Abstract
Background
Evidence for an association of alcohol consumption with prognosis after a diagnosis of breast cancer has been inconsistent. We have reviewed and summarized the published evidence and evaluated the association using individual patient data from multiple case cohorts.
Methods
A MEDLINE search to identify studies published up to January 2013 was performed. We combined published estimates of survival time for “moderate drinkers” versus nondrinkers. An analysis of individual participant data using Cox regression was carried out using data from 11 case cohorts.
Results
We identified 11 published studies suitable for inclusion in the meta-analysis. Moderate post-diagnosis alcohol consumption was not associated with overall survival [HR, 0.95; 95% confidence interval (CI), 0.85–1.05], but there was some evidence of better survival associated with prediagnosis consumption (HR, 0.80; 95% CI, 0.73–0.88). Individual data on alcohol consumption for 29,239 cases with 4,839 deaths were available from the 11 case cohorts, all of which had data on estrogen receptor (ER) status. For women with ER-positive disease, there was little evidence that pre- or postdiagnosis alcohol consumption is associated with breast cancer–specific mortality, with some evidence of a negative association with all-cause mortality. On the basis of a single study, moderate postdiagnosis alcohol intake was associated with a small reduction in breast cancer–specific mortality for women with ER-negative disease. There was no association with prediagnosis intake for women with ER-negative disease.
Conclusion
There was little evidence that pre- or post-diagnosis alcohol consumption is associated with breast cancer–specific mortality for women with ER-positive disease. There was weak evidence that moderate post-diagnosis alcohol intake is associated with a small reduction in breast cancer–specific mortality in ER-negative disease.
Impact
Considering the totality of the evidence, moderate postdiagnosis alcohol consumption is unlikely to have a major adverse effect on the survival of women with breast cancer.
Introduction
Many studies have investigated the association of alcohol consumption and prognosis in women diagnosed with breast cancer. However, the results of these studies have been inconsistent. Many studies have reported no significant association between pre- or postdiagnosis alcohol consumption and overall survival (OS; refs. (1–10), whereas other studies showed a protective effect (11–16) or an adverse effect (17). Fewer studies have examined the association of alcohol consumption with breast cancer-specific survival (BCSS; refs. (1, 2, 7, 12, 14, 18–22) and even fewer studies investigated disease-free survival (DFS; refs. (1, 2, 7, 9, 13, 19, 20). Two reviews have been published and concluded that alcohol intake was not associated with survival in patients with breast cancer (23, 24). More recently, Kwan and colleagues reported the results of a joint analysis of data from three large cohorts with information on postdiagnosis alcohol consumption (1). Overall they found no association between regular alcohol consumption and breast cancer mortality, but regular alcohol consumption was associated with an increased risk of recurrence in postmenopausal women.
There are several possible reasons for the heterogeneity of the published evidence: different studies used different endpoints, many studies had a small number of events and limited statistical power, the timing of the exposure varied and included both pre- and postdiagnosis alcohol intake, the range of alcohol consumption was limited in some studies, the classification of the exposure variable varied widely, and finally different studies adjusted for different covariates.
It is important to clarify the impact of alcohol intake on prognosis in women with breast cancer because alcohol is a well-established risk factor for breast cancer (25), and public health advice to women is to limit alcohol consumption. It is not clear, however, whether it is safe to continue with moderate alcohol consumption after breast cancer diagnosis. Thus, the key question relates to the influence of postdiagnosis alcohol consumption on outcome.
The aim of this study was to carry out a systematic review and meta-analysis of published data to provide more precise estimates of mortality risk after breast cancer diagnosis and, if possible, to identify the causes of heterogeneity across published studies. In addition, we evaluated the association between alcohol consumption and prognosis in large case cohorts: the Studies of Epidemiology and Risk Factors in Cancer Heredity breast cancer cohort (SEARCH), the European Prospective Investigation into Cancer and Nutrition (EPIC), and nine studies from the Breast Cancer Association Consortium (BCAC). The association between self-reported postdiagnosis alcohol consumption and all-cause mortality has been previously reported for SEARCH (11), but the sample size is now considerably larger, data on breast cancer-specific mortality are now available, and data on other variables such as tumor estrogen receptor (ER) status are more complete.
Materials and Methods
Systematic review and meta-analysis of the published studies
A MEDLINE search of the literature up to and including January 2013 was performed using the following search terms: [“survival” (Mesh) or “mortality” (Mesh) or “survival rate” (Mesh) or “DFS” (Mesh) or “recurrence” (Mesh)] or “prognosis” (Mesh) or “death” (Mesh) or survival or mortality or relapse or recurrence or outcome or prognosis or death) and [“ethanol” (Mesh) or “alcohols” (Mesh) or “alcohol drinking” (Mesh) or “alcoholic beverages” (Mesh) or alcohol or wine or spirits or beer] and [“breast neoplasms” (MeSH) or breast cancer or breast neoplasm]. Overall 1,096 hits were retrieved; of these 50 articles were relevant based on skimming the titles and the abstracts. Each article was reviewed and included in the analysis if the following criteria were met: (i) case cohort published as an original article and (ii) findings expressed as HRs. In addition, the bibliographies of all retrieved articles were reviewed for any relevant publications missed by the search. Overall 22 original studies were relevant and were systematically reviewed. Only the most recent and complete article of studies published more than once was included in the meta-analysis. From each article, we abstracted the HR and 95% confidence limits for different exposure categories of alcohol consumption associated with any of the following endpoints: BCSS, OS, and DFS. If adjusted HR estimates were reported, we used the maximally adjusted estimates.
Different studies used different units to measure alcohol consumption, including grams, milliliters, ounces, or drinks consumed per day, week, or month. We converted these to units per week as a standard measure of ethanol intake according to the following equivalencies: 1 mL = 0.8 g, 1 oz = 28 g, 1 drink = 12.5 g, and 1 U = 8 g. We defined patients who consumed not more than 2 U of alcohol per day (14 U per week) as moderate drinkers, and compared them with nondrinkers in the meta-analysis. However, some studies used different cutoff points; we excluded reported categories that included women with alcohol consumption of more than 2 U per day (Supplementary Fig. S1). Where a single study included more than one moderate drinker category, these estimates were pooled.
We performed fixed-effect meta-analysis using the inverse-variance weighting method (26). Statistical heterogeneity between studies was assessed using the among-study variance (τ2) and the I2 statistic (27). When two estimates were used from the same study, they were combined in the same way.
SEARCH, EPIC, and BCAC breast cancer cohorts
SEARCH cohort
SEARCH is an ongoing, population-based study of breast cancer in the region covered by the Eastern Cancer Registration and Information Centre (ECRIC). A detailed description of the study has previously been published (11). The study was set up to investigate genetic susceptibility to breast cancer. All patients diagnosed with invasive breast cancer before the age of 55 years since 1991 and still alive at the start of the study in 1996 (prevalent cases; median age, 48 years) together with all those diagnosed under 70 years of age between 1996 and the present (incident cases; median age, 54 years), are eligible to take part. The study was approved by the Cambridgeshire Research Ethics Committee. The present analysis is based on data from 8,446 participants (98% of whom were White British) with a diagnosis of invasive breast cancer.
