Abstract
Setting: Tuberculosis (TB) patients in Mekelle Zone, Tigray Region, in Ethiopia.
Objective: To investigate adherence to anti-tuberculosis treatment.
Design: A cross-sectional study in health facilities providing anti-tuberculosis treatment was conducted. Adherence was measured in three ways: through self-reported missed doses, by visual analogue scale whereby patients rate their own adherence and by record review. A patient was considered to be adherent if 90% or more of the prescribed medication was taken.
Result: Of 278 TB patients included, 101 were in the intensive and 177 in the continuation phase. Respectively 67 (24.1%), 130 (46.8%) and 80 (28.8%) patients had smear-positive, smear-negative and extra-pulmonary TB. Self-report of missed doses and record review indicated adherence of respectively 273 (97.3%) and 271 (97.5%) patients. By visual analogue scale, 250 (91.6%) patients rated themselves as adherent. History of drug side effects (aOR 0.25, 95%CI 0.08–0.77) and knowledge about TB prevention (aOR 0.19, 95%CI 0.05–0.8) were independently associated with being adherent in this setting.
Conclusion: Adherence to anti-tuberculosis treatment was high in our study. Adherence support should be given to the poor, the elderly, patients co-infected with the human immunodeficiency virus, alcohol abusers and smokers. Health education on TB prevention should be given to all TB patients regularly.
Keywords: intensive phase, continuation phase, visual analogue scale, record review, risk factor
Abstract
Contexte : Patients tuberculeux dans la zone de Mekelle, région du Tigray en Ethiopie.
Objectif : Examiner l'adhésion au traitement antituberculeux.
Schéma : Une étude transversale a été réalisée dans des centres de santé offrant un traitement antituberculeux. L'adhésion a été mesurée de trois manières : par la déclaration des doses manquées par les patients eux-mêmes, par une échelle visuelle analogue grâce à laquelle les patients notent leur propre adhésion et en étudiant les dossiers. Un patient était considéré comme adhérent s'il avait pris au moins 90% des doses prescrites.
Résultats : Un total de 278 patients tuberculeux ont été inclus, 101 dans la phase intensive et 177 dans la phase de continuation. Respectivement 67 (24,1%), 130 (46,8%) et 80 (28,8%) patients avaient une tuberculose à frottis positif, à frottis négatif et extra-pulmonaire. La déclaration par les patients sur les doses manquées et la consultation des dossiers ont indiqué une adhésion de 273 patients (97,3%) et 271 patients (97,5%), respectivement. Avec l'échelle visuelle analogue, 250 (91,6%) patients se considéraient comme adhérents. Des antécédents d'effets secondaires (aOR ajusté [aOR] 0,25 ; IC95% 0,08–0,77) et des connaissances en matière de prévention de la TB (aOR 0,19 ; IC95% 0,05–0,8) étaient indépendamment associés à l'adhésion dans ce contexte.
Conclusion : L'adhésion au traitement antituberculeux était élevée dans notre étude. Un soutien à l'adhésion devrait être offert aux patients pauvres, âgés, co-infectés par le virus de l'immunodéficience humaine, consommateurs excessifs d'alcool et fumeurs. Tous les patients devraient bénéficier régulièrement d'une éducation sanitaire à la prévention de la TB.
Abstract
Marco de referencia: Los pacientes con diagnóstico de tuberculosis (TB) de la zona de Mekelle en la región Tigray en Etiopía.
Objetivo: Evaluar el cumplimiento del tratamiento antituberculoso.
Método: Se practicó un estudio transversal en los centros de atención sanitaria que suministran tratamiento antituberculoso. La medición de la observancia terapéutica se obtuvo mediante tres mecanismos, a saber la referencia de los propios pacientes sobre las dosis omitidas, una escala visual analógica en la cual los pacientes calificaban su propio cumplimiento y el examen de las historias clínicas. Se consideró que un paciente cumplía con el tratamiento cuando tomaba como mínimo 90% de los medicamentos recetados.
