17 | 1.63 | <0.001 |
|
| ||||
| COMORBIDITIES | ||||
|
| ||||
| Cardiac disease | ||||
| Yes (v No) | 1.12 | 0.85 | 1.46 | 0.43 |
|
| ||||
| Diabetes | ||||
| Yes (v No) | 1.15 | 0.91 | 1.44 | 0.24 |
|
| ||||
| SOCIAL DEMOGRAPHIC | ||||
|
| ||||
| Radiation oncologist density | ||||
| 9.7 to 13.9 vs. ≤ 9.6 | 1.14 | 0.97 | 1.35 | 0.12 |
| 14.0 to 19.5 vs. ≤ 9.6 | 1.14 | 0.97 | 1.34 | 0.12 |
| > 19.5 vs. ≤ 9.6 | 1.15 | 0.98 | 1.37 | 0.10 |
Abbreviations: CI (Confidence Interval), HR (Hazard Ratio), WBI (whole breast irradiation
Despite the independent effects of age and endocrine therapy status, there were no statistically significant interactions between radiation treatment type with age, endocrine therapy status, or risk group (Pinteraction >0.05). Therefore, there were no statistically significant relative differences in subsequent mastectomy risks after treatment with brachytherapy vs. WBI, either stratified by age or by endocrine therapy status or risk group. However, absolute differences in outcomes after radiation treatment varied, with the highest absolute 5-year subsequent mastectomy risks occurring in 6,229 Endocrine−/Age<50 patients (24.4% [95% CI 11.6% – 39.6%] after brachytherapy vs. 9.0% [7.7% – 10.1%] after WBI; HR=2.18, 95% CI 1.37–3.47; P=0.001). Intermediate subsequent mastectomy risks occurred in 13,863 Endocrine−/Age≥50 patients (8.6% [5.0% – 13.5%] vs. 4.9% [4.2% – 5.6%]; HR=1.76, 95% CI 1.26–2.46; P<0.001). Lowest risks occurred in Endocrine+ patients of any age: in 7,858 patients Endocrine+/Age<50 (5.5% [2.4% – 10.6%] vs. 4.5% [3.7% – 5.2%]; HR=1.18, 95% CI 0.61–2.31; P=0.63); in 17,934 patients Endocrine+/Age≥50 (4.2% [2.1% – 7.5%] vs. 2.4% [2.1% – 2.9%]; HR=1.71, 95% CI 1.16–2.51; P<0.001) (Figure 3). We observed no significant differences in subsequent mastectomy outcomes by type of brachytherapy applicator (HR=0.77, 95% CI 0.49–1.20; P=0.25 for multi-channel brachytherapy, with single-channel brachytherapy as referent)
Treatment costs
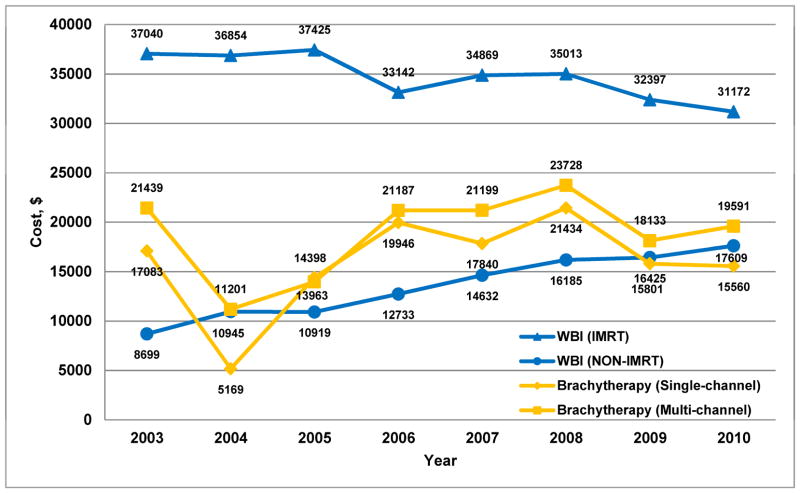
For diagnosis year 2010, median costs in 2013 dollars were $31,172 for WBI with IMRT, $19,591 for multi-channel brachytherapy, $17,609 for WBI without IMRT, and $15,560 for single-channel brachytherapy (P<0.001 for all pairwise comparisons). Generally, the absolute cost differences between treatment types narrowed over time; however, WBI with IMRT persistently remained the most costly treatment option (Figure 4).
Figure 4. Median cost of radiation treatment by year and type of radiation.