A self-administered questionnaire was used to collect information on lifestyle factors, including height, weight, smoking history, and current (postdiagnosis) alcohol intake. Reported weekly alcohol intake was converted into standard units. The local area Index of Multiple Deprivation was used as a proxy for socioeconomic status (SES; ref. 28). Age at diagnosis, vital status, and data on tumor characteristics were obtained through the ECRIC. The registry actively follows up individuals at 3 and 5 years after diagnosis and every 5 years afterwards with continuous passive follow-up through notifications of death received from the Office for National Statistics.
EPIC cohort
Data from incident breast cancer cases from EPIC were included in the analysis. EPIC is an ongoing multicenter prospective cohort study designed to investigate the associations between diet, lifestyle, genetic and environmental factors, and various types of cancer. A detailed description of the methods has previously been published (29). In summary, 521,448 participants from 10 European countries (~70% women) mostly ages 35 years or above were recruited between 1992 and 2000. Written informed consent was provided by all study participants. Ethical approval for the EPIC study was provided from the Review Boards of the International Agency for Research on Cancer and local participating centers. Self-administered lifestyle questionnaires were used to obtain information on alcohol consumption, smoking status, and education, which was used as a proxy for SES. Height and weight were measured by trained research staff.
Incident cancer cases were identified through record linkages with regional cancer registries in all EPIC study centers except those in France, Germany, Greece, and Naples (Italy) where follow-up is conducted by review of health insurance records, contacts with cancer and pathology registries, and/or direct contact with cohort members. Vital status was ascertained through linkages with regional and national mortality registries and data collected by active follow-up (Germany and Greece). For the present study, the latest dates of complete follow-up for cancer incidence and vital status in the EPIC centers ranged from 2002 to 2006.
BCAC studies
BCAC comprises multiple studies investigating inherited susceptibility to breast cancer susceptibility (30). Many of these have detailed pathologic data on the breast cancer cases linked to follow-up data. All BCAC studies that had collected data on alcohol intake, and had data available on survival time were eligible for inclusion in this analysis. In all the studies, the prediagnosis alcohol intake was estimated using a self-reported questionnaire that was filled in after diagnosis. A multistep data, harmonization procedure was used to reconcile differences in individual study questionnaires (31). In total, nine studies from Europe, North America, Japan, and Australia contributed unpublished data on 10,232 cases. A full description of all studies included is given in Supplementary Table S1.
Statistical analysis
Cox regression was used to assess the association of alcohol consumption and survival for the SEARCH, EPIC, and BCAC breast cancer case cohorts. Because cases were enrolled at variable times after diagnosis, analyses were conducted allowing for left-truncated data. Time to failure was considered from the date of diagnosis. Time at risk began on the date of receipt of the completed questionnaire and ended at the date of death from any cause or, if death did not occur, at date of last follow-up. Follow-up of all breast cancer cases was censored at 15 years. EPIC data were stratified by country and BCAC data were stratified by study. We modeled alcohol consumption both as a categorical and as an ordinal variable in four categories: nondrinkers, up to 7, >7 to 14, and >14 U per week. We also carried out multivariable analyses adjusting for body mass index (BMI; in quartiles), smoking status (never, former, and current), menopausal status at diagnosis (<45 years, premenopausal; 45–55 years, perimenopausal, ≥55 years, menopausal), and SES. SES was categorized into five groups (1 = least deprived in SEARCH and the most educated in EPIC). Tumor characteristics considered included clinical stage (I–IV), histopathological grade (well, moderately and poorly differentiated), and ER status (negative/positive). Stage and grade were modeled as ordinal variables in the multivariable analysis. ER-positive and ER-negative disease were analyzed separately. HRs with 95% confidence intervals (CI) were estimated. For categorical variables, a likelihood-ratio test for heterogeneity of risk between groups was carried out by comparing the fit of the full model with the intercept-only model. A similar procedure was used for a trend test for ordinal and continuous variables. All tests were two sided. The assumption of proportional hazards was assessed using standard log–log plots and tested using Schoenfeld residuals. ER status and stage were time dependent (in addition to grade in SEARCH) and were therefore treated as time-dependent variables in an extended Cox model. Intercooled Stata version 12 (STATA statistical software, release 12; Stata Corporation) was used for all analyses.
Results
Published data meta-analysis
We identified 22 studies that investigated the relationship between alcohol consumption and survival (Table 1). Half of these studies had been conducted in the United States. Sample size varied considerably (range: 125–9,325 cases), with 12 studies including more than 1,000 cases. Median follow-up ranged from 3 to 13 years. Ten studies reported findings based on alcohol intake before breast cancer diagnosis and 12 studies measured alcohol intake after diagnosis.
Table 1.