Resultados: Se incluyeron en el estudio 278 pacientes con diagnóstico de TB, de los cuales 101 se encontraban en la fase intensiva del tratamiento y 177 en la fase de continuación. Sesenta y siete pacientes presentaron una baciloscopia positiva (24,1%) y 130 una baciloscopia negativa (46,8%); en 80 pacientes la TB fue de localización extrapulmonar (28,8%). Con base en la autorreferencia sobre las dosis omitidas se observó cumplimiento terapéutico en 273 pacientes (97,3%) y en 271 (97,5%) cuando se examinaron los registros clínicos. En la prueba de la escala visual analógica, 250 pacientes consideraron que eran cumplidos (91,6%). Los factores que se asociaron de manera independiente con la observancia del tratamiento en este entorno fueron los antecedentes de reacciones adversas a los medicamentos (OR ajustada [aOR] 0,25; IC95% 0,08–0,77) y los conocimientos en materia de prevención de la TB (aOR 0,19; IC95% 0,05–0,8).
Conclusión: Se observó una alta tasa de cumplimiento del tratamiento antituberculoso. Es importante respaldar la observancia terapéutica de las personas con escasos recursos, los ancianos, los pacientes aquejados de coinfección por el virus de la inmunodeficiencia humana o abuso de alcohol y los fumadores. Se recomienda impartir educación en materia de prevención de la enfermedad de manera sistemática a todos los pacientes que reciben tratamiento antituberculoso.
Tuberculosis (TB) remains a major public health problem in Ethiopia, with an estimated incidence of 378 per 100 000 population for all forms and 163/100 00 for smear-positive pulmonary TB.1 Ethiopia started implementing the DOTS strategy under its National TB Control Programme in 1992. Current DOTS coverage is estimated at 100% geographically and 95% at health facility level.2 Reported treatment success and case detection rates of all forms of TB in Ethiopia are respectively 83% and 72%.3 The Tigray Region, in northern Ethiopia, initiated the DOTS strategy in 1995.4 According to the Regional Health Bureau 2013 report, the case detection, default, death, treatment success and cure rates were respectively 58.1%, 2.5%, 5.0%, 92.4% and 47.2%.5
Although complete treatment interruption is routinely reported, intermittent interruptions are not included in routine quarterly reports, although they contribute to the development of multidrug-resistant tuberculosis (MDR-TB).6 Anecdotal data from hospital reports have indicated that the number of MDR-TB patients in the region is highest in the Mekelle Zone, which hosts 40% of the 38 registered MDR-TB cases in the region. To our knowledge, no studies on adherence to anti-tuberculosis treatment have been conducted in Tigray.
The objective of the present study was to assess adherence to anti-tuberculosis treatment and factors influencing adherence among TB patients in the Mekelle Zone of the Tigray Region in Ethiopia.
METHODS
A facility-based cross-sectional study was conducted from 18 November to 16 December 2013 in the Mekelle Zone of the Tigray Region, northern Ethiopia. Mekelle, the capital city of the Tigray Region, had an estimated population of 307 304 in 2013–2014. Residents use intercity minibuses and three-wheeler motorcycles for transport, trade and small scale industry are the main means of income, and public and private health institutions and schools, including colleges, are readily available.
TB patients were sampled from all 12 public health facilities in the zone providing anti-tuberculosis treatment services, which include four public hospitals and eight health centres. TB patients aged <15 years, hospitalised patients and patients who had taken TB medicines for <2 weeks were excluded. The targeted sample size was 275, as determined by a single population formula with the following assumptions: 95% confidence interval (CI), 80% power, a margin of error of 5%, assumed 81.5% patient adherence based on a study from Kenya,7 and a non-response rate of 10%. The number of patients to be studied from each health facility was allocated proportionate to the patient load in the selected facilities. Data were collected at the facilities every other day until the allocated number for each institution was attained. Structured questionnaires, document review and interviews were used to collect baseline characteristics and information on adherence. The data collection tools were developed in English and translated into Tigrigna, the local language. The TB patients were interviewed by trained data collectors. Additional qualitative data were gathered through key informant interviews with TB focal persons in the health institutions.
