Abstract
Background
Hepatitis E virus (HEV) infection has become relevant to blood transfusion practice because isolated cases of blood transmission have been reported and because HEV has been found to cause chronic infection and severe liver disease in immuno-compromised patients.
Study design and Methods
We tested for IgG and IgM antibodies to the hepatitis E virus (HEV) and for HEV RNA in 1939 unselected volunteer US blood donors. Subsequently, we tested the same parameters in pre- and serial post-transfusion samples from 362 prospectively followed blood recipients to assess transfusion risk.
Results
IgG anti-HEV seroprevalence in the total 1939 donations was 18.8%: 916 of these donations were made in 2006 at which time the seroprevalence was 21.8% and the remaining 1023 donations were in 2012 when the seroprevalence had decreased to 16.0% (p<0.01). A significant (P<0.001) stepwise increase in anti-HEV seroprevalence was seen with increasing age. Eight of 1939 donations (0.4%) tested anti-HEV IgM positive; no donation was HEV RNA positive. Two recipients had an apparent anti-HEV seroconversion, but temporal relationships and linked donor testing showed that these were not transfusion transmitted HEV infections.
Conclusion
No transfusion-transmitted HEV infections were observed in 362 prospectively followed blood recipients despite an anti-HEV seroprevalence among donations exceeding 16%.
Keywords: Hepatitis E virus, transfusion transmitted, blood donor, recipient, seroconversion
Introduction
Hepatitis E virus (HEV) infection has been recognized as an important cause of acute, often epidemic, hepatitis in Asia and was believed to be rare in industrialized countries.1,2 However, indigenous HEV infections are increasingly reported in developed nations, and most are caused by HEV genotypes 3 or 4 as compared to genotypes 1 and 2 that are associated with large outbreaks due to contaminated water supplies.3,4 In addition, HEV seroprevalence among blood donors and the general population in industrialized countries has been found to be much higher than expected and accumulating evidence suggests that the clinical importance of HEV infection in non-endemic regions has been underestimated. 5–7 The routes of transmission in countries with safe water supplies are not well defined, though transmission from contaminated pork products has been demonstrated in southwestern France and other regions.5,8–10
The high seroprevalence of infection in asymptomatic individuals raises the potential risk of HEV transmission through blood transfusion. Though such transmission appears to be rare, a small number of transfusions related cases have been reported and confirmed by molecular identity of the agent in donor and recipient.11–13 Importantly, this infection, once thought to be universally self-limiting, has now been shown to result in chronic infection and cirrhosis in immune-compromised patients and to exacerbate fibrosis progression and liver-related mortality in infected subjects with pre-existing liver disease.13–15 The potential risk of blood transmission is compounded by the high proportion of blood recipients who are immunosuppressed and repeatedly transfused.
In United States, HEV seroprevalence was found to be 21% in a national health survey (NHANES III) conducted from 1988 to 1994 16 and then to have fallen to 6.4 % in a similar survey (NHANES IV) conducted by the Centers for Disease Control from 2009 to 201017. The reason for the fall in HEV seroprevalence between these two surveys is currently unexplained. To better assess the risk of HEV transmission by blood transfusion we investigated HEV seroprevalence among healthy US blood donors and tracked transmission rates among blood recipients enrolled in an ongoing prospective study of transfusion transmitted infections (TRIPS). We utilized a commercial anti-HEV assay that performed well in comparative studies18 and a sensitive in-house PCR assay validated with plasma from persons confirmed to have HEV genotype 3 infection.
MATERIALS AND METHODS
HEV specimens
In our study, all tests were performed on plasma samples. In the donor study, we used unselected NIH volunteer blood donor samples obtained in two different time periods, specifically 2006 and 2012. In the recipient study, samples were tested pre-transfusion and then at 4 and/or 8 weeks post-transfusion, and at the end of the study (ES, 24 or 36 weeks post-transfusion): 21% of recipients had a pre-sample and 3 post-transfusion samples and all recipients had a pre-sample and at least one sample obtained 8 or more weeks after transfusion. Linked donor samples were available for most recipients. Donor samples used for determination of HEV seroprevalence were not linked to specific recipients.
