Abstract
Setting: Health centres in Southern Ethiopia.
Objective: To determine factors contributing to the large variations in the notification of smear-positive tuberculosis (TB) cases.
Design: A cross-sectional study in selected health centres from areas with high and low case notification was conducted and health system and health care worker (HCW) related determinants were examined.
Results: A total of 50 (61.0%) health centres and 172 (65.2%) HCWs from high case notification areas, and 32 (39.0%) health centres and 92 (33.8%) HCWs from low case notification areas were included in the study. Assignment of a full-time TB focal person at the TB clinic (adjusted odds ratio [aOR] 5.8, 95%CI 1.5–22.4) and availability of TB recording tools (aOR 7.0, 95%CI 1.5–32.5) were independent predictors of high case notification rates. HCW knowledge about TB screening, diagnosis and treatment was positively associated with case notification (aOR 2.53, 95%CI 1.42–4.48).
Conclusion: Increased TB case notification was associated with the presence of a full-time focal person, availability of TB recording tools and good knowledge about TB among HCWs. Putting in place these measures in all health centres could increase TB notification in the region.
Keywords: case finding, case notification, TB-related training, supervision
Abstract
Contexte : Centres de santé dans le Sud de l'Ethiopie.
Objectif : Déterminer les facteurs contribuant aux importantes variations dans la déclaration des cas de tuberculose (TB) à frottis positif.
Schéma : Une étude transversale a été réalisée dans des centres de santé sélectionnés dans des régions à déclaration de cas élevée ou faible et a examiné les déterminants liés au système de santé et au personnel.
Résultats : Un total de 50 (61,0%) centres de santé et de 172 (65,2%) membres du personnel de santé ont été inclus dans la région à déclaration élevée de cas, tandis que 32 (39,0%) centres de santé et 92 (33,8%) membres du personnel de santé venaient de la région à faible déclaration de cas. Le recrutement d'une personne focale TB à temps plein dans le centre anti-tuberculeux (odds ratio ajusté [aOR] 5,8 ; IC95% 1,5–22,4) et la disponibilité d'outils d'enregistrement de la TB (aOR 7,0 ; IC95% 1,5–32,5) étaient des facteurs prédictifs indépendants d'un taux élevé de déclaration des cas. Les connaissances du personnel de santé en matière de dépistage, diagnostic et traitement de la TB étaient positivement associées à la déclaration des cas (aOR 2,53 ; IC95% 1,42–4,48).
Conclusion : Une augmentation de la déclaration des cas de TB était associée à la présence d'une personne focale à plein temps, à la disponibilité des outils d'enregistrement de la TB et aux bonnes connaissances du personnel de santé. La mise en place de ces mesures dans tous les centres de santé pourrait augmenter la déclaration des cas dans cette région.
Abstract
Marco de referencia: Algunos centros de salud en el sur de Etiopía.
Objetivo: Determinar los factores que contribuyen a la amplia variación observada en materia de notificación de casos de tuberculosis (TB) con baciloscopia positiva.
Método: Fue este un estudio transversal llevado a cabo en centros de salud escogidos en zonas con diferentes tasas de notificación de casos de TB, en el cual se examinaron los factores que determinan el grado de notificación y que dependen del sistema y de los profesionales sanitarios.
Resultados: Se incluyeron en el estudio 50 centros de salud (61,0%) y 172 profesionales sanitarios (65,2%) de la zona con altas tasas de notificación y 32 centros (39,0%) y 92 profesionales (33,8%) de la zona con bajas tasas de notificación. Aparecieron como factores pronósticos independientes de una alta notificación, la asignación de una persona de contacto en materia de TB en jornada completa en el consultorio (OR ajustado [aOR] 5,8; IC95% 1,5–22,4) y la existencia de materiales de registro de la TB (ORa 7,0; IC95% 1,5–32,5). El grado de conocimiento de los profesionales de salud en materia de detección, diagnóstico y tratamiento de la TB se relacionó positivamente con la tasa de notificación (ORa 2,53; IC95% 1,42–4,48).
