Summary
Background
The high allelic frequency of the prothrombotic Leiden polymorphism in human blood coagulation factor V (fV) has been speculated to reflect positive selection during evolution. Heterozygous Leiden carriers enrolled in the placebo arm of the PROWESS sepsis trial, and heterozygous Leiden mice challenged with endotoxin both showed reduced mortality, whereas homozygous Leiden mice were not protected from lethal endotoxemia. Follow-up analyses of clinical outcomes, and of mouse models of infection with various pathogens remained inconclusive.
Objective
To establish whether aPC-resistance of fV Leiden modifies the outcome of bacterial infection in murine sepsis models.
Methods
Homozygous and heterozygous fV Leiden mice were subjected to gram-positive (S.aureus) or gram-negative (Y.pestis; E.coli) septic peritonitis, or polymicrobial, focal septic peritonitis induced by cecal ligation and puncture (CLP); and the effect of fV Leiden on 7-day survival and bacterial dissemination was assessed. Outcomes were compared to the sepsis survival of mice with genetically impaired hemostasis (hemophilia A, thrombocytopenia, thrombin receptor PAR4 deficiency, protein C receptor ProcR/EPCR-deficiency).
Results
Heterozygous, but not homozygous Leiden mice were protected from lethal infection with highly virulent S.aureus and Y.pestis strains. FV Leiden did not affect the outcome of sepsis induced by CLP, staphylokinase-deficient S.aureus, Pla-deficient Y.pestis, or E.coli. Thrombocytopenia, deficiency of PAR1 or PAR4 did not affect S.aureus sepsis survival, whereas hemophilia A increased mortality. ProcR-deficiency selectively abolished the survival advantage of heterozygous Leiden mice.
Conclusions
In mice, heterozygous fV Leiden carriers are protected from sepsis mortality after infection with clinically relevant human bacterial pathogens.
Keywords: APC-resistance, Blood coagulation, Factor V Leiden, EPCR, Sepsis
Introduction
The Leiden mutation (Arg506Gln) in coagulation factor V (fV) is the most common genetic cause of venous thrombosis in Caucasians. The mutation eliminates one of several sites in activated fV (fVa) that are substrates for proteolysis by the endogenous serine protease, activated protein C (aPC). APC resistance of fV Leiden results in diminished inactivation of fVa by aPC, and prevents the formation of the anticoagulant form of fV (fVac). The latter is generated by cleavage of intact fV at R506 by aPC and serves as a cofactor for the protein S-dependent inactivation of fVIIIa by aPC. APC resistance of fV Leiden thereby enhances the thrombosis risk of heterozygous and homozygous carriers by 4–6-fold, and 30–80-fold, respectively (reviewed in (1–4)). The high allelic frequency of the fV Leiden polymorphism in Caucasians (5–15%), together with genomic features indicating its origin in a single individual approximately 40,000 years ago (5, 6), have led to suggestions that aPC-resistant fV may have been positively selected during human evolution. Possible benefits exerted by fV Leiden may include enhanced reproductive fitness attributable to improved embryo implantation or male fertility, and reduced maternal fatality caused by peri-partum hemorrhage and puerperal sepsis (reviewed in (7, 8)). We reported that heterozygous carriership for the fV Leiden allele was indeed associated with reduced mortality of septic patients enrolled in the placebo arm of the PROWESS sepsis trial, and in mouse models of sterile lethal inflammation triggered by infusion of bacterial lipopolysaccharide (LPS) (9). While fV Leiden may reduce mortality of ARDS (10) and ameliorate the pathology of type 2 diabetes in humans (11, 12), clinical sepsis outcome studies, as well as animal experiments did not provide evidence for a beneficial effect of fV Leiden in sepsis (reviewed in (13)). One single study described improved survival of homozygous fV Leiden mice undergoing antibiotic treatment in a mouse model of pneumococcal pneumonia (14). In contrast, in mouse endotoxemia studies, only heterozygous fV Leiden mice were protected, whereas homozygous carriers were identical to carriers of the normal fV allele (9). Assuming that therapeutic options were rather limited at the time when the fV Leiden mutation occurred, it remains unclear to what extent analyses of contemporary clinical outcomes can be informative about the potential selective pressures that might have resulted in the evolutionary establishment of the fV Leiden allele. Also, sterile endotoxemia is an extremely unlikely context in which selection of the fV Leiden allele might have occurred. In the current study we show that heterozygous fV Leiden carriers have a distinct survival advantage in mouse models of infection with highly virulent human pathogens.