The TRIPS repository was initiated in November 2001 and is composed of linked donor-recipient specimens from transfusion recipients enrolled at the NIH Clinical Center (Bethesda, MD) and the Children’s National Medical Center (Washington, DC) and from Suburban Hospital (Bethesda, MD). Informed consent was obtained from all donors and recipients in accordance with the Declaration of Helsinki for participation in NIH-sponsored and institutional review board–approved protocols (NIH Protocol 01-CC-0231; Children’s National Medical Center, Protocol 2540). Human subjects were assigned a code number, and all TRIPS patient samples and both linked and unlinked donor samples were identified only by that code; the testing laboratories had no capability of linking the code number to the study participant’s name.
Anti-HEV serology
Anti-HEV IgG and IgM antibodies were detected by ELISA using assays manufactured by Wantai Pharmaceutical Co., Beijing, China (Research use only). This assay utilizes a recombinant peptide corresponding to open reading frame 2 (ORF2) of the HEV genome.19 According to the package insert, this assay has a specificity of 98.6% in testing 9012 healthy Asian subjects. It is to be noted that confirmatory assays for HEV IgM and IgG antibodies are not available rendering positive findings only presumptive. Testing was performed according to the manufacturer’s instructions. Briefly, samples were diluted 1:10, added to antigen coated plates and incubated for 30 minutes. Then HRP-conjugate was added and followed by another 30 minutes incubation. After incubation, color development was measured at an absorbance of 450nm. Results were calculated as sample/cutoff ratios: values of 1.0 or above were considered reactive. Each sample was tested in duplicate and all positive samples were confirmed in a repeat duplicate assay. Only samples reactive in at least 3 of the 4 quadruplicate assays were considered positive.
HEV RNA detection
Pools of 7–8 donor samples were tested for HEV RNA by both Real-time RT PCR20 and a nested PCR 21. The 95% limit of detection (LOD) for the real time PCR assay was 400 IU/mL and the 50% LOD was 200 IU/mL. For the nested PCR, the 95% LOD was 200 IU/mL and the 50% LOD was 50 IU/mL. IgM positive samples were tested individually by both PCR methods. Nucleic acid isolation was performed using the QIAamp Viral RNA Mini kit (Qiagen) according to the manufacturer’s instructions. For Real-time PCR, highly conserved sequences in the open reading frame (ORF) 3 region of all HEV genotypes were targeted: forward primer (JVHEVF; 5′-GGTGGTTTCTGGGGTGAC-3′), reverse primer (JVHEVR: 5′-AGGGGTTGGTTGGATGAA-3′) and probe (JVHEVP: FAM-5′-TGATTCTCAGCCCTTCGC-3′-TAMRA). For nested PCR, a 150-nt segment of ORF2, was amplified with primers E1 (5′-CTGTTTAAYCTTGCTGA CAC- 3′) and E5 (5′-WGARAGCCAAAGCACATC-3′ ′ ′) in the first round of PCR and primers E2 (5′-GACAGAATTGATTTCGTCG-3′) and E4 (5′-TGYTGGTTRTCRTAATCCTG-3′) in the second round. PCR cycling conditions for both rounds consisted of 35 cycles of denaturation at 94°C for 30 s, annealing at 53°C for 30 s, and extension at72°C for 40 s. A positive HEV RNA control (200 IU/ml) was included in each assay.
Statistical methods
All statistical analysis was performed using IBM SPSS Statistics 19 where P<0.05 was consider as significant.
