Introduction
Autism is a complex and heterogeneous neurodevelopmental disorder with widely varied clinical characteristics and a multitude of possible etiologic features. A wide variety of neurobiological processes may fundamentally alter neural development in autism. This pervasive developmental disorder first manifests in early development and is characterized by deficits in three core domains: social interaction, communication or language use, and restricted or repetitive behaviors and interests. Social interaction deficits may be expressed through a variety of behaviors, including a lack of theory of mind, which is the ability to recognize that others have a point of view. Children may also resist touch and direct eye contact and often prefer to play alone rather than with others. Communication deficits include a lack of age-appropriate language development, with language use either underdeveloped or, in some cases, completely absent. When children with autism do develop language, it is often atypical and may include features such as a reliance on echolalia, or the repetition of words or phrases. In addition to social and communication difficulties, children with autism tend to exhibit a preference for sameness, which often manifests through a variety of restricted or repetitive behaviors or interests. Children with autism may react aversely to even small changes in routine and may engage in repetitive behaviors such as spinning, arm flapping, or arranging and rearranging objects or toy. Deficits in all three symptom domains are required for a diagnosis of autism, but individuals on the spectrum reflect varying degrees of abnormalities in each of the core functions. Given this complexity, investigators have recently referred to the broad array of deficits associated with these variations as “the autisms.”
The aim of this review is to present a novel way of studying “the autisms,” namely by deconstructing and refining the autism phenotype and identifying intermediate brain phenotypes that might offer plausible neural substrates for the three domains of deficits. After a brief introduction, we will review electrophysiological and neuroimaging methods for exploring the neural circuitry associated with two of the core domains of deficits in autism, namely the difficulties with social-emotional processing and the repetitive and restrictive behaviors. We will further indicate value of exploring interactions between these previously individually studied domains of deficit. We will then examine recent evidence of aberrant neural connectivity in autism. Finally we will discuss new directions in studying the neurobiological underpinnings of autism through familial studies of high-risk individuals, and parents of children with autism expressing the broad autism phenotype (Adolphs et al 2008; Losh et al 2009).
I. The Autisms: a constellation of complex and domain-clustered deficits
Autism is a syndrome, not an etiologically defined disorder. The term autism spectrum disorder (ASD) encompasses a wide variability of clinical presentation, ranging from individuals who are extremely high-functioning to individuals who are severely affected. Furthermore, autism spectrum disorders have been associated with numerous possible etiological features (genetic and environmental heterogeneity) and a broad clinical or syndromic heterogeneity.
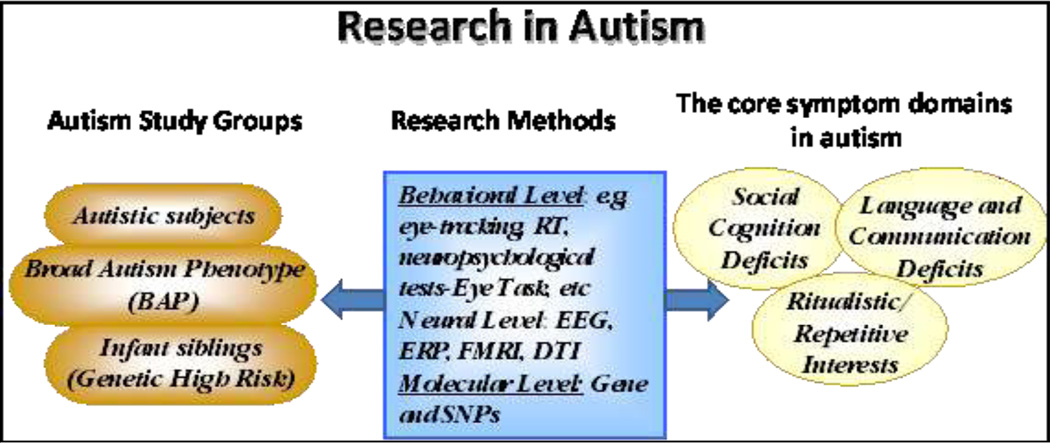
While the etiology of autism is thought to be highly heterogeneous, it is know that genetics confer a significant risk for autism, with typical estimates ranging between 70% and 90%. Genetic mutations causing autism can be identified in about 10%–20% of cases using current methods, many of which detect copy number variants (CNVs). Despite the genetic heterogeneity, a recent review of the literature reveals that a number of these mutations converge on a common neurodevelopmental pathway involved in neurogenesis, axon guidance, and synapse formation, all of which are critical for proper neural connectivity (Geschwind 2009). (Figure 1). Despite the recent advances in understanding the genetic factors that may be contributing to autism, the specific relationship between the etiologies, mechanisms and the ensuing behavioral abnormalities remains unclear.
Figure1.
A schematic illustration of research in autism. EEG, electroencephelogram; ERP, event-related potentials; FMRI, functional magnetic resonance imaging; DTI, diffusion tensor imaging; SNP, single-nucleotide polymorphism
Consistent with a neurodevelopmental model of autism, the three core domains of deficits emerge early in childhood and become more apparent as early development proceeds. Early markers for ASD are present and detectable by twelve months of age. Social and communication deficits may be evident as early as the second to third year of life. By four to five years, restricted and repetitive behaviors increase. While one third of ASD individuals achieve some degree of independence, two thirds will require supervision throughout their lives, bringing the average estimated cost of ASD to society to three million per person over a lifetime (Ganz 2007).
While most studies on autism have focused on symptom clusters and phenomenology in young children, the expression of autism changes over time. Early in development, in the preschool age range (2–3), the most prominent deficits seem to emerge in the domains of social communication. Around age 4–5 stereotopies increase, and seem to be followed by onset of seizures in a subgroup on children. The adolescent phase appears predominantly marked by inappropriate social emotional responses and behaviors. In adulthood, approximately 1–2 % of individuals with autism live independently, 1/3 with some degree of independence and 2/3 requiring supervision.
Early diagnosis and intervention appear to be critical and are most effective during preschool years (Bryson et al 2003; Patten and Watson 2010). Unfortunately there are a number of diagnostic issues that interfere with early detection. First, as mentioned above, autism is a highly heterogeneous disorder. Second, the neurobiological underpinnings remain poorly understood, and hence restrict the ability to generate reliable biomarkers. Finally, the current methods of diagnosis rely on behavioral observation and hence depend upon the ability to detect subtle differences in complex behaviors that may not surface until later in development. As a result, many children are not diagnosed until they are 2–3 years of age, well after first symptoms appear.
II. Deconstructing the Neural Circuitry of Autism: Electrophysiological and Neuroimaging studies
Studies exploring the neural circuitry of autism have utilized various methodologies to gather converging evidence for the disruption of selective pathways and domains of information processing. Advances in the development of non-invasive methods enable the study of biological markers in individuals with autism from infants to adults, as well as first degree family members.
Electrophysiological Studies of the Neural Circuitry of Autism
Currently, the most effective method of examining real-time changes with good temporal resolution in the brain is the examination of event related potentials (ERPs), or voltage changes in the brain that are time-locked to a particular stimulus or response (Picton and Taylor 2007). ERP studies may examine normal or abnormal neurodevelopment, both of which are valuable in the understanding of ASD because a strong knowledge of normal ERP signatures is critical in recognizing abnormal ones. For this reason, longitudinal studies of “high risk” participants may be particularly effective.
Deficits in communication and language development lend themselves to investigation with ERP studies. Speech production and verbal communication necessitate the ability to distinguish and process minute changes in auditory stimuli, such as the difference between similar consonant sounds. Individuals with ASD often have difficulty processing novelty (Courchesne et al 1985) and also have greater difficulty processing auditory or verbal information than visual information (Duncan et al 2009).These challenges may account for the deficits in language production and communication seen in ASD. Therefore, studies that examine the components of auditory processing in ASD children may help to uncover the neurological nature of these language deficits.
Mismatch negativity (MMN), or the brain’s response to rare changes in a series of repetitive auditory stimuli (Naatanen and Escera 2000), provides an important avenue for investigating auditory processing in ASD individuals because it measures response to novelty. MMN is typically characterized as an unconscious component of the ERP that automatically detects differences between current stimuli and previously-detected stimuli (Duncan et al 2009). Findings of mismatch negativity paradigms suggest that ASD individuals have difficulty perceiving small changes in auditory stimuli, which may account for their subsequent language deficits. A number of studies (Gomot et al 2002; Oram Cardy et al 2005) found decreased MMN for vowel perception in ASD children compared to typically developing controls, and shortened MMN latencies to pitch change respectively. However, others (Ferri et al 2003) reported larger MMN for pitch-deviant tones in ASD children affected with mental retardation compared to typically developing controls, indicating a difference between the experience of MMN in ASD children and that of ASD children with mental retardation. Yet others (Ceponiene et al 2003) have reported normal MMNs to simple tones in individuals with autism compared to age matched controls, while others (Dunn et al 2008) found smaller MMN amplitudes. These inconsistent findings most likely are due to heterogeneity of the subjects as well as paradigms used across studies.
