Abstract
Cancerous cells can originate in a number of different tissues such as prostate, breast and lung, yet often go undetected and are non-painful. Many types of cancers will metastasize toward the bone microenvironment first. Tumor burden within the bone causes excruciating breakthrough pain with properties of continual pain inadequately managed with current analgesics. Part of this failure is due to the poor understanding of the etiology of cancer pain. Animal models of cancer-induced bone pain (CIBP) have revealed that the neurochemistry of cancer has features distinctive from other chronic pain states. For example, preclinical models of metastatic cancer often result in the upregulation of neurotrophins, such as NGF and BDNF that can lead to nociceptive sensitization. Preclinical cancer models demonstrate nociceptive neuronal expression of acid sensing receptors, such as ASIC1 and TRPV1 that respond to a significant increase in an acidic cancer-induced environment within the bone. CIBP is correlated with a significant increase in pro-inflammatory mediators acting peripherally and centrally, contributing to neuronal hypersensitive states. And finally, cancer cells generate high levels of oxidative molecules that are thought to significantly increase extracellular glutamate, thus activating primary afferent neurons. Knowledge of the unique neuro-molecular profile of cancer pain will ultimately lead to the development of novel and superior therapeutics for CIBP.
Keywords: Cancer, tumor, bone, pain, metastasis, TRPV1, ASIC, BDNF, NGF, inflammatory, cytokines, chemokines, glutamate, oxidative stress, nitric oxide
1. Cancer-induced bone pain
The most common cancer types, including breast cancer, prostate cancer, and lung cancer, have a propensity to metastasize to bone. Metastasis to bone subsequently induces cancer-induced bone pain (CIBP) [66, 77] and can be characterized as ongoing or breakthrough pain. Ongoing pain is described as dull in character, persistent, and increases over time [49, 76]. Breakthrough pain can be further characterized as “spontaneous pain,” ensuing without an apparent triggering event, as well as a “movement-evoked pain,” initiated by movement of the tumor-bearing bone [66, 77]. Breakthrough pain is often more intense and irregular than ongoing cancer pain, and can severely compromise a patient’s quality of life [77].
Cancer-induced bone pain depicts unique neurochemical changes from other chronic pain states, such as inflammatory or neuropathic pain. The bone itself is innervated with Aβ, Aδ, and C fibers [62]. Interestingly, the majority of human tumors arising in bone lack detectable nerve fibers within the tumor mass or peripheral bone in close proximity [69]. Rather it is the acidic environment and secretion of substances, such as growth factors, cytokines, chemokines, from tumor cells themselves reported to stimulate nearby primary afferent nociceptors inducing pain [67, 86, 102]. The principal challenge in understanding the mechanism of cancer pain was the development of an animal model of pain, displaying similar characteristics to human cancer-induced bone pain. The first in vivo model of cancer-induced bone destruction entailed tail vein injection of human plasma leukemia cells in severe combined immunodeficiency (SCID) mice resulting in the development of bone disease similar to clinical profile of multiple myeloma assessed by blood ionized calcium levels, x-ray, and histology [2]. However, myeloma cells infiltrated vital organs such as liver and spleen in additional to vertebrate and long bones [2]. Tumor burden within the spinal cord caused hind leg paralysis [2]. The degree of metastasis in this model does not allow for a localized assessment of nociceptive behaviors.
Another cancer model resulting in observable bone destruction involved inoculation of sarcoma cells inside the intramedullary space of a mouse’s femur, sealed with dental amalgam, allowing for cancer cells to remain localized [86]. This model allows researchers to correlate cancer-induced bone remodeling, behavioral signs of pain, and neurochemical changes in the spinal cord and primary afferent neurons. Following cancer-induced bone destruction in this model, increases in spontaneous and evoked pain behaviors over time can be correlated with primary afferent neurons sensitization, as evidenced by substance P receptor internalization and C-Fos expression [86]. In tumor bearing femur, sarcoma also induces peripheral changes, such as up-regulation of activating transcription factor-3 (ATF-3), a marker for injured neurons, and macrophage infiltration in dorsal root ganglion (DRG) [86, 88]. Additionally, the spinal cord is reorganized in a manner similar to central sensitization observed in other chronic pain states, including dynorphin up-regulation and astrocyte hypertrophy [39, 86, 88]. These data suggest changes in astrocyte activity in spinal cord are unique characteristics of CIBP.
Consistent with human tumors, mouse neoplasms contain few nerve fibers, particularly those expressing calcitonin gene related peptide (CGRP) [69] [88]. However, human studies have indicated that the tumor microenvironment causes an abnormal remodeling of nearby sensory nerve fibers perceived as painful by patients [56], [9] [18]. Similarly, in this established mouse model, tumor does not become innervated [86]. Rather, changes occur in the periosteum that surrounds the bone extracellular matrix. The periosteum expresses CGRP and substance P (SP), neuropeptides expressed by a subgroup of small neurons in DRG, trigeminal, and vagal ganglia. These small neurons expressing CGRP and SP respond to noxious, thermal, or visceral input and are described as peptidergic [92]. In naïve animals, CGRP and SP fibers are found in mineralized bone, marrow, and periosteum enveloping bone extracellular matrix. Of these three tissues, the periosteum contains the highest amounts of CGRP and SP [88]. However, unlike skin innervation, bone does not have significant Mrgprd(+) and P(2)X(3)(+) C-fiber innervation, suggesting that unique therapies are needed to target skeletal pain versus skin pain [47]. Interestingly, sarcoma, prostate, and breast cancer induce ectopic sprouting of sensory nerve fibers and the formation of neuroma like-features in perioseum [11, 30, 45, 46, 65]. These nociceptive sensory nerve fibers innervating the bone appeared reorganized and increased in density compared to control animals. [11, 30, 45, 46, 65].