Summary of studies that investigated the relationship between alcohol consumption and survival after breast cancer diagnosis
| Study | Year | N | Year of diagnosis |
Country | Age at diagnosis range (mean) |
Exposure time (pre/ postdiagnosis) |
Stage | Follow-up (mean/ median) |
All- cause deaths |
Breast deaths |
Relapses | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barnett et al. (11) | 2008 | 4,560 | 1991–2005 | UK | 23–69 | Post | All | 6.8 | 564 | Age, stage, grade, ER status | ||
| Beasley et al. (12) | 2011 | 4,441 | 1988–2001 | USA | 20–79 | Post | Local and regional | 5.5 | 525 | 137 | BMI, physical activity, energy intake, age, state of residence, menopausal status, smoking, stage, HRT and treatment | |
| Dal et al. (37) | 2008 | 1,453 | 1991–1994 | Italy | 23–74 | Post | All | 12.6 | 503 | 398 | Region of residence, age, year of diagnosis, TNM stage and ER/PR status | |
| Ewertz et al. (8) | 1991 | 1,744 | 1983–1984 | Denmark | <70 | Post | All | Up to 7 | 805 | Tumor size, skin invasion, number of positive LNs and grade | ||
| Flatt et al. (13) | 2010 | 3,088 | 1991–2000 | USA | 18–70 | Post | Early | 7.3 | 315 | 518 | Stage, grade, years between diagnosis and study entry, physical activity, smoking, education, parity, body weight, and ethnicity. | |
| Franceschi et al. (5) | 2009 | 1,453 | 1991–1994 | Italy | 23–74 | Post | 12.6 | 503 | Region of residence, age at diagnosis, year of diagnosis, tumor, node and metastasis stage, estrogen and progesterone receptor status, BMI, and smoking habit. | |||
| Goodwin et al. (18) | 2003 | 477 | 1989–1996 | Canada | 26–74 (50) | Post | Early | 6.1 | 52 | BMI, age, stage, nodal status, adjuvant hormone and chemotherapy, and total energy. | ||
| Hebert et al. (19) | 1998 | 472 | 1982–1984 | USA | 20–80 | Post | Early | Up to 10 | 73 | 109 | Stage, ER status, age, BMI, menopausal status, meat and fat intake | |
| Holmes et al. (10) | 1999 | 1,982 | 1976–1990 | USA | (54) | Post | All | 18 | 378 | 326 | Age, diet interval, diagnosis year, BMI, OCP, menopausal status, postmenopausal hormone use, smoking, age at first birth and parity, number of metastatic LNs. | |
| Kwan et al. (7) | 2010 | 1,897 | 1997–2002 | USA | 18–70 | Post | Early | 7.4 | 273 | 154 | 293 | Age, BMI, folate intake, stage, hormone receptor status, tamoxifen use, treatment, and positive LN |
| Kwan et al. (1) | 2012 | 9,325 | 1990–2006 | USA | 58.8 | Post | I, II, III | 10.3 | 1,542 | 911 | 1,487 | Age, stage, race, education, menopausal status, hormone status, treatment, smoking, physical activity, BMI, and comorbidity |
| McDonald et al. (21) | 2002 | 125 | 1989–1994 | USA | (64.2) | Post | All | 5.4 | 45 | 33 | Stage, radiotherapy, cigarette smoking | |
| Rohan et al. (22) | 1993 | 412 | 1982–1984 | Australia | Post | 5.5 | 123 | 112 | BMI and energy intake | |||
| Allemani et al. (17) | 2011 | 264 | 1987–2001 | Italy | 35–70 | Pre | All | 7.6 | 43 | BMI and non-alcoholic energy intake | ||
| Harris et al. (14) | 2012 | 3,146 | 1987–2008 | Sweden | N/R | Pre | All | up to 21 | 860 | 385 | Age, energy intake, education level, marital status, menopausal status, BMI, calendar year of diagnosis, stage, grade and treatment | |
| Hellmann et al. (6) | 2010 | 528 | 1976–2003 | Denmark | 33.1–95.4 | Pre | All | 7.8 | 323 | Smoking, physical activity, BMI, HRT, age, stage, menopausal status, parity, education, adjuvant treatment | ||
| Holm et al. (20) | 2012 | 1,028 | 1993–2006 | Denmark | 53–71 | Pre | Early | 6.3 | 178 | 106 | 110 | Tumor size, LN status, hormone receptor status, grade. BMI, smoking, menopausal status, HRT use, education level, physical activity, folate intake |
| McEligot et al. (3) | 2006 | 516 | 1994–1995 | USA | (65) | Pre | All | 6.7 | 96 | 41 | Not reported | |
| Pierce et al. (15) | 2007 | 1,490 | 1991–2000 | USA | 70 | Pre | Early | 8.7 | 135 | 118 | 236 | Age, stage, adjuvant treatment, menopausal state, parity, smoking, physical activity, BMI, and HRT |
| Reding et al. (16) | 2008 | 1,286 | 1983–1992 | USA | 45 | Pre | All | 10þ | 364 | Age, diagnosis year, mammography | ||
| Saxe et al. (9) | 1999 | 149 | 1989–1991 | USA | 26–95 (57.8) | Pre | All | 5þ | 26 | 28 | Energy intake | |
| Vrieling et al. (2) | 2012 | 2,522 | 2001–2005 | Germany | 50–74 | Pre | All | 5.5 | 316 | 235 | 247 | Age, study centre, tumor size, nodal status, metastases, tumor grade, ER/PR status, radiotherapy, HRT use at diagnosis, mode of detection |
| Zhang et al. (4) | 1995 | 698 | 1986–1991 | USA | 55–56 | Pre | All | 3 | 56 | Age |
Seventeen studies reported the relationship between alcohol consumption and OS. Most studies reported no significant association between pre- or postdiagnosis alcohol consumption and OS (1–10). However, some studies found a protective effect of alcohol (11–16) and one showed an adverse effect (28). We excluded six of these studies from the meta-analysis as follows. One study reported a protective effect of alcohol but provided HRs without 95% CIs and an overall P value across multiple levels of exposure (15). Another study reported no association for a comparison of drinkers with nondrinkers (3). Two studies (9, 11) modeled alcohol intake as a continuous variable, one of which used data from SEARCH that we have updated in the new analysis reported in this paper (11). The other study reported no association between alcohol intake and prognosis (9). One study was excluded because there were large overlaps with the data reported in other publications (7). Finally one study was excluded because of lack of details about levels of exposure, although a trend toward lower risk of death from any cause with higher alcohol consumption was reported (Ptrend = 0.01; ref. 12).
Thus, 11 studies were included in the meta-analysis. For the purposes of this meta-analysis, we pooled the results for the reported comparison that we considered most closely approximating moderate alcohol consumption (up to 14 U per week) compared with nondrinkers. Six studies reported the association between prediagnosis alcohol intake and OS. Moderate alcohol consumption was associated with better survival (Fig. 1; HR, 0.80; 95% CI, 0.73–0.88). Five studies reported the association with postdiagnosis alcohol intake which was not associated with all-cause mortality (Fig. 2; HR, 0.95; 95% CI, 0.85–1.05).
Figure 1.
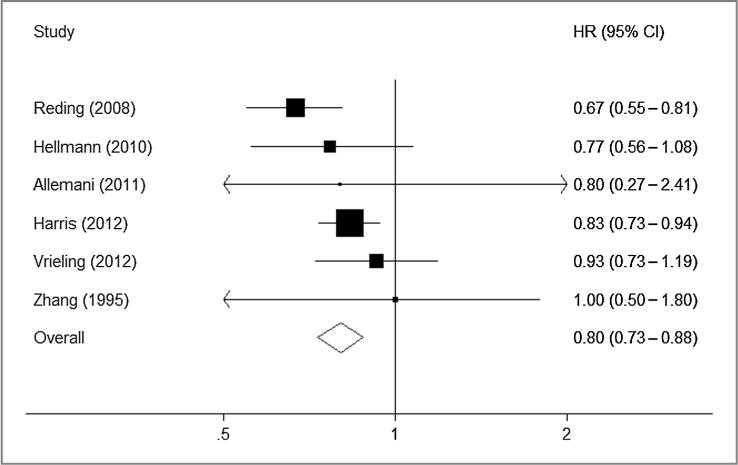
HRs for prediagnosis alcohol consumption and overall mortality (moderate drinkers vs. nondrinkers); Pheterogeneity = 0.36.
Figure 2.