While MMN is considered an early sensory change detection component, the P300, a large positive waveform occurring about 300 milliseconds after the onset of a rare, task-relevant stimulus is an accepted indicator of attention orienting and higher order processing. A variation of the P300 waveform, the P3a occurs in response to task-irrelevant stimuli and is typically slightly earlier in latency at around 250–300 ms after stimulus onset (Dunn et al 2008). When utilizing auditory target stimuli, ASD children’s P300s are generally smaller in amplitude and later in latency compared to typically-developing children (Courchesne et al 1989; Kemner et al 1999; Oades et al 1988) potentially indicating a delay in neural information transmission. Reports of enlarged P3as in young children with autism but smaller P3as in older children with autism (Ferri et al 2003) further support the observation that age effects contribute significantly to both normal and abnormal development.
While the MMN and the P300 are used as indices for early and late attention orienting and target detection, other ERP components have been used to index the neural substrates of social cognitive and emotional processing in autism. For example, the ability to process faces is an important evolutionary mechanism that allows humans to interact optimally with their environment and respond appropriately. Because faces are salient cues that tell individuals about people they encounter in their environment and how to respond to those people, it is evolutionarily beneficial to distinguish between faces and objects, to recognize familiar faces as compared to unfamiliar faces, and to determine the emotional state of a particular face. This ability develops in early infancy and is frequently impaired in individuals with ASD (Dawson and Zanolli 2003)
As in auditory information processing, face processing has been associated both with early evoked potential indices of sensory processing (N290, P100, N170), as well as later cognitive categorization (e.g. P400). Typically developing individuals automatically distinguish faces from objects, generating N170 component specific to faces. They further generate N290 components specific to familiar faces, relative to unfamiliar faces. This ability to distinguish faces form objects, and familiar and unfamiliar faces, greatly increases success at social interaction and is often impaired in individuals with ASD. Adolescents and adults with autism show longer N170 latencies to faces than controls but respond to objects on par with controls. Adults with autism also show no difference in N170 when a face is inverted, whereas there is a clear “face-inversion” effect in typically developing participants (McPartland et al 2004). Studies have also reported that infants at a genetic high risk for ASD show faster responses to objects than faces,comparable to low risk controls, as measured through both the N290 latency and the P400 latency. These findings may reflect disruptions in the neural circuitry of facial processing. Low risk infants showed asymmetry across hemispheres at P100, N290 and P400, but high risk infants did not (McCleery et al 2009). This lack of distinct responses to faces versus objects suggests an impaired ability to recognize faces as a special stimulus. Another study found that typically developing children show differences in the P300 waveforms in response to familiar, unfamiliar and their own faces. However, children with autism do not (Gunji et al 2009). While typically developing children exhibit differences in the P400 waveforms in response to their mother’s faces versus an unfamiliar face, children with autism do not. However, children with autism respond identically to typically-developing children when presented with a favorite toy versus an unfamiliar one (Dawson et al 2002). These studies suggest that children with autism have specific deficits related to distinguishing between familiar and unfamiliar faces, but these abilities do not differ from controls with respect to recognizing and responding to objects.
Determining the emotional constitution and salience of a face allows individuals to respond appropriately to social interaction, but evidence suggests that individuals with ASD have difficulty with this ability. One of the most salient indicators of emotional state besides facial expression is direction of eye gaze and children with autism have exhibited deficits in utilizing information from eye gaze. For example, typically developing children exhibited more pronounced N170s when gaze direction was congruent with emotion (fearful/averted gaze, angry/direct gaze, etc) but children with ASD did not, suggesting impairment in correlating type of gaze with emotional content of a face (Akechi et al 2010). One study found that children with autism ranging in age form 42 to 87 months had eye gaze patterns equivalent to that of four-month-old infants, whereas typically developing children displayed eye gaze patterns equivalent to that seen in adults (Grice et al 2005)
In addition to difficulties utilizing eye gaze to infer the mental state of others, children with autism also exhibit a general decrease in ERP response to face detection, processing, and determination of emotional states such as fear, anger or sadness (Wong et al 2008). Typically developing children show larger N300s in response to fearful than to neutral faces, while children with autism do not (Dawson et al 2004). A lack of distinction in neural response to emotional versus neutral faces could account for the difficulties in facial processing observed in autism.
Many ERP studies of facial processing in ASD yield inconsistent results. For example, many of the studies described above noted differences in ERPs in response to facial processing. However, another study (Webb et al 2010) found that adults with autism perform poorly on facial recognition tests, but the waveforms associated with early facial processing and identification of faces are not significantly different from controls. Difficulty in researching ASD lies in the heterogeneity of abnormalities, but differences in IQ may underlie the known differences in ERP response. Children with autism scoring low on IQ tests exhibited longer P3a latencies and smaller P3b amplitudes, waveforms associated with novelty detection. However, children with autism scoring high on IQ tests performed similarly to typically developing children (Salmond et al 2007). This suggests that, at least on some measures, IQ may explain some of the heterogeneity observed in ERP studies.
While current research has progressed significantly toward understanding the neurological components of social and auditory deficits in ASD, research has yet to uncover a reliable ERP signature for ASD. Further study is necessary to continue to define abnormal auditory processing in ASD.
Neuroimaging Studies of the Neural Circuitry of Autism
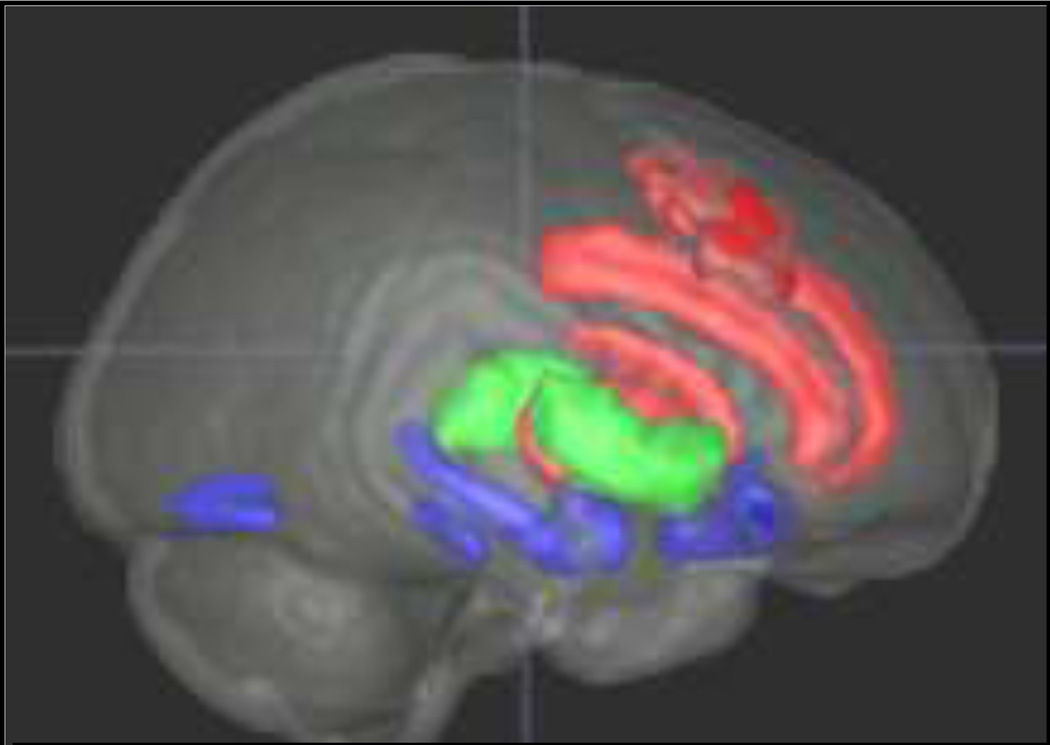
While electrophysiological studies enable the measure of neural responses with high temporal resolution, functional magnetic resonance imaging studies allow for the examination of neural networks associated with specific domains of processing deficits with great anatomical spatial resolution. Figure 3 depicts a schematic representation of brain regions that have been postulated to be involved in the mediation of the three core behaviors impaired in autism. For the purpose of this review, we will focus on regions implicated in the first two domains. Regions in red depict sub-nodes associated with repetitive restrictive behaviors, including the anterior cingulate, the dorsolateral prefrontal cortex, the caudate and the dorsal striatum. We refer to these areas as the dorsal executive control system (DECS). Regions in blue highlight subnodes associated with social-affective processing and include the amygdala-hippocampus complex, fusiform gyrus, and orbitofrontal cortex, which form the ventral social-affective processing system (VSAPS). Areas in green represent subcortical nodes, such as the thalamus and basal ganglia that are heavily engaged by both processing systems.
Figure 3.
Dorsal and ventral regions implicated in executive attention (red) and social-affective processing (blue). Green regions interface these circuits.