Median cost in 2013 dollars of brachytherapy (including both radiation costs and cost to place brachytherapy catheters; classified as single- or multi-channel delivery) and whole breast irradiation (WBI) either with or without intensity modulated radiation therapy (IMRT).
Discussion
In this contemporary cohort of breast cancer patients ages 64 and younger, patterns of breast brachytherapy treatment selection were incongruent with brachytherapy treatment outcomes, as determined by absolute rates of subsequent mastectomy. Overall treatment selection patterns suggest that breast brachytherapy has gradually replaced standard WBI in nearly 10% of patients, with use of brachytherapy stabilizing at this level after 2008. Age was one of the most important clinical variables impacting selection for brachytherapy use, among the clinical variables measured in this dataset. From 2008 to 2010, approximately 5% of patients <50 years received brachytherapy versus approximately 11% of patients ≥50 years. In contrast, patient selection appeared only minimally impacted by endocrine therapy status, with approximately 10% of Endocrine+ patients receiving brachytherapy compared to 9% of Endocrine− patients. These utilization patterns reflect the prevailing guidelines available during our study period (specifically ABS and ASBS guidelines), which recommended consideration of age but not ER status in selecting patients for brachytherapy.
We were surprised to find that though endocrine therapy status was less important for treatment selection, endocrine therapy status was the most important, among all the measured variables, predictor of differences in early subsequent mastectomy outcomes for brachytherapy versus WBI. Among Endocrine− patients, the absolute 5-year risk of subsequent mastectomy was 15.4% higher with brachytherapy compared to WBI for those Age<50 and 3.7% higher for those Age≥50. Among Endocrine+ patients, however, the absolute 5-year risk of subsequent mastectomy was only 1.0% higher with brachytherapy compared to WBI for those Age<50 and 1.7% higher for those Age≥50. These findings suggest that contemporary trends in patient selection for brachytherapy may not be appropriately risk-based, influenced strongly by age but only minimally by endocrine therapy status.
In light of consensus statements and guidelines written by professional societies to that currently guide use of breast brachytherapy, our results are hypothesis-generating, particularly as mature data from phase III trials are awaited. In particular, Endocrine- status in our study is a surrogate variable for pathological hormone receptor status. Additional validating studies with detailed biological marker information, including actual receptor status as well as the influence of other clinico-pathologic variables such as Her2/neu status, triple negative status, margins, and grade, along with studies with detailed information on hormone therapy use and compliance may further influence future consensus recommendations based on hormone receptor status. With this caution in mind, regarding the ASTRO consensus statement, our findings may suggest brachytherapy as a suitable treatment for certain patients with ER+ disease, ages 50 to 60 years, particularly if treated with endocrine therapy. For patients under age 50 years, brachytherapy may also be an acceptable option, with only small absolute differences in early subsequent mastectomy risks for this group, for example 3.7% found in our study. However, careful long-term follow up in such patients remains warranted. In particular, given that the median follow-up in our study was 2.4 years, our results reflect early subsequent mastectomy event rates, which could change over time especially in patients taking endocrine therapy who may experience later failures (4). With regard to the ABS and GEC-ESTRO recommendations, our findings commend a more cautious stance on use of brachytherapy in patients age<50 not treated with endocrine therapy. It should be noted that both groups do not recommend brachytherapy for women age<50, but our data suggest that this recommendation may need to be restricted to those younger patients who do not receive endocrine therapy, or by extrapolation, ER− patients, who had the highest risk differential between brachytherapy and WBI in our study (5,6). The ASBS considers brachytherapy acceptable in women age 45 and older regardless of ER status; our findings would suggest inclusion of ER status may be additive to the current criteria (7).
While randomized trials are the gold standard to inform clinical decision making, the recently completed trials evaluating brachytherapy may be insufficient to clarify indications in younger patients with high risk features. Specifically, the Radiation Therapy and Oncology Group (RTOG) 0413 trial likely enrolled fewer than 600 patients treated with brachytherapy, of whom only a fraction would have had ER− tumors or age under 50 (22). In contrast, 1,606 similar patients are included in the present analysis, patients who did not receive endocrine therapy (and who were presumably more likely ER−), allowing the current study substantial power to measure effect sizes relative to endocrine therapy. Similarly, the GEC-ESTRO randomized trial has limited ability to inform US brachytherapy practice patterns, because this study used interstitial brachytherapy, which treats 2 to 3 cm of tissue beyond the tumor bed, considerably greater than the 1 cm of tissue beyond the tumor bed treated with single-entry catheters commonly utilized in the US (23).