Furthermore, the model developed by Schwei et al. [86] allows for development of pain behaviors similar to cancer-induced bone pain exhibited in humans. It can be modified to include inoculation into other bone types (i.e. tibia), model host variation (i.e. mice or rats), and investigation of different tumor types in bone microenvironment. Utilization of this model in the last 14 years has provided significant insight to understanding etiology of cancer pain and the possibility of more efficacious therapeutics for this distinct pain profile.
2. Mechanisms of cancer pain
2.1 Neurotrophins
Nerve growth factor (NGF), brain-derived neurotrophic factor, (BDNF) and neurotrophin-3 (NT-3) are a family of molecules whose cognate receptors are tropomyosin-related kinase (Trk) A, B and C, respectively [91] Neurotrophins also bind the receptor, p75. These proteins regulate survival, development, and function of subsets of sensory and sympathetic neurons, essential to the maintenance and generation of pain [78]; [64]. Neurotrophins are normally expressed in low levels in the majority of adult tissues, becoming up-regulated with inflammatory or injurious states, particularly NGF.
ARRY-470 (Array BioPharma, Boulder, CO) is a selective inhibitor of neurotrophin receptors, exhibiting nanomolar cellular inhibition of TrkA (6.5 nM), TrkB, (8.1 nM), and TrkC (10.6 nM) and a high level of selectivity over a panel of kinases and non-kinase receptors [30]. Doses between 10–100 mg/kg ARRY-470 can obtain high concentrations in plasma and peripheral tissues, while the brain concentrations remain negligible, implying limited crossing of the blood brain barrier [30]. In a mouse model of CIBP, ARRY-470 attenuates spontaneous and evoked pain behaviors compared to untreated animals [30]. ARRY-470 can also effectively diminish nerve fiber sprouting and neuroma formation in the periosteum [30]. Although these data suggests neurotrophins can reduce pain, it does not allow distinguishing between receptors.
Once bound to its cognate receptor, NGF can modulate the function of proteins expressed by nociceptors, such as neurotransmitters (substance P and CGRP), receptors (bradykinin), channels (P2X3, TRPV1, ASIC-3, and sodium), and structural molecules (neurofilaments and p11), namely by up-regulating their expression [35] and resulting in increased nociceptive activity. NGF can also be expressed by tumor, inflammatory, and immune cells [88] that may also add to CIBP. The monoclonal antibody, MAb 911, (Rinat/Pfizer) is a sequestering antibody that inhibits the selective binding of NGF to TrkA and p75 over TrkB or TrkC receptors in rodents and humans [38]. The antibody also blocks TrkA autophosphorylation [38] and has a half-life of approximately five days in mice [88]. In mouse models of CIBP, nociceptive sensory nerve fibers innervating the bone express TrkA receptors, and treatment with MAbs towards NGF in this model attenuates cancer-induced behavioral signs of pain [35, 45, 46] [11, 65]. Treatment with anti-NGF modulates peripheral changes in DRG and central changes in spinal cord [88]. Early and sustained administration of MAb 911 induces marked reduction of sprouting (indicated by CGRP+ expression) in nerve fibers in the tumor-bearing bone and without affecting the density of CGRP+ sensory nerve [11, 35, 45]. This provides evidence that NGF/TrkA signaling over other neurotrophin subtypes may be more important in cancer pain [46, 65] Recent studies on BDNF have demonstrated a role in the induction and maintenance of behavioral hypersensitivity in a rat model of CIBP by BDNF modulating the NMDA subunit 1 (NR1) at the level of the spinal cord and DRG [99]. Upregulation of BDNF in descending pain modulating areas of the rostral ventromedial medulla (RVM) have been reported in a rat model of CIBP [59] suggesting that BDNF may also play a role in CIBP, yet the clinical evidence of whether anti- NGF or anti-BDNF can attenuate CIBP are unknown.
2.2 Acid sensing ion channels
Neoplasms are comprised of a heterogeneous population of cells, essential to the functionality of tumor microenvironment. Besides the prototypical cancer cells, containing oncogenic and tumor suppressor mutations characterizing cancer, there are cancer stem cells, pericytes, cancer-associated fibroblast, stem and progenitor cells of the tumor stroma, and immune pro-inflammatory cells [36]. These, particular the inflammatory cells, are tumor promoting cells that may include macrophage subtypes, mast cells, neutrophils, T and B lymphocytes [36]. Furthermore, they release many different signaling molecules, including protons, which promote an acidic environment. Cancer cells exhibit a reversed pH gradient, compared to normal cells, having a higher intracellular pH (~7.4) and a lower extracellular pH (~6.7–7.2) [36]. Advantages of reversed pH gradient include growth factor independent proliferation, evasion of apoptosis, migration and invasion, and reprogramming energy metabolism [14–16, 36]. Excessive cancer cell proliferation and the typically poor vasculature of the central tumor mass results in hypoxia ([14–16]. As an adaptation to hypoxia, cells can up-regulate glucose transporters, such as GLUT1, and enzymes of glycolytic pathway [36]; [14–16, 36] This increase in glycolysis results in increased acid production [14–16, 36].
Upregulation of growth factors, cytokines and chemokines in the tumor microenvironment, disrupt normal bone metabolism maintained by osteoclasts, bone resorbing cells, and osteoblasts, bone forming cells. As extracellular pH increases, mineralization of pre-osteoblasts and transcription of the osteoblastic gene, osteopontin increases in vitro [44]. Decrease in extracellular pH as seen in metabolic acidosis, reduces osteoblast mediated collagen synthesis and alkaline phosphatase activity [52]. Osteoclasts mediate bone resorption through secretion of proteases, such as cathepsin K, B-glucuronidase, and the generation of protons [20] [52]. Extracellular protons effectively sensitize primary afferent neurons [8, 53, 80] More specifically, in CIBP, breast cancer upregulates ASIC1a/b expression in the primary sensory neuron, which likely contributes to hyperalgesia [68]. In normal and cancer-bearing bone, sensory fibers of mineralized bone and bone marrow express the Transient Receptor Potential Channel, Vanilloid subfamily member 1 (TRPV1) [31]. Thus, persistent activation of acid-sensing channels may not be the only channels that contribute to CIBP.