Studies of Executive functions, restrictive/repetitive behaviors, and Dorsal Pathway abnormalitie s in autism
Regions in the frontal and parietal cortices, which make up the DECS network, are involved in a number of cognitive operations, including planning, working memory, impulse control, inhibition, and set-shifting. These cognitive domains are often referred to under the umbrella term of “executive functions,” which broadly refers to the set of processes that are employed when an individual is involved in a goal-directed activity. Damage to the frontal cortex, which is considered the “seat” of executive functioning, interrupts the ability of individuals to complete many goal-directed tasks and has been shown to result in the emergence of perseverative and repetitive behaviors, insistence for sameness, and impulsivity, all of which are clinical manifestations of autism spectrum disorders (Hill 2004a; Hill 2004b; Ozonoff 1995; Russo et al 2007; Schmitz et al 2006). The striking similarity between the behavioral profiles of individuals with autism and individuals with damage to the frontal lobes has led many researchers to investigate whether individuals with autism exhibit deficits on various tasks of executive function. This research has shown that, much like the clinical manifestation of autism, the profile of executive capabilities seen in individuals with autism is highly heterogeneous in that it is characterized by deficits on some measures of executive function, such as cognitive flexibility, planning, and prepotent inhibition, and yet there are “islets of ability” in other measures including rote memory and interference inhibition (Eigsti and Shapiro 2003).
While there is clear evidence that individuals with autism do exhibit deficits in some domains of executive function, it is believed that these deficits are secondary to the disorder, meaning that they are not a cause of autism, but instead may be a consequence of having the disorder and as such they develop over time (Yerys et al 2007). While the executive deficits may not be a primary cause of the disorder, they have been shown to be linked to the restricted and repetitive behaviors that are a primary characteristic of autism (Lopez et al 2005; Rinehart et al 2002; South et al 2007). In particular, it has been shown that impairments in inhibitory control and cognitive flexibility may be associated with the perseverative behaviors and obsessionality that is emblematic of autism (Schmitz et al 2006).
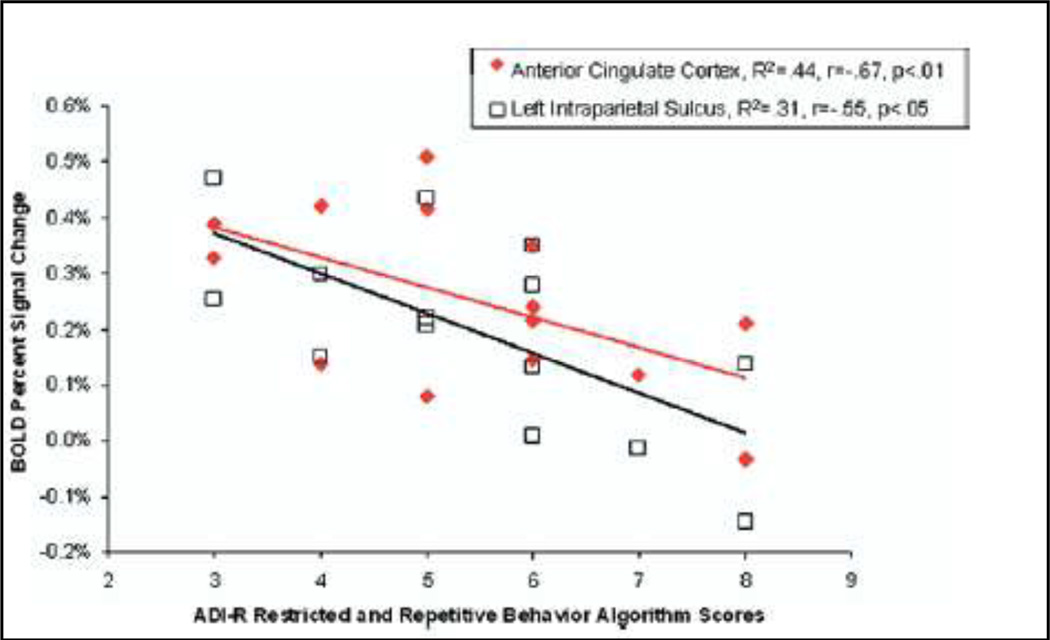
Several studies have set out to identify the neural circuitry associated with executive deficits to gain a better understanding of the neurobiological underpinnings of restricted and repetitive behaviors. For example, it has been reported that the cognitive inflexibility and perseverative behaviors especially prevalent in individuals with autism may be linked to a difficulty with disengaging from previous task demands after a paradigm shift. This has been shown to be related to an uncoupling of the inhibition network, comprised of the cingulate gyri and the insula, from the frontal-parietal processes in individuals with high-functioning autism (Kana et al 2007). In addition, it has been shown that when cognitive set-shifting abilities are explored in individuals with autism, the autism group tends to make more errors and has slower reaction times than the typically developing participants. This behavioral finding is coupled with hypoactivation in the executive circuitry of the individuals with autism, specifically in the dorsal lateral prefrontal cortex, anterior cingulate gyrus, intraparietal sulcus, thalamus, and basal ganglia. Moreover, correlational analyses reveal that the hypoactivation in the anterior cingulate gyrus and left intraparietal sulcus is significantly negatively correlated with the clinical manifestation of restricted and repetitive behaviors as measured in the ADI-R (Shafritz et al 2008) (Figure 4).
Figure 4.
Association between cingulated and parietal actovation and RRBs Adapted form Shafritz, 2008 (Shafritz et al 2008)
While studies of cognitive set-shifting suggest that the dorsal network of individuals with autism is hypoactive, studies investigating inhibitory control, have reported hyperactivation of this network in autism as compared to typically developing participants. This hyperactivation is seen in the frontal cortex, insula, anterior cingulate, and the inferior parietal cortex. It is not yet known whether increased activity in these brain regions is due to inefficient recruitment of the dorsal neural network, or whether it reflects compensatory changes associated with their reliance on alternative cognitive strategies to complete the tasks (Schmitz et al 2006).
In addition to alterations in focal regions within the dorsal network, recent research has demonstrated that there are circuit-level alterations in this system using functional connectivity analyses, which are measures of the level of synchronization between separate brain regions within a broader neural network. Utilizing this method, multiple studies have shown that there is decreased functional connectivity between frontal brain regions and areas in the parietal and temporal cortex in individuals with autism. For example, decreased functional connectivity between regions associated with language processing, specifically Broca’s and Wernicke’s areas, and regions associated with integration of information, such as the dorsal lateral prefrontal cortex has been reported in individuals with autism (Just et al 2004). Similar findings have been reported for the connectivity between frontal and parietal regions on a task of sentence imagery (Kana et al 2006), as well as on the Tower of London task, which is a task of executive function (Just et al 2007).
In summary, research suggests that the dorsal network in individuals with autism functions differently than that of typically developing individuals. Additionally, alterations in these frontal-parietal and frontal-striate systems appear to be associated with secondary deficits in executive functions and have been linked to the restrictive and repetitive behaviors, insistence for sameness, and impulsivity that are characteristic of autism.
Studies of Social-affective processing Deficits and Ventral pathway abnormalities in autism
Abnormalities in social-affective processing are a striking feature of autism. As previously reviewed, children with autism have difficulty identifying facial expressions of emotion (Adolphs et al 2001a; Pelphrey et al 2002) such as fear and anger. There is evidence that these difficulties are associated with differences in the way individuals with autism scan faces as compared to typically developing individuals. Individuals with autism have been reported to spend significantly less time looking at the eyes and more time on a speaker’s mouth or body (Klin et al 2002a; Pelphrey et al 2002). Whereas the behavioral and cognitive characteristics of impaired social cognition have been extensively documented, much less is known about the neural basis of social perception dysfunction in autism. Key brain regions involved in aspects of social perception and cognition, including the amygdala, the fusiform gyrus, and the orbito-frontal cortex, have now been identified as critical components of the VSAPS in healthy controls and have been shown to be differentially activated in individuals with autism.
One of the primary nodes within the VSAPS network is the amygdala (AMY), which plays a critical role in the detection of threat and mobilization of appropriate behavioral responses (Adolphs et al 1994; Amaral et al 2003; Amaral and Corbett 2003; Anderson and Phelps 2000; Anderson et al 2000; Breiter et al 1996; Calder et al 1996; Emery et al 1999; Emery et al 2001; Kling et al 1992; Kluver and Bucy 1937; Morris et al 1996; Rosvold et al 1954; Whalen et al 2001; Young et al 1996),,and is also critical for recognizing social emotions from faces (Adolphs 2010). Recent post-mortem, structural, and functional imaging studies(Bauman and Kemper 1985; Pelphrey et al 2004; Schumann et al 2004) have suggested AMY dysfunction in autism, mostly reflecting reduced activation of the AMY (Baron-Cohen et al 1997; Baron-Cohen et al 1999; Critchley et al 2000; Wang et al 2004) In contrast, other studies have reported increased activation of the AMY in response to viewing facial photographs (Dalton et al 2005b) and have attributed this pattern to the heightened emotional response elicited by gaze fixation in autism. Although these studies provide evidence that the functioning of the AMY is abnormal in autism during social perception, the direction, magnitude, and meaning of the pathological findings is unclear.