Conclusión: Una mayor notificación de los casos de TB se asoció con la presencia en horario completo de una persona de contacto en el consultorio, con la disponibilidad de instrumentos de registro de la TB y con el buen conocimiento de los profesionales sanitarios sobre la enfermedad. La puesta en práctica de estas medidas en todos los establecimientos de salud aumentaría la notificación de los casos de TB de la región.
Case finding and prompt treatment are considered key methods of tuberculosis (TB) control.1 In 2012, it was estimated that a third of all TB cases worldwide were missed by national tuberculosis programmes (NTPs).2 Studies suggest that most non-identified or non-notified cases were from South-East Asia and Africa.3
In 2012, Ethiopia ranked seventh among the 22 high TB burden countries that account for 80% of the global TB burden.2 The results of the first national prevalence study in 2011 indicated that the prevalence of smear-positive TB was respectively 108 and 63 per 100 000 population among those aged ⩾15 years4 and the total population.5 The prevalence of bacteriologically confirmed TB was 277/100 000,4 and the estimated case detection rate was 72%.6 In 2012, Ethiopia notified 51.5 smear-positive TB cases/100 000, which was less than expected.2 High variations in case notification rates (CNRs) were observed, both among and within regions. For example, in the Southern Nations, Nationalities and People Region (SNNPR), the CNR varied between 22 and 117/100 000 in 2012–2013.7 It is not known whether these variations are due to operational (recording and reporting), logistical (access to diagnosis and care) or patient-related (health-seeking behaviour) factors, or whether they represent true differences in the TB burden.
The present study aimed to investigate operational and logistic factors contributing to the large variations in CNR of new smear-positive pulmonary TB (PTB+) cases reported by the zones and special districts of SNNPR.
STUDY POPULATION, DESIGN AND METHODS
A health facility-based comparative cross-sectional study was conducted from 23 September to 22 October 2013 in SNNPR (estimated population 15 million).8 In 2011–2012, a total of 643 health facilities, 623 health centres (HCs) and 20 hospitals were operational in the region. A single HC services on average 25 000 people residing within a 10 km radius. TB diagnostic and treatment services are provided free of charge for patients at all government facilities.
The study target population consisted of all HCs with TB diagnosis and treatment facilities and all health care workers (HCWs) engaged in providing TB services in SNNPR. Sample size was calculated using the formula for a one-tailed comparative cross-sectional study design assuming a 95% confidence level and 80% power. This resulted in a sample size of 51 HCs for each comparison group. Zones and special districts in the region were ranked from the lowest to the highest CNR based on 2012–2013 routine surveillance data. HCs in the lowest quartile were then classified as low CNR area and those in the highest as high CNR area. Zones with TB-related interventions by non-governmental organisations (NGOs), pastoralist zones with mainly mobile nomadic populations and one highly urbanised area (Hawassa City) were excluded from the study, as they differed greatly from the general population. Three zones were categorised as high CNR areas, with a CNR >77/100 000 (Bench Maji, Gurage and Gedeo), while three were categorised as low CNR areas, with a CNR <28/100 000 (Segen People Zone, Yem and Konta special districts). In the low CNR areas, all 35 HCs were included, as the number was less than the targeted sample size of 51. Three HCs were excluded from the study, as they did not provide TB diagnosis and treatment services. For the high CNR areas, the 51 HCs were allocated to the zones proportionate to the number of HCs in each zone; the required number of HCs was selected using simple random sampling.
In each of the 82 HCs selected, nurses or health officers were interviewed to assess HCW-related factors. Using the sampling manual for facility surveys,9 a sample size of 328, i.e., four HCWs per HC, was targeted. If more than the required number of staff were available in an HC, simple random sampling was applied to select four of these.
Data were collected using a pre-tested structured questionnaire filled out by trained data collectors. Data were entered and analysed using IBM SPSS version 20 (IBM, Armonk, NY, USA) statistical software.