TRPV1 channel is a member of the six transmembrane domain heterotetramer TRP ion channel super family. It is modulated by not only low pH but also by capsaicin, resiniferatoxin (a capsaicin analogue), noxious heat (>43 °C), voltage, and endovanilloids [17, 94]. TRPV1 is expressed by variety of cell types including astrocytes, perivascular structures, and neurons [73]. TRPV1 has been found in DRG, small C and Aδ sensory fibers, and co-localize with TrKA [73]. In human osteoclasts in vitro, biomolecular and functional experiments showed that resiniferatoxin (RTX), a selective TRPV1 receptor agonist, increased the expression and activity of the osteoclast biomarkers, TRAP and cathepsin K [73]. Capsazepine has been shown to inhibit osteoclastic bone resorption, osteoblast activity and bone formation [42]. Inhibition of TRPV1 also protects against ovariectomy induced bone loss in mice [42]. This implies pharmacological blockade of TRPV1 may interfere with osteoclastogenesis, reducing one source of extracellular proton production and ensuing pain.
In rat and mouse models of squamous cell carcinoma, upregulation of TRPV1-positive large- sized neurons were observed in the dorsal root ganglion [5, 90]. Administration of the competitive TRPV1 antagonist, capsazepine, reduced the thermal hyperalgesia and mechanical allodynia induced by the cancer [5, 90]. Utilization of sarcoma-induced bone cancer model provided evidence that a significant portion of sensory neurons that innervate the tumor-bearing bone express TRPV1 [31]. JNJ-17203212 is a selective, potent antagonist of both rodent and human TRPV1, with an IC50 value of 38±10 nM [93] was used in a murine bone cancer model. In TRPV1 wildtype animals (+/+) and TRPV1 heterozygotes (+/−), spontaneous- and movement-evoked pain were inhibited through chronic treatment with the TRPV1 antagonist, JNJ-17203212. In contrast, TRPV1 null mice (−/−), exhibited reduction in pain behaviors that was not further attenuated with JNJ-1720312 treatment, implying attenuation of pain was mediated by TRPV1 [31]. Sarcoma cells in vitro were found to release a lipophilic substance that activates TRPV1 in dorsal root ganglion neurons [55]. Activation of TRPV1 with this releasing substance was blocked by a TRPV1 antagonist [55]. These data suggest not only is the acidic environment created by cancer promoting sensitization of TRPV1, but some cancer cells may also be producing endogenous agonists for TRPV1 [55].
One acid particularly important to TRPV1 sensitization may be lysophosphatidic acid (LPA), a lipid metabolite released by cancer cells and blood platelets after tissue injury known to induce proliferation, adhesion, migration, morphogenesis, differentiation and survival [12, 27]. Ascitic fluid and plasma of cancer patients contain high levels of LPA and the fluids or the LPA has been shown to facilitate bone metastasis by stimulating secretion of IL-6 and IL-8 [12, 26, 55]. In naïve animals, TRPV1 and LPA1 receptors in DRG co-localized, enabling possible crosstalk [74]. This was verified by TRPV1 currents being potentiated by LPA, and blocked by the TRPV1 antagonist, capsazepine, and LPA1 antagonist, VPC32183 [74]. In a rat model of CIBP, VPC32183, an LPA1 receptor antagonist, attenuated mechanical allodynia and thermal hyperalgesia, suggesting release of LPA from cancer cells and cross-talk between LPA1 and TRPV1 in CIBP [74].
ASICs belong to the epithelial sodium channel (ENaC)/degenerin (DEG) superfamily of ion channels [23]. Seven subtypes (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, ASIC4 and ASIC5) have been identified in rodents [23]. They can form homotetamers or heterotetramers, each depicting a distinctive functional heterogeneity. These channels are characterized by their sensitivity to an extracellular decease in pH [98]. In rodents, ASICs are expressed throughout the nervous system [70, 98]. ASIC1b and ASIC3 were identified exclusively in dorsal root ganglion primary afferent neurons [70, 98]. ASIC channels are also expressed by osteoblasts and osteoclasts, and may explain how bone cell function can be modulated by environmental pH under physiological (e.g., bone resorption) and pathological (e.g. bone metastases) conditions [44].
ASIC3 is unique within its family since the channel produces a rapidly inactivating peak current and a sustained current [23, 98]. This sustained current is thought to contribute to non-adapting pain induced by a range of acidities [23]. ASIC3, is important for inflammatory pain in the peripheral nervous system [23]. In DRG neuron primary culture, pro-inflammatory mediators, specifically NGF, serotonin, interleukin-1, and bradykinin, reportedly up-regulate the expression of ASIC3 and leads to their hyperexcitability. This finding suggests a mechanism by which ASIC3 channels contribute to hyperalgesia in vivo [63].
Investigation of ASIC3 knockout mice in presence of nociceptive stimuli indicates ASIC3 channel can have pro-nociceptive or modulatory role in pain sensation, dependent on location and heterotetramer composition. Furthermore, in a neuropathic pain model [51] sciatic nerve ligation of L5 (SNL) induces hyperalgesia to mechanical and thermal stimuli. In this model, ASIC currents are modulated and the expression of ASIC subunit mRNA is altered in DRGs from injured animals, providing evidence of ASIC channels in neuropathic pain [75]. Omori et al. [71] demonstrated injection of acetic acid to SNL rats evoked an increase in spontaneous pain and mechanical allodynia compared to sham animals, providing evidence that ASIC3 is associated with hyperalgesia in response to an acidic stimulus in this neuropathic pain model. Additionally, application of acetic acid to neuropathic animals increased the total ASIC3-ir neurons in the L4 DRG and modified the cell size of ASIC3-ir neurons in the L4/5 DRGs [71]. These studies suggest ASIC3 modulation contributes to maintenance and generation of acute inflammatory and chronic neuropathic pain. With the unique expression of ASIC channels solely in the bone and nervous system to date, pharmacological modulation of ASICs warrant attention for bone degenerative diseases, including bone metastases.