Neuropsychological studies reporting impairments in social and emotional judgments from faces in high-functioning individuals with autism (HFAs) and in their parents (Spezio et al 2005) are remarkably similar to those of individuals with AMY lesions (Adolphs et al 2001b; Losh et al 2009). New evidence has revealed a strong positive correlation between the level of AMY activity and gaze fixation upon the eye region in individuals with and without autism (Dalton et al 2005b). It is unclear, however, whether this correlation reflects a role for the AMY in directing the visual system to seek out and attend to the eyes as a socially important stimulus or, conversely, a contribution of abnormal scan-paths in autism and AMY lesion individuals (Adolphs et al 2005; Klin et al 2002b; Pelphrey et al 2002).
Aberrant AMY function in response to emotional faces has recently been linked social anxiety in autism spectrum disorders (Kleinhans et al 2010a). Recent studies investigated whether abnormal habituation characterizes amygdala dysfunction in autism spectrum disorders and whether the rate of amygdala habituation is related to social impairment (Kleinhans et al 2009). Measures of change over time in activation of the amygdala and fusiform gyrus to neutral facial stimuli in adults with autism spectrum disorders and healthy comparison adults revealed no group differences in overall fusiform habituation. In contrast, the comparison group evidenced significantly greater amygdala habituation bilaterally than the autism spectrum group. In addition, lower levels of habituation of the amygdala to the face stimuli in individuals with autism were associated with more severe social impairment. These results suggest that amygdala hyperarousal in autism spectrum disorders in response to socially relevant stimuli may contribute to the social deficits observed in autism spectrum disorders. Finally, evidence is also accumulating suggesting that the fusiform-amygdala system may play a critical role in the emergence of the pathophysiology of autism (Dziobek et al 2010).
Structural, morphologic, and cytoarchitectural abnormalities of the AMY in autism have also been reported. While some researchers showed a bilateral increase in AMY size (Howard et al 2000; Hrdlicka et al 2005; Sparks et al 2002), others reported reduced or normal AMY volume (Haznedar et al 2000; Herbert et al 2003; Rojas et al 2004; Schumann et al 2004). Recent findings (Hazlett et al 2010) replicate the AMY enlargement observed in autistic subjects compared to controls, beyond that explained by total brain enlargement.
The fusiform gyrus (FG) is another region that is critically involved in social processing. Functional imaging and electrophysiological studies have documented the importance of the FG in processing social stimuli such as faces (Allison et al 1994; Kanwisher et al 1997; Kanwisher and Yovel 2006; McCarthy and Gazzaniga 1999; McCarthy et al 1997; Puce et al 1996; Puce et al 1999) and individual facial features such as the eyes (Haxby et al 2000; McCarthy and Gazzaniga 1999). Behavioral studies of autism have consistently demonstrated face processing deficits (Hobson et al 1988; Loveland et al 1997; Schultz et al 2000), although the specific mechanisms underlying these deficits remain elusive. Although three studies have replicated findings of aberrant FG hypoactivation (Critchley et al 2000; Hubl et al 2003; Pierce et al 2001), recent data indicates activation in the FG is strongly correlated with gaze fixation on the eyes (Dalton et al 2005b). This finding raised the possibility that abnormal scanpaths while viewing faces in autistic individuals (Dalton et al 2005b; Klin et al 2002b; Pelphrey et al 2002) may account for the observed hypoactivation of both the FG and the AMY. Autonomic hyper-activity caused by dysregulation of affective processes in the AMY in response to salient social stimuli, such as eyes, may explain this aberrant AMY-FG interaction. Recent evidence supports autonomic hyper-activity in response to facial stimuli with direct eye gaze in autism (Kylliainen and Hietanen 2006).
Recent studies have further supported the proposition of abnormal development along face-processing pathways in autism (Kleinhans et al 2010b; Scherf et al 2010). Aberrant activation in cortical and subcortical regions involved in face processing have been reported, including the left amygdala, bilateral fusiform gyrus, right pulvinar, and bilateral superior colliculi. Thus while the basic rapid face processing modules appear to be functional in ASD, individuals with ASD failed to engage the subcortical brain regions involved in face detection and automatic emotional face processing, suggesting a core mechanism for impaired socioemotional processing in ASD. Thus, aberrant neurodevelopment along these pathways may contribute to early-emerging deficits in social orienting and attention, the putative precursors to abnormalities in social cognition and cortical face processing specialization (Kleinhans et al 2010b).
Anomalous functioning of a third VSAP region, the orbito-frontal cortex (OFC), has also been identified as a critical contributor to social and emotional cognition deficits in individuals with autism. (Baron-Cohen et al 1994). An early postmortem study reported cellular abnormalities not only in AMY but also in orbito-frontal regions (Hof et al 1991). Furthermore, bilateral damage to orbito-frontal regions also yields significant social cognitive impairments and deficits in judging the intentions of others (Stone et al 1998). Most recently, impaired social cognition measures in HFA adults were associated with significantly reduced activation of orbito-frontal regions (Dalton et al 2005b), lending further support to the critical contribution of orbito-frontal limbic connections in the successful implementation of social and emotional cognitive processes (Ashwin et al 2006) (Dalton et al 2005a; Morris et al 1996).
Interaction between executive/social-affective processing in autism
Though often studied separately, social processing and executive function (EF) are not isolated constructs in the natural environment. EF allows us to adapt our behavior and inhibit inappropriate responses within the context of an ever-changing social environment. As such, properly functioning EF processes are necessary for the development of socially appropriate behaviors (Jurado and Rosselli 2007). Similarly, social awareness may contribute to EF abilities, implicating strong interconnections between the two DECS and VSAPS neural circuits.
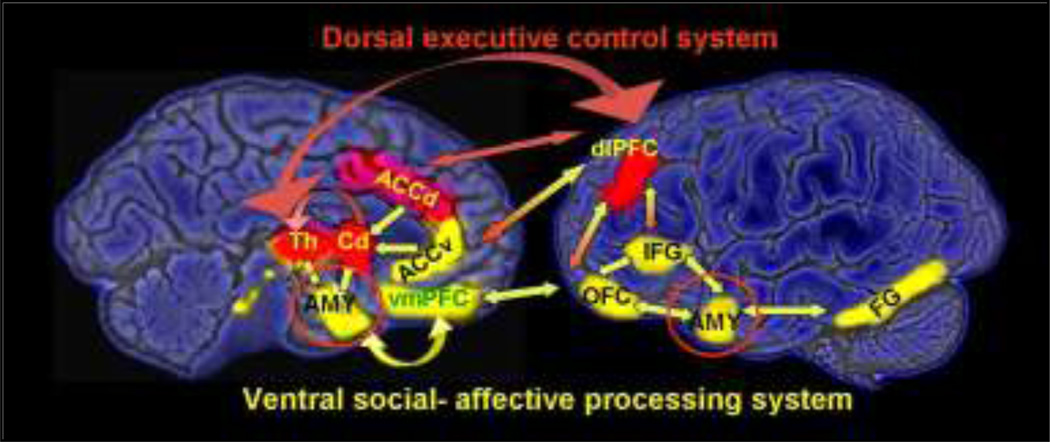
Several brain regions may be involved in mediating the interaction between EF and social processing (Figure 5). For example, the anterior cingulate cortex (ACC) may be an important brain region for the linkage of the ventral social cognitive network and the dorsal executive network possible related to their role in social orientation (Mundy 2003). The ACC is likely involved in deciding what response is appropriate with respect to incoming stimuli. Further, it has been shown that ACC ablation results in decreased social interactions due to its role in regulating affective behaviors (Bachevalier and Loveland 2006). The ACC is involved in emotional self-control, focused problem solving, error recognition, and cognitive flexibility. This is probably achieved through the widespread network between the ACC spindle cells and their ability to coordinate other brain regions (Allman et al 2001). The dorsal ACC is involved in cognition, and the ventral ACC is involved in emotion (Allman et al 2001).
Figure 5.
Schematic pathways of executive control (red) and social-affective processing (green). Red-yellow arrows indicate interaction nodes.