A patient was considered to be adherent if ⩾90% of the prescribed medication was taken.8,9 Three different adherence measures were used: 1) self-reported missed doses, whereby patients were asked whether they had ever missed the recommended dose, and if so, how often this had happened in the previous week and month; 2) using the visual analogue scale (VAS), patients were asked ‘How much of your prescribed TB medication have you taken until now in the course of your treatment (on a scale from 1 to 10)?’ The ends of the scale were defined as the extreme limits of adherence orientated from the left (1 = 10% for the worst) to the right (10 = 100% for the best). The VAS method was chosen as it can be used to assess adherence for the whole duration of anti-tuberculosis treatment rather than at a specific time. VAS was used to assess adherence to anti-tuberculosis treatment in another study, and was found to be accurate.10 3) The TB Unit register was reviewed, and the number of doses that were noted as missed since the beginning of treatment was counted for each patient.
Data were coded, cleaned, entered and validated using Epi Info™ version 3.5.2 (Centers for Disease Control and Prevention, Atlanta, GA, USA). SPSS version 20 (Statistical Package for the Social Sciences, Chicago, IL, USA) was used for data analysis. Descriptive statistics with mean, frequency and percentage were used to describe and summarise the quantitative data. Association between dependent and independent categorical variables was assessed using the χ2 test. Independent variables significant in bivariate analysis were included in the multivariate logistic regression analysis. Qualitative data were mainly analysed on the basis of content analysis and identification of major themes. These data were used to ascertain observations made in the quantitative part of the study.
The study was approved by the Institutional Review Board of the College of Health Sciences at Mekelle University (ERC No: 0288/2013), Mekelle, Ethiopia. A support letter was obtained from the Tigray Regional Health Bureau and the Mekelle Zonal Health Office.
RESULTS
Study participant characteristics
A total of 278 registered TB patients participated in this study. Their age ranged from 15 to 82 years, with a median of 30 years; 171 (63%) were males (Table 1). Nearly one third (n = 101) of the patients were in the intensive phase and 177 (64%) were in the continuation phase of treatment. The median duration of the study participants on anti-tuberculosis treatment was 71 days (in-terquartile range [IQR] 38–136). Respectively 46 (16.9%), 11 (4.0%), 2 (0.8%) and 3 (1.1%) patients had human immunodeficiency virus (HIV) infection, diabetes mellitus, liver disease and renal disease, in addition to TB. Fifty (18%) had received medicines for >2 weeks to treat comorbidities other than TB. Eleven (4%) patients reported having been informed by the health institution that TB medicines were out of stock. More than half (n = 163, 58.6%) of the patients experienced adverse drug effects, presumably due to TB medicines. Nearly three quarters (n = 200, 72%) indicated they had received health education about TB at enrolment.
TABLE 1.