2.3 Inflammatory Mediators
A variety of small proteins, produced by immune cells that facilitate intercellular communication can be termed cytokines [4]. Two cytokine subtypes, the pro-inflammatory cytokines and the chemokines, have been shown to contribute to inflammatory and neuropathic pain (for review see [97]). This has been demonstrated through use of cytokine inhibitors, KO mice, and direct application of cytokines administered within the central and peripheral nervous system [97], [107]. Osteoclasts are derived from monocyte/macrophage hematopoietic lineage [13]. Thus, it is unsurprising that osteoclasts have receptors for IL-lβ, TNFα, IL-6, and TGFβ and induce osteoclastogenesis [13]. Furthermore, it is well established that cytokines play a role in tumor development and metastasis and their effects vary depending on the type and location of the tumor [25]. With the ability to modulate pain both centrally and peripherally and stimulate bone resorption, up-regulation of cytokines in bone microenvironment may therefore contribute to peripheral and central components of CIBP.
Pro-inflammatory cytokines include IL-lβ, TNFα, IL-6, and TGFβ. Baamonde and colleagues [6] demonstrate in an osteosarcoma model, cancer-bearing animals demonstrated a decrease of IL-1 β levels at the site of tumor and in the spinal cord and that IL-1R-receptor antagonist attenuated thermal and mechanical hyperalgesia [6]. Studies utilizing bone cancer models in wildtype or transgenic animals, lacking TNFα receptors TNFR1 and TNFR2, implicate the involvement of TNF gene in cancer-induced hyperalgesia, spinal astrogliosis, and tumor growth [29, 32]. Studies by Constantine et. al suggest TNFα sensitized TRPV1 channels via p38/MAP kinase- and PKC-dependent pathways and unregulated TRPV1 in cultured DRG neurons [21]. In a soft tissue cancer/metastasis model, cancer-induced heat hyperalgesia correlates with an increased expression of TRPV1 protein [21].
Evidence supports roles for chemokines in addition to cytokines in CIBP. Development of co- cultures with femur and cancer cells, up-regulate MCP-1 and TGFβ expression and secretion in sarcoma, but down-regulates MCP-1 and TGFβ expression and secretion in breast cancer cultures [85]. However, recent studies measuring cytokines and chemokines in vivo in a murine model of breast cancer demonstrated a significant increase in MCP-1, MIP-1A, IL-6 and TNFα [61]. These cytokines and chemokines were significantly reduced by the systemic administration of a cannabinoid-2 receptor agonist suggesting a novel treatment in bone cancer pain [61]. These results provide further evidence that cancer in the bone environment modify cytokines and chemokines present. Additionally, intrathecal administration of an anti-MCP-1 neutralizing antibody attenuated the mechanical allodynia established in a rat model carcinoma induced bone pain [41]. Over an 18 day period of carcinoma implanted in the tibia, the expression level of CX3CR1, the receptor for fractalkine, in the spinal cord gradually increased [40] [104] suggesting chemokine-receptor interactions rise centrally that may promote pain in a tibia model of cancer pain. Hence, intrathecal injection of a neutralizing antibody against CX3CR1 reduced mechanical allodynia in cancer-induced rats with further studies suggesting a CX3CR1 mediated pain through microglia and p38 mitogen-activated protein kinase (MAPK) activation in the spinal cord [40].
2.4 Oxidative Stress
Oxidative stress is an established hallmark of tumor burden and decidedly plays a role in multiple types of chronic pain, including those of inflammatory and neuropathic pathology [58, 100, 48, 57, 34, 84]. In murine models of breast cancer and in human cancers, studies detect systemic and tissue-specific elevations of lipid markers of oxidative stress and associated changes in detoxification enzymes including glutathione peroxidase (GPx) and superoxide dismutase (SOD) isoforms [33, 37]. Specific antioxidants including SOD mimetics have been utilized to relieve pain in preclinical models [34, 50, 24, 103, 83, 79], however little work to date has investigated the therapeutic potential of antioxidant therapy for CIBP. Sources of oxidative stress in the tumor-bone microenvironment include cancerous cells and a diverse population of tumor stromal cells (including monocytes and T-lymphocytes) that elicit stress response from both local cells involved in bone homeostasis and innervating sympathetic and nociceptive fibers. The pro-nociceptive action of oxidative stress in CIBP can be understood as a function of stress-induced production of glutamate as an algogenic substance by cancer cells, and secondly as a result of tumor-derived nitrating species altering the response to glutamate by primary afferent neurons. Glutamatergic transmission plays a key role in several types of pain including cancer pain [72, 19, 96]. It is also vital for normal bone homeostasis [22].