Information collected from the facilities included access to electricity, availability of a functional microscope, laboratory reagents, recording and reporting formats, availability of supportive supervision and an external quality assurance (EQA) system for microscopy services. HCW-related variables collected included socio-demographic characteristics, 31 questions on HCW knowledge and 20 questions on HCW practices in TB prevention and control.
Using these questions, composite scores were constructed as proxy for knowledge and practice levels among HCWs. To calculate the knowledge score, each correct answer was awarded one point, and the points were added. The scores of all respondents were summed to obtain the median knowledge score. The median score was then used to categorise respondents' knowledge level as above or below the median. The practice score was similarly calculated. The list of variables (questions) used to create the composite scores for knowledge and practices are presented in Appendix Tables A.1 and A.2.
A binary logistic regression model was fitted to the data set to identify predictors for low and high CNRs for both HC and HCW data separately. Variables with a P value <0.25 in bivariate analysis were included in the multivariate analysis to identify independent predictors. P < 0.05 was considered statistically significant.
Ethical clearance was obtained from the Regional Ethical Committee of the SNNP Regional Health Bureau, Hawassa, Ethiopia; verbal permission to collect data was obtained from the health offices and centres in the study area. Verbal consent was provided by all study participants.
RESULTS
Health centre-related factors
Respectively 50 (61.0%) and 32 (39.0%) HCs from the high and low CNR areas were included in the study. One HC in the high CNR area had to be excluded due to inaccessible road conditions because of heavy rain. Respectively 43 (86.0%) and 17 (53.1%) HCs had access to electricity in the high and low CNR areas. In the high CNR area, the majority of the HCs had a dedicated TB clinic and a TB focal person assigned to work in the clinic (88.0% and 90.0%, respectively). This was not the case in the low CNR area, where only 56.2% and 37.5% of the HCs had a TB clinic and focal person, respectively. The availability of an EQA system for microscopy differed between the high and low CNR areas (37, 74.0% vs. 16, 50.0%). Respectively 53% and 6.0% of the HCs in the low and high CNR areas did not have TB registration tools in the last quarter before data collection. In both areas, almost half of the HCs reported the absence of one or more laboratory reagents for acid-fast bacilli testing in the 3 months preceding data collection (Table 1).
TABLE 1.
Availability of TB-related services, infrastructure, utilities and associated factors in selected health centres in high and low case notification areas in SNNPR, October 2013
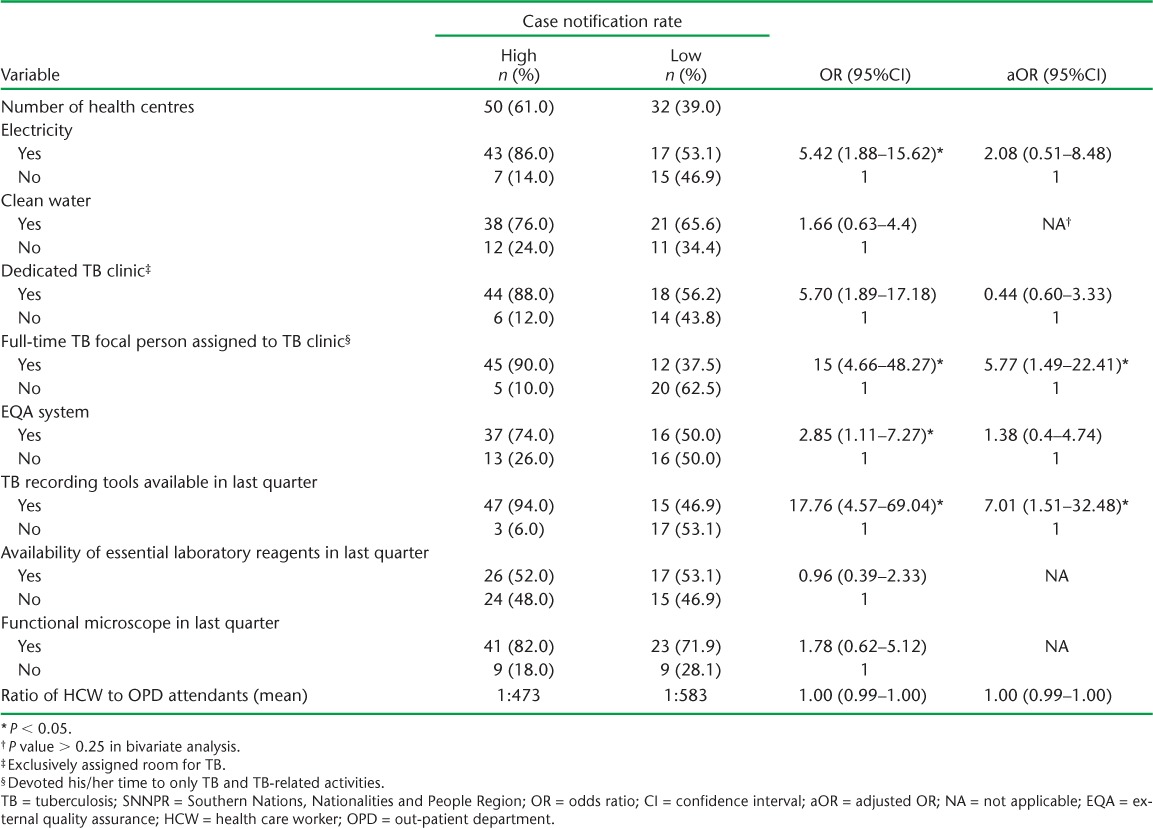
In bivariate logistic regression, availability of electricity, assignment of a full-time TB focal person, and availability of an EQA system and of TB recording tools in the HCs differed significantly between the high and low CNR areas. In multivariate analysis, assignment of a full-time TB focal person (adjusted odds ratio [aOR] 5.77, 95%CI 1.49–22.41) and the availability of TB recording tools (aOR 7.01, 95%CI 1.51–32.48) were independent predictors of a high CNR after controlling for all other factors (Table 1).
Health care worker-related factors