Our findings regarding the relative cost of single and multi-channel brachytherapy in comparison to WBI +/− IMRT are uniquely suited to facilitate assessment of the value of radiation modalities in younger women with private insurance. Brachytherapy confers value to patients as total treatment time is decreased and radiation-related fatigue may be lessened (24), yet such gains could be offset by higher recurrence risks or much higher costs, either from brachytherapy itself or salvage treatment (such as for recurrent disease, though this outcome was not directly measured in our current study) (25). Prior literature has sought to estimate value using costs derived from Medicare reimbursement (25,26), but such approaches are dated given changes to brachytherapy reimbursement that occurred in 2009 (27) and further do not extend to younger patients with private insurance.
Several limitations of this work are worthy of consideration. First, results only apply to younger patients with private insurance. Second, patients with ductal carcinoma in situ were excluded; findings should not be extended to this population. Third, follow up is inherently limited in studies of patients with private insurance, for whom insurance changes are not uncommon. Fourth, while subsequent mastectomy is a clinically relevant endpoint, it is not necessarily equivalent to local failure, as mastectomy may be performed for other reasons such as treatment of severe complications, contralateral breast cancer, risk-reduction against future breast cancers, or even as a component of surgery for a regional recurrence. Nevertheless, the literature indirectly validates subsequent mastectomy as an outcome whose risk is reduced by standard WBI (28–31). Additionally, endocrine therapy status was defined by filling a prescription for endocrine therapy, not by actual testing for ER expression. Thus, the Endocrine− group includes both ER+ positive who did take endocrine therapy in addition to ER− patients for whom endocrine therapy is not beneficial. Nevertheless, our finding of a two-fold reduction in subsequent mastectomy risk attributed to Endocrine+ status underscores the clinical relevance of this definition (32). Future studies with detailed pathologic variables, for example tumor size, lymphovascular invasion status, and grade, may also seek to quantify the degree to which each of these clinical features potentially modifies the risk associated with endocrine therapy status. Use of these surrogate variables as well as the possibility of residual confounding remain limitations of a claims-based, retrospective cohort analysis (33), and thus our results require interpretation also in the context of additional validating cohorts and awaited prospective data. Moreover, the range of absolute differences in subsequent mastectomy outcomes emphasizes the need to consider both the statistical and clinical significance of these results, as well as individual patient preference, when potentially extrapolating these findings to the clinical setting. Finally, the years of this study, from 2003 to 2010, span a time period when the new technology of breast brachytherapy had more recently been FDA approved, so this study tends to reflect the learning curve of early-adopters. Future studies may serve to reflect continuing temporal trends in outcomes of experts or experienced practitioners.
In summary, while age drives current guidelines and clinical practice patterns for brachytherapy treatment selection, endocrine therapy status appears to be a more valuable discriminatory factor predicting subsequent mastectomy risk after brachytherapy treatment as compared with WBI for younger patients. Clinicians and guidelines should acknowledge endocrine therapy status as a key factor relevant to brachytherapy selection criteria.
Supplementary Material
eTable 1. Cohort selection (CONSORT format)
eTable 2. Claims codes for variable definitions
eTable 3. National Drug Codes used to define systemic therapies
eTable 4. Multivariate logistic regression: Predictors of use of brachytherapy
Summary.
In working-age women with incident invasive breast cancer, treated with lumpectomy plus either whole breast irradiation or brachytherapy, subsequent mastectomy risk was similar for patients treated with endocrine therapy and either radiation strategy. In contrast, for patients treated without endocrine therapy, the risk was notably higher in those receiving brachytherapy. Endocrine therapy status, and by extrapolation, hormone receptor status, may be a helpful discriminatory factor when contemplating brachytherapy in such patients.
Acknowledgments
This work was supported in part by the Conquer Cancer Foundation (Career Development Award, BD Smith), American Society for Radiation Oncology (Comparative Effectiveness Award, BD Smith), Cancer Prevention & Research Institute of Texas (RP101207; SH Giordano, BD Smith), the Duncan Family Institute, and a philanthropic gift from Ann and Clarence Cazalot. It was also supported by the NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group.