In cancer cells, glutamate secretion is tightly coupled to the uptake of antioxidant precursors [89]. Several types of cancer cells have adapted to express the glutamate-cystine antiporter, system xc, which passively exchanges intracellular glutamate as a byproduct of metabolism for extracellular cystine[60]. In the cell, cystine is cleaved into two cysteine residues for incorporation into the potent antioxidant glutathione [43]. As such it plays a profound role in resilience against oxidative challenge. Accordingly, elevated cysteine consumption can be detected in chemoresistant subgroups of human MCF-7 breast cancer cells [82]. Tumoral expression of system xc is thought to confer protection against oxidative stress as an adaptation to a metabolic abnormality known as the Warburg effect, or the observation that cancer cells rely upon anaerobic glycolysis for ATP generation in low-glucose, hypoxic environments [101, 54]. The Warburg effect results in the inefficient use of glucose and excessive generation of reactive oxygen species as the normal byproducts of respiration [7]. Therefore, system xc facilitates the removal of both metabolic waste and harmful oxidants from cancer cells at the expense of extracellular glutamate in the bone-tumor microenvironment. AMPA receptors, NMDA receptors and mGluR1 and 5 are expressed at the peripheral terminals of primary afferent nociceptive neurons [10]. Therefore, glutamate derived from cancer cells may evoke nociceptive signaling and lead to persistent nociceptive states found in CIBP [96]. Furthermore, because glutamate is a vital communicator in bone homeostasis and function, elevated glutamate may lead to further dysregulation of bone and add-on mechanisms of pain. Indeed, extracellular glutamate released from bone tumor cells induces osteoclastogenesis and modulates osteoblast function [87].
Tumor burden also generates oxidative stress in the bone-tumor microenvironment. In pathological states, induction of a constitutively active form of nitric oxide synthase iNOS results in the generation of both nitric oxide (NO) and peroxynitrite (ONOO-) as reaction product of NO with byproducts of cellular respiration. Pathological generation of these radicals results in nitration modification of protein function and stress signaling events. In the context of CIBP, both cancer cells and tumor stromal cells act as a source of NO. The viability of breast cancer lines MCF-7, T47D and 4T1 is promoted by the uptake of NO precursor, L-arginine, and its conversion to nitric oxide [1]. Our own laboratory has demonstrated basal release of NO from the murine 4T1 and 66.1 mammary carcinoma cell lines in vitro (unpublished). Furthermore, in human breast cancer tumors, aberrant iNOS expression and production of NO is thought to activate oncogenic pathways, select for mutant tumor suppressor loss, induce stem cell-like tumor characteristics and promote tumor viability [3]. Because NO diffuses easily from its site of release, primary afferent neurons innervating the bone-tumor microenvironment are vulnerable to reaction with NO and subsequent nitration events. Exposure to nitrating agents derived from NO increases the sensitivity of the NMDA receptor to glutamate in vivo by increasing the receptor affinity for glutamate [105] and calcium conductance. In cortical synaptosomes, it was demonstrated that nitration events may be specific to the NR1, NR2a and NR2b subunits of the NMDAR [106]. Accordingly, NMDA antagonists have demonstrated efficacy in preclinical models of pain associated with nitration events [28] and in a model of CIBP [81]. It has also been posited that nitration of glutamate recycling mechanisms may contribute to enhanced glutamatergic neurotransmission in the bone-tumor microenvironment: the nitration of glutamate transporters including GLT-1 and GLAST reduces uptake of glutamate, which may lead prolonged synaptic glutamate concentrations [95]. Understanding mechanisms of nitration-mediated enhancements in glutamatergic transmission in the context of CIBP models is an on-going effort that requires research attention.
2.5 Conclusion
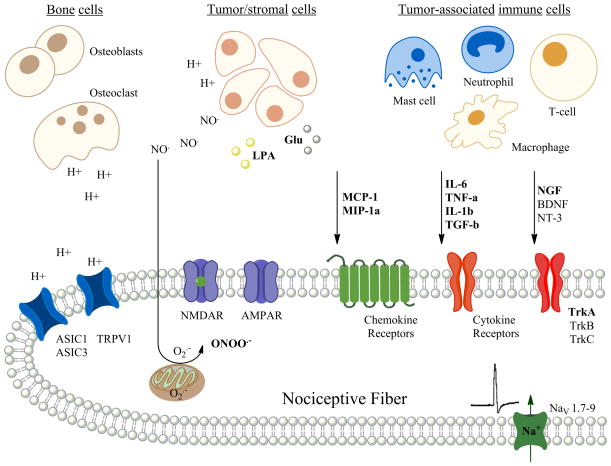
Currently there is little known about how cancers that have metastasized to bone can produce both chronic- and breakthrough pain. It is odd that while a primary tumor such as breast cancer or prostate cancer in its tissue of origin results in very little or no pain whatsoever, but when it metastasizes to bone it can produce excruciating pain. Preclinical studies have just begun to scratch the surface on how such cancers may interact with the bone microenvironment to result in pain. Ideas, of course, include growth factors, cytokines, chemokines, acidic environments, enzymes, excessive glutamate and oxidative species (Figure 1). Yet opioids and compounds that inhibit osteoclast activity such as bisphosphonates and denosumab are the only pharmacological therapy used to treat the pain. Further studies are desperately needed at both the preclinical and clinical level to determine whether the targets mentioned in this review are viable and feasible for patient populations. Cancer patients are living longer while being treated with chemotherapeutic agents; however their quality of life is severely diminished due to the pain and the side effects of current opioid pain medication.
Figure 1.
Diagram of a nociceptive fiber innervating the bone microenvironment demonstrating a number of nociceptive stimuli that act via their receptors that may cause bone cancer pain. Preclinical models have demonstrated that the nociceptive stimuli are released from either the tumor cells themselves or from a number of different tumor-associated immune cells within the bone microenvironment. An addition source of protons and proteases includes the osteoclasts. Preclinical models predict that such nociceptive stimuli may act directly to cause pain via ion channels on the nociceptive fibers or may act via second messengers resulting in the sensitization and increased excitability of the nociceptive fiber within and around the bone. (H+ = proton; NO = nitric oxide; O2− = superoxide; ONOO = peroxynitrate, LPA = lysophosphatidic acid; Glu = glutamate; MCP-1 = monocyte chemottractant protein-1; MIP-1a = macrophage inflammatory protein-1α; IL-6 = interleukin-6; TNF α = tumor necrosis/factor- α; IL-1β = interleukin-1β; TGF-β = transforming growth factor-β; NGF = nerve growth factor; BDNF = brain-derived neurotrophic growth factor; NT-3 = neurotrophin-3; ASIC1 – acid sensing ion channel-1; ASIC3 = acid sensing ion channel-3; TRPV1 = transient receptor potential cation channel-subfamilyV1; NMDAR = N-methyl-D-aspartate receptor; AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; TrkA, B, & C = tyrosine kinase receptors A, B & C)(figure made by AM Symons-Liguori).
