Abstract
Accumulating evidence suggests that dopamine (DA) is involved in altering neural activity and gene expression in a zebra finch cortical–basal ganglia circuit specialized for singing, upon the shift between solitary singing and singing as a part of courtship. Our objective here was to sample changes in the extracellular concentrations of DA in Area X of adult and juvenile birds, to test the hypothesis that DA levels would change similarly during presentation of a socially salient stimulus in both age groups. We used microdialysis to sample the extracellular milieu of Area X in awake, behaving adult and juvenile male zebra finches, and analysed the dialysate using high-performance liquid chromatography coupled with electrochemical detection. The extracellular levels of DA in Area X increased significantly during both female presentation to adult males and tutor presentation to juvenile males. DA levels were not correlated with the time spent singing. We also reverse-dialysed Area X with pharmacologic agents that act either on DA systems directly or on norepinephrine, and found that all of these agents significantly increased DA levels (3- to 10-fold) in Area X. These findings suggest that changes in extracellular DA levels can be stimulated similarly by very different social contexts (courtship and interaction with tutor), and influenced potently by dopaminergic and noradrenergic drugs. These results raise the possibility that the arousal level or attentional state of the subject (rather than singing behavior) is the common feature eliciting changes in extracellular DA concentration.
Keywords: behavior, juvenile, microdialysis, monoamine, striatum
Introduction
In songbirds, both song learning and adult song production are powerfully modulated by social factors and attentional cues (Tchernichovski & Nottebohm, 1998; Hessler & Doupe, 1999; Cousillas et al., 2006; Hauber et al., 2007; Adar et al., 2008; Lehongre et al., 2009; Kojima & Doupe, 2011). Numerous recent studies of song learning reveal the influences of social cues – for example, adolescent male zebra finch song performance decreases markedly in variability and increases in stereotypy in the presence of an adult female (Kojima & Doupe, 2011), and sound processing in a starling central auditory nucleus depends on the social interactions occurring during development (Cousillas et al., 2006). In addition to sensory and motor areas that process and produce song, the male zebra finch brain contains a cortico-basal ganglia circuit specialized for song that is affected by social cues. This rostral circuit, known as the anterior forebrain pathway (AFP; Fig.1A), is composed of the lateral magnocellular nucleus of the anterior nidopallium (LMAN, a cortex-like output structure), the medial dorsolateral nucleus of the thalamus (a thalamic nucleus) and Area X (a striatopallidal nucleus). Neural activity (Hessler & Doupe, 1999; Kao et al., 2008) and immediate early gene (IEG) expression (Jarvis et al., 1998) in LMAN and Area X are higher in male birds singing alone than in males singing to females. The importance of social cues beyond courtship has not been investigated as extensively (e.g. Nordeen et al., 2009).
Figure 1.
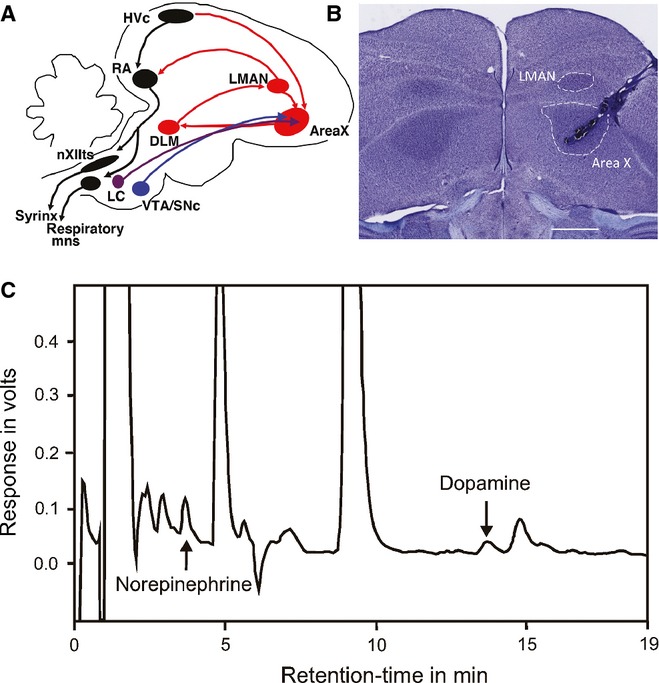
(A) Schematic representation of important song-related circuits in the zebra finch brain, including catecholaminergic projections (in purple and blue) to the anterior forebrain pathway (AFP), in red. HVC, letter-based proper name; RA, robust nucleus of the arcopallium; nXIIts, cranial nerve XII; LC, locus coeruleus; VTA/SNc, ventral tegmental area/substantia nigra pars compacta; DLM, dorsolateral nucleus of the medial thalamus; LMAN, lateral magnocellular nucleus of the anterior nidopallium. (B) Photomicrograph of representative coronal section of male zebra finch brain, illustrating location of microdialysis probe. Note that placement of probe avoids damage to LMAN. Image obtained on a Nikon Eclipse E800 microscope, with 2× objective and 10× ocular, captured with Zeiss AxioCamMRc5 and Axiovision 4.4 software. Scale bar = 1 mm. (C) Representative chromatogram for a single microdialysis sample collected from Area X of an adult zebra finch. Signal amplitude (in volts) is plotted on the y-axis and retention time (in minutes) is plotted on the x-axis. NE and DA peaks are designated with arrows. A clear separation from interfering peaks is apparent for both NE and DA.
Much evidence suggests that both dopamine (DA) (Sasaki et al., 2006; Heimovics & Riters, 2008; Huang & Hessler, 2008; Leblois et al., 2010) and norepinephrine (NE) (Barclay et al., 1996; Castelino et al., 2007; Riters & Pawlisch, 2007) are involved in courtship-mediated changes in basal ganglia activity. Area X receives dopam-inergic projections from the substantia nigra (SNc) and ventral tegmental area (VTA), as well as NE from locus coeruleus (Lewis et al., 1981; Bottjer et al., 1989; Soha et al., 1996; Castelino et al., 2007; Person et al., 2008). Depletion of either NE (Castelino & Ball, 2005) or DA (Hara et al., 2007) results in altered IEG expression in the song system. DA levels in Area X increase significantly when males sing song directed at females but not when they sing alone (Sasaki et al., 2006). The D1 receptor antagonist chronically delivered to Area X prevents the usual decrease in song variability seen during directed (courtship) singing (Leblois et al., 2010). It remains unclear, however, whether artificially raising DA levels with direct agonist treatment would reproduce levels seen in response to social cues.