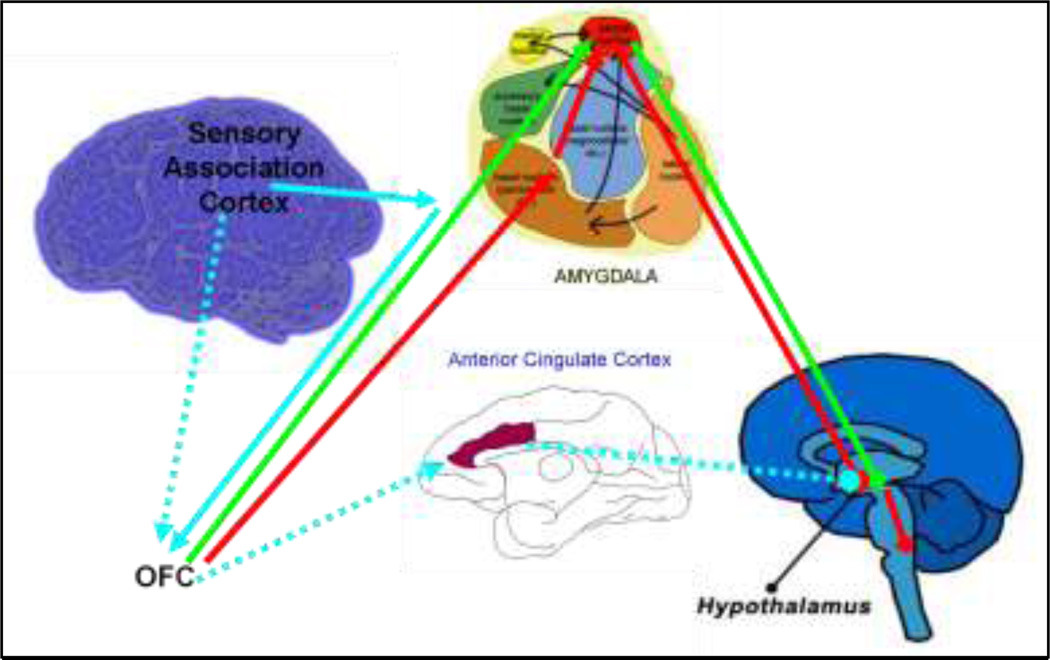
In addition to the ACC, the interconnections between the basolateral amygdala and the OFC may be associated both with the modulation of behavior in response to changes in social situations and with the formation of a set of expectation concerning reinforcers that help guide goal-directed behaviors (Bachevalier and Loveland 2006). Information from the sensory association cortex is sent to the amygdala, where its emotional significance is assessed. If the sensory information is deemed threatening (red pathway in Figure 6), projections from the posterior OFC activate inhibitory neurons in the intercalated masses of the amygdala. These neurons then inhibit activation of the central nucleus of the amygdala, disinhibiting the hypothalamus and beginning a cascade of responses including increased heart rate and respiration. If the sensory information is deemed non-threatening (green pathway Figure 6) projections from the OFC that innervate directly into the central nucleus of the amygdala activate the central nucleus, reinstating the inhibition of the hypothalamus and resulting in a return to homeostasis.
Figure 6.
Sensory information from the sensory association cortex is sent to the amygdala, where its emotional significance is assessed. If the sensory information is deemed threatening (red pathway), projections from the posterior OFC activate GABAergic neurons. Derived from Barbas 2007 (Barbas 2007).
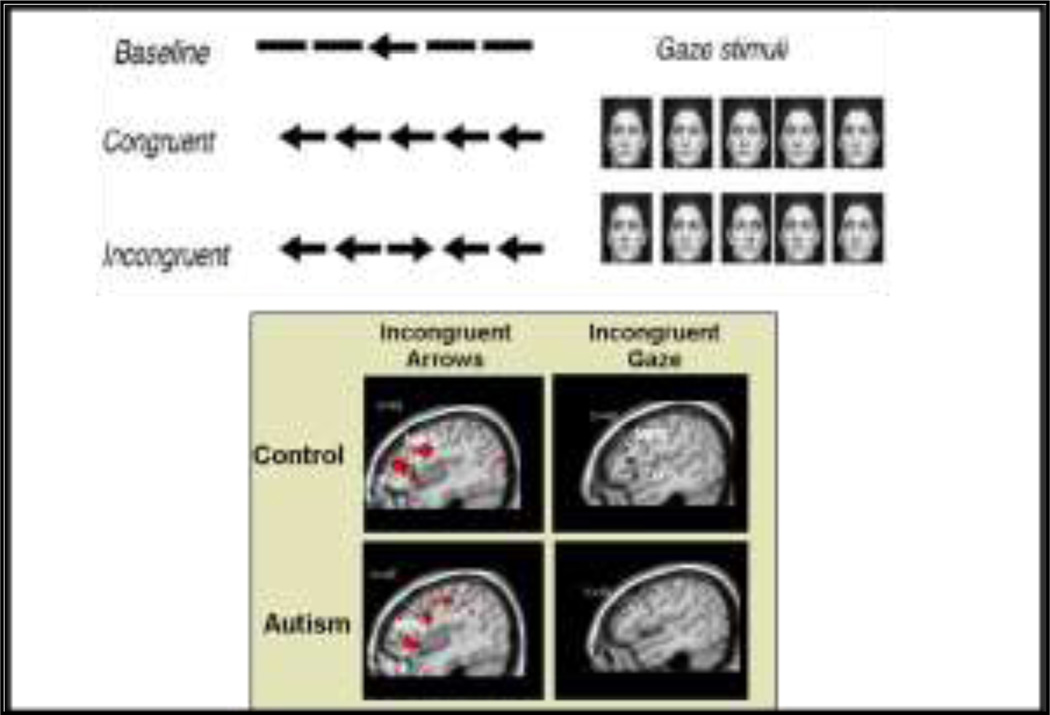
Recent fMRI research has examined the neural substrates of the interaction between EF and SC in individuals with ASD as compared to typically developing (TD) individuals (Figure 7). In this task, subjects identified the direction of a central arrow or gaze stimulus in the presence of flanker stimuli oriented in the same (“congruent”) or opposite (“incongruent”) direction (Dichter and Belger 2007). Behaviorally, the TD group made more errors to incongruent as opposed to congruent arrows, but performed similarly with both congruent and incongruent gaze stimuli. In contrast, the HFA group made more overall errors that were unrelated to arrow congruency, suggesting that they did not benefit from using gaze instead of arrows as in the TD group. Both the TD and HFA groups activated a similar EF neural network, comprised of the MFG, IFC, IPS, and the ACG, in response to incongruent versus congruent arrow stimuli. In TDs the incongruent gaze stimuli activated the same neural network as the incongruent arrow trials; however HFAs only activated the IPS. This, combined with the behavioral data, suggests that the presence of social stimuli may adversely impact cognitive flexibility and control in HFA (Dichter and Belger 2007).
Figure 7.
Frontal activation is attenuated by social context in individuals with Autism. Reprinted with permission from the American Journal of Psychiatry, (Copyright 2009).
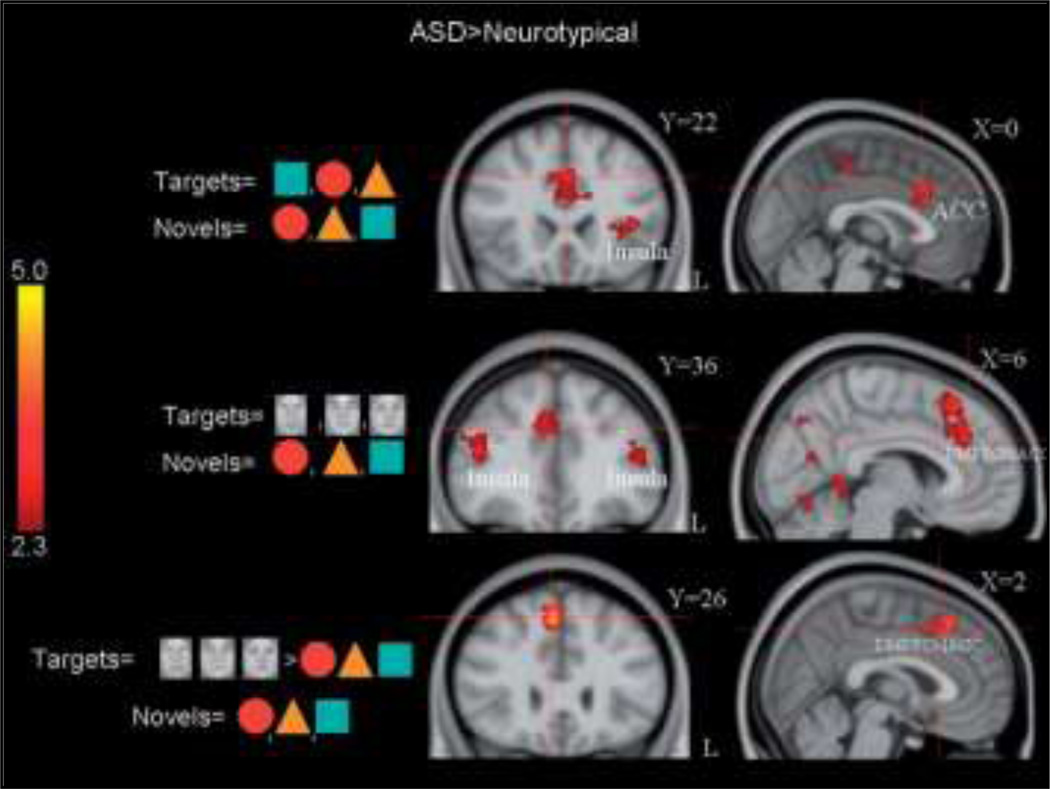
In a follow-up to the previous study, the same group investigated how socially relevant target stimuli were processed when presented alone (Dichter et al 2009). This study found that individuals with autism recruited the executive neural network to a greater extent than the TD group in response to both social and non-social target events (Figure 8). These findings contrast those from the previous study; however it may suggest that the hypoactivity in the previous study was associated with the presence of distracting flanker stimuli more than with the task stimuli.