Characteristics of study participants on anti-tuberculosis treatment from health facilities, Mekelle Zone, Northern Ethiopia, 2013 (n = 278)
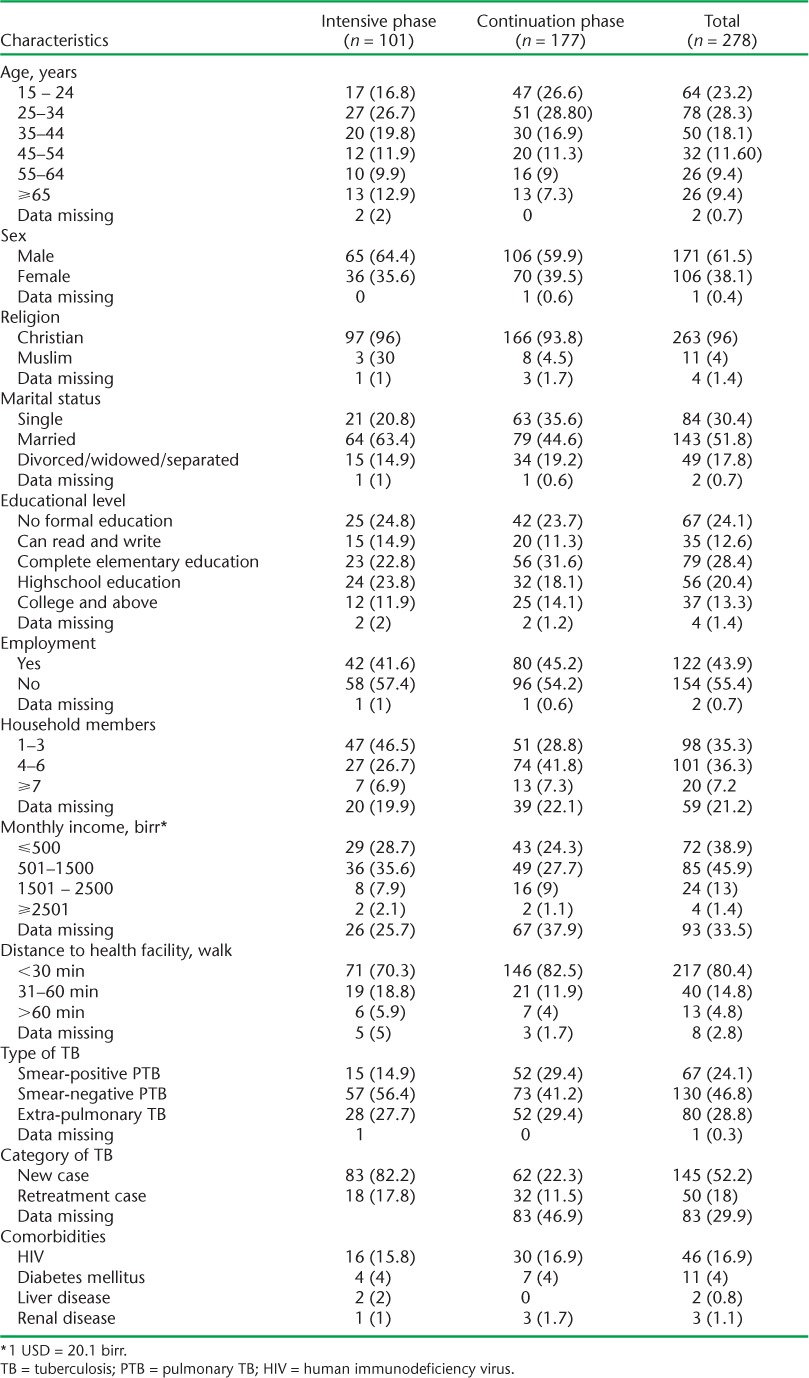
The majority (92.4%) of the patients knew the duration of their anti-tuberculosis treatment, and 96.8% were convinced that they would complete their treatment. Asked about their impression about the services provided by the health facility, respectively 10 (3.6%), 142 (51.1%) and 126 (45.4%) considered the services poor, good and very good. One hundred and forty six (82.5%) patients in the continuation phase and 71 patients (70.3%) in the intensive phase lived within 30 min walking distance of the health institution.
Adherence to anti-tuberculosis treatment
The different approaches used to measure adherence provided very similar results (Table 2). Self reports on missed doses and the record review indicated that respectively 273 (97.3%) and 271 (97.5%) patients were adherent. Using VAS, 250 (91.6%) patients scored themselves as adherent for the entire duration of anti-tuberculosis treatment. As the VAS scores adherence for the treatment received to date and results were similar, VAS scoring was used for all subsequent analyses. Adherence was higher among patients in the continuation phase (93%) than in the intensive phase (89.1%). Adherence varied by health facility, with respectively 28.3%, 12.8% and 6.7% of non-adherent patients coming from health centre 1, health centre 2 and hospital 1.