The goal of the present study was to determine whether changes in extracellular DA concentration in Area X differed during two different social interactions, courtship and tutoring. We monitored fluctuations in extracellular DA levels using microdialysis. We also assessed how behaviorally-induced changes in DA concentration compared with those induced by pharmacological manipulation. Here, we report that similar changes in DA levels are associated with different types of social interactions, and that these changes are much smaller than those induced by pharmacologic agents.
Methods
Subject animals
Male zebra finches (Taeniopygia guttata) were born and raised in our colony, and kept on a 14/10-h light–dark cycle with access to seed, grit, mash and water ad libitum. Adult males were all > 180 days old, while juvenile males ranged in age from 42 to 81 days old (average age was 61.7 days old) at the time of the behavior experiments. All procedures were performed in accordance with protocols approved by the University of California, San Francisco Institutional Animal Care and Use Committee. Experiments were conducted on a total of 22 birds. For behavior experiments, 13 zebra finches were used (data for two birds were excluded due to incorrect probe placement). These 13 birds and an additional nine adult birds were also used for pharmacology experiments; pharmacology experiments were conducted several days after the behavior experiments.
Chemicals
All salts for artificial cerebrospinal fluid (ACSF) except CaCl2 were obtained from Fluka (Devon, UK). CaCl2 and all other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA).
Surgical procedures
Birds were prepared for surgery by pre-anesthetizing them with a mixture of ketamine (40 mg/kg) and midazolam (1.5 mg/kg) injected intramuscularly. Under isofluorane anesthesia (0.5–2%) a guide cannula (CMA 7; CMA Microdialysis, Stockholm, Sweden) was stereotaxically implanted just above Area X and 45° from vertical (to avoid LMAN) several days prior to experiments (with coordinates relative to the posterior bifurcation of the saggital sinus: AP = 5.1–5.2 mm, ML = 4.0 mm, at a depth of 2 mm from the dorsal surface of the brain). For experiments that involved baclofen infusion into the VTA a second guide cannula was implanted; this VTA-directed cannula was implanted in a similar fashion but with VTA-specific coordinates: AP = 3.65 mm, ML = 0.45 mm, depth = 6 mm. Guide cannulae were secured to the top of the skull with epoxy.
Behavior protocol
Each bird was isolated for several days prior to the experiment by housing it alone in a sound attenuating chamber (Acoustic Systems, Austin, TX, USA). Approximately 15–18 h before the beginning of the experiment a microdialysis probe (CMA 7, active membrane length = 1 mm) was prepared by flushing the membrane with 70% EtOH, and then HPLC-grade water, before attaching it to the liquid swivel (375/D/22QM; Instech, Plymouth Meeting, PA, USA) of our microdialysis apparatus. The other component of the microdialysis apparatus was a CMA syringe pump (model 100), housed outside the sound attenuating chamber. ACSF (145 mm NaCl, 2.7 mm KCl, 2.4 mm CaCl2, 1.0 MgCl2, 2.0 mm Na2HPO4, 5 mm glucose) was pumped through the inlet channel of the swivel, through the probe, out through the outflow channel, and collected outside the sound attenuating chamber in small vials (SciPro, Sanborn, NY, USA). Once attached to the swivel, the microdialysis probe was inserted into Area X through the guide cannula and allowed to equilibrate with ACSF overnight (at a pump rate of 0.3 μL/min) while the dialysate was discarded. Following probe equilibration and while the subject was awake and alone in the light, baseline samples were collected at 10- or 15-min intervals at a pump rate of 1.5 μL/min. After baseline samples were collected a ‘stimulus’ zebra finch (a female for the adult males or the tutor for the juveniles) was presented to the subject (by placing the stimulus individual’s cage alongside the subject’s cage in the sound attenuating chamber), and samples continued to be collected. Following removal of the stimulus bird, additional samples (‘post-baseline’ or ‘recovery’) were collected; for adults, this recovery period was sampled for 60–75 min and for juveniles, up to 130 min. Samples were collected on ice, and then stored at −80 °C until analysed.
All experiments were digitally recorded to capture songs and behavior for review and analysis following microdialysis sample collection. Amount of singing was quantified as the number of seconds elapsed from the first call note to the end of the song bout. Pauses within the song were not included in the quantification; for example, if a bout lasted 5 s, but within the bout there was a 0.5-s pause, the amount of singing was quantified as 4.5 s. Subject behavior was observed and recorded in general descriptive terms (preening, courtship, approach, aggression, etc.) during each collection interval.
Pharmacology protocol
The effects of a GABA-ergic (baclofen), a dopaminergic (amphetamine) and noradrenergic (nomifensine, reboxetine and NE) pharmacologic agents on neurotransmitter release were tested using reverse microdialysis. Baclofen is an agonist at GABAB receptors; it decreases the firing rate of neurons possessing these receptors. VTA neurons possess GABAB receptors, and at moderate to high doses of baclofen the effect on DA projection neurons predominates, and decreases DA release from the VTA (Cruz et al., 2004). Amphetamine reverses the action of the dopamine transporter (DA-T) and causes release of DA from neurons that express DA-T (Sulzer et al., 2005). Nomifensine inhibits the function of the NE/DA-T and theoretically results in an increase in the extracellular levels of both NE and DA (Gianutsos et al., 1982), although in birds it has been used as a DA reuptake inhibitor (Sasaki et al., 2006). In mammals reboxetine blocks reuptake of NE by inhibiting the action of the synaptic NE transporter (NE-T; Kasper et al., 2000) and results in an increase in extracellular levels of NE.