Respectively 172 (65.2%) and 92 (34.9%) HCWs from the HCs in the high and low CNR areas were interviewed. About two thirds in both areas reported never having received training on TB; 58 (33.7%) HCWs interviewed in the high and 22 (23.9%) in the low CNR areas indicated that they had been supervised specifically for TB activities in the last 6 months (Table 2).
TABLE 2.
Characteristics of HCWs and associated factors in health centres with high and low case notification rates in SNNPR, October 2013
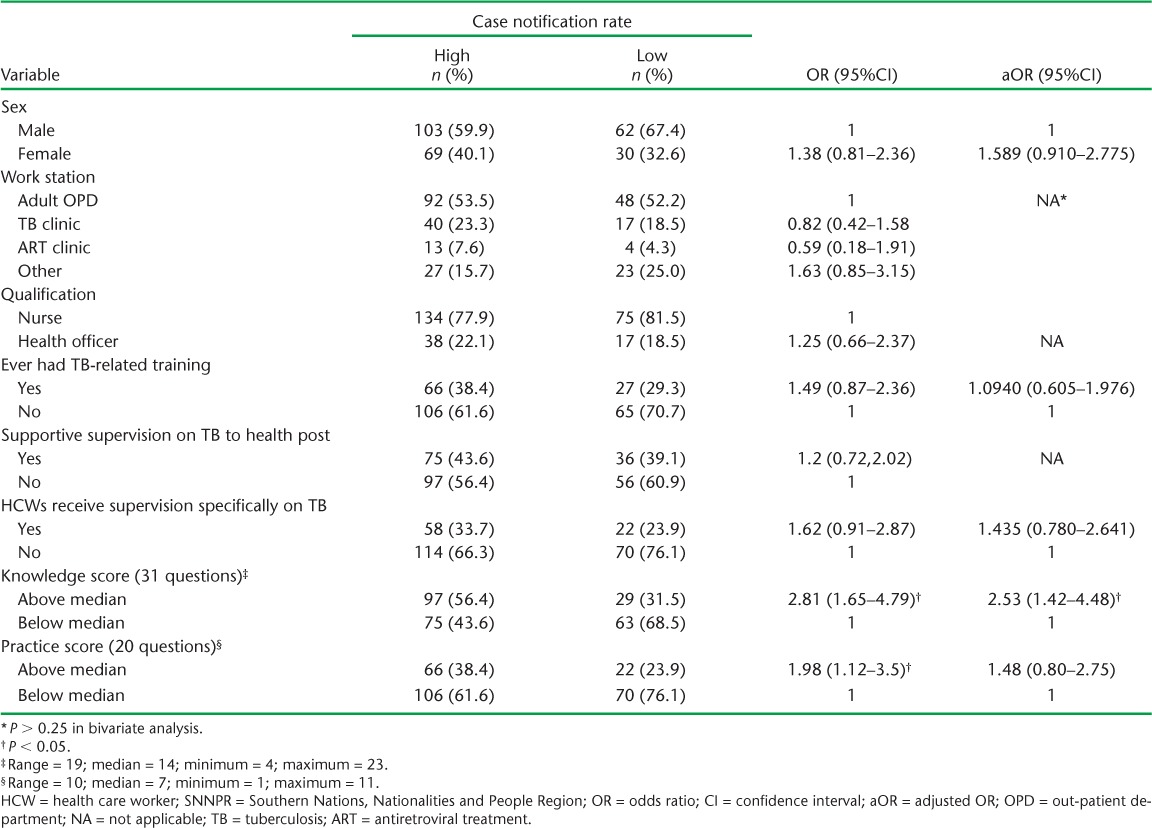
In bivariate analysis, having an above-median knowledge or practice score was associated with a high CNR. However, in multivariate analysis only an above-median knowledge score had a statistically significant association with a high CNR (aOR 2.53, 95%CI 1.42–4.48, Table 2).
Effect of TB-related training on knowledge and practice scores
Among the HCWs who had ever undergone TB-related training, respectively 63% and 43% had above-median knowledge and practice scores. Receiving training on TB was significantly associated with both above-median knowledge and practice scores (Table 3). Furthermore, serving in an HC for ⩾3 years and currently working in TB clinics were also independently associated with ever having received TB-related training (Table 4).
TABLE 3.
Cross-tabulation of TB-related training vs. knowledge and practice scores among health care workers, SNNPR, October 2013
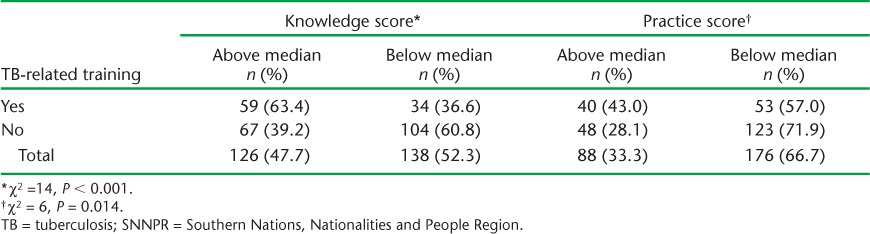
TABLE 4.
Factors associated with receiving TB training, SNNPR, October 2013

DISCUSSION
In southern Ethiopia, health system- and HCW-related factors likely contributed to observed differences in case notification. The assignment of a full-time TB focal person and availability of TB recording tools were significantly associated with greater notification of TB cases in the study area. A higher knowledge score among HCWs was also positively associated with case notification.