TABLE 2.
Agreement between adherence measurement tools in patients on anti-tuberculosis treatment from health facilities, Mekelle Zone, Northern Ethiopia, 2013 (n = 278)

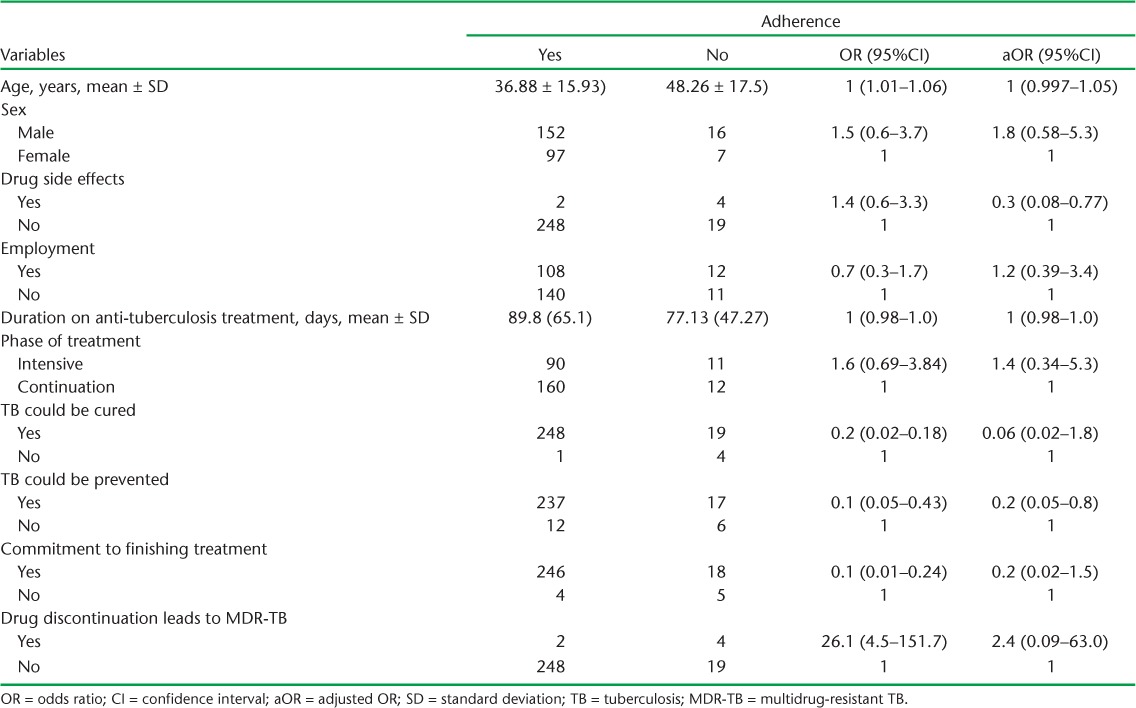
Using χ2 analysis, belief that TB cannot be cured, that TB cannot be prevented, or that discontinuation of anti-tuberculosis treatment will not result in a form of TB difficult to treat and/or a significant health problem, lack of knowledge about the duration of anti-tuberculosis treatment, lack of confidence about whether one was going to complete anti-tuberculosis treatment, experience of a drug side effect, distance from the health institution, monthly income and age were associated with lower adherence (Table 3). In multivariate analysis, having experienced drug side effects (adjusted odds ratio [aOR] 0.25, 95%CI 0.08–0.77) and good knowledge about TB prevention (aOR 0.19, 95%CI 0.05–0.8) were the only variables that were independently associated with being adherent. The association with HIV as a possible risk factor could not be analysed further, as information on HIV status was obtained from the patients themselves, and this could introduce bias.