Figure 8.
Cortical Activation Patterns to social and non-social targets in neurotypical and Autism Spectrum Disorder individuals. Adapted from Dichter et al., 2009.
Beyond localized deficits: Abnormal connectivity in autism
More recently numerous studies have proposed that autism is primarily a disconnection syndrome, associated with aberrant connections between networks of brain regions as opposed to more focal deficits in particular regions.
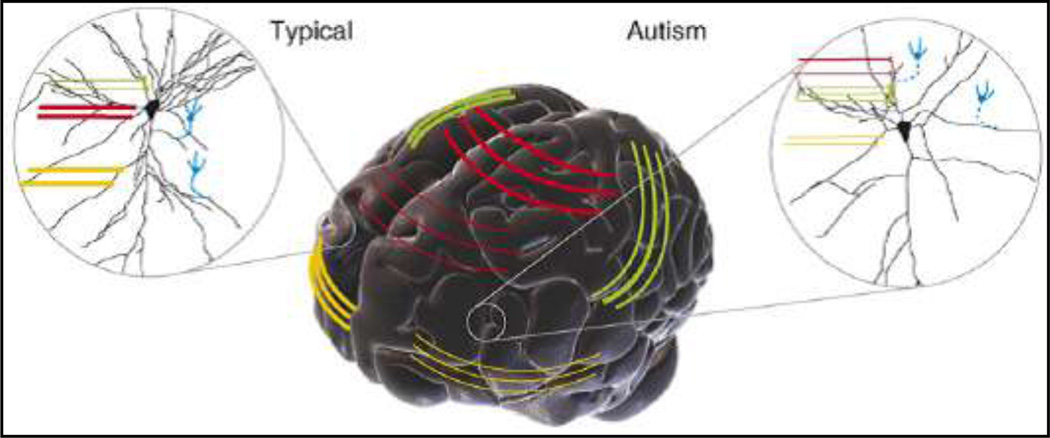
In a model proposed by Geshwind and Levitt (Geschwind and Levitt 2007), the key disconnection has been proposed to involve several frontal lobe and temporal lobe multi-modal higher-order association cortices (Figure 9). These may include frontotemporal, frontolimbic, frontoparietal and interhemispheric connection. As such, disconnection between cortical areas in the autisms can be heterogeneous and is represented by reduced size (illustrated by thinner lines) of certain callosal tracts (red) and frontotemporal connections (yellow). Over-connectivity (illustrated by thick green lines, Figure 9) between certain cortical areas might also lead to enhanced function in certain domains. At the level of local circuits (insets Figure 9), the effects of disruption to long-range inputs can also be influenced by altered inhibitory input (blue broken lines, Figure 9), which is essential for the proper maturation and stabilization of connectivity.
Figure 9.
Adapted from Geschwind and Levitt (Geschwind and Levitt 2007)
In support of the Geshwind and Levitt model, findings of aberrant limbic ventral fiber pathways in autism have been reported using diffusion tensor tracking studies. Conturo et al (Conturo et al 2008) reported abnormal microstructure in the individuals with autism in the hippocampo-fusiform and amygdalo-fusiform pathways, despite normal size and shape. Functional imaging studies have further provided evidence consistent with reduced functional and intrinsic connectivity in autism both during verbal processing (Just et al 2004) and during executive processing (Just et al 2007).
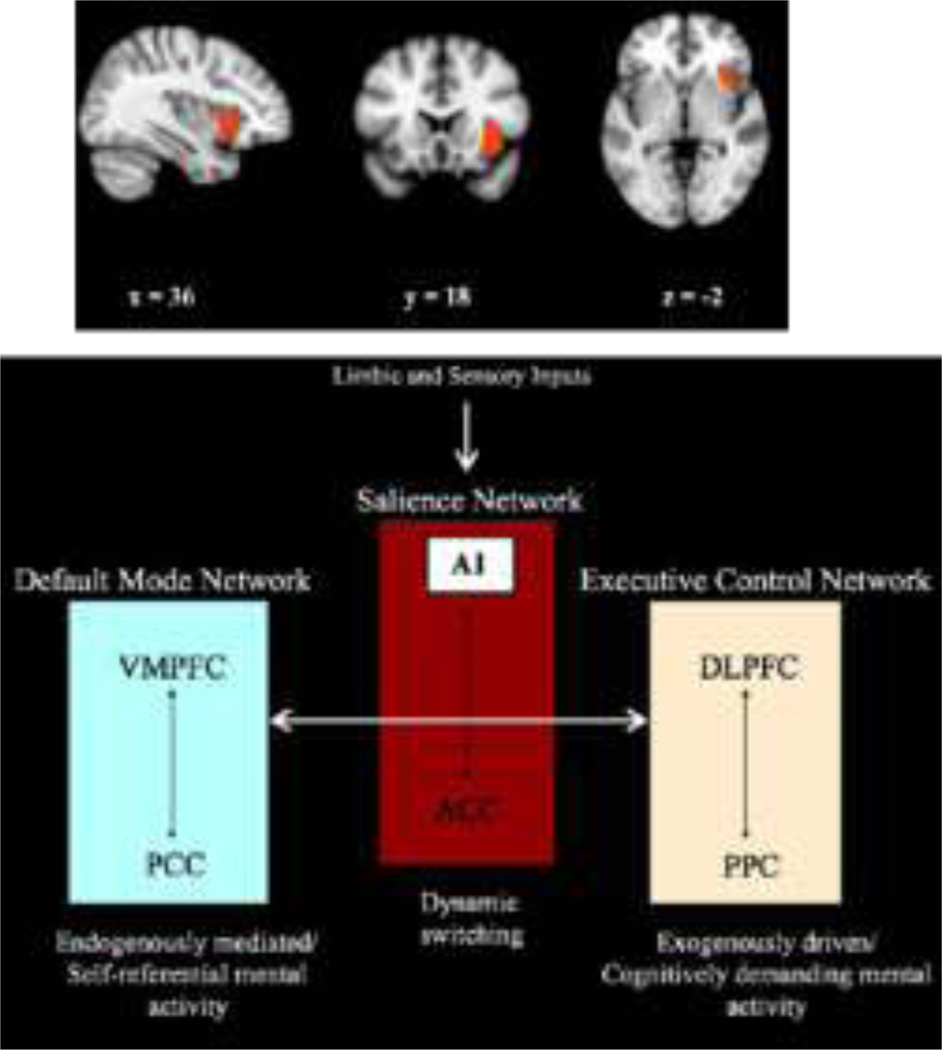
Recent studies have also drawn attention to the potentially critical role of the anterior insula in initiating dynamic switched between intrinsic connectivity states and task-dependent functional connectivity states. The anterior insula is proposed to play a critical role in a salience network. According to this model (Uddin et al 2009) limbic and sensory inputs may be inadequately processed by the anterior insula during social cognition in autism, leading to disruption of the anterior insula’s role in coordination of these large-scale brain networks (Figure 10).
Figure 10.
Representation of the role of the anterior Insula in task-adaptive response generation. Reprinted with permission from Uddin, 2009.
In sum, there is a mounting body of evidence suggesting that autism is associated with altered functional and structural connectivity patterns, most pointing to a pattern of under-connectivity between frontal and parietal and frontal and temporo-limbic regions. These findings have shifted thinking away from autism as a disorder of regional brain dysfunction to a model of autism as a large-scale neural network disorder.
III. Deconstructing the autism phenotype: Inherited phenotypic features in autism – new research directions
Although research into the biological and genetic basis of autism is in its early days, twin and family studies have provided strong evidence that autism is a highly heritable disorder (Bailey et al 1995; Folstein and Rosen-Sheidley 2001; Freitag 2007; Gupta and State 2007). The first observation of inherited traits in children with autism and their parents were reported by Leo Kanner, M.D. in 1943 (Kanner 1943), and further supported by observations by Leon Eisenberg, M.D. in 1957 (Kanner and Eisenberg 1957). While Kanner’s observations gave us the first clue to the heritability of autistic traits, twin studies and recent family studies have suggested that underlying genes that cause autism may also cause milder phenotypes. According to this proposition, the full autism syndrome may emerge if an individual has all the high-risk genes. In contrast, if an individual only has a subset of the genes, they may demonstrate some of the autism-associated deficits, such as poor social cognition.