The protocol for this type of experiment was as described above, with the following exceptions: (i) the subject remained isolated throughout the experiment; and (ii) after baseline samples were collected, while ACSF was pumped through the probe, the dialysate was changed to one containing the pharmacologic agent (in an ACSF vehicle). Given the pump rate and tubing length from pump to probe, the lag time for the drug to reach the probe was approximately 15 min; the effects of the drug were apparent within an 15 additional minutes, the lag time from the probe to the collection vials. For each drug, three subjects were tested (i.e. n = 3 for each pharmacology experiment) and each subject was drug-naïve for each such experiment (i.e. no subject was exposed to more than one drug nor was used more than once for any pharmacology experiment).
For the purpose of graphically representing group mean changes within experiments, we determined change from baseline using the following calculation – the microdialysis value obtained for each collection interval during and after behavioral or pharmacologic stimulation was divided by the average of the 3–5 previous baseline values and then averaged across the three experimental time bins (baseline, during, after) and multiplied by 100 to generate a percentage of change relative to baseline.
High-performance liquid chromatography and electrochemical detection (HPLC-ED)
All dialysate samples from behavioral stimulation experiments, as well as the reboxetine and NE pharmacology experiments, were analysed for neurotransmitter content using HLPC-ED as described by Cremers et al. (2004) and detailed below. The effects of baclofen, amphetamine and nomifensine were analysed using the HPLC-ED method described by Becker & Cha (1989).
DA and NE detection
Separation
Microdialysis aliquots (20 μL) were injected onto the analytical column (BDS Hypersil C18 column, 150 × 2.1 mm, 3 μm particle size; Thermo Scientific, Waltham, MA, USA) by a refrigerated microsampler system, consisting of a syringe pump (model 402; Gilson, France) and a multi-column injector (model 233 XL; Gilson). The column was held at a constant temperature of 32.5 °C by a column oven (Intro; Antec Leyden, the Netherlands). Chromatographic separation was isocratic at a flow rate of 0.35 mL/min by an HPLC pump (model LC-10AD vp; Shimadzu, Tokyo, Japan). The mobile phase consisted of an NaAc buffer (4.1 g/L) with MeOH (2.5% v/v), EDTA (150 mg/L), OSA (150 mg/L) and TMA (150 mg/L) and adjusted with glacial acetic acid to pH 4.1 (Cremers et al., 2007).
Electrochemical detection
DA and NE were detected electrochemically using a potentiostat (VT-03 flowcell; Antec Leyden) fitted with a glassy carbon electrode set at +500 mV vs. Ag/AgCl reference electrode. This potential was selected to maximize sensitivity while minimizing noise.
Data were analysed using Shimadzu Chromatography Data System (class-vp) software. Concentrations of NE and DA were quantified by the external standard method. The average retention time for NE was 4.1 min and the detection limit was 0.7 fmol per sample [signal-to-noise ratio (S/N) > 5]. The average retention time for DA was 16.2 min with a detection limit of 1 fmol per sample (S/N > 5).
Histology
At the conclusion of all experiments, subjects’ brains were harvested to determine the location of the microdialysis probe (Fig.1B). Following anesthesia with isofluorane, subjects were perfused transcardially with filtered, room temperature PBS followed by perfusion with room temperature 3.7% formalin. Brains were cryoprotected in a 30% sucrose solution, and then sectioned (40 μm thickness) on a microtome in the coronal plane. Subjects whose probes were not located within Area X (n = 2) were excluded from analysis.
Statistical analyses
To compare mean extracellular DA levels in adult male finches in the presence of a female with those in the absence of a female, we created a three-level categorical variable for experimental condition. Baseline measurements (prior to introduction of female) served as the reference category for both ‘stimulus’ measurements (during the time that the female was present) and ‘recovery’ measurements (immediately after removal of the female). Similar comparisons were made in the juvenile group, with tutor substituting for female in the stimulus condition. Changes in extracellular DA concentration in response to pharmacologic manipulation were also examined for the pharmacologic agents baclofen, amphetamine, nomifensine, reboxetine and NE, with similar comparisons made between referent baseline levels and both stimulus (pharmacologic agents) and recovery periods.
All analyses were conducted using random effects models, a type of repeated-measures analysis. Because, during the course of one experiment, repeated measurements were taken on the same subjects over extended periods of time (i.e. hours), they were probably correlated. To accommodate this correlation, mixed model regression (specifically, a random effects model) was used (sas proc mixed, SAS Ver 9.2; SAS Institute, Cary, NC, USA). Unlike repeated-measures anova, mixed model regression controls for repeated measures (multiple samples comprising a single condition) as well as repeated trials within subjects (pre- and post-stimulation conditions), while also allowing for unbalanced data, use of all available data when some are missing, and for incorporation of predictors that change from one time to the next. In particular, this model was also able to assess whether any amount of change was accounted for by time (given the long duration of the experiments) by modeling time as a potential predictor of DA level. Potential correlations between amount of singing and DA content during the different time periods were examined using non-parametric Spearman correlations.
Results
Extracellular DA levels in Area X increase in response to different social contexts
We collected dialysate from Area X of adult males zebra finches using a technique similar to one described previously (Sasaki et al., 2006), except that we did not manipulate lighting conditions. For all subjects extracellular DA levels increased in the presence of a female (see Fig.2A, top panel, for a representative adult experiment of behavioral manipulation of DA levels). Compared with baseline (set to zero), DA levels during time spent with a female were increased by 5.4 fmol per sample (P < 0.0001, n = 5; Table1). All males sang directed song and engaged in courtship displays in the presence of a female. Some males sang exclusively during female presentation, while others sang as much during time alone as with a female (see Fig.2A, bottom panel, for a representative bar graph quantifying singing). The amount of singing for each 15-min interval, as measured by seconds of singing, was not correlated with the DA content of the sample collected during that interval, using Spearman (non-parametric) correlation (r = −0.29, P = 0.15), consistent with the results of Sasaki et al. (2006). The effect of this social stimulation, namely a 26.5 ± 8.4% increase in the normalized group mean DA content (Fig.2B), partially reversed during the time period immediately following the stimulus; compared with baseline, DA levels after the female was removed remained increased but only by 2.5 fmol per sample (P = 0.003, n = 5, Table1).