RESULTS
Donor Testing
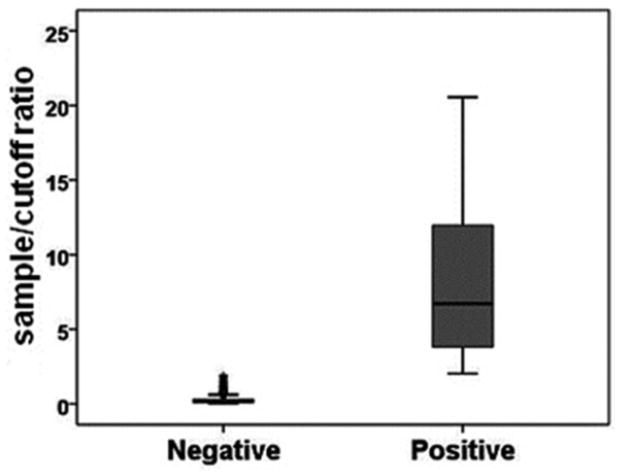
General characteristics of the 1939 blood donations examined are summarized in Table 1. The first 916 samples were collected from 01/03/2006 to 05/19/2006. The remaining 1023 donations were collected from 01/03/2012 to 03/21/2012. HEV IgG was detected in 364 (18.8%) of the total 1939 blood donations (95% confidence interval [CI], 17.0%–20.5%). The ranges of sample/cutoff ratios for positive and negative samples showed a clear bimodal distribution (Fig. 1). The mean ± SD sample/cutoff ratio of the positive samples was 8.15 ± 5.06. The seroprevalence was 21.8% (95% confidence interval [CI], 19.2%–24.5%) in year 2006 samples and 16.0% (95% confidence interval [CI], 13.8%–18.3%) in year 2012 samples (P<0.01).
Table 1.
Hepatitis E markers in NIH volunteer blood donors.
| Collection Year | No. Tested | Anti-HEV IgG+ | Anti-HEV IgM+ | HEV RNA+ |
|---|---|---|---|---|
| 2006 | 916 | 200 (21.8%) 95% CI: 19.2%–24.5% |
3 (0.3%) 95% CI: −0.0%–0.7% |
0 (0%) |
|
| ||||
| 2012 | 1023 | 164 (16.0%) 95% CI: 13.8%–18.3% |
5 (0.5%) 95% CI: −0.0%–0.9% |
0 (0%) |
| Total | 1939 | 364 (18.8%) 95% CI: 17.0%–20.5% |
8 (0.4%) 95% CI: 0.1%–0.7% |
0 (0%) |
CI: confidence interval.
Figure 1.
Distribution of sample/cut off ratios for hepatitis E virus IgG in positive and negative samples from 1939 NIH volunteer blood donors.
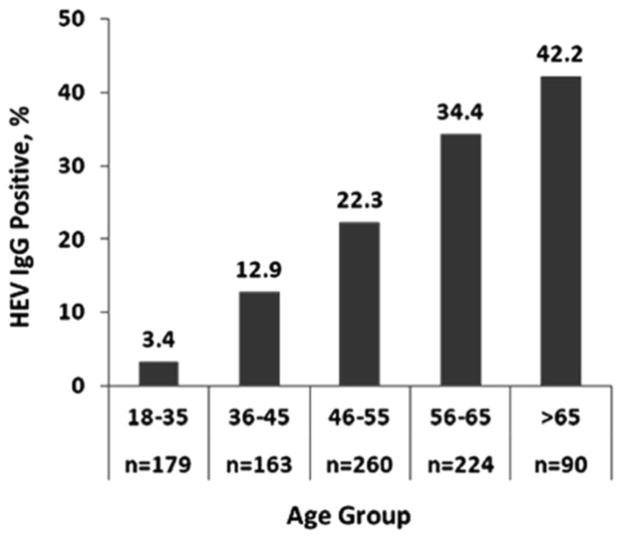
We analyzed three demographic variables for their association with IgG seroprevalence using the samples obtained in 2006. No statistically significant association was found in regard to gender or race (data not shown). There was a strong statistical association with age (P<0.001): the seroprevalence of anti-HEV IgG was 3.4% (95% confidence interval [CI], 0.7% – 6.0%) in ages below 25, and then increased linearly to 42.2% (95% confidence interval [CI], 31.8% – 52.6%) in those over age 65 (Fig. 2). Age data were not available for samples obtained in 2012.
Figure 2.
Prevalence of anti-hepatitis E virus (HEV) IgG in 916 NIH volunteer blood donors by age group.
There were 8 donations that tested anti-HEV IgM reactive among the 1939 donations (0.4%). HEV RNA was not detected in any donations.