Highlights.
Non-painful primary tumors often yield severely painful bone metastases in cancer patients.
Tumor-derived growth factors stimulate neuronal sprouting of nociceptors in the bone microenvironment.
Acid-sensing ion channels are activated by acidic tumor-induced osteolysis and local tumor acidity.
Cytokines and chemokines released in the bone microenvironment lead to nociceptive signaling and sensitization in bone.
Oxidative stress produces an increase in glutamate signaling in bone through a glutamate-cystine antiporter on tumor cells.
Acknowledgments
We want to thank Dr. William Staatz for his help in proofreading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdelmagid SA, Rickard JA, McDonald WJ, Thomas LN, Too CK. CAT-1- mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J Cell Biochem. 2011;112:1084–1092. doi: 10.1002/jcb.23022. [DOI] [PubMed] [Google Scholar]
- 2.Alsina M, Boyce B, Devlin RD, Anderson JL, Craig F, Mundy GR, Roodman GD. Development of an in vivo model of human multiple myeloma bone disease. Blood. 1996;87:1495–1501. [PubMed] [Google Scholar]
- 3.Ambs S, Glynn SA. Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer. Cell Cycle. 2011;10:619–624. doi: 10.4161/cc.10.4.14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai KI, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 5.Asai H, Ozaki N, Shinoda M, Nagamine K, Tohnai I, Ueda M, Sugiura Y. Heat and mechanical hyperalgesia in mice model of cancer pain. Pain. 2005;117:19–29. doi: 10.1016/j.pain.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Baamonde A, Curto-Reyes V, Juarez L, Meana A, Hidalgo A, Menendez L. Antihyperalgesic effects induced by the IL-1 receptor antagonist anakinra and increased IL-1beta levels in inflamed and osteosarcoma-bearing mice. Life Sci. 2007;81:673–682. doi: 10.1016/j.lfs.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 8.Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 9.Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol. 2008;64:644–653. doi: 10.1002/ana.21527. [DOI] [PubMed] [Google Scholar]
- 10.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Bloom AP, Jimenez-Andrade JM, Taylor RN, Castaneda-Corral G, Kaczmarska MJ, Freeman KT, Coughlin KA, Ghilardi JR, Kuskowski MA, Mantyh PW. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain. 2011;12:698–711. doi: 10.1016/j.jpain.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 14.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med (Berl) 2007;85:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 15.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Brahimi-Horn MC, Pouyssegur J. Oxygen, a source of life and stress. FEBS Lett. 2007;581:3582–3591. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 18.Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, Muller MW, Giese T, Buchler MW, Giese NA, Friess H. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186. e171. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Chiechio S, Nicoletti F. Metabotropic glutamate receptors and the control of chronic pain. Curr Opin Pharmacol. 2012;12:28–34. doi: 10.1016/j.coph.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RE, Purohit OP. Osteoclast inhibition for the treatment of bone metastases. Cancer Treat Rev. 1993;19:79–103. doi: 10.1016/0305-7372(93)90028-p. [DOI] [PubMed] [Google Scholar]
- 21.Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28:5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowan RW, Seidlitz EP, Singh G. Glutamate signaling in healthy and diseased bone. Frontiers in endocrinology. 2012;3:89. doi: 10.3389/fendo.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, Lingueglia E. Acid- sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Doyle T, Chen Z, Muscoli C, Bryant L, Esposito E, Cuzzocrea S, Dagostino C, Ryerse J, Rausaria S, Kamadulski A, Neumann WL, Salvemini D. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci. 2012;32:6149–6160. doi: 10.1523/JNEUROSCI.6343-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6:2482–2491. [PubMed] [Google Scholar]
- 27.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, Jaffe R, Erickson J, Mills GB. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 28.Fundytus ME. Glutamate receptors and nociception: implications for the drug treatment of pain. CNS drugs. 2001;15:29–58. doi: 10.2165/00023210-200115010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Geis C, Graulich M, Wissmann A, Hagenacker T, Thomale J, Sommer C, Schafers M. Evoked pain behavior and spinal glia activation is dependent on tumor necrosis factor receptor 1 and 2 in a mouse model of bone cancer pain. Neuroscience. 2010;169:463–474. doi: 10.1016/j.neuroscience.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, Mantyh PW. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain. 2010;6:87. doi: 10.1186/1744-8069-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu X, Zheng Y, Ren B, Zhang R, Mei F, Zhang J, Ma Z. Intraperitoneal injection of thalidomide attenuates bone cancer pain and decreases spinal tumor necrosis factor-alpha expression in a mouse model. Mol Pain. 2010;6:64. doi: 10.1186/1744-8069-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo CH, Hsia S, Chen PC. Distribution of selenium and oxidative stress in breast tumor-bearing mice. Nutrients. 2013;5:594–607. doi: 10.3390/nu5020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL., Jr Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res. 2006;173:211–216. doi: 10.1016/j.bbr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65:9426–9435. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Hasan HR, Mathkor TH, Al-Habal MH. Superoxide dismutase isoenzyme activities in plasma and tissues of Iraqi patients with breast cancer. Asian Pac J Cancer Prev. 2012;13:2571–2576. doi: 10.7314/apjcp.2012.13.6.2571. [DOI] [PubMed] [Google Scholar]