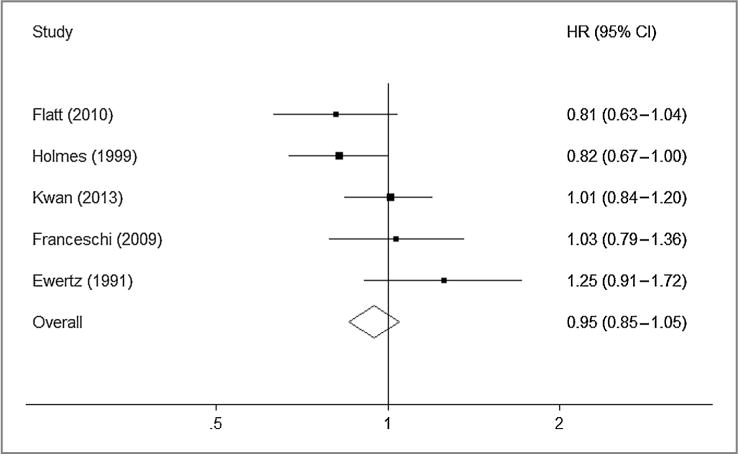
HRs for postdiagnosis alcohol consumption and overall mortality (moderate drinkers vs. nondrinkers); Pheterogeneity = 0.12.
Of the 11 studies that reported on BCSS, six studies reported no association (1, 12, 14, 18, 20, 22) and four studies reported that increased alcohol intake was associated with increased breast cancer-specific mortality (2, 7, 19, 21). The reporting of alcohol consumption in these studies was very heterogeneous. Therefore, it was not possible to combine estimates in a formal meta-analysis. One study did not report details about levels of exposure (12), one study did not use non- or minimal drinkers as a reference group (20), and two studies modeled alcohol intake as a continuous variable (19, 21). Both of these reported that alcohol consumption was associated with a poorer prognosis, but the sample sizes were small and in one, the effect was limited to beer drinkers (19). Kwan and colleagues (7) showed that drinking ≤6 g/day of alcohol compared with no drinking was associated with an increased risk of death due to breast cancer (HR, 1.51; 95% CI, 1.00–2.29). However, in a more recent analysis with a large sample size, this association was not observed (1). One study (2) showed increased risk of death from breast cancer in drinkers especially among those who consumed 12 or more grams of alcohol per day in comparison with nondrinkers (HR, 1.74; 95% CI, 1.13–2.67). Holm and colleagues (20) showed no statistically significant association (HR, 1.06; 95% CI, 0.66–1.72), but their reference group was women who consumed one unit or less per day. Similarly, Harris and colleagues (14) showed no significant association between alcohol intake and BCSS. In that study, women who consumed 10 g per day or more of alcohol had an adjusted HR of breast cancer-specific death of 1.36 (95% CI, 0.82–2.26; Ptrend: 0.47) compared with nondrinkers. Similar conclusions of null association were made by others (12, 18, 22).
Three studies examined the relationship between prediagnosis alcohol consumption and breast cancer recurrence and four examined postdiagnosis alcohol consumption; four showed no association (1, 2, 9, 13) and three showed increased risk of recurrence (7, 19, 20). Again, heterogeneity prevented the combining of these studies. Two studies modeled alcohol intake as a continuous variable (9, 19), one study did not use non- or minimal drinkers as a reference group (20) and there was an overlap between two studies (1, 7). In one of the studies (19), a statistically significant increased risk of recurrence with beer drinking (drinks/day) was reported (HR, 1.41; 95% CI, 1.02–1.97). Similarly, Kwan and colleagues (7) showed that drinking ≥6 g/day of alcohol compared with no drinking was associated with an increased risk of breast cancer recurrence (HR, 1.35; 95% CI, 1.00–1.83). This association has been confirmed only in postmenopausal women in their recent analysis (1); in which postmenopausal women who regularly consumed alcohol had an increased risk of recurrence (HR, 1.19; 95% CI, 1.01–1.40). Like other studies (9, 13), Holm and colleagues (20) showed no statistically significant association with moderate drinking (HR, 1.31; 95% CI, 0.81–2.11). However, they found a modest but significant association among heavy drinkers between prediagnostic alcohol consumption and recurrence, both when using baseline measures of alcohol intake (HR, 1.65; 95% CI, 1.02–2.67) and cumulated alcohol intake (HR, 2.02; 95% CI, 1.06–3.85).
SEARCH, EPIC, and BCAC
Postdiagnosis alcohol intake data were available for 8,446 patients with breast cancer in SEARCH with 55,684 person-years of follow-up (median 6 years). Age at diagnosis varied from 23 to 73 years (mean 54 years). There were 1,506 deaths of which 1,213 were due to breast cancer. The annual mortality rate was 2 per 100 and the 5-, 10-, and 15-year breast cancer survival rates were 88% (95% CI, 87%–89%), 75% (95% CI, 74%–77%), and 43% (95% CI, 36%–50%), respectively. Supplementary Table S2 shows the characteristics of the SEARCH study by post-diagnosis alcohol consumption. Alcohol intake tended to be higher for women with low-grade and early-stage tumors and varied by ER status, BMI, SES, smoking, and menopausal status.
Prediagnosis alcohol intake data were available for 10,561 patients in EPIC with 69,383 person-years of follow-up (median 7 years). Age at diagnosis varied from 25 to 93 years (mean 60 years). There were 1,422 deaths of which 749 were due to breast cancer. The 5-, 10-, and 15-year breast cancer survival rates were 90% (95% CI, 90%–91%), 81% (95% CI, 80%–82%), and 71% (95% CI, 69%–75%), respectively. Supplementary Table S3 shows the characteristics of the EPIC study by prediagnosis alcohol consumption. Alcohol consumption varied by menopausal status, BMI, and SES. There was a trend for alcohol consumption to decrease with increasing BMI. There were missing data in most of the variables studied, especially ER status (35%). Supplementary Table S4 shows the distribution of alcohol consumption by country in the EPIC cohort.
Prediagnosis alcohol intake data were available for 10,232 patients in BCAC with 69,710 person-years of follow-up (median 6 years). Age at diagnosis varied from 18 to 95 years (mean 54 years). There were 1,911 deaths of which 860 were due to breast cancer. The 5-, 10-, and 15-year breast cancer survival rates were 88% (95% CI, 87%–88%), 77% (95% CI, 76%–78%), and 66% (95% CI, 64%–67%), respectively. Supplementary Table S5 shows the characteristics of the BCAC data by study.
The associations between self-reported alcohol intake and prognosis in women with ER-positive breast cancer are shown in Table 2. There was a weak inverse association between postdiagnosis alcohol intake and all-cause mortality for women with ER-positive tumors (SEARCH data) with an apparent dose-response effect in univariate analysis. However, the association was substantially attenuated after adjustment for stage, grade, BMI, smoking status, SES, and menopausal status. There was also an inverse association between postdiagnosis alcohol intake and BCSS in ER-positive disease, but the effect was weaker than for OS and no association remained in the multivariate analysis. There was a very similar pattern for the inverse association of prediagnosis alcohol intake and both OS and BCSS in women with ER-positive breast cancer (EPIC and BCAC datasets).
Table 2.