Figure 2.
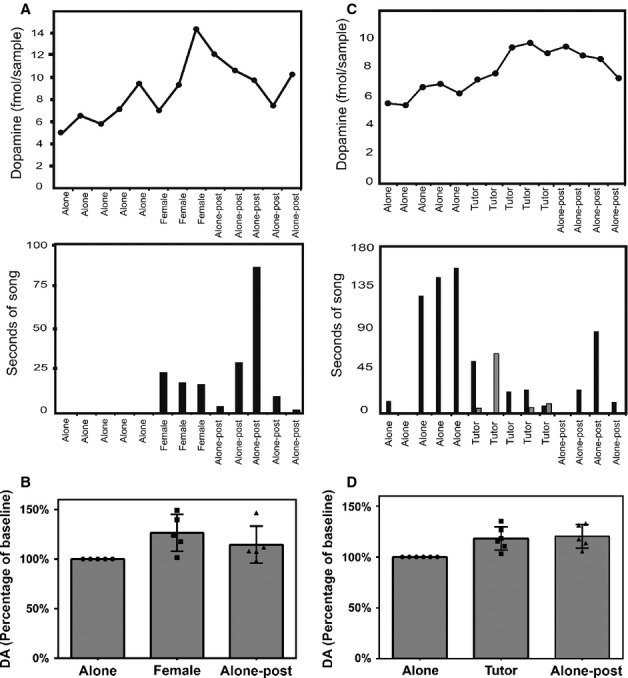
Social interaction is associated with increases in extracellular dopamine levels. (A) Representative experimental time course (top panel) for an individual adult male zebra finch, illustrating extracellular DA levels (on the y-axis) at 15-min intervals throughout the course of one experiment, with histogram (bottom panel) illustrating amount of singing (in seconds, on the y-axis) produced during the same time intervals. Different experimental conditions [baseline state (‘alone’), social stimulation (‘female’) and post-stimulation (‘alone-post’)] are indicated on the x-axis. (B) Bar graph illustrating change in extracellular DA levels in adult male zebra finch Area X during courtship behavior. Group (n = 5) mean DA levels are expressed as normalized values on the y-axis, and the experimental conditions (as described in A) are indicated on the x-axis. The individual values of the five data points composing the group means are plotted within the bars for each condition. Error bars indicate SEM. (C) Representative experimental time course for an individual juvenile male zebra finch, illustrating extracellular DA levels at 10-min intervals throughout the course of one experiment, with histogram illustrating amount of singing (in seconds) produced by both the juvenile (black bars) and the adult tutor (gray bars) zebra finch. Different experimental conditions [baseline state (‘alone’), social stimulation (‘tutor’) and post-stimulation (‘alone-post’)] are indicated on the x-axis. Because of the duration of the experiment, every other interval is plotted. (D) Bar graph illustrating change in extracellular DA levels in juvenile male zebra finch Area X during observation of tutor. Group (n = 6) mean DA levels are expressed as normalized values on the y-axis, and the experimental conditions are indicated on the x-axis. The individual values of the six data points composing the group means are plotted within the bars for each condition. Error bars indicate SEM.
Table 1.
Comparison of behavioral conditions, using a random effects model [solution for fixed effects, adults (n = 5)]
| Characteristic | Estimate [DA, (fmol) per sample] | 95% CI | P | ||
|---|---|---|---|---|---|
| Condition | Baseline | 0 | n/a | n/a | n/a |
| Stimulus* | +5.4003 | 3.8753 | 6.9253 | < 0.0001 | |
| Recovery | +2.5013 | 0.8786 | 4.1239 | < 0.003 | |
| Time | 0.02807 | −0.00476 | 0.06090 | 0.0926 | |
Presence of female.
We then extended this study to examine the effect of social interaction in juvenile male zebra finches. We measured extracellular DA levels in dialysate samples collected at 10-min intervals in the presence of the juvenile birds’ tutors (fathers) (see Fig.2C, top panel, for a representative juvenile experiment of behavioral manipulation of DA levels). We found a significant increase in Area X DA concentration in juvenile males during tutor presentation that was almost as large as that seen for the social stimulus of courtship in adult males. Compared with baseline, DA levels during tutor presentation were increased by 3.3 fmol per sample (P < 0.0001, n = 6, Table2). Unlike adults, juveniles’ singing behavior varied widely in the presence of the stimulus (their tutor); some sang in response to tutor song while others decreased their song output, quieting in the presence of their tutor when they previously had been singing frequently while alone (see Fig.2C, bottom panel, for a representative bar graph quantifying singing both by the juvenile (black bars) and by the tutor (gray bars)). All juveniles changed their behavioral repertoire from solitary activities (e.g. eating and preening) to interactive activities upon presentation of the tutor (alerting to, approaching and occasionally even sparring with the tutor). All juveniles displayed these behaviors at some point during the presentation of the tutor, even when the tutor did not sing.
Table 2.
Comparison of behavioral conditions, using a random effects model [solution for fixed effects, juveniles (n = 6)]
| Characteristic | Estimate [DA, (fmol) per sample] | 95% CI | P | ||
|---|---|---|---|---|---|
| Condition | Baseline | 0 | n/a | n/a | n/a |
| Stimulus* | +3.2774 | 1.8679 | 4.6869 | < 0.0001 | |
| Recovery | +5.0826 | 3.5859 | 6.5793 | < 0.0001 | |
| Time | 0.02057 | 0.0004123 | 0.03703 | 0.0146 | |
Presence of tutor.
Strikingly, unlike the situation with adults, in juveniles recovery of DA levels was not evident within the first hour following removal of the tutor; in fact, DA continued to increase following removal of the tutor (5.0 fmol per sample increase in DA over baseline; P < 0.0001, n = 6). Whereas the tutor presentation was associated with a 21.4 ± 7.3% increase in the normalized group mean DA content compared with baseline, removal of the tutor was associated with a 24.1 ± 8.1% increase in the normalized group mean DA content compared with baseline (Fig.2D). Recovery only became evident in later samples, 90–130 min following removal of the tutor, but samples from the first hour post-stimulation were still used in the analysis, to be consistent with the parameters established in the experiments with adult subjects. In this experimental condition there was a very small effect of time; for each additional minute of the experiment, the DA level increased by 0.02 fmol per sample. As with adults, the amount of DA increase did not appear to be correlated with the amount of singing observed in subjects. Statistical correlation analysis could not be used because, for multiple subjects, a similar change in DA was seen with very different changes in behavior. For example, the three subjects with mid-range changes in DA (increases of 15–27%) had no change in song frequency, either because they did not sing at all throughout the experiment or because the number of songs did not differ between baseline and stimulation conditions.