According to the national guidelines for clinical and programmatic management of TB, TB-HIV and leprosy, a TB focal person is responsible for the delivery of DOTS services and coordination of all TB-related activities, such as health education and contact tracing of infectious index cases. He/she also maintains TB registers and prepares quarterly reports that are submitted to the next level.10 Failure to have an HCW assigned for TB could have both direct and indirect effects on CNR. First, patients diagnosed with TB are not notified, resulting in underreporting. Second, indirect failure to provide TB-related services may lead to underdiagnosis and lower CNR.11 Assigning a TB focal person in an HC with TB diagnosis and treatment services in high TB burden countries would likely increase case notification. For Ethiopia, this is seen to be feasible and effective, as there is an adequate number of diploma nurses who could act as TB focal persons after undergoing short-term TB-related in-service training. This would improve quality of service, as they will be able to coordinate all TB and TB-related activities in HCs, including DOTs services, in an organised manner. The observed significant role of assigning a TB focal person could be due to the fact that HCs with high TB caseloads and/or facilities with good infrastructures may attract HCWs. Moreover, preferential visits by patients for different reasons may increase the TB caseload and finally enforce the assignment of a TB focal person; however, this point was not addressed by our study.
It is clear that the unavailability of standard TB recording and reporting tools adversely affects the CNR, as HCWs are unable to report the patients they have diagnosed and started on treatment.2,12 Other factors not investigated in this study may also have contributed to the observed differences in case notification. For example, residing in rural areas and high population density per DOTS site in districts with more than 25 000 people were associated with low case notification in a study conducted in central Ethiopia that analysed 15 years of TB case notification trends in 25 districts.13
Other health system-related factors, such as EQA, having a well-functioning sputum microscopy facilities and an uninterrupted electricity supply were not independent predictors of high CNR in this study. As EQA evaluates the most important steps in microscopy procedures, it contributes significantly to accurate TB diagnoses.14 Meanwhile, an uninterrupted electricity supply is also necessary for TB diagnosis and notification.15 In this study, the power of the test to detect statistically significant differences could have played a role. The fact that some HCs used solar microscopes instead of light microscopes was not taken into account.
Studies have shown that poor knowledge about TB and lack of TB training are associated with low case detection and notification rates.11 In this study, with the exception of the knowledge score, no other HCW-related factors showed a significant association with CNR. HCWs in the high CNR area had good knowledge (above median value) compared to HCWs in the low CNR area (aOR 2.53, 95%CI 1.42–4.48). Most of the questions on TB knowledge referred to the identification of patients with presumed TB and TB screening procedures, which have a direct impact on the detection of TB cases.
The fact that ever having received TB-related training was not statistically significant in multivariate analysis could indicate a limited impact of training and raises questions regarding the quality of the training and retention of training content. However, the lack of a statistically significantly positive association between having received TB-related training and CNR does not necessarily mean that training does not contribute to improving CNR, as this could be due to principles of equity. From observations, all training is offered on an equitable basis for each HCW; all HCWs in HCs therefore have an equal chance of receiving training. Moreover, in HCs with TB diagnosis and treatment services, HCWs who work in the TB clinic are expected to take additional training. However, as we observed, staff trained in TB may not be fully engaged in TB-related activities. In addition, as this information was based on self report, it may have been influenced by expectations of further training among respondents, thus biasing the responses. Although not associated with high or low CNR, having received TB-related training was significantly associated with both knowledge (more impact) and practice (less impact); respectively 63% and 43% of trained individuals had above-median knowledge and practice scores.
The strengths of this study are its wide geographic coverage in the region and the relatively large sample size (82 HCs). Limitations include the fact that all factors were assessed using interviews, and data on practice-related questions were self-reported. Although the objectives of the study were clearly explained to the study participants, social desirability bias could have influenced responses. This study did not include observation of HCWs and we did not assess patient-related factors. Moreover, the study findings may not be generalisable to the whole region, as a city and certain zones were excluded.
In conclusion, assigning a dedicated TB focal person and addressing the shortage of TB recording tools is likely to improve CNRs. Meanwhile, pre-service and in-service training should be strengthened to enhance HCW knowledge and practices so that a high degree of clinical suspicion of TB is maintained and patients are screened for TB.
Acknowledgments
The authors would like to thank the Southern Nations, Nationalities and Peoples Regional (SNNPR) State Health Bureau, College of Medicine and Health Sciences Hawassa University, Hawassa, and the Tuberculosis Research Advisor Committee of the Ethiopian Federal Ministry of Health (TRAC/FMoH, Addis Ababa, Ethiopia) for facilitating this study. We would also like to thank R Dlodlo of The Union Against Tuberculosis and Lung Disease, Paris, France, for her valuable comments during the development of the study proposal and manuscript.