The recent description of the Broad autism phenotype (BAP) support the tenet that features of autism can be present in unaffected family members at varying degrees of severity (Losh et al 2009; Losh et al 2008a; Losh and Piven 2007; Losh et al 2008b; Piven 2001; Piven 2002)]. Piven and colleagues (Piven et al 1997a; Piven et al 1997b) have conducted a systematic assessment of traits thought to be present in the BAP and have found that social aloof personality and rigid or perfectionistic personality are thought to parallel the social symptom as well as ritualistic and repetitive domains of autism, respectively. These traits did not appear when the BAP group was studied as a whole, but rather when the subjects were subdivided into the specific domain of deficits. In particular, parents of children with autism who had reliable evidence of the aloof personality (“BAP +” Group) appeared to show affective and social cognitive blunting akin to the deficits observed in those domains in individuals with autism (Adolphs et al 2008; Losh et al 2009). A recent BAP study (Adolphs et al 2008) examined face processing among three groups: parents of individuals with autism with the socially aloof personality (“BAP +”); parents of individuals with autism with a non-aloof personality (“BAP −”); and parents with a TD child (“Controls”). The results showed that the three groups differed in their face performance strategies. Both the BAP + group and the BAP − group showed less use of the eye region of the face matching compared to the control group. Moreover, BAP + group showed more use of the mouth region of the face than the BAP – group whereas the BAP − group made more use of the eyes than did BAP + group. These findings in parents of individuals with autism are consistent with the pattern of face processing (diminished use of the eye region and increased use of the mouth region) characteristic of individuals with autism.
Shared aberrant phenotype and neural circuitry associated with social and emotional processing have further been reported in studies examining pairs of adolescents with autism and their fathers. The recent FMRI study (Greimel et al 2010) has explored the neural correlates of empathy in adolescents with autism and their fathers. Both adolescents with autism and their fathers showed abnormal activation of the fusiform and amygdala. The results of such studies may identify neurobiological endophenotypes of autism and provide future directions for genetic studies.
The observation of overlapping behavioral, emotional and cognitive features between the parents with the BAP and individuals with the full autism spectrum further supports the role of genetics in the emergence of autism but mostly reflects the heritability of specific phenotypes. Indeed, vulnerability in specific neural pathways may disrupt the development of domain-specific functions, such as emotional, social, executive or language operations, which can selectively alter the severity of the deficits in core domains associated with autism. Using this approach, studies exploring the neural circuitry of autism can benefit from converging evidence from studies of individuals with autism, as well as first degree relatives of individuals with autism. Accordingly, these studies should focus on domains of dysfunction that can also be modeled in the BAP.
In summary, autism is a complex and heterogeneous syndrome with distinct endophenotypes. Both in autism and in the BAP, these endophenotypes are, at least in part, associated with altered affective/executive processing and abnormal interactions between ventral and dorsal pathways and critical neural nodes (OFC/AMY) along these pathways. Studies of the Broad Autism Phenotype may provide insights into neural components of the genetic liability to autism, and may provide tractable, quantitative phenotypes for genetic and neuroimaging studies.
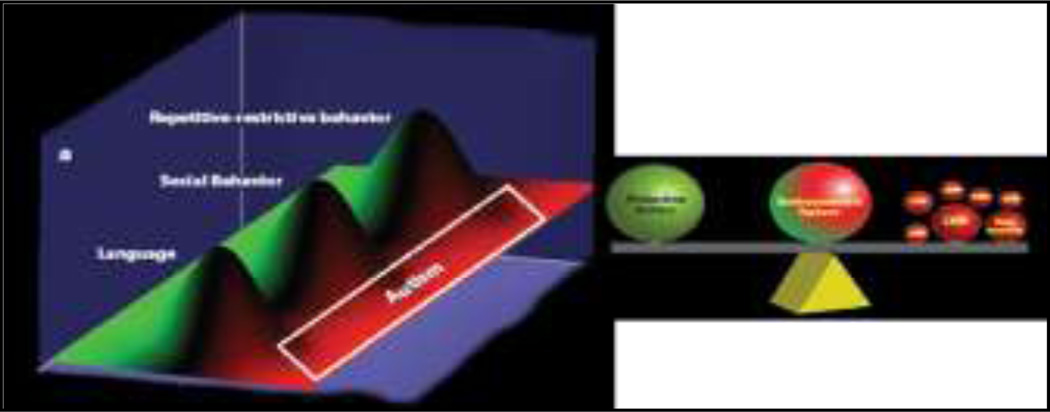
Figure 2.
Model of autisms: reproduced from Geschwind 2009 (Geschwind 2009)
Aknkowlegements
Preparation of this manuscript was supported by a NIMH Grants HD40127 and U54MH66418, NC TRACS Institute Research Funds – National Center for Research Awards # UL1RR025747, NICHD 2R01-HD042168-05A, and the UNC University Council Small Grants Program.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal Processing of Social Information from Faces in Autism. Journal of Cognitive Neuroscience. 2001a;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001b;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Spezio ML, Parlier M, Piven J. Distinct face-processing strategies in parents of autistic children. Curr Biol. 2008;18:1090–1093. doi: 10.1016/j.cub.2008.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Akechi H, Senju A, Kikuchi Y, Tojo Y, Osanai H, Hasegawa T. The effect of gaze direction on the processing of facial expressions in children with autism spectrum disorder: an ERP study. Neuropsychologia. 2010;48:2841–2851. doi: 10.1016/j.neuropsychologia.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. CerebCortex. 1994;4:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Corbett BA. The amygdala, autism and anxiety. Novartis Found Symp. 2003;251:177–187. discussion 187–197, 281–197. [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Expression without recognition: contributions of the human amygdala to emotional communication. Psychol Sci. 2000;11:106–111. doi: 10.1111/1467-9280.00224. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Spencer DD, Fulbright RK, Phelps EA. Contribution of the anteromedial temporal lobes to the evaluation of facial emotion. Neuropsychology. 2000;14:526–536. doi: 10.1037//0894-4105.14.4.526. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the 'social brain' during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2006 doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological medicine. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. Journal of anatomy. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. J Child Psychol Psychiatry. 1997;38:813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Clinical findings in children with autism and a functional neuroimaging study of normal adults. Br J Psychiatry. 1994;165:640–649. doi: 10.1192/bjp.165.5.640. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Rogers SJ, Fombonne E. Autism spectrum disorders: early detection, intervention, education, and psychopharmacological management. Can J Psychiatry. 2003;48:506–516. doi: 10.1177/070674370304800802. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL. Facial Emotion Recognition after Bilateral Amygdala Damage: Differentially Severe Impairment of Fear. Cognitive Neuropsychology. 1996;13:699–745. [Google Scholar]
- Ceponiene R, Lepistö T, Shestakova A, Vanhala R, Alku P, Näätänen R, et al. Speech–sound-selective auditory impairment in children with autism: They can perceive but do not attend. Proc Natl Acad Sci U S A. 2003;100:5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ. Neuronal fiber pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J Int Neuropsychol Soc. 2008;14:933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Lincoln AJ, Kilman BA, Galambos R. Event-related brain potential correlates of the processing of novel visual and auditory information in autism. Journal of autism and developmental disorders. 1985;15:55–76. doi: 10.1007/BF01837899. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Lincoln AJ, Yeung-Courchesne R, Elmasian R, Grillon C. Pathophysiologic findings in nonretarded autism and receptive developmental language disorder. Journal of autism and developmental disorders. 1989;19:1–17. doi: 10.1007/BF02212714. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. J Cogn Neurosci. 2005a;17:969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005b;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Dev Sci. 2004;7:340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Zanolli K. Early intervention and brain plasticity in autism. Novartis Found Symp. 2003;251:266–274. discussion 274–280, 281–297. [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. NeuroImage. 2007;35:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Social cognitive and affective neuroscience. 2009;4:215–226. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. Journal of autism and developmental disorders. 2008;38:52–71. doi: 10.1007/s10803-007-0359-3. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Bahnemann M, Convit A, Heekeren HR. The role of the fusiform-amygdala system in the pathophysiology of autism. Arch Gen Psychiatry. 2010;67:397–405. doi: 10.1001/archgenpsychiatry.2010.31. [DOI] [PubMed] [Google Scholar]
- Eigsti IM, Shapiro T. A systems neuroscience approach to autism: biological, cognitive, and clinical perspectives. Ment Retard Dev Disabil Res Rev. 2003;9:205–215. doi: 10.1002/mrdd.10081. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Amaral DG, Nadel RDLaL. Cognitive Neuroscience of Emotion. Oxford: Oxford University Press; 1999. The role of the amygdala in primate social cognition; pp. 156–191. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) BehavNeurosci. 2001;115:515–544. [PubMed] [Google Scholar]
- Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology. 2003;114:1671–1680. doi: 10.1016/s1388-2457(03)00153-6. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Molecular psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- Ganz ML. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med. 2007;161:343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annual review of medicine. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard MH, Adrien JL, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Greimel E, Schulte-Ruther M, Kircher T, Kamp-Becker I, Remschmidt H, Fink GR, et al. Neural mechanisms of empathy in adolescents with autism spectrum disorder and their fathers. NeuroImage. 2010;49:1055–1065. doi: 10.1016/j.neuroimage.2009.07.057. [DOI] [PubMed] [Google Scholar]
- Grice SJ, Halit H, Farroni T, Baron-Cohen S, Bolton P, Johnson MH. Neural correlates of eye-gaze detection in young children with autism. Cortex; a journal devoted to the study of the nervous system and behavior. 2005;41:342–353. doi: 10.1016/s0010-9452(08)70271-5. [DOI] [PubMed] [Google Scholar]