The maximum amount of change from baseline in DA concentration in response to physiologic (behavioral) manipulation was 49% in the adult experiments, and 35% in the juvenile experiments. The passage of experimental time was found to have an effect only in juveniles, and even then only accounted for < 1% of the change in DA levels from baseline. Given this effect of behavioral stimulation we then undertook to determine the degree to which DA levels could change in Area X by pharmacologically manipulating changes in extracellular DA concentration.
Extracellular DA levels in Area X can be rapidly modulated by a number of pharmacologic agents
Baclofen
As Area X is known to be innervated by VTA, we directly infused a small volume of a high-dose baclofen solution (0.1 μL of a 0.1 μg/μL solution) into the midbrain, targeting the VTA, and measured the resultant change in [DA] in Area X from baseline. Compared with baseline, DA levels decreased by 1.69 pg per sample during baclofen infusion (P = 0.002, n = 3, Table3, Fig.3A), and subsequently recovered to levels not significantly different from baseline (P = 0.51).
Table 3.
Analysis of effects of pharmacologic agents, using a random effects model [solution for fixed effects, pharmacologic agents (n = 3 subjects for each drug tested)]
| Stimulus | Recovery | |||||
|---|---|---|---|---|---|---|
| Estimate* | 95% CI | P | Estimate* | 95% CI | P | |
| Baclofen | −1.69 | −2.48, −0.90 | 0.0002 | −0.26 | −1.08, 0.56 | 0.51 |
| Amphetamine | +45.33 | 19.19, 71.47 | 0.0024 | n/a | n/a | n/a |
| Nomifensine | 0.55 | 0.42, 0.68 | < 0.0001 | n/a | n/a | n/a |
| Reboxetine | +113.88 | 65.34, 162.42 | < 0.0001 | n/a | n/a | n/a |
| Norepinephrine | +2.28 | 0.90, 3.65 | 0.0018 | –1.36 | –2.74, 0.009 | 0.05 |
Unit change in [DA] per sample, relative to baseline.
Figure 3.
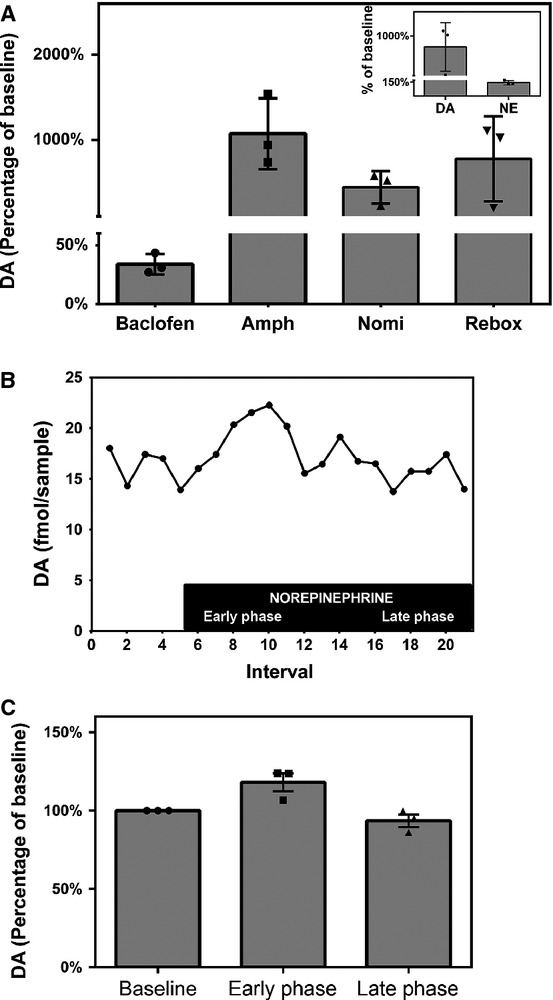
Extracellular DA levels can be pharmacologically manipulated by drugs that target dopaminergic and noradrenergic synapses. (A) Bar graph illustrating the effects of pharmacologic agents on Area X DA levels. The GABAergic agent baclofen (0.1 μL of a 0.1 μg/μL solution) was infused into VTA, and the dopaminergic [amphetamine (‘Amph’, 10 μm) and nomifensine (‘Nomi’, 10 μm)] and noradrenergic [reboxetine (‘Rebox’, 250 μm)] agents were reverse-dialysed into Area X. Bars represent normalized group mean DA values, and error bars indicate SEM. n = 3 for each pharmacologic agent. The individual values of the three data points composing the group means are plotted within the bars for each condition. The effect of reboxetine on NE is demonstrated with the inset, where bars represent normalized group mean DA values on the left and normalized group mean NE values on the right. Error bars indicate SEM (n = 3). (B) Representative experimental time course for an individual zebra finch, illustrating extracellular DA levels at 10-min intervals throughout the course of one experiment as exogenous NE (500 nm, indicated by the black box) was reverse-dialysed into Area X. (C) Bar graph illustrating the mean change in extracellular DA in Area X that occurred in the presence of NE (n = 3). Bars represent normalized group mean DA levels (y-axis) at baseline, in the first 50 min (‘early phase’), and last 50 min (‘late phase’) of NE reverse dialysis, as indicated on the x-axis. Error bars indicate SEM. The individual values of the three data points composing the group means are plotted within the bars for each condition.
Amphetamine
We used reverse dialysis to deliver 10 μm amphetamine to Area X and measured the change in [DA] from baseline. Compared with baseline, levels of DA increased by 45.3 pg per sample during amphetamine (P = 0.002, n = 3, Table3, Fig.3A), a level 10-fold higher than the increases triggered physiologically.