Materials and methods
Animals
C57Bl/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Transgenic fV Leiden mice, fVIII−/−, Nfe2−/−, EPCRLow, Par1−/−, and Par4−/− mice have been described earlier (15–21). Mouse strains used for the current study had been backcrossed onto an inbred C57Bl/6J background for more than 20 generations. All experiments involving animals were performed in adherence to the National Institutes of Health guidelines on the use of laboratory animals and approved by the Medical College of Wisconsin’s Institutional Animal Care and Use Committee. Bacterial sepsis models were performed as previously described (22).
Infection models
Single colony isolates of S.aureus (ATCC 29523, Manassas, VA), or S.aureus LS1 (23, 24) were expanded in tryptic soy broth to logarithmic growth phase, and titrated by dilution plating on tryptic soy agar. Mice were infected via intra-peritoneal injection of 1×108 (S. aureus) or 2×109 bacteria (S.aureus LS1), based on previous dose finding (LD50) experiments. E.coli (ATCC 12014) was prepared as above using Luria-Bertani (LB) Broth and Agar. 2.5 ×108 bacteria were used for infection based on previous LD50 experiments. Single colony isolates of Yersinia pestis Kim5 pla+ and pla− strains (25) were expanded in 5 ml of heart infusion broth at 26°C for 7 h, diluted to an absorbance of 0.002 in 10 ml of HIB, and grown overnight at 26°C with shaking. Mice were infected with 1×102 bacteria via an intravenous injection into the retro-orbital venous plexus.
Cecal Ligation and Puncture
The Cecal Ligation and Puncture (CLP) procedure was performed as previously described (26), without antibiotic treatment. In brief, the abdominal wall was opened under general anesthesia through a 1 cm midline incision and the cecum and ascending colon were exteriorized. A puncture hole via an 18-gauge needle was created into the antimesenteric wall of the colon (approximately 10 mm from the ileocaecal valve). To initiate bacteremia, stool was milked from the cecum into the ascending colon until a small drop of stool appeared at the puncture. The abdominal cavity was flushed with 0.5 ml of sterile saline and the wound was closed by suture.
Inhibition of fibrinolysis with Tranexamic acid
Tranexamic acid (Sigma, St. Louis, MO) was added at 5g/100 ml to the drinking water 72 h prior to infection. This dosing inhibits the fibrinolytic potential in plasma to less than 10% of wildtype levels (27). Prior to infection, animals had free access to bottled water ad libidum. Following infection, chow pellets soaked with 5 ml water containing tranexamic acid were placed in petri dishes in the cages to facilitate access to wetted food.
Enumeration of bacteria
Bacterial numbers (colony forming units: cfu) were determined by triplicate dilution plating on TSA or LB agar media. Peritoneal lavage was conducted with 10 ml of sterile PBS. Spleen, liver and lung were collected aseptically 18 h after infection, weighed, and homogenized in sterile PBS. Blood was sampled from the vena cava 15 min after i.p. administration of 200 U of unfractionated heparin.
Hematological analysis and assays
Differential blood analysis and platelet counts were determined in a HEMAVET counter (Drew Scientific, Dallas TX). Plasma thrombin-antithrombin complex (TAT) and D-dimer were measured by ELISA (Enzygnost TAT Micro, Siemens, Deerfield, IL; D-di Asserachrome, Diagnostica Stago, Parsippany, NJ). Fibrinogen levels were measured by ELISA as described (28).
Histology and TUNEL staining
Hepatic apoptosis was visualized by TUNEL assay on paraformaldehyde-fixed tissue sections using an In Situ cell death detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s directions. Images were captured with a Zeiss Axioskop Fluorescent microscope (Zeiss, Thornwood, NY) coupled to a Sensys camera and analyzed using the Metamorph software (Molecular Devices, Sunnyvale, CA).
Statistical Methods
Statistical differences between groups were analyzed by 2-tailed Student t test and survival curves were analyzed by the Kaplan-Meyer log-rank test using StatView (Version 5.0, SAS Institute Inc., Cary, NC).