- 38.Hongo JS, Laramee GR, Urfer R, Shelton DL, Restivo T, Sadick M, Galloway A, Chu H, Winslow JW. Antibody binding regions on human nerve growth factor identified by homolog- and alanine-scanning mutagenesis. Hybridoma. 2000;19:215–227. doi: 10.1089/02724570050109611. [DOI] [PubMed] [Google Scholar]
- 39.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 40.Hu JH, Yang JP, Liu L, Li CF, Wang LN, Ji FH, Cheng H. Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res. 2012;1465:1–9. doi: 10.1016/j.brainres.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Hu JH, Zheng XY, Yang JP, Wang LN, Ji FH. Involvement of spinal monocyte chemoattractant protein-1 (MCP-1) in cancer-induced bone pain in rats. Neurosci Lett. 2012;517:60–63. doi: 10.1016/j.neulet.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Idris AI, Landao-Bassonga E, Ralston SH. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone. 2010;46:1089–1099. doi: 10.1016/j.bone.2010.01.368. [DOI] [PubMed] [Google Scholar]
- 43.Ishii T, Sugita Y, Bannai S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J Cell Physiol. 1987;133:330–336. doi: 10.1002/jcp.1041330217. [DOI] [PubMed] [Google Scholar]
- 44.Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337:349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30:14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152:2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14:R101. doi: 10.1186/ar3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallenborn-Gerhardt W, Schroder K, Geisslinger G, Schmidtko A. NOXious signaling in pain processing. Pharmacol Ther. 2013;137:309–317. doi: 10.1016/j.pharmthera.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Kerba M, Wu JS, Duan Q, Hagen NA, Bennett MI. Neuropathic pain features in patients with bone metastases referred for palliative radiotherapy. J Clin Oncol. 2010;28:4892–4897. doi: 10.1200/JCO.2010.28.6559. [DOI] [PubMed] [Google Scholar]
- 50.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 52.Krieger NS, Sessler NE, Bushinsky DA. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol. 1992;262:F442–448. doi: 10.1152/ajprenal.1992.262.3.F442. [DOI] [PubMed] [Google Scholar]
- 53.Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- 54.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Lautner MA, Ruparel SB, Patil MJ, Hargreaves KM. In vitro sarcoma cells release a lipophilic substance that activates the pain transduction system via TRPV1. Ann Surg Oncol. 2011;18:866–871. doi: 10.1245/s10434-010-1328-1. [DOI] [PubMed] [Google Scholar]
- 56.Lindqvist A, Rivero-Melian C, Turan I, Fried K. Neuropeptide- and tyrosine hydroxylase-immunoreactive nerve fibers in painful Morton’s neuromas. Muscle Nerve. 2000;23:1214–1218. doi: 10.1002/1097-4598(200008)23:8<1214::aid-mus9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 57.Little JW, Chen Z, Doyle T, Porreca F, Ghaffari M, Bryant L, Neumann WL, Salvemini D. Supraspinal peroxynitrite modulates pain signaling by suppressing the endogenous opioid pathway. J Neurosci. 2012;32:10797–10808. doi: 10.1523/JNEUROSCI.6345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little JW, Doyle T, Salvemini D. Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids. 2012;42:75–94. doi: 10.1007/s00726-010-0633-0. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Bu H, Liu C, Gao F, Yang H, Tian X, Xu A, Chen Z, Cao F, Tian Y. Inhibition of glial activation in rostral ventromedial medulla attenuates mechanical allodynia in a rat model of cancer-induced bone pain. J Huazhong Univ Sci Technolog Med Sci. 2012;32:291–298. doi: 10.1007/s11596-012-0051-5. [DOI] [PubMed] [Google Scholar]
- 60.Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 61.Lozano-Ondoua AN, Hanlon KE, Symons-Liguori AM, Largent-Milnes TM, Havelin JJ, Ferland HL, 3rd, Chandramouli A, Owusu-Ankomah M, Nikolich-Zugich T, Bloom AP, Jimenez-Andrade JM, King T, Porreca F, Nelson MA, Mantyh PW, Vanderah TW. Disease modification of breast cancer-induced bone remodeling by cannabinoid 2 receptor agonists. J Bone Miner Res. 2013;28:92–107. doi: 10.1002/jbmr.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 63.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology. 2011;115:189–204. doi: 10.1097/ALN.0b013e31821b1ac5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–598. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 67.Mundy GR. Pathophysiology of cancer-associated hypercalcemia. Semin Oncol. 1990;17:10–15. [PubMed] [Google Scholar]
- 68.Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007;25:99–104. doi: 10.1007/s00774-006-0734-8. [DOI] [PubMed] [Google Scholar]
- 69.O’Connell JX, Nanthakumar SS, Nielsen GP, Rosenberg AE. Osteoid osteoma: the uniquely innervated bone tumor. Mod Pathol. 1998;11:175–180. [PubMed] [Google Scholar]
- 70.Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport. 1998;9:1109–1113. doi: 10.1097/00001756-199804200-00028. [DOI] [PubMed] [Google Scholar]
- 71.Omori M, Yokoyama M, Matsuoka Y, Kobayashi H, Mizobuchi S, Itano Y, Morita K, Ichikawa H. Effects of selective spinal nerve ligation on acetic acid-induced nociceptive responses and ASIC3 immunoreactivity in the rat dorsal root ganglion. Brain Res. 2008;1219:26–31. doi: 10.1016/j.brainres.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 72.Osikowicz M, Mika J, Przewlocka B. The glutamatergic system as a target for neuropathic pain relief. Exp Physiol. 2013;98:372–384. doi: 10.1113/expphysiol.2012.069922. [DOI] [PubMed] [Google Scholar]