Nomifensine
We used reverse dialysis to deliver 10 μm nomifensine [half of the dose used by Bert et al. (2002) to achieve a 30-fold increase in rat striatal DA efflux] to Area X and measured the change in [DA] from baseline. Compared with baseline, DA levels increased by 0.55 pg per sample during nomifensine (P < 0.0001, n = 3, Table3, Fig.3A). Because this subset of experiments was analysed using an HPLC system adapted to detect only DA (Becker & Cha, 1989) we could not examine the effect of nomifensine on NE levels.
Reboxetine
We used reverse dialysis to deliver 250 nm reboxetine (M. van der Hart, personal communication; ∼0.1× the dose used in rodent studies; Linnér et al., 2001) into Area X. We found that reboxetine changed the extracellular concentration of NE to a much lesser extent (44.3 ± 12.7%) than for DA (775.2 ± 290%); see inset to Fig.3A. In three experiments, the [DA] level in the presence of 250 nm reboxetine was increased by 113.9 fmol per sample (P < 0.0001, n = 3, Table3, Fig.3A), although the magnitude of the increase varied between birds (from two- to 10-fold).
Norepinephrine
To determine whether the effect of reboxetine on DA levels was direct (acting on the DA-T in a promiscuous fashion) or the downstream result of increased NE levels (which then modulate DA levels through an as yet undetermined mechanism), we reverse-dialysed a supraphysiologic concentration of NE (500 nm; M. van der Hart, personal communication) into Area X and measured the change in [DA] from baseline. This approach allowed us to monitor the onset of the [DA] change in relation to the time at which NE became detectable in the dialysate (Fig.3B). Early in the experiment, during the period of the greatest change in [NE], the extracellular [DA] began to rise. After 40 min, when the [NE] had plateaued at a mean level of 2116 fmol per sample, the [DA] began to decrease. After another 40 min, the extracellular [DA] also plateaued, but to a slightly lower level than baseline (late in the experiment). Compared with baseline, DA levels increased by 2.3 fmol per sample during NE (P = 0.002 vs. baseline, n = 3, Table3, Fig.3C), and decreased to 1.4 fmol per sample in the late phase, back to a level not different from baseline (P = 0.052). Thus, supraphysiologic levels of NE transiently increase [DA] in Area X.
As in the adult behavioral experiments, time was not a significant predictor of DA level in the pharmacology experiments.
Discussion
Using in vivo microdialysis we have determined that the content of extracellular DA in Area X increases during different social interactions. Not only were the social stimuli very different (courtship and exposure to a tutor), but subjects’ behavioral responses were also divergent (directed singing and courtship displays in adults, in contrast to quieting or aggression in juveniles). The end result in terms of DA levels was similar, however, suggesting that a salient social signal probably brought about changes in extracellular DA in both cases. These findings also suggest that similar brain circuits are involved, whether an adult male responds to a female or a juvenile male responds to a tutor. Our study deliberately focused on only two conditions of social stimulation, to investigate the predictions that arousal may be the contextual cue in the neural changes seen in directed singing (Hessler & Doupe,1999) and that increased VTA activity in response to tutor song may be associated with increased DA release in Area X (Nordeen et al., 2009). Hessler & Doupe (1999) performed the control experiment of using a male to elicit directed singing and found that neural activity recorded during directed song in the presence of a male companion was not different from directed singing produced as part of the courtship display (both conditions resulted in a decrease in neural activity in the song circuit). Although courtship behavior has been demonstrated previously to result in increases in extracellular DA concentration in Area X (Sasaki et al., 2006), the finding that interaction with a tutor similarly influences extracellular neurotransmitter fluctuations in this key nucleus of the juvenile AFP is a novel result.
The likely source of the DA we measured in Area X is VTA/SNc. VTA (area A10) neurons of adult males, which have robust projections to the AFP, show both enhanced IEG induction and increased firing in the presence of a female (Heimovics & Riters, 2005; Yanagihara & Hessler, 2006; Hara et al., 2007; Huang & Hessler, 2008; Riters, 2011). Similarly, Nordeen et al. (2009) reported that presentation of the bird’s tutor increased the density of IEG expression in the VTA and SNc (area A9) of juvenile male zebra finches relative to unfamiliar controls, again suggesting tutor-driven VTA neuronal activation. Such activation could reflect the social salience of the signal or a possible function of the VTA in song memorization or evaluation during learning.
Our finding that singing is neither necessary nor sufficient for causing a change in extracellular DA in Area X is consistent with the results of Yanagihara & Hessler (2006) and Huang & Hessler (2008), who observed VTA activation in the presence of a female regardless of whether the adult male bird sang or not, and with the finding of Sasaki et al. (2006) that undirected singing did not significantly increase extracellular DA levels. It remains unclear what aspects of the social stimuli mediate the increase in extracellular DA concentration or what suite of behaviors DA may regulate. In our experiments, both adults and juveniles engaged in behaviors that reflected changes in attention and activity level. The findings reported in our study suggest that changes in DA levels in Area X can result from components of behavior that are associated with, but are not the same as, vocalization – even juvenile subjects who did not sing in the presence of their tutors, but whose behavioral arousal levels changed markedly when the tutor appeared, had elevated DA levels, and these elevations persisted for longer than those in adults in response to females. This conclusion extends those reached by Leblois et al. (2010), who focused primarily on the role of DA in shaping motor stereotypy, by decoupling DA from song and other behaviors associated with adult singing. Our study also suggests that the phenomenon of juvenile song performance enhancement in the presence of an adult female (Kojima & Doupe, 2011) could result from the female-triggered elevation of DA in Area X, in a manner similar to the social cues provided by the presence of a tutor; this increased DA could then modulate singing-related neuronal activity. Our work, along with many previous studies (e.g. Gale et al., 2008), lends further support for the idea that dopaminergic mechanisms involved in reward and motivation and/or salience influence the function of the AFP.