Results
Pathogen-selective modulation of sepsis survival by fV Leiden
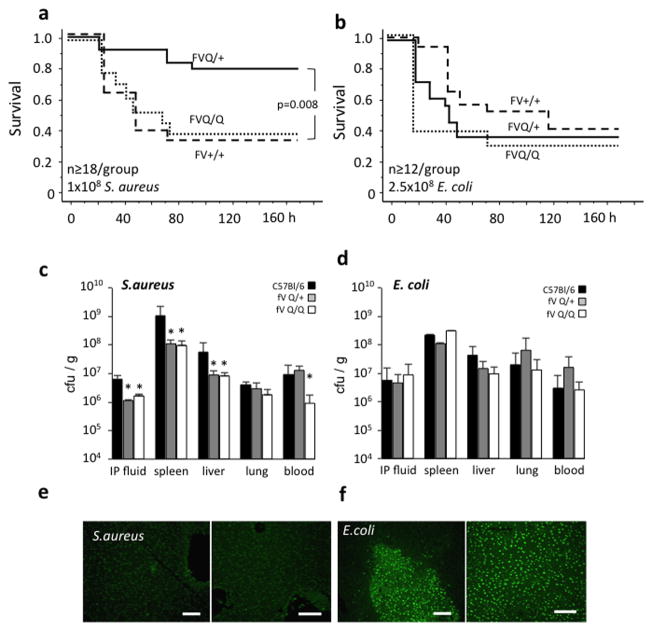
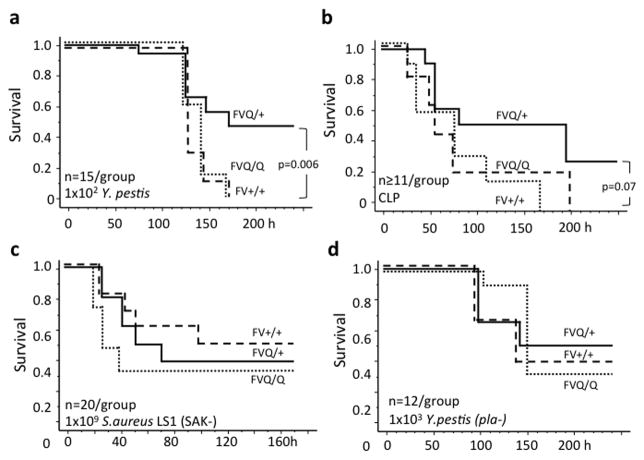
Intraperitoneal infection of wildtype mice with 1 × 108 S.aureus (Newman strain) elicited approximately 60% lethality by day 4 after infection. The survival of homozygous fV Leiden mice was identical to wildtype controls, whereas heterozygous carriers showed significantly less mortality (Figure 1a). In contrast, confirming earlier reports (29), fV Leiden carrier status did not affect survival following infection with E.coli (serotype B55:0020) (Figure 1b). Compared to wildtype mice, acute pathogen clearance from the site of infection (peritoneum) and bacterial dissemination into organs were attenuated to a similar extent in heterozygous and homozygous fV Leiden carriers infected with S.aureus, whereas bacterial load in blood was suppressed selectively in homozygous carriers (Figure 1c). FV Leiden had no significant effect on clearance or dissemination of E.coli (Figure 1d). Infection with E.coli was associated with marked hepatocyte apoptosis as reported earlier (29), independent of fV Leiden carrier status, whereas S.aureus elicited little if any liver necrosis (Figure 1e,f). To determine whether the selective survival advantage of heterozygous fV Leiden carriers was strictly limited to gram-positive pathogens, such as S.aureus, we tested the effect of fV Leiden on survival of infection with the gram-negative pathogen of the bubonic plague, Y.pestis (KIM5). In wildtype mice, intravenous infection with 102 Y.pestis (KIM5) bacteria elicited a delayed onset of sepsis, with 100% mortality by day 6 after infection. As in the S.aureus model, heterozygous fV Leiden mice showed substantially improved 10-day survival, whereas the survival of homozygous fV Leiden mice was indistinguishable from wildtype controls (Figure 2a). In a model of focal polymicrobial sepsis induced by cecal ligation and puncture, heterozygous fV Leiden mice showed a non-significant trend towards improved survival, as compared to homozygous carriers or wildtype mice (Figure 2b).
Figure 1