TABLE 3.
Logistic bivariate and multivariate regression analysis of factors associated with adherence among patients on anti-tuberculosis treatment, Mekelle Zone, Northern Ethiopia, 2013 (n = 278)
DISCUSSION
TB control largely depends on optimal treatment adherence leading to cure. Adherence needs to be in the order of 85–90%11 to achieve an acceptable cure rate of at least 85%.12 Based on this we defined being adherent as taking ⩾90% of the prescribed medication. We found that in the Mekelle Zone of Tigray, 91.6% of TB patients were adherent to anti-tuberculosis treatment. This finding is comparable to the findings of a recently published study from North Western Ethiopia, which reported a level of 90%,13 defining adherence, as in this study, as an intake of at least 90% of the prescribed dose during a given month of treatment. A study from Kenya, also using the VAS method, reported 92.5% (95%CI 88.0–95.6)10 of patients to be adherent, defined as intake of at least 80% of the prescribed dose. Studies among HIV-infected TB patients in South West Ethiopia (79.2%),14 Uganda (75%),15 India (59%)16 and China (88%)17 reported lower adherence; these studies defined adherence as the intake of respectively 90%, 90%, ⩾95% and >90% of prescribed medications. The lower level of adherence in HIV co-infected patients might be attributed to the high pill burden of these patients, who were also undergoing antiretroviral therapy (ART). Although, in our study, 16.9% of the patients disclosed that they were HIV-positive, adherence in this group did not significantly differ from that of HIV-negative patients or those with unknown HIV status.
The results of this study suggest that patients were less adherent during the intensive phase (89.1%) than during the continuation phase (93.0%), as has also been reported in a study from India.16 Daily clinic visits are recommended during the intensive phase of treatment; however, this could be an obstacle for patients due to challenges in transport, resources or time. It may be easier for patients to comply with the weekly clinic visits during the continuation phase of treatment. Taking >90% of the prescribed medication, especially in the intensive phase of treatment, is crucial given the high bacillary loads,8 and should be encouraged in all patients.
In our study, elderly patients were more likely not to adhere, in line with a study from Thailand.18 The authors of that study believe that physical infirmity, resulting in difficulty in attending daily clinic visits, could play a role in the lower adherence among elderly patients. A recent study from Ethiopia reported low income level, presence of comorbidities and forgetfulness as being responsible for non-adherence in TB patients.13 During the key informant interviews, it was mentioned that daily labourers find it difficult to attend the TB clinic during regular opening hours, as they start work before the clinics open. From this experience, one of the health centres revised its working hours and was opening at 6 am instead of 8 am to accommodate the needs of daily labourers.
The time needed to reach the health facility also influenced adherence: patients residing beyond a 30-min walk from the clinic were more likely to interrupt treatment than patients within a walking distance of <30 min (P = 0.002). However, age, low income and travel time were not significant in multivariate analysis. This could be attributed to sampling power. Much more people were adherent than anticipated during the sampling design, resulting in lower power for the analysis.
The effect of drug side effects on adherence, as observed in this study, has also been reported in several other studies.19–22 Drug side effects can be unpleasant, and could cause patients to fear that they would suffer further health problems if they continued to take their treatment. Patient knowledge of the fact that TB is preventable was significantly associated with being adherent. However, contrary to our expectation, the association was negative, and patients who knew that TB is a preventable illness were less likely to adhere to treatment than patients who were not aware of this fact (aOR 0.19, 95%CI 0.05–0.8). The belief that TB can be prevented may have led to complacency, whereas patients who were unaware of this might have made an effort not to miss their treatment.