RECIPIENT TESTING
The anti-HEV IgG seroprevalence of the 362 recipients in their pre-transfusion samples was 21.5% (95% confidence interval [CI], 17.3% – 25.8%) and thus very similar to the blood donor population. One recipient (0.3%) was IgM anti-HEV positive in the pre-transfusion sample, but no recipient was IgM anti-HEV positive in any post-transfusion sample.
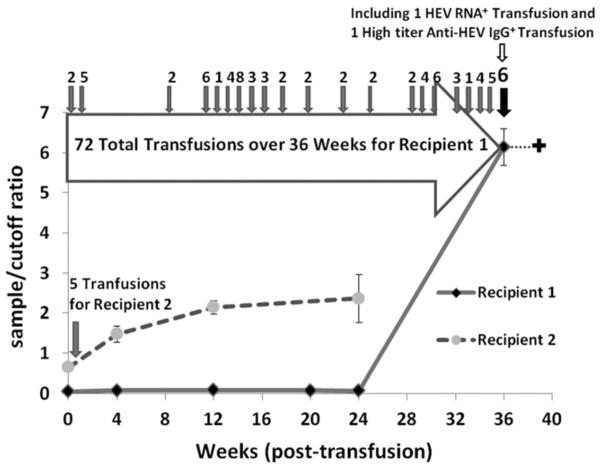
Among the 362 recipients there were two instances (0.6%) where the last post-transfusion sample was anti-HEV positive while the pre-transfusion sample was anti-HEV negative. These potential anti-HEV seroconversions were further investigated. In recipient 1, we tested all available samples from the time of transfusion to the end of study sample that tested anti-HEV positive. Each of these interim samples, obtained at 4, 12 and 20 weeks post-transfusion, tested negative for anti-HEV IgM and IgG and for HEV RNA (Fig. 3). Thus, the finding of anti-HEV in the late post-transfusion sample was an isolated, albeit reproducible finding. Next we tested linked donations: such samples were available for 69 of the 72 blood products received by this patient during the course of the study. In the 69 linked donations tested, the anti-HEV IgG seroprevalence was 14.5% (95% confidence interval [CI], 6.0% – 23.0%). One of IgG positive donations was also anti-HEV IgM reactive. Four days before the recipient sample that tested anti-HEV IgG positive, this repeatedly transfused patient received blood from a donation which was HEV RNA positive by nested PCR and from a second donation which had high titer anti-HEV antibody (sample/cutoff ratio > 20). Since the interval from transfusion of these HEV reactive blood donations to the detection of HEV antibody in the recipient was only four days, we conclude that IgG antibody detected in the patient was passively transferred from the donated unit with high titer anti-HEV and hence did not indicate a true seroconversion or a transfusion associated HEV infection. Unfortunately, the patient died soon after receipt of the HEV RNA positive blood donation so that we could not trace whether that blood unit would have transmitted HEV infection.
Figure 3. Suspected, but unconfirmed anti-HEV IgG seroconversion in two blood recipients.
Recipient 1 received one HEV RNA+ and one high titer anti-HEV IgG+ blood unit 4 days before the sample collected at 36 weeks after the index study transfusion. The patient died shortly after receiving the HEV RNA+ unit and thus the consequences of that transfusion could not be assessed.
Recipient 2 could be interpreted as having a very early seroconversion post-transfusion, but more likely was infected prior to the index transfusion based on the timing and on the pre-transfusion sample having a relatively high, albeit below cut-off.
In the potential anti-HEV seroconversion recipient 2, all post-transfusion samples showed low level anti-HEV reactivity with sample/cutoff ratios gradually increasing from 1.5 at week 4 to 2.5 at week 24 post-transfusion (Fig. 3). Neither IgM anti-HEV nor HEV RNA was detected in any post-transfusion sample. Although the pre-transfusion sample was below the assay cut-off and thus interpreted as negative, in retrospect it was just below the cut-off and probably the onset of an anti-HEV antibody response that preceded study enrollment. The cause of this HEV antibody response is unknown, but we conclude it was not related to the index transfusion in this study. None of the linked donations tested in this case had HEV markers, but we only had access to 2 of 5 donations to this patient.