In our experiments here, microdialysis coupled with sensitive HPLC-ED allowed us to detect fluctuations in [DA] in response to social cues that, averaged over a small number of subjects, revealed statistically significant increases from baseline. The same was not true, however, for 5-HT or NE. It is possible that 5-HT could not be measured reliably because the 5-HT content of the extracellular milieu in Area X is below the detection limit for the HPLC-ED system we used. Alternatively, this nucleus may have a dearth of serotonergic and noradrenergic input. Previous reports documenting the total neurotransmitter content of Area X have found lower levels of 5-HT and NE, relative to DA, in canaries (Barclay & Harding, 1988) and zebra finches (Gale & Perkel, 2005); however, total tissue content of neurotransmitter is not equivalent to extracellular concentrations (the latter is likely to be less than the former).
In addition to using microdialysis to assess physiologic changes in DA levels after behavioral stimuli, we also used it to simultaneously deliver pharmacologic agents to Area X and measure their effects on extracellular DA levels. This approach allowed us to better understand the capacity of Area X for changes in extracellular DA content. Our pharmacologic manipulation of Area X with dopaminergic agonists revealed that changes in extracellular DA concentration could be much greater than those occurring during behavioral manipulations, consistent with what has been described for natural rewards (Bassareo et al., 2011). These results suggest that the capacity of the system to modulate DA is quite large, even though the behavioral manipulations, as we applied them, did not elicit such extensive increases in extracellular DA concentration. We also expected to be able to detect pharmacologically induced changes in extracellular NE levels, given the potential role of NE in Area X function (Barclay & Harding, 1988; Barclay et al., 1996), as well as the reports of cross-talk between catecholamines (Cornil et al., 2008; Ball & Balthazart, 2010). While reboxetine did increase the NE concentration of the extracellular fluid, this change was considered to be negligible compared with the substantial increase in DA concentration it caused.
The finding that extracellular DA content in Area X could be modulated by a pharmacologic agent (reboxetine) that blocks reuptake of NE via NE-Ts (Wong et al., 2000) was surprising, albeit consistent with findings that locally applied reboxetine causes release of DA in the nucleus accumbens of awake, behaving rats (Linnér et al., 2001; Valentini et al., 2004). Our finding that supraphysiologic levels of NE could transiently increase [DA] in Area X underscores the point that avian NE-Ts may be like mammalian NE-Ts in their capacity for transporting DA. Although our results cannot provide insight into monoamine transporter function or cross-talk in Area X, more characterization of the monoamine system is needed to understand the differences and similarities of avian monoamine transporters compared with those in mammalian basal ganglia.
Our findings suggest directions for future research. It will be useful to determine the effect of other social interactions [juvenile male with adult female, juvenile male with juvenile male, dummy/video female (stimulus) presented to an adult male (subject)] to attempt to determine which social stimuli are salient and rewarding. Closer observation of animal behavior (of both the subject and the stimulus birds) during the experiment would also be warranted, to more deliberately evaluate arousal, attention, and orienting behaviors. Although microdialysis in birds has been focused on the AFP, much could be learned from sampling from other structures, particularly those associated with motivated behaviors (e.g. POA, nucleus accumbens) or those with projections to the midbrain. Determining what triggers the changes in extracellular levels of DA as we have measured them can give insight into the circuitry of the song system (and beyond). It remains unclear whether VTA activity alone is sufficient to increase DA levels in Area X, or whether VTA activity must be coordinated with another nucleus (e.g. amygdala), song circuit structure or cortical input into Area X. Finally, it would be a logical extension of our pharmacologic findings to attempt to influence song learning by administering dopaminergic agents to juveniles during both the sensory and the sensorimotor periods; we hypothesize that learning (as measured by recognition of tutor song during the sensory period) might be accelerated by the administration of dopaminergic agents.
Song is a complex behavior learned during a critical period in the development of male zebra finches, with parallels to the development of human speech (Doupe & Kuhl, 1999). In both species, vocal learning and adult vocal production are modulated by social context. In humans, learning depends on social (and affective) influences (see Vinogradov et al., 2012; for a review). Small animal models provide additional insight into the mechanisms underlying these influences, and, hence, potentially how to target such mechanisms in more complex animals (see Acheson et al., 2013). The actions of conspecifics must be perceived, interpreted and further processed by the individual observer before it can respond in an appropriate way. Tractable animal models such as songbirds allow us to probe the fundamental neurobiological mechanisms of behavioral modulation in response to social context. With such models we may eventually shed light on the pathophysiology of disorders such as autism and schizophrenia, neuropsychiatric syndromes that have a strong locus in frontal–striatal circuits and that are particularly devastating because of their impact on social functioning.
Conflict of interests
The authors assert that the research was conducted in the absence of any commercial or financial relationships that could be considered to be posing a potential conflict of interest.