The US Agency for International Development (USAID) Ethiopia through The Global Health Bureau, Office of Health, Infectious Disease and Nutrition (HIDN), USAID, Washington DC, USA, financially supported this study as part of the Ethiopia operational research capacity building initiative through TB CARE I under the terms of Agreement No AID-OAA-A-10-00020. This study was made possible by the generous support of the American people through USAID (Ethiopia mission). The contents of this paper are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
This manuscript is the result of an operational research study conducted by the SNNPR regional team during their operational research training under the TBCARE I/USAID funded initiative to build operational research capacity in Ethiopia.
APPENDIX
TABLE A.1.
Health care worker (nurse/health officer) knowledge assessing questions on TB
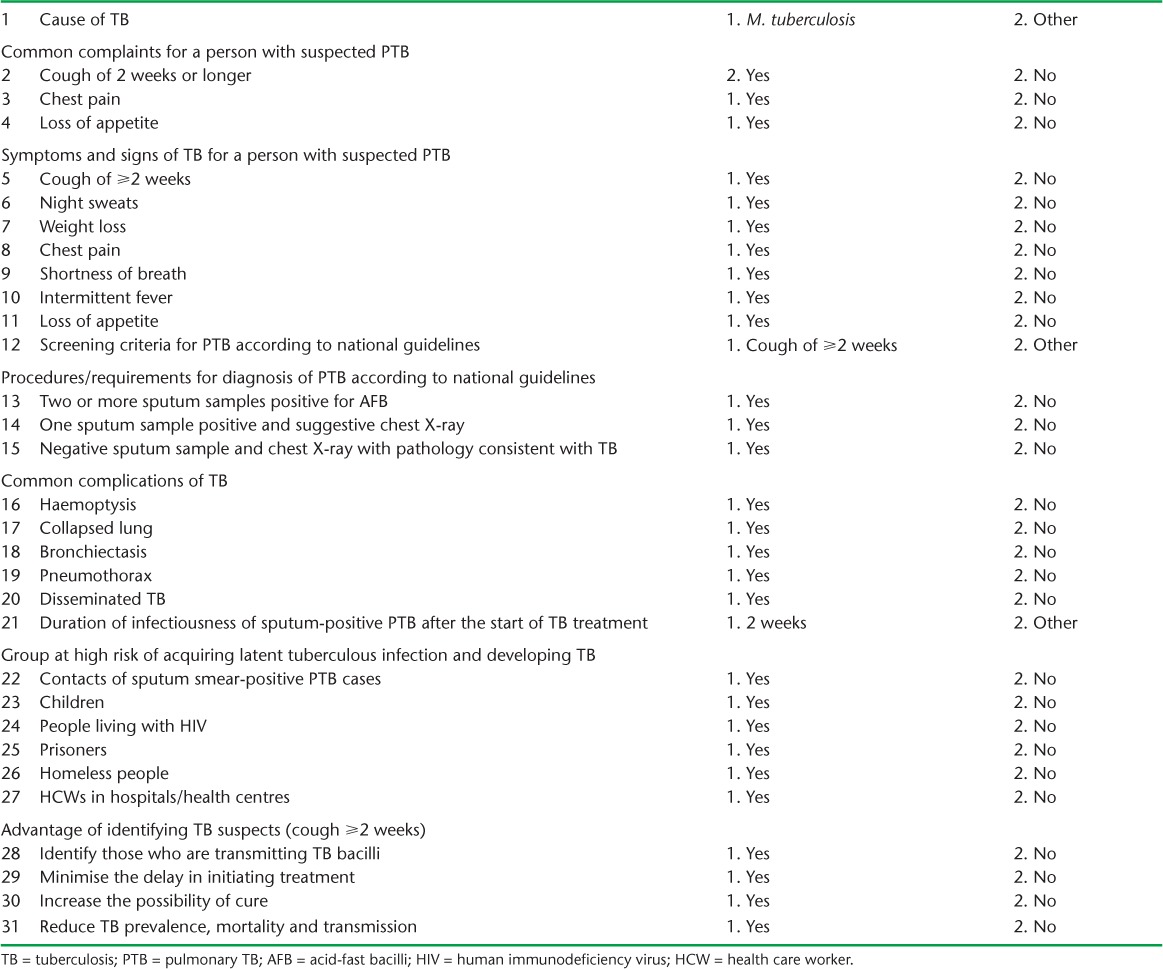
TABLE A.2.