Association of alcohol consumption and mortality in breast cancer cases with ER-positive tumors
| Study | Alcohol intakea (U/wk) | N | Univariate
|
Multivariateb
|
||
|---|---|---|---|---|---|---|
| Events | HR (95% CI) | Events | HR (95% CI) | |||
| Overall mortality | ||||||
| SEARCH | ||||||
| Nondrinker | 1,273 | 218 | 1 (Ref.) | 180 | 1 (Ref.) | |
| ≤7 | 2,300 | 390 | 0.96 (0.82–1.14) | 332 | 1.02 (0.84–1.22) | |
| >7– ≤14 | 861 | 132 | 0.85 (0.68–1.05) | 112 | 0.90 (0.71–1.14) | |
| >14 | 396 | 56 | 0.77 (0.58–1.03) | 47 | 0.89 (0.64–1.23) | |
| Linear (per category) | 4,830 | 796 | 0.92 (0.85–0.99) | 671 | 0.95 (0.87–1.04) | |
| EPIC | ||||||
| Nondrinker | 807 | 87 | 1 (Ref.) | 50 | 1 (Ref.) | |
| ≤7 | 2,674 | 256 | 0.88 (0.69–1.14) | 119 | 1.08 (0.76–1.53) | |
| >7– ≤14 | 1,066 | 97 | 0.80 (0.59–1.08) | 43 | 0.92 (0.60–1.41) | |
| >14 | 1,206 | 116 | 0.83 (0.62–1.12) | 51 | 0.92 (0.61–1.39) | |
| Linear (per category) | 5,753 | 556 | 0.94 (0.86–1.03) | 263 | 0.95 (0.84–1.08) | |
| BCAC | ||||||
| Nondrinker | 2,209 | 423 | 1 (Ref.) | 219 | 1 (Ref.) | |
| ≤7 | 2,725 | 402 | 0.64 (0.54–0.75) | 313 | 0.56 (0.47–0.68) | |
| >7– ≤14 | 920 | 131 | 0.72 (0.59–0.88) | 79 | 0.68 (0.52–0.88) | |
| >14 | 982 | 147 | 0.74 (0.61–0.91) | 84 | 0.64 (0.49–0.83) | |
| Linear (per category) | 6,836 | 1,103 | 0.90 (0.84–0.95) | 695 | 0.85 (0.78–0.93) | |
| Breast cancer–specific mortality | ||||||
| SEARCH | ||||||
| Nondrinker | 1,273 | 158 | 1 (Ref.) | 131 | 1 (Ref.) | |
| ≤7 | 2,300 | 317 | 1.08 (0.89–1.31) | 274 | 1.15 (0.93–1.42) | |
| >7– ≤14 | 861 | 101 | 0.90 (0.70–1.15) | 84 | 0.94 (0.71–1.24) | |
| >14 | 396 | 47 | 0.90 (0.65–1.24) | 38 | 1.01 (0.70–1.46) | |
| Linear (per category) | 4,830 | 623 | 0.95 (0.87–1.04) | 527 | 0.98 (0.89–1.09) | |
| EPIC | ||||||
| Nondrinker | 807 | 45 | 1 (Ref.) | 24 | 1 (Ref.) | |
| ≤7 | 2,674 | 121 | 0.90 (0.62–1.29) | 52 | 1.33 (0.78–2.27) | |
| >7– ≤14 | 1,066 | 42 | 0.76 (0.49–1.19) | 17 | 1.18 (0.61–2.29) | |
| >14 | 1,206 | 46 | 0.76 (0.49–1.18) | 17 | 1.12 (0.57–2.17) | |
| Linear (per category) | 5,753 | 254 | 0.91 (0.79–1.04) | 110 | 1.01 (0.83–1.24) | |
| BCAC | ||||||
| Nondrinker | 2,209 | 203 | 1 (Ref.) | 74 | 1 (Ref.) | |
| ≤7 | 2,725 | 164 | 0.65 (0.51–0.83) | 116 | 0.58 (0.41–0.81) | |
| >7– ≤14 | 920 | 52 | 0.83 (0.61–1.14) | 22 | 0.97 (0.59–1.58) | |
| >14 | 982 | 53 | 0.84 (0.62–1.14) | 21 | 0.76 (0.46–1.26) | |
| Linear (per category) | 6,836 | 472 | 0.93 (0.84–1.02) | 233 | 0.92 (0.78–1.09) | |
SEARCH reported postdiagnosis alcohol consumption, whereas EPIC and BCAC reported prediagnosis alcohol consumption.
Adjusted for stage, grade, BMI, smoking status, SES, and menopausal status (EPIC estimates were also adjusted for country).
The associations between self-reported alcohol intake and prognosis in women with ER-negative breast cancer are shown in Table 3. In unadjusted analyses, there was a strong inverse relationship in SEARCH between post-diagnosis alcohol intake and all-cause mortality (P = 0.0003) with an apparent dose-response effect (per-category HR, 0.74; 95% CI, 0.65–0.87; Ptrend = 0.0001). A slightly weaker inverse association was observed for breast cancer-specific mortality (P = 0.006) with a similar dose-response effect (per-category HR, 0.78; 95% CI, 0.67–0.91; Ptrend = 0.001). This association was attenuated slightly in a multivariable adjusted model (per-category HR, 0.81; 95% CI, 0.69–0.96; Ptrend = 0.013). However, no association between prediagnosis alcohol intake and mortality was observed in EPIC or the BCAC dataset. Supplementary Table S6 shows the association of alcohol consumption and mortality without stratification by ER status. We also carried out analyses stratified by BMI and menopausal status but no significant differences were found (data not shown). We also restricted the analysis stage I and II disease for SEARCH and again there was no significant difference in the findings (Supplementary Table S7).
Table 3.