Acknowledgments
The considerable contributions to this work made by our co-author, Allison J. Doupe, are specifically acknowledged and commemorated; she passed away on 24 October 2014. We thank A. Arteseros, G. Carrillo, D. Romero and J. Becker for technical assistance, and M. Brainard, L. Stepanek, J. Young and N. Hills for thoughtful comments on this manuscript. Experiments were supported by NIH grant MH 77824 and a NARSAD Distinguished Investigator Award to A.J.D. The data analysis in this publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Glossary
- ACSF
artificial cerebrospinal fluid
- AFP
anterior forebrain pathway
- DA
dopamine
- DA-T
dopamine transporter
- HPLC-ED
high-performance liquid chromatography with electrochemical detection
- IEG
immediate early gene
- LMAN
lateral magnocellular nucleus of the anterior nidopallium
- NE
norepinephrine
- NE-T
norepinephrine transporter
- SNc
substantia nigra, pars compacta
- VTA
ventral tegmental area
References
- Acheson DT, Twamley EW. Young JW. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: a roadmap for preclinical development. Front. Neurosci. 2013;7:103. doi: 10.3389/fnins.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adar E, Lotem A. Barnea A. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav. Brain Res. 2008;187:178–184. doi: 10.1016/j.bbr.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Ball GF. Balthazart J. Introduction to the chemical neuroanatomy of birdsong. J. Chem. Neuroanat. 2010;39:67–71. doi: 10.1016/j.jchemneu.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Barclay SR. Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: steroid effects on brain monoamines. Brain Res. 1988;459:333–343. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol. Biochem. Be. 1996;53:213–220. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Musio P. Di Chiara G. Reciprocal responsiveness of nucleus accumbens shell and core dopamine to food- and drug-conditioned stimuli. Psychopharmacology. 2011;214:687–697. doi: 10.1007/s00213-010-2072-8. [DOI] [PubMed] [Google Scholar]
- Becker JB. Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav. Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Bert L, Parrot S, Robert F, Desvignes C, Denoroy L, Suaud-Chagny MF. Renaud B. In vivo temporal sequence of rat striatal glutamate, aspartate and dopamine efflux during apomorphine, nomifensine, NMDA and PDC in situ administration. Neuropharmacology. 2002;43:825–835. doi: 10.1016/s0028-3908(02)00170-3. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA. Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J. Comp. Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Castelino CB. Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata. Eur. J. Neurosci. 2005;21:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Diekamp B. Ball GF. Noradrenergic projections to the song control nucleus area X of the medial striatum in male zebra finches (Taeniopygia guttata. J. Comp. Neurol. 2007;502:544–562. doi: 10.1002/cne.21337. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Castelino CB. Ball GF. Dopamine binds to α2-adrenergic receptors in the song control system of zebra finches (Taeniopygia guttata. J. Chem. Neuroanat. 2008;35:202–215. doi: 10.1016/j.jchemneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousillas H, George I, Mathelier M, Richard JP, Henry L. Hausberger M. Social experience influences the development of a central auditory area. Naturwissenschaften. 2006;93:588–596. doi: 10.1007/s00114-006-0148-4. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mørk A, Honig G, Bøgesø KP, Westerink BH, den Boer H, Wikstrom HV. Tecott LH. Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology. 2004;29:1782–1789. doi: 10.1038/sj.npp.1300474. [DOI] [PubMed] [Google Scholar]
- Cremers TI, Rea K, Bosker FJ, Wikström HV, Hogg S, Mørk A. Westerink BH. Augmentation of SSRI effects on serotonin by 5-HT2C antagonists: mechanistic studies. Neuropsychopharmacology. 2007;32:1550–1557. doi: 10.1038/sj.npp.1301287. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA. Lüscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat. Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Doupe AJ. Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Gale SD. Perkel DJ. Properties of dopamine release and uptake in the songbird basal ganglia. J. Neurophysiol. 2005;93:1871–1879. doi: 10.1152/jn.01053.2004. [DOI] [PubMed] [Google Scholar]
- Gale SD, Person AL. Perkel DJ. A novel basal ganglia pathway forms a loop linking a vocal learning circuit with its dopaminergic input. J. Comp. Neurol. 2008;508:824–839. doi: 10.1002/cne.21700. [DOI] [PubMed] [Google Scholar]
- Gianutsos G, Morrow G, Light S. Sweeney MJ. Dopaminergic properties of nomifensine. Pharmacol. Biochem. Be. 1982;17:951–954. doi: 10.1016/0091-3057(82)90478-6. [DOI] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA. Jarvis ED. Role of midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur. J. Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber ME, Cassey P, Woolley SM. Theunissen FE. Neurophysiological response selectivity for conspecific songs over synthetic sounds in the auditory forebrain of non-singing female songbirds. J. Comp. Physiol. A. 2007;193:765–774. doi: 10.1007/s00359-007-0231-0. [DOI] [PubMed] [Google Scholar]
- Heimovics SA. Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J. Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA. Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol. Behav. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA. Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat. Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Huang YC. Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS One. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA. Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD. Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J. Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, el Giamal N. Hilger E. Reboxetine: the first selective noradrenaline re-uptake inhibitor. Expert Opin. Pharmaco. 2000;1:771–782. doi: 10.1517/14656566.1.4.771. [DOI] [PubMed] [Google Scholar]
- Kojima S. Doupe AJ. Social performance reveals unexpected vocal competency in young songbirds. Proc. Natl. Acad. Sci. USA. 2011;108:1687–1692. doi: 10.1073/pnas.1010502108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ. Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J. Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehongre K, Aubin T. Del Negro C. Influence of social conditions in song sharing in the adult canary. Anim. Cogn. 2009;12:823–832. doi: 10.1007/s10071-009-0241-0. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP. Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J. Comp. Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Linnér L, Endersz H, Ohman D, Bengtsson F, Schalling M. Svensson TH. Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J. Pharmacol. Exp. Ther. 2001;297:540–546. [PubMed] [Google Scholar]
- Nordeen EJ, Holtzman DA. Nordeen KW. Increased Fos expression among midbrain dopaminergic cell groups during birdsong tutoring. Eur. J. Neurosci. 2009;30:662–670. doi: 10.1111/j.1460-9568.2009.06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Gale SD, Farries MA. Perkel DJ. Organization of the songbird basal ganglia, including area X. J. Comp. Neurol. 2008;508:840–866. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- Riters LV. Pleasure seeking and birdsong. Neurosci. Biobehav. R. 2011;35:1837–1845. doi: 10.1016/j.neubiorev.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. Pawlisch BA. Evidence that norepinephrine influences responses to male courtship song and activity within song control regions and the ventromedial nucleus of the hypothalamus in female European starlings. Brain Res. 2007;1149:127–140. doi: 10.1016/j.brainres.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR. Jarvis ED. Social context-dependent singing-regulated dopamine. J. Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soha JA, Shimizu T. Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J. Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW. Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog. Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O. Nottebohm F. Social inhibition of song imitation among sibling male zebra finches. Proc. Natl. Acad. Sci. USA. 1998;95:8951–8956. doi: 10.1073/pnas.95.15.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini V, Frau R. Di Chiara G. Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J. Neurochem. 2004;88:917–927. doi: 10.1046/j.1471-4159.2003.02238.x. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M. de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Sonders MS, Amara SG, Tinholt PM, Piercey MF, Hoffmann WP, Hyslop DK, Franklin S, Porsolt RD, Bonsignori A, Carfagna N. McArthur RA. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol. Psychiat. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]
- Yanagihara S. Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur. J. Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]