The strength of our study is the use of different measures to assess adherence, including one method measuring adherence for the entire duration of treatment, which strengthened the findings. Limitations of our study were recall bias and inclusion of patients from urban communities only. The inclusion of only patients attending the health centre may also have affected our study findings, as those not adherent at all would be missed. However, our aim was not to investigate default but adherence to taking all prescribed doses of anti-tuberculosis drugs for those on treatment. The questions concerning belief as to whether TB can be cured, whether TB can be prevented, that discontinuation of anti-tuberculosis treatment will result in a form of TB that is difficult to treat and/or significant health problems, and confidence as to whether one was going to complete anti-tuberculosis treatment, are difficult, and might have resulted in more confirmative answers due to the nature of the question. Furthermore, the collection of qualitative data from key informant interviews after the quantitative data limited the use of the information to guide the quantitative study. Key informant interviews were only conducted with health care staff and not with patients, which may have provided less information on the possible reasons for missed doses. However, despite these limitations, the authors believe the study reveals important findings.
In conclusion, our study demonstrated optimal adherence to anti-tuberculosis treatment in Mekelle Zone. Experience of drug side effects and lack of overall knowledge about TB, except for knowledge on the modes of prevention, were observed to be barriers to adherence. Health institution of follow-up, old age, alcoholism, smoking, HIV co-infection and food insecurity were iden-tified as affecting adherence. All TB patients should be given regular health education on TB prevention. Special attention should be given to HIV-positive patients, alcohol abusers, smokers, the poor and the elderly. We suggest a study on adherence among rural communities in the Mekelle Zone to complement this study on the links between adherence and the development of MDR-TB.
Acknowledgments
The authors would like to thank the Tuberculosis Research Advisory Committee (TRAC) of the Federal Ministry of Health for organising the operational research (OR) training of which this project was part of. We are grateful to the Tigray Regional Health Bureau and Mekelle University, Mekelle, Ethiopia, for permitting us to be away from office for extended periods to work on the study. We thank R Dlodlo of the International Union Against Tuberculosis and Lung Disease, Paris, France, for constructive support during protocol development and manuscript writing.
United States Agency for International Development (USAID) Ethiopia through The Global Health Bureau, Office of Health, Infectious Disease and Nutrition, US Agency for International Development, Washington DC, USA, financially supported this study as part of the Ethiopia operational research capacity building initiative through TB CARE I under the terms of Agreement No AID-OAA-A-10-00020. This study was made possible by the generous support of the American people through USAID (Ethiopia mission). The contents of this paper are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
This manuscript is the result of an operational research study conducted by the Tigray regional team during their operational research training under the TB-CARE I/USAID funded initiative to build operational research capacity in Ethiopia.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis control: epidemiology, strategy, financing. Geneva, Switzerland: WHO; 2009. WHO/HTM/TB/2009411. [Google Scholar]
- 2.Federal Ministry of Health, Ethiopia. Tuberculosis, Leprosy and TB/HIV Prevention and Control Programme Manual. 4th ed. Addis Ababa, Ethiopia: FMoH; 2008. [Google Scholar]
- 3.Federal Ministry of Health. Overview of national TB control implementation status: Sixth National TB Research Workshop. Gondar, Ethiopia: Gondar University; 2011. [Google Scholar]