Association of alcohol consumption and mortality in patients with ER-negative tumors
| Study | Alcohol intakea (U/wk) | N | Univariate
|
Multivariateb
|
||
|---|---|---|---|---|---|---|
| Events | HR (95% CI) | Events | HR (95% CI) | |||
| Overall mortality | ||||||
| SEARCH | ||||||
| Nondrinker | 321 | 108 | 1 (Ref.) | 97 | 1 (Ref.) | |
| ≤7 | 529 | 147 | 0.75 (0.59–0.97) | 137 | 0.84 (0.64–1.09) | |
| >7– ≤14 | 187 | 30 | 0.43 (0.29–0.64) | 27 | 0.51 (0.33–0.78) | |
| >14 | 74 | 16 | 0.58 (0.34–0.98) | 13 | 0.54 (0.30–0.97) | |
| Linear (per category) | 1,111 | 301 | 0.74 (0.65–0.87) | 274 | 0.77 (0.66–0.90) | |
| EPIC | ||||||
| Nondrinker | 175 | 39 | 1 (Ref.) | 18 | 1 (Ref.) | |
| ≤7 | 739 | 154 | 0.74 (0.52–1.07) | 68 | 0.97 (0.56–1.67) | |
| >7– ≤14 | 240 | 46 | 0.65 (0.42–1.01) | 15 | 0.78 (0.38–1.60) | |
| >14 | 281 | 62 | 0.78 (0.51–1.20) | 31 | 1.11 (0.59–2.08) | |
| Linear (per category) | 1,435 | 301 | 0.94 (0.83–1.08) | 132 | 1.02 (0.85–1.25) | |
| BCAC | ||||||
| Nondrinker | 777 | 187 | 1 (Ref.) | 84 | 1 (Ref.) | |
| ≤7 | 772 | 192 | 0.96 (0.76–1.20) | 121 | 0.78 (0.57–1.06) | |
| >7– ≤14 | 295 | 73 | 0.97 (0.73–1.28) | 33 | 0.83 (0.55–1.26) | |
| >14 | 232 | 61 | 1.03 (0.76–1.38) | 30 | 0.87 (0.56–1.33) | |
| Linear (per category) | 2,076 | 513 | 1.00 (0.92–1.10) | 268 | 0.95 (0.82–1.09) | |
| Breast cancer–specific mortality | ||||||
| SEARCH | ||||||
| Nondrinker | 321 | 91 | 1 (Ref.) | 81 | 1 (Ref.) | |
| ≤7 | 529 | 127 | 0.78 (0.59–1.02) | 120 | 0.87 (0.66–1.16) | |
| >7– ≤14 | 187 | 28 | 0.48 (0.31–0.73) | 25 | 0.58 (0.37–0.92) | |
| >14 | 74 | 15 | 0.65 (0.38–1.12) | 12 | 0.60 (0.32–1.11) | |
| Linear (per category) | 1,111 | 261 | 0.78 (0.67–0.91) | 238 | 0.81 (0.69–0.96) | |
| EPIC | ||||||
| Nondrinker | 175 | 23 | 1 (Ref.) | 11 | 1 (Ref.) | |
| ≤7 | 739 | 93 | 0.70 (0.44–1.13) | 30 | 0.62 (0.29–1.32) | |
| >7– ≤14 | 240 | 23 | 0.52 (0.28–0.94) | 6 | 0.57 (0.20–1.64) | |
| >14 | 281 | 36 | 0.76 (0.43–1.33) | 17 | 1.30 (0.57–2.99) | |
| Linear (per category) | 1,435 | 175 | 0.93 (0.78–1.12) | 64 | 1.19 (0.90–1.56) | |
| BCAC | ||||||
| Nondrinker | 777 | 110 | 1 (Ref.) | 34 | 1 (Ref.) | |
| ≤7 | 772 | 111 | 1.05 (0.77–1.43) | 65 | 0.85 (0.53–1.37) | |
| >7– ≤14 | 295 | 31 | 0.82 (0.55–1.23) | 10 | 0.87 (0.42–1.80) | |
| >14 | 232 | 31 | 1.12 (0.75–1.68) | 13 | 1.05 (0.54–2.05) | |
| Linear (per category) | 2,076 | 283 | 1.00 (0.88–1.13) | 122 | 1.00 (0.80–1.24) | |
SEARCH reported postdiagnosis alcohol consumption, whereas EPIC and BCAC reported prediagnosis alcohol consumption.
Adjusted for stage, grade, BMI, smoking status, SES, and menopausal status (EPIC estimates were also adjusted for country).
Discussion
We have evaluated the association between alcohol consumption and mortality after a diagnosis of breast cancer using a combination of meta-analysis of previously published studies and an analysis of individual participant data from 11 breast cancer case cohorts (SEARCH, EPIC, and nine BCAC studies). The exposure of primary interest was postdiagnosis alcohol consumption, but many studies only obtained data on prediagnosis alcohol consumption. It is likely that pre- and postdiagnosis consumption are correlated, and therefore prediagnosis intake may be a proxy for postdiagnosis intake (14). However, prediagnosis alcohol consumption could be associated with prognosis through complex mechanisms in addition to any correlation with postdiagnosis consumption. For example, alcohol intake is a well-established risk factor for breast cancer, but risk is higher for hormone receptor-positive disease than for hormone receptor-negative disease (25, 32–34). Thus, any association of prediagnosis consumption with prognosis may reflect differences in the risk of the different subtypes.
There are other potential weaknesses in the current study. We relied on information on lifestyle factors collected mostly at point of time and, therefore, cannot address the issue of lifestyle modifications subsequent to cancer, including weight loss after diagnosis. Similarly, changing drinking habits over time as well as pre/post-diagnosis need to be investigated. Prospective studies with follow-up of incident cases and repeat measures of alcohol intake and other lifestyle factors, before and after diagnosis, may offer the best approach for resolving the issue. A very recently published large study has shown that moderate alcohol consumption before diagnosis was associated with modest improvement in BCSS. There was no evidence for an association with postdiagnosis alcohol intake and breast cancer survival. The main strength in this study was that all women reported on prediagnostic intake and a subsample of 4,881 reported on postdiagnostic intake (35).
The previously published data are consistent with a small reduction in all-cause mortality with moderate alcohol intake both pre- and postdiagnosis. However, these differences may reflect a reduction in mortality from breast cancer or a reduction in mortality from other causes (2). The studies reporting results for breast cancer-specific mortality or recurrence were too heterogeneous to be combined in a meta-analysis. A major limitation of the published studies, with only few exceptions (1, 2), is that they did not stratify the analysis by ER status. The clinical behavior of ER-positive and ER-negative breast cancers differ significantly (36), and it is plausible that they respond differently to alcohol and its metabolites. Furthermore, any observed association may be confounded by the different risks of the different subtypes. Publication bias should always be considered in a review of published data, but, given the heterogeneity of results with reported effects in both directions, it seems unlikely to be important here.
The SEARCH, EPIC, and BCAC case cohort results are considerably less heterogeneous. We used consistent definitions of alcohol intake, and the analysis approach was the same for all three datasets. In particular, we performed separate analyses for ER-positive and ER-negative disease, separately and we adjusted for multiple potential confounders. For women with ER-positive disease, there was little evidence that pre- or postdiagnosis alcohol consumption is associated with breast cancer-specific mortality, with some evidence of a reduction in all-cause mortality. On the basis of these findings, it is unlikely that alcohol intake has an adverse effect on survival of women with ER-positive breast cancer. For women with ER-negative disease, the SEARCH data suggest that moderate postdiagnosis alcohol intake may be associated with a small reduction in breast cancer-specific mortality, whereas the EPIC and BCAC results suggest no association for prediagnosis intake. These differences may be because if there is a true association with postdiagnosis alcohol intake, it may have been missed when using prediagnosis alcohol intake as a proxy for postdiagnosis intake. Alternatively, reverse causality, for example, alcohol intake being associated with general well being, may explain the findings for the SEARCH data. Measurement error in estimating pre- and postdiagnosis alcohol intake is likely to result in an underestimation of any association. Self-reported intake is likely to underestimate alcohol consumption, and the conversion of reported consumption of different types of alcohol into a standardized quantity may result in additional error.