These two cases illustrate the importance of having serial recipient samples and linked donations in interpreting apparent recipient seroconversion after blood transfusion. In sum, we did not observe any cases of transfusion-transmitted HEV infection among 362 prospectively followed blood recipients despite the high seroprevalence of anti-HEV IgG in our population. The observation of zero HEV infections among 362 recipients could represent a true infection rate up to 0.8% based on the upper bound of the 95% confidence interval according to the “rule of 3”.22,23
Discussion
The results of our study show an anti-HEV IgG seroprevalence approaching 19% in testing 1939 blood donations. This proportion is similar to previous studies16 and confirms that exposure to HEV is common in the US blood donor population. Although the seroprevalence appears to have decreased over the 6-year interval between 2006 and 2012, evidence of recent or past HEV exposure remains very high. In general population surveys conducted by CDC (National Health and Nutrition Survey, NHANES), a more striking decline in anti-HEV seroprevalence was observed between 1988–1994 and 2009–2010 with a fall from 21% to 6%.16,17 Continued monitoring of HEV seroprevalence will be important to elucidate the root causes of transmission.
Estimates of HEV seroprevalence are highly dependent on assay sensitivity and more recently employed assays appear to be both more sensitive and specific. The anti-HEV assay utilized in this study was manufactured by Wantai Pharmaceutical Co. and is now widely used in Asia and Europe where its enhanced sensitivity has resulted in large increases in anti-HEV seroprevalence as compared with earlier assays.5,18,24–26 The difference between the HEV seroprevalence observed in our study of blood donors in 2012 (16%) compared to the NHANES IV population survey in 2009–2010 (6%) may in part reflect test sensitivity rather than true differences in seroprevalence in the tested populations. Although preliminary studies indicate that the increased sensitivity of the Wantai assay does not come at the expense diminished specificity, there is great need to develop HEV standards and pedigreed panels to allow valid comparisons among the competing assays to determine whether differences in seroprevalence are geographic or epidemiologic or simply assay dependent. Such panels are now under development.
No HEV viremia was detected in the approximate 2000 donations tested in this study, but this number of donations is too small to detect a low occurrence event and much larger surveys are needed. We did, however find a small percentage of donations with IgM reactivity suggesting recent infection and posing the potential for relevant incident infections in the donor population. Indeed, although we did not find an HEV RNA positive donation in the donor testing phase of this study, we did find an HEV RNA positive donation when retrospectively testing linked donations to a case of potential transfusion-associated anti-HEV seroconversion. These indications of recent HEV infections in blood donors indicate the need for continued vigilance for HEV in the blood supply and the need for recipient tracing through prospective studies and look-back investigations. In our study, as in others5,16, advancing age is a key correlate of anti-HEV seroprevalence. It is probable that this represents cumulative exposure over time, but an alternate explanation is that there is a cohort effect wherein the population had an unidentified, non-lethal HEV exposure in the remote past and the resulting antibody response has been carried forward into old age.
Sporadic cases of transfusion related HEV infection have been reported recently11–13 and have been confirmed by molecular linkage between donor and recipient. Thus, the issue at hand is not whether HEV can be transmitted by transfusion, but rather how often and with what consequences?
In the prospective arm of our study, we tested pre- and post-transfusion samples from 362 recipients. The HEV seroprevalence of the recipients before transfusion was very similar to that of the blood donors we tested suggesting that HEV exposure and infection is widespread in the US population. Most recipients in our study received blood from at least 5 donations enhancing the possibility that a patient would receive blood from a donor previously infected with HEV. Based on the average transfusion number and the 0.4% seroprevalence of IgM anti-HEV, recipients in this study would have had a 1%–2% chance of receiving blood from recently infected, IgM positive blood donor.
Two recipients (0.6%) among the 362 total recipients prospectively followed had an apparent anti-HEV IgG seroconversion suggesting transfusion-transmitted HEV infection. However, in one of these recipients, IgG anti-HEV was detected in only the last study sample at 36 weeks after his first within-study transfusion. No IgM antibody or HEV RNA was detected in post-transfusion samples obtained prior to the appearance of IgG anti-HEV. Investigation of linked donations to this repeatedly transfused recipient revealed that the patient was transfused with a strongly reactive anti-HEV positive donation just prior to the detection of anti-HEV in his last follow-up sample. Thus, the antibody detected in this recipient was passively transferred and not indicative of HEV infection followed by anti-HEV seroconversion. Interestingly, the patient also received an HEV RNA positive and an IgM anti-HEV positive blood unit four days before our last study sample. The patient died soon thereafter and thus we could not trace the outcome of HEV positive blood donations. However, this does demonstrate that HEV RNA positive donors exist in our donor population even though none were detected in the approximate 2000 donations tested in the donor sero-survey.