Footnotes
Conflicts of interest: Dr. B Smith receives research support from Varian Medical Systems, but this support was not used for the current project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Czechura T, Winchester DJ, Pesce C, et al. Accelerated partial-breast irradiation versus whole-breast irradiation for early-stage breast cancer patients undergoing breast conservation, 2003–2010: a report from the national cancer data base. Ann Surg Oncol. 2013;20:3223–3232. doi: 10.1245/s10434-013-3154-8. [DOI] [PubMed] [Google Scholar]
- 2.BLINDED
- 3.Presley CJ, Soulos PR, Herrin J, et al. Patterns of use and short-term complications of breast brachytherapy in the national medicare population from 2008–2009. J Clin Oncol. 2012;30:4302–4307. doi: 10.1200/JCO.2012.43.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 5.Shah C, Vicini F, Wazer DE, et al. The American Brachytherapy Society consensus statement for accelerated partial breast irradiation. Brachytherapy. 2013;12:267–277. doi: 10.1016/j.brachy.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Polgar C, Van Limbergen E, Potter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 8.Shah C, Wilkinson JB, Lyden M, et al. Predictors of local recurrence following accelerated partial breast irradiation: a pooled analysis. Int J Radiat Oncol Biol Phys. 2012;82:e825–830. doi: 10.1016/j.ijrobp.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Stull TS, Catherine Goodwin M, Gracely EJ, et al. A single-institution review of accelerated partial breast irradiation in patients considered “cautionary” by the American Society for Radiation Oncology. Ann Surg Oncol. 2012;19:553–559. doi: 10.1245/s10434-011-1941-7. [DOI] [PubMed] [Google Scholar]
- 10.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–3891. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 12.Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a Higher Radiation Dose on Local Control and Survival in Breast-Conserving Therapy of Early Breast Cancer: 10-Year Results of the Randomized Boost Versus No Boost EORTC 22881–10882 Trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 13.Hattangadi JA, Taback N, Neville BA, et al. Accelerated partial breast irradiation using brachytherapy for breast cancer: patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012;104:29–41. doi: 10.1093/jnci/djr495. [DOI] [PubMed] [Google Scholar]
- 14.BLINDED
- 15.Pan IW, Smith BD, Shih YC. Factors contributing to underuse of radiation among younger women with breast cancer. J Natl Cancer Inst. 2014;106:djt340. doi: 10.1093/jnci/djt340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–1749. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BLINDED
- 18.Harris JR, Halpin-Murphy P, McNeese M, et al. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:989–990. doi: 10.1016/s0360-3016(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40:IV-26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 20.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV-104–117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ, Greenland S, Lash L. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 22.Wolmark N, Curran WJ, Vicini F, et al. Response to “Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation” (Int J Radiat Oncol Biol Phys 2010;76:71–78) and “Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation” Int J Radiat Oncol Biol Phys 2009;75:1290–1296) Int J Radiat Oncol Biol Phys. 2010;77:317. doi: 10.1016/j.ijrobp.2009.12.033. author reply 318. [DOI] [PubMed] [Google Scholar]
- 23.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 24.Albuquerque K, Tell D, Lobo P, et al. Impact of partial versus whole breast radiation therapy on fatigue, perceived stress, quality of life and natural killer cell activity in women with breast cancer. BMC Cancer. 2012;12:251. doi: 10.1186/1471-2407-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sher DJ, Wittenberg E, Suh WW, et al. Partial-breast irradiation versus whole-breast irradiation for early-stage breast cancer: a cost-effectiveness analysis. Int J Radiat Oncol Biol Phys. 2009;74:440–446. doi: 10.1016/j.ijrobp.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suh WW, Pierce LJ, Vicini FA, et al. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;62:790–796. doi: 10.1016/j.ijrobp.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Lanni T, Keisch M, Shah C, et al. A cost comparison analysis of adjuvant radiation therapy techniques after breast-conserving surgery. Breast J. 2013;19:162–167. doi: 10.1111/tbj.12075. [DOI] [PubMed] [Google Scholar]
- 28.Punglia RS, Saito AM, Neville BA, et al. Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. BMJ. 2010;340:c845. doi: 10.1136/bmj.c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.BLINDED
- 30.BLINDED
- 31.Gold HT, Do HT, Dick AW. Correlates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancer. Cancer. 2008;113:3108–3115. doi: 10.1002/cncr.23923. [DOI] [PubMed] [Google Scholar]
- 32.Davies C, Godwin J, et al. Early Breast Cancer Trialists’ Collaborative G. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuske RR, Young SS. Breast brachytherapy versus whole-breast irradiation: reported differences may be statistically significant but clinically trivial. Int J Radiat Oncol Biol Phys. 2014;88:266–268. doi: 10.1016/j.ijrobp.2013.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cohort selection (CONSORT format)
eTable 2. Claims codes for variable definitions
eTable 3. National Drug Codes used to define systemic therapies
eTable 4. Multivariate logistic regression: Predictors of use of brachytherapy