- 4.Mesfin M M. Community-based directly observed treatment short-course strategy in pilot districts of Tigray: overview of baseline studies. Ethiop J Health Dev. 2005;19:3–6. [Google Scholar]
- 5.Tigray Health Bureau. Annual TB performance report of Tigray region. Mekkelle, Ethiopia: Tigray Health Buro; 2013. [Google Scholar]
- 6.California Department of Health Services. CDHS/CTCA Joint Guidelines for the treatment of active tuberculosis. Fresno, CA, USA: CDHS; 2003. www.ctca.org/fileLibrary/file_65.pdf. Accessed September 2014. [Google Scholar]
- 7.Ong'ang'o J R, Mwachari C, Kipruto H. The effects on tuberculosis treatment adherence from utilising community health workers: a comparison of selected rural and urban settings in Kenya. PLOS ONE. 2014;9:e88937. doi: 10.1371/journal.pone.0088937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harries A D, Maher D, Graham S. TB/HIV: a clinical manual. 2nd ed. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 9.Awofeso N. Anti-tuberculosis medication side effects constitute major factor for poor adherence to tuberculosis treatment. Bull World Health Organ. 2008;86 doi: 10.2471/BLT.07.043802. B–D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nackers F, Huerga H, Espié E et al. Adherence to self-administered tuberculosis treatment in a high hiv-prevalence setting: a cross-sectional survey in Homa Bay, Kenya. PLOS ONE. 2012;7:e32140. doi: 10.1371/journal.pone.0032140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray C J L, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Tuberc Lung Dis. 1990;65:1–24. [PubMed] [Google Scholar]
- 12.World Health Organization. Tuberculosis control and research strategies for the 1990s: memorandum from a WHO meeting. Bull World Health Organ. 1992;70:17–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Adane A A, Alene K A, Koye D N, Zeleke B M. Non-adherence to anti-tuberculosis treatment and determinant factors among patients with tuberculosis in Northwest Ethiopia. PLOS ONE. 2013;8:e78791. doi: 10.1371/journal.pone.0078791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kebede A, Wabe N T. Medication adherence and its determinants among patients on concomitant tuberculosis and antiretroviral therapy in South West Ethiopia. N Am J Med Sci. 2012;4:67–71. doi: 10.4103/1947-2714.93376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amuha M G, Kutyabami P, Kitutu F E, Odoi-Adome R, Kalyango J N. Non-adherence to anti-TB drugs among TB/HIV co-infected patients in Mbarara Hospital Uganda: prevalence and associated factors. Afr Health Sci. 2009;9(Suppl 1):S8–S15. [PMC free article] [PubMed] [Google Scholar]
- 16.Sardar P, Jha A, Roy D, Roy S, Guha P, Bandyopadhyay D. Intensive phase noncompliance to anti tubercular treatment in patients with HIV-TB coinfection: a Hospital-based cross-sectional study. J Community Health. 2010;35:471–478. doi: 10.1007/s10900-009-9215-z. [DOI] [PubMed] [Google Scholar]
- 17.Xu W, Lu W, Zhou Y, Zhu L, Shen H, Wang J. Adherence to anti tuberculosis treatment among pulmonary tuberculosis patients: a qualitative and quantitative study. BMC Health Serv Res. 2009;9:169. doi: 10.1186/1472-6963-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anunnatsiri S, Chetchotisakd P, Wanke C. Factors associated with treatment outcomes in pulmonary tuberculosis in northeastern Thailand. Southeast Asian J Trop Med Public Health. 2005;36:324–330. [PubMed] [Google Scholar]
- 19.Jaiswal A, Singh V, Ogden J A et al. Adherence to tuberculosis treatment: Lessons from the urban setting of Delhi, India. Trop Med Int Health. 2003;8:625–633. doi: 10.1046/j.1365-3156.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 20.Greene J A. An ethnography of non-adherence: culture, poverty, and tuberculosis in urban Bolivia. Cult Med Psychiatry. 2004;28:401–425. doi: 10.1023/b:medi.0000046429.55801.c8. [DOI] [PubMed] [Google Scholar]
- 21.Edginton M E, Sekatane C S, Goldstein S J. Patients' beliefs: do they affect tuberculosis control? A study in a rural district of South Africa. Int J Tuberc Lung Dis. 2002;6:1075–1082. [PubMed] [Google Scholar]
- 22.Watkins R E, Rouse C R, Plant A J. Tuberculosis treatment delivery in Bali: A qualitative study of clinic staff perceptions. Int J Tuberc Lung Dis. 2004;8:218–225. [PubMed] [Google Scholar]