In the second recipient demonstrating suspected seroconversion, retrospective testing revealed that all his post-transfusion samples were weakly reactive for anti-HEV beginning with the 4 week post-transfusion sample (Fig. 3). No sample was IgM anti-HEV or HEV RNA positive. Linked donations did not reveal an HEV infected donor, but some donations were not available for testing. Further examination of this patient’s pre-transfusion sample showed that although it fell below the cut-off of the assay and hence was interpreted as anti-HEV negative, in fact, the OD reading was just below the cut-off and might have represented the onset of anti-HEV seroconversion which then increased incrementally throughout follow-up. This scenario is compatible with an HEV exposure that occurred prior to the index transfusion in this study. We conclude from these two cases that neither patient was HEV infected as the result of blood transfusion administered within the time frame of the study. More importantly, we conclude that in the proper interpretation of apparent antibody seroconversions, it is vital to examine serial recipient samples and to have linked donor samples. Access to such samples is only possible in a prospective study design.
Overall, this study concludes that past exposure to HEV is common in the US donor population, that a small proportion of such donors have IgM antibody suggestive of recent exposure and that a very small proportion harbor HEV RNA. Thus, the potential for transfusion-transmitted HEV infection clearly exists and indeed has been confirmed in several case reports.11–13 Therefore the issue at hand is not whether HEV can be transmitted by transfusion, but rather whether the frequency of such transmission and the clinical consequences of the infection warrant donor screening for this agent? HEV fulfills the essential criteria for an agent that might justify blood donor screening in that it can be found in asymptomatic individuals, it has been proven to be transmitted by transfusion and it can cause significant disease in immuno-compromised patients who now constitute a large segment of hospitalized blood recipients. What we do not know is the magnitude of the problem and the likelihood that an asymptomatic HEV carrier would present to a donor center and be otherwise eligible to donate. Although prolonged asymptomatic viremia has been observed in immuno-compromised individuals, it has not been documented in healthy blood donors. We know from studies of other blood transmitted agents that it is the duration of asymptomatic viremia that best defines the transfusion risk. Agents such as hepatitis A virus that have an extremely brief duration of asymptomatic viremia pose little risk whereas HBV and HCV, which have prolonged periods of asymptomatic viremia, pose major threats and necessitate donor screening by both serology and nucleic acid testing.
Although we did not detect any transfusion associated HEV infections among 362 prospectively followed recipients in our study, calculation of the upward bound for zero observations indicates that HEV transmission could have occurred in up to 0.8% of recipients. Further, the finding of IgM anti-HEV in the absence of HEV RNA in 0.4% of donors, allows for the possibility that these donors could have been infectious had they donated days to weeks earlier when they might have been HEV RNA positive. In addition, although we did not find an HEV RNA positive donor in a sero-survey of 1939 donors, we did detect one HEV RNA positive donor during investigation of an apparent anti-HEV seroconversion in a recipient. Thus, it is probable that HEV positive US blood donors will be detected when much larger sero-surveys are performed. The most immediate need for the study of HEV and blood transfusion is to test tens of thousands of donors for HEV RNA and then to follow those found positive to determine the duration of viremia. If few RNA positive donors are identified, as in our study, and if the duration of viremia in immune-competent individuals is very short, then routine donor screening will not be necessary. In contrast, if the occurrence of viremia is frequent and the duration of viremia prolonged, then donor screening would be indicated since the disease consequences in those recipients who are immune-suppressed and in those with pre-existing liver diseases can be dire.