- Gunji A, Inagaki M, Inoue Y, Takeshima Y, Kaga M. Event-related potentials of self-face recognition in children with pervasive developmental disorders. Brain Dev. 2009;31:139–147. doi: 10.1016/j.braindev.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Gupta AR, State MW. Recent advances in the genetics of autism. Biological psychiatry. 2007;61:429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Mosconi M, Gerig G, Gimple RS, Piven J. Enlargement of Subcortical Structures in Two Year Olds with Autism: An MRI Study. 2010 [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. The American journal of psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Lange N, Bakardjiev A, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Developmental Review. 2004a;24:189. [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004b;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. What's in a face? The case of autism. BrJ Psychol. 1988;79(Pt 4):441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hof PR, Knabe R, Bovier P, Bouras C. Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathol (Berl) 1991;82:321–326. doi: 10.1007/BF00308819. [DOI] [PubMed] [Google Scholar]
- Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, et al. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Hrdlicka M, Dudova I, Beranova I, Lisy J, Belsan T, Neuwirth J, et al. Subtypes of autism by cluster analysis based on structural MRI data. Eur Child Adolesc Psychiatry. 2005;14:138–144. doi: 10.1007/s00787-005-0453-z. [DOI] [PubMed] [Google Scholar]
- Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychology review. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biological psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic Disturbances of Affective Contact. The Nervous Child. 1943;2:217–250. [Google Scholar]
- Kanner L, Eisenberg L. Early infantile autism, 1943–1955. Psychiatric research reports. 1957:55–65. doi: 10.4159/harvard.9780674367012.c2. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, van der Gaag RJ, Verbaten M, van Engeland H. ERP differences among subtypes of pervasive developmental disorders. Biological psychiatry. 1999;46:781–789. doi: 10.1016/s0006-3223(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. The American journal of psychiatry. 2009;166:467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G, et al. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. NeuroImage. 2010a doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Weaver K, Johnson LC, Greenson J, Dawson G, et al. Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia. 2010b doi: 10.1016/j.neuropsychologia.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. The American journal of psychiatry. 2002a;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002b;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers LA, Aggleton JP. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York: Wiley-Liss; 1992. The amygdala and social behavior. [Google Scholar]
- Kluver H, Bucy PC. "Psychic blindness" and other symptoms following bilateral temporal lobectomy in rhesus monkeys. Americal Journal of Physiology. 1937;119:352–353. [Google Scholar]
- Kylliainen A, Hietanen JK. Skin conductance responses to another person's gaze in children with autism. Journal of autism and developmental disorders. 2006;36:517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. Journal of autism and developmental disorders. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, et al. Neuropsychological profile of autism and the broad autism phenotype. Arch Gen Psychiatry. 2009;66:518–526. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: a comparison across parents of multiple- and single-incidence autism families. Am J Med Genet B Neuropsychiatr Genet. 2008a;147B:424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Piven J. Social-cognition and the broad autism phenotype: identifying genetically meaningful phenotypes. J Child Psychol Psychiatry. 2007;48:105–112. doi: 10.1111/j.1469-7610.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol. 2008b;67:829–837. doi: 10.1097/NEN.0b013e318184482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KA, Tunali-Kotoski B, Chen YR, Ortegon J, Pearson DA, Brelsford KA, et al. Emotion recognition in autism: verbal and nonverbal information. Dev Psychopathol. 1997;9:579–593. doi: 10.1017/s0954579497001351. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Gazzaniga MS. The New Cognitive Neurosciences, 2nd ed. Cambridge: MIT Press; 1999. Physiological Studies of Face Proecessing in Humans; pp. 393–410. [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. J Cogn Neurosci. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological psychiatry. 2009;66:950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol Psychiatry. 2004;45:1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J Child Psychol Psychiatry. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Escera C. Mismatch negativity: clinical and other applications. Audiology & neuro-otology. 2000;5:105–110. doi: 10.1159/000013874. [DOI] [PubMed] [Google Scholar]
- Oades RD, Walker MK, Geffen LB, Stern LM. Event-related potentials in autistic and healthy children on an auditory choice reaction time task. Int J Psychophysiol. 1988;6:25–37. doi: 10.1016/0167-8760(88)90032-3. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TP. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005;16:521–525. doi: 10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Reliability and validity of the Wisconsin Card Sorting Test in studies of autism. Neuropsychology. 1995;9:491–500. [Google Scholar]
- Patten E, Watson L. Interventions Targeting Attention in Young Children with Autism. American Journal of Speech and Language Pathology. 2010 doi: 10.1044/1058-0360(2010/09-0081). [DOI] [PubMed] [Google Scholar]
- Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. MentRetardDev DisabilRes Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual Scanning of Faces in Autism. Journal of autism and developmental disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Picton TW, Taylor MJ. Electrophysiological evaluation of human brain development. Developmental neuropsychology. 2007;31:249–278. doi: 10.1080/87565640701228732. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform 'face area' in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Piven J. The broad autism phenotype: a complementary strategy for molecular genetic studies of autism. Am J Med Genet. 2001;105:34–35. [PubMed] [Google Scholar]
- Piven J. The genetics of personality: the example of the broad autism phenotype. In: Benjamin J, Ebstein R, Belmaker R, editors. Molecular Genetics and the Human Personality. Washington, DC: American Psychiatric Publishing Inc; 2002. pp. 43–62. [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. The American journal of psychiatry. 1997a;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Santangelo S, Jacobi D, Childress D. Personality and Language Characteristics in Parents From Multiple-Incidence Autism Families. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1997b;74:398–411. [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. CerebCortex. 1999;9:445–458. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Tonge BJ, Brereton AV, Bellgrove MA. A neurobehavioral examination of individuals with high-functioning autism and Asperger's disorder using a fronto-striatal model of dysfunction. Behav Cogn Neurosci Rev. 2002;1:164–177. doi: 10.1177/1534582302001002004. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Smith JA, Benkers TL, Camou SL, Reite ML, Rogers SJ. Hippocampus and amygdala volumes in parents of children with autistic disorder. The American journal of psychiatry. 2004;161:2038–2044. doi: 10.1176/appi.ajp.161.11.2038. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. J Comp Physiol Psychol. 1954;47:173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Russo N, Flanagan T, Iarocci G, Berringer D, Zelazo PD, Burack JA. Deconstructing executive deficits among persons with autism: Implications for cognitive neuroscience. Brain Cogn. 2007;65:77–86. doi: 10.1016/j.bandc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Vargha-Khadem F, Gadian DG, de Haan M, Baldeweg T. Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: evidence from ERP and MRI. Cortex; a journal devoted to the study of the nervous system and behavior. 2007;43:686–699. doi: 10.1016/s0010-9452(08)70498-2. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Luna B, Minshew N, Behrmann M. Location, Location, Location: Alterations in the Functional Topography of Face- but not Object- or Place-Related Cortex in Adolescents with Autism. Front Hum Neurosci. 2010;4:26. doi: 10.3389/fnhum.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biological psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. JNeurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biological psychiatry. 2008;63:974–980. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. 2007;11:437–451. doi: 10.1177/1362361307079606. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RSE, Piven J. Analysis of face gaze in autism using "bubbles". Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human brain mapping. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am AcadChild AdolescPsychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Jones EJ, Merkle K, Murias M, Greenson J, Richards T, et al. Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. Int J Psychophysiol. 2010;77:106–117. doi: 10.1016/j.ijpsycho.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Wong TK, Fung PC, Chua SE, McAlonan GM. Abnormal spatiotemporal processing of emotional facial expressions in childhood autism: dipole source analysis of event-related potentials. The European journal of neuroscience. 2008;28:407–416. doi: 10.1111/j.1460-9568.2008.06328.x. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Hepburn SL, Pennington BF, Rogers SJ. Executive function in preschoolers with autism: evidence consistent with a secondary deficit. Journal of autism and developmental disorders. 2007;37:1068–1079. doi: 10.1007/s10803-006-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AW, Hellawell DJ, Van De WC, Johnson M. Facial expression processing after amygdalotomy. Neuropsychologia. 1996;34:31–39. doi: 10.1016/0028-3932(95)00062-3. [DOI] [PubMed] [Google Scholar]