- 73.Palazzo E, Luongo L, de Novellis V, Rossi F, Marabese I, Maione S. Transient receptor potential vanilloid type 1 and pain development. Curr Opin Pharmacol. 2012;12:9–17. doi: 10.1016/j.coph.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 74.Pan HL, Zhang YQ, Zhao ZQ. Involvement of lysophosphatidic acid in bone cancer pain by potentiation of TRPV1 via PKCepsilon pathway in dorsal root ganglion neurons. Mol Pain. 2010;6:85. doi: 10.1186/1744-8069-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 77.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 78.Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, Diogenes A, Trott AA, Hargreaves KM, Flores CM. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci. 2005;6:4. doi: 10.1186/1471-2202-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rausaria S, Ghaffari MM, Kamadulski A, Rodgers K, Bryant L, Chen Z, Doyle T, Shaw MJ, Salvemini D, Neumann WL. Retooling manganese(III) porphyrin-based peroxynitrite decomposition catalysts for selectivity and oral activity: a potential new strategy for treating chronic pain. J Med Chem. 2011;54:8658–8669. doi: 10.1021/jm201233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res. 1996;113:143–151. doi: 10.1016/s0079-6123(08)61085-7. [DOI] [PubMed] [Google Scholar]
- 81.Ren BX, Gu XP, Zheng YG, Liu CL, Wang D, Sun YE, Ma ZL. Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology. 2012;116:122–132. doi: 10.1097/ALN.0b013e31823de68d. [DOI] [PubMed] [Google Scholar]
- 82.Ryu CS, Kwak HC, Lee JY, Oh SJ, Phuong NT, Kang KW, Kim SK. Elevation of cysteine consumption in tamoxifen-resistant MCF-7 cells. Biochem Pharmacol. 2013;85:197–206. doi: 10.1016/j.bcp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Salvemini D, Neumann W. Targeting peroxynitrite driven nitroxidative stress with synzymes: A novel therapeutic approach in chronic pain management. Life Sci. 2010;86:604–614. doi: 10.1016/j.lfs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Scheid T, Bosco LD, Guedes RP, Pavanato MA, Bello-Klein A, Partata WA. Sciatic Nerve Transection Modulates Oxidative Parameters in Spinal and Supraspinal Regions. Neurochem Res. 2013 doi: 10.1007/s11064-013-1000-9. [DOI] [PubMed] [Google Scholar]
- 85.Schiller KR, Zillhardt MR, Alley J, Borjesson DL, Beitz AJ, Mauro LJ. Secretion of MCP-1 and other paracrine factors in a novel tumor-bone coculture model. BMC Cancer. 2009;9:45. doi: 10.1186/1471-2407-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seidlitz EP, Sharma MK, Singh G. Extracellular glutamate alters mature osteoclast and osteoblast functions. Can J Physiol Pharmacol. 2010;88:929–936. doi: 10.1139/y10-070. [DOI] [PubMed] [Google Scholar]
- 88.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, Kubota K, Kuskowski MA, Boustany L, Shelton DL, Mantyh PW. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115:128–141. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Sharma MK, Seidlitz EP, Singh G. Cancer cells release glutamate via the cystine/glutamate antiporter. Biochem Biophys Res Commun. 2010;391:91–95. doi: 10.1016/j.bbrc.2009.10.168. [DOI] [PubMed] [Google Scholar]
- 90.Shinoda M, Ogino A, Ozaki N, Urano H, Hironaka K, Yasui M, Sugiura Y. Involvement of TRPV1 in nociceptive behavior in a rat model of cancer pain. J Pain. 2008;9:687–699. doi: 10.1016/j.jpain.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- 92.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swanson DM, Dubin AE, Shah C, Nasser N, Chang L, Dax SL, Jetter M, Breitenbucher JG, Liu C, Mazur C, Lord B, Gonzales L, Hoey K, Rizzolio M, Bogenstaetter M, Codd EE, Lee DH, Zhang SP, Chaplan SR, Carruthers NI. Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1- carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J Med Chem. 2005;48:1857–1872. doi: 10.1021/jm0495071. [DOI] [PubMed] [Google Scholar]
- 94.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 95.Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- 96.Ungard RG, Seidlitz EP, Singh G. Oxidative stress and cancer pain. Can J Physiol Pharmacol. 2013;91:31–37. doi: 10.1139/cjpp-2012-0298. [DOI] [PubMed] [Google Scholar]
- 97.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 99.Wang LN, Yang JP, Ji FH, Zhan Y, Jin XH, Xu QN, Wang XY, Zuo JL. Brain- derived neurotrophic factor modulates N-methyl-D-aspartate receptor activation in a rat model of cancer-induced bone pain. J Neurosci Res. 2012;90:1249–1260. doi: 10.1002/jnr.22815. [DOI] [PubMed] [Google Scholar]
- 100.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 101.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 103.Yeo JF, Ling SF, Tang N, Ong WY. Antinociceptive effect of CNS peroxynitrite scavenger in a mouse model of orofacial pain. Exp Brain Res. 2008;184:435–438. doi: 10.1007/s00221-007-1211-x. [DOI] [PubMed] [Google Scholar]
- 104.Yin Q, Cheng W, Cheng MY, Fan SZ, Shen W. Intrathecal injection of anti- CX3CR1 neutralizing antibody delayed and attenuated pain facilitation in rat tibial bone cancer pain model. Behav Pharmacol. 2010;21:595–601. doi: 10.1097/FBP.0b013e32833e7e2a. [DOI] [PubMed] [Google Scholar]
- 105.Zanelli SA, Ashraf QM, Delivoria-Papadopoulos M, Mishra OP. Peroxynitrite-induced modification of the N-methyl-D-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci Lett. 2000;296:5–8. doi: 10.1016/s0304-3940(00)01608-6. [DOI] [PubMed] [Google Scholar]
- 106.Zanelli SA, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neuroscience. 2002;112:869–877. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- 107.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]