Breast cancer treatment has changed over time and the use of hormonal treatment, chemotherapy, and trastuzumab has improved survival for some patients. It is possible that changes in alcohol intake in populations over time may correlate with changes in treatment and thus the observed association may be the result of confounding by treatment. Screening is another potential confounder as screen-detected cases show longer survival. Data on mode of detection (clinical or screening) were available for patients in SEARCH. In the SEARCH study, the effect of reported alcohol intake was the same in women with screen detected and clinically detected cancers (data not shown).
Considering the totality of the evidence, moderate post-diagnosis alcohol consumption is unlikely to have a major adverse effect on survival of women with breast cancer. Given the methodologic problems of studying alcohol intake and breast cancer prognosis in simple observational studies, the best approach for resolving the issue would be to embed serial dietary and lifestyle assessment within randomized controlled treatment trials.
Supplementary Material
Acknowledgments
The authors thank the following individuals without whom this study would not have been possible: the participating patients; Stephen Kaptoge, Robert Luben, Amit Bhaniani (EPIC); the SEARCH team (SEARCH); Ursula Eilber, Sabine Behrens (GESBC); Karl von Smitten, Sofia Khan, Irja Erkkilä, Virpi Palola (HEBCS); and Maggie Angelakos, Judi Maskiell, and Gillian Dite (ABCFS).
Grant Support: A.M.G. Ali was supported by a studentship from the Citadel Capital Foundation, Egypt. M. Garcia-Closas was funded by the Breakthrough Breast Cancer Research Centre, London, United Kingdom. J.L. Hopper is a National Health and Medical Research Council (NHMRC) Australia Fellow and a Victorian Breast Cancer Research Consortium Group Leader.
Funding of the constituent studies was provided by the Botin Foundation Fund; Cancer Research UK (C490/A10119, C490/A10124, C1287/ A10118 and C1287/A12014); the Dutch Cancer Society (NKI-2007-3839; NKI-2009-4363); BBMRI-NL (NWO 184.021.007); Dutch Ministry of Public Health; Welfare and Sports, Netherlands Cancer Registry, LK Research Funds, Dutch Prevention Funds, Zorg Onderzoek Nederland; World Cancer Research Fund, Statistics Netherlands; the Dutch National Genomics Initiative; the Deutsche Krebshilfe e.V. (70492); European Community’s Seventh Framework Programme under grant agreement 223175 (HEALTH-F2-2009-223175); FIS Intrasalud (PS09/02368); Grant-in-Aid for Scientific Research on Priority Areas and on Innovative Area, Ministry of Education, Science, Sports, Culture and Technology of Japan; Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control, Ministry of Health, Labour and Welfare, Japan; Health Strategic Action Instituto de Salud Carlos III (FIS PI12/02125); the Hellenic Health Foundation (Greece); the Helsinki University Central Hospital Research Fund, the Academy of Finland (132473), Norwegian Research Council and Norwegian Cancer Society; the Sigrid Juselius Foundation, the Finnish Cancer Society; Herlev Hospital, the Danish Medical Research Council, Chief Physician Johan Boserup and Lise Boserup’s Fund.; Intramural Research Funds of the National Cancer Institute, Department of Health and Human Services; NIH (CA58860, CA92044) and the Lon V Smith Foundation (LVS39420); the National Health and Medical Research Council of Australia, the New South Wales Cancer Council, Norwegian Research Council and Norwegian Cancer Society; the Victorian Health Promotion Foundation (Australia) and the Victorian Breast Cancer Research Consortium; the UK National Institute for Health Research Biomedical Research Centre at the University of Cambridge; and the United States National Cancer Institute, NIH (RFA-CA06503, U01-CA69638, UM1-CA164920).
Footnotes
Authors’ Contributions
Conception and design: A.M.G. Ali, A. Carracedo, H. Flyger, J. Chang-Claude, H. Anton-Culver, J.L. Hopper, H.B. Bueno-de-Mesquita, V. Krogh, E. Weiderpass, P.H.M. Peeters, K. Overvad, A. Barricarte, P.D.P. Pharoah
Development of methodology: A.M.G. Ali, A. Carracedo, J.L. Hopper, H.B. Bueno-de-Mesquita, E. Weiderpass, P.H.M. Peeters
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A.M.G. Ali, M.K. Schmidt, Q. Wang, M. Gago-Dominguez, J.E. Castelao, A. Carracedo, V.M. Garzón, S.E. Bojesen, B.G. Nordestgaard, H. Flyger, J. Chang-Claude, A. Rudolph, P. Seibold, H. Nevanlinna, T.A. Muranen, K. Aaltonen, C. Blomqvist, K. Matsuo, H. Ito, H. Iwata, A. Horio, E.M. John, M. Sherman, J. Lissowska, J.D. Figueroa, M. Garcia-Closas, H. Anton-Culver, M. Shah, J.L. Hopper, A. Trichopoulou, H.B. Bueno-de-Mesquita, V. Krogh, E. Weiderpass, A. Andersson, F. Clavel-Chapelon, P.H.M. Peeters, D.F. Easton, S. Borgquist, K. Overvad, A. Barricarte, M.-J. Sanchez, P. Amiano, T.J. Key, P.D.P. Pharoah
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A.M.G. Ali, A. Carracedo, J. Chang-Claude, K. Matsuo, M. Sherman, J. Lissowska, J.L. Hopper, E. Weiderpass, P.H.M. Peeters, G.C. Wishart, D.F. Easton, P.D.P. Pharoah
Writing, review, and/or revision of the manuscript: A.M.G. Ali, M.K. Schmidt, M. Gago-Dominguez, J.E. Castelao, A. Carracedo, V.M. Garzón, S.E. Bojesen, B.G. Nordestgaard, H. Flyger, J. Chang-Claude, A. Vrieling, A. Rudolph, H. Nevanlinna, T.A. Muranen, C. Blomqvist, H. Iwata, E.M. John, M. Sherman, J. Lissowska, J.D. Figueroa, M. Garcia-Closas, H. Anton-Culver, M. Shah, J.L. Hopper, A. Trichopoulou, H.B. Bueno-de-Mesquita, V. Krogh, E. Weiderpass, A. Andersson, F. Clavel-Chapelon, L. Dossus, G. Fagherazzi, P.H.M. Peeters, A. Olsen, G.C. Wishart, D.F. Easton, S. Borgquist, K. Overvad, A. Barricarte, C.A. González, M.-J. Sanchez, P. Amiano, E. Riboli, T.J. Key, P.D.P. Pharoah
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A.M.G. Ali, M. Bolla, B.G. Nordestgaard, A. Rudolph, P. Seibold, A. Horio, J. Lissowska, M. Shah, E. Weiderpass, P.H.M. Peeters, S. Borgquist, M.-J. Sanchez
Study supervision: A. Carracedo, E.M. John, E. Weiderpass, C.A. González
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
References
- 1.Kwan ML, Chen WY, Flatt SW, Weltzien EK, Nechuta SJ, Poole EM, et al. Postdiagnosis alcohol consumption and breast cancer prognosis in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2013;22:32–41. doi: 10.1158/1055-9965.EPI-12-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vrieling A, Buck K, Heinz J, Obi N, Benner A, Flesch-Janys D, et al. Pre-diagnostic alcohol consumption and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res Treat. 2012;136:195–207. doi: 10.1007/s10549-012-2230-2. [DOI] [PubMed] [Google Scholar]