Acknowledgments
This study was supported by grant R01 HL67229 from The National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) and by the NIH Clinical Center Intramural Program, TRIPS Protocol 01-CC-0231.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 2.Labrique AB, Thomas DL, Stoszek SK, Nelson KE. Hepatitis E: an emerging infectious disease. Epidemiol Rev. 1999;21:162–79. doi: 10.1093/oxfordjournals.epirev.a017994. [DOI] [PubMed] [Google Scholar]
- 3.Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. doi: 10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 4.Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, Farrington L, Hamad N, Sieberhagen C, Ellis V, Mitchell J, Hussaini SH, Banks M, Ijaz S, Bendall RP. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–90. doi: 10.1097/MEG.0b013e3282f5195a. [DOI] [PubMed] [Google Scholar]
- 5.Mansuy JM, Bendall R, Legrand-Abravanel F, Saune K, Miedouge M, Ellis V, Rech H, Destruel F, Kamar N, Dalton HR, Izopet J. Hepatitis E virus antibodies in blood donors, France. Emerg Infect Dis. 2011;17:2309–12. doi: 10.3201/eid1712.110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo QS, Yan Q, Xiong JH, Ge SX, Shih JW, Ng MH, Zhang J, Xia NS. Prevalence of hepatitis E virus in Chinese blood donors. J Clin Microbiol. 2010;48:317–8. doi: 10.1128/JCM.01466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baylis SA, Gartner T, Nick S, Ovemyr J, Blumel J. Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sang. 2012;103:89–90. doi: 10.1111/j.1423-0410.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 8.Barnaud E, Rogee S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol. 2012;78:5153–9. doi: 10.1128/AEM.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–34. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 10.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–88. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 11.Boxall E, Herborn A, Kochethu G, Pratt G, Adams D, Ijaz S, Teo CG. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsubayashi K, Kang JH, Sakata H, Takahashi K, Shindo M, Kato M, Sato S, Kato T, Nishimori H, Tsuji K, Maguchi H, Yoshida J, Maekubo H, Mishiro S, Ikeda H. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion. 2008;48:1368–75. doi: 10.1111/j.1537-2995.2008.01722.x. [DOI] [PubMed] [Google Scholar]
- 13.Tamura A, Shimizu YK, Tanaka T, Kuroda K, Arakawa Y, Takahashi K, Mishiro S, Shimizu K, Moriyama M. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol Res. 2007;37:113–20. doi: 10.1111/j.1872-034X.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 14.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–7. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 15.Ollier L, Tieulie N, Sanderson F, Heudier P, Giordanengo V, Fuzibet JG, Nicand E. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med. 2009;150:430–1. doi: 10.7326/0003-4819-150-6-200903170-00026. [DOI] [PubMed] [Google Scholar]
- 16.Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmberg SD. Hepatitits E in the United States. http://www3.niddk.nih.gov/fund/other/HepE2012/HepatitisE2012ProgramBook.pdf.
- 18.Dalton H. Diagnostic Assays for HEV. http://www3.niddk.nih.gov/fund/other/HepE2012/HepatitisE2012ProgramBook.pdf.
- 19.Zhang J, Ge SX, Huang GY, Li SW, He ZQ, Wang YB, Zheng YJ, Gu Y, Ng MH, Xia NS. Evaluation of antibody-based and nucleic acid-based assays for diagnosis of hepatitis E virus infection in a rhesus monkey model. J Med Virol. 2003;71:518–26. doi: 10.1002/jmv.10523. [DOI] [PubMed] [Google Scholar]
- 20.Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Gu Y, Ge SX, Li SW, He ZQ, Huang GY, Zhuang H, Ng MH, Xia NS. Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine. 2005;23:2881–92. doi: 10.1016/j.vaccine.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA. 1983;249:1743–5. [PubMed] [Google Scholar]
- 23.Lilienfeld D, Stolley P. Foundations of Epidemiology. 3. New York: Oxford University Press; 1994. [Google Scholar]
- 24.Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 25.Park HK, Jeong SH, Kim JW, Woo BH, Lee DH, Kim HY, Ahn S. Seroprevalence of anti-hepatitis E virus (HEV) in a Korean population: comparison of two commercial anti-HEV assays. BMC Infect Dis. 2012;12:142. doi: 10.1186/1471-2334-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]