Health care worker (nurse/health officer) practice assessing questions on TB

Footnotes
Conflicts of interest: none declared.
References
- 1.Raviglione M C. The new Stop TB strategy and the Global Plan to Stop TB, 2006–2015. Bull World Health Organ. 2007;85:327. doi: 10.2471/06.038513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report, 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 3.Lönnroth K, Jaramillo E, Williams B G, Dye C, Raviglione M. The role of risk factors and social determinants. In: Blas E, Sivasankara A K, editors. Priority public health conditions: from learning to action on social determinants of health. Geneva, Switzerland: World Health Organization; 2010. pp. 219–241. [Google Scholar]
- 4.Kebede A H, Alebachew Z, Tsegaye F et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010–2011. Int J Tuberc Lung Dis. 2014;18:635–639. doi: 10.5588/ijtld.13.0417. [DOI] [PubMed] [Google Scholar]
- 5.Federal Ministry of Health of Ethiopia. First Ethiopian national population-based tuberculosis prevalence survey. Addis Ababa, Ethiopia: FMOH; 2011. [Google Scholar]
- 6.World Health Organization. Global tuberculosis report, 2012. Annex 2: Country Profile, Ethiopia. Geneva, Switzerland: WHO; 2012. p. 113. WHO/HTM/TB/2012.6. p. [Google Scholar]
- 7.Southern Nation Nationalities and People Regional Health Bureau. Annual TB report. Hawassa, Ethiopia: SNNP-RHB; 2013. [Google Scholar]
- 8.Federal Democratic Republic of Ethiopia Population Census Commission. Statistical report of 2007 population and housing census. Addis Ababa, Ethiopia: FDRE-PCC; 2008. [Google Scholar]
- 9.Turner A G, Angeles G, Tsui A O, Wilkinson M, Magnani R. Sampling manual for facility surveys for population, maternal health, child health and STD programs in developing countries. Chapel Hill, NC, USA: University of North Carolina, Carolina Population Center; 2001. MEASURE Evaluation Manual Series, No.3. [Google Scholar]
- 10.Federal Ministry of Health of Ethiopia. Guidelines for clinical and programmatic management of TB, TB/HIV and Leprosy in Ethiopia. Addis Ababa, Ethiopia: FMOH; 2013. [Google Scholar]
- 11.Chatarina U, Budiono, Lutfia D et al. Obstacles for optimal tuberculosis case detection in primary health centers (PHC) in Sidoarjo District, East Java, Indonesia. BMC Health Serv Res. 2007;7:135. doi: 10.1186/1472-6963-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunbar R, Lawrence K, Verver S et al. Accuracy and completeness of recording of confirmed tuberculosis in two South African communities. Int J Tuberc Lung Dis. 2011;15:337–343. [PubMed] [Google Scholar]
- 13.Hamusse S D, Demissie M, Lindtjorn B. Trends in TB case notification over fifteen years: the case notification of 25 districts of Arsi Zone of Oromia Regional State, Central Ethiopia. BMC Public Health. 2014;14:304. doi: 10.1186/1471-2458-14-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simsek H, Ceyhan I, Tarhan G, Guner U. Quality assessment of microscopic examination in tuberculosis diagnostic laboratories: a preliminary study. Mikrobiyol Bul. 2010;44:561–569. [PubMed] [Google Scholar]
- 15.Parsons L M, Somoskövi A, Gutierrez C et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