- 3.McEligot AJ, Largent J, Ziogas A, Peel D, Anton-Culver H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55:132–40. doi: 10.1207/s15327914nc5502_3. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat. The Iowa Women’s Health Study. Cancer. 1995;76:275–83. doi: 10.1002/1097-0142(19950715)76:2<275::aid-cncr2820760218>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Franceschi S, Dal ML, Zucchetto A, Talamini R. Alcohol consumption and survival after breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1011–2. doi: 10.1158/1055-9965.EPI-08-0904. [DOI] [PubMed] [Google Scholar]
- 6.Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19:366–73. doi: 10.1097/CEJ.0b013e32833b4828. [DOI] [PubMed] [Google Scholar]
- 7.Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, et al. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol. 2010;28:4410–6. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewertz M, Gillanders S, Meyer L, Zedeler K. Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int J Cancer. 1991;49:526–30. doi: 10.1002/ijc.2910490409. [DOI] [PubMed] [Google Scholar]
- 9.Saxe GA, Rock CL, Wicha MS, Schottenfeld D. Diet and risk for breast cancer recurrence and survival. Breast Cancer Res Treat. 1999;53:241–53. doi: 10.1023/a:1006190820231. [DOI] [PubMed] [Google Scholar]
- 10.Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999;86:826–35. doi: 10.1002/(sici)1097-0142(19990901)86:5<826::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–6. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- 12.Beasley JM, Newcomb PA, Trentham-Dietz A, Hampton JM, Bersch AJ, Passarelli MN, et al. Post-diagnosis dietary factors and survival after invasive breast cancer. Breast Cancer Res Treat. 2011;128:229–36. doi: 10.1007/s10549-010-1323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flatt SW, Thomson CA, Gold EB, Natarajan L, Rock CL, Al-Delaimy WK, et al. Low to moderate alcohol intake is not associated with increased mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:681–8. doi: 10.1158/1055-9965.EPI-09-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris HR, Bergkvist L, Wolk A. Alcohol intake and mortality among women with invasive breast cancer. Br J Cancer. 2012;106:592–5. doi: 10.1038/bjc.2011.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–51. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reding KW, Daling JR, Doody DR, O’Brien CA, Porter PL, Malone KE. Effect of prediagnostic alcohol consumption on survival after breast cancer in young women. Cancer Epidemiol Biomarkers Prev. 2008;17:1988–96. doi: 10.1158/1055-9965.EPI-07-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allemani C, Berrino F, Krogh V, Sieri S, Pupa SM, Tagliabue E, et al. Do pre-diagnostic drinking habits influence breast cancer survival? Tumori. 2011;97:142–8. doi: 10.1177/030089161109700202. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Trudeau ME, Hood N. Diet and breast cancer: evidence that extremes in diet are associated with poor survival. J Clin Oncol. 2003;21:2500–7. doi: 10.1200/JCO.2003.06.121. [DOI] [PubMed] [Google Scholar]
- 19.Hebert JR, Hurley TG, Ma Y. The effect of dietary exposures on recurrence and mortality in early stage breast cancer. Breast Cancer Res Treat. 1998;51:17–28. doi: 10.1023/a:1006056915001. [DOI] [PubMed] [Google Scholar]
- 20.Holm M, Olsen A, Christensen J, Kroman NT, Bidstrup PE, Johansen C, et al. Pre-diagnostic alcohol consumption and breast cancer recurrence and mortality: Results from a prospective cohort with a wide range of variation in alcohol intake. Int J Cancer. 2012;132:686–94. doi: 10.1002/ijc.27652. [DOI] [PubMed] [Google Scholar]
- 21.McDonald PA, Williams R, Dawkins F, Adams-Campbell LL. Breast cancer survival in African American women: is alcohol consumption a prognostic indicator? Cancer Causes Control. 2002;13:543–9. doi: 10.1023/a:1016337102256. [DOI] [PubMed] [Google Scholar]
- 22.Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer. 1993;20:167–77. doi: 10.1080/01635589309514283. [DOI] [PubMed] [Google Scholar]
- 23.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66:5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–16. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lew JQ, Freedman ND, Leitzmann MF, Brinton LA, Hoover RN, Hollenbeck AR, et al. Alcohol and risk of breast cancer by histologic type and hormone receptor status in postmenopausal women: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;170:308–17. doi: 10.1093/aje/kwp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12:2468–75. doi: 10.1158/1078-0432.CCR-05-2719. [DOI] [PubMed] [Google Scholar]
- 28.Lyratzopoulos G, Barbiere JM, Gajperia C, Rhodes M, Greenberg DC, Wright KA. Trends and variation in the management of oesophago-gastric cancer patients: a population-based survey. BMC Health Serv Res. 2009;9:231. doi: 10.1186/1472-6963-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 30.Breast Cancer Association Consortium. Commonly studied single-nucleotide polymorphisms and breast cancer: results from the Breast Cancer Association Consortium. J Natl Cancer Inst. 2006;98:1382–96. doi: 10.1093/jnci/djj374. [DOI] [PubMed] [Google Scholar]
- 31.Nickels S, Truong T, Hein R, Stevens K, Buck K, Behrens S, et al. Evidence of gene-environment interactions between common breast cancer susceptibility loci and established environmental risk factors. PLoS Genet. 2013;9:e1003284. doi: 10.1371/journal.pgen.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gapstur SM, Potter JD, Drinkard C, Folsom AR. Synergistic effect between alcohol and estrogen replacement therapy on risk of breast cancer differs by estrogen/progesterone receptor status in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 1995;4:313–8. [PubMed] [Google Scholar]
- 33.Kwan ML, Kushi LH, Weltzien E, Maring B, Kutner SE, Fulton RS, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sellers TA, Vierkant RA, Cerhan JR, Gapstur SM, Vachon CM, Olson JE, et al. Interaction of dietary folate intake, alcohol, and risk of hormone receptor-defined breast cancer in a prospective study of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1104–7. [PubMed] [Google Scholar]
- 35.Newcomb PA, Kampman E, Trentham-Dietz A, Egan KM, Titus LJ, Baron JA, et al. Alcohol consumption before and after breast cancer diagnosis: associations with survival from breast cancer, cardiovascular disease, and other causes. J Clin Oncol. 2013;31:1939–46. doi: 10.1200/JCO.2012.46.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dal ML, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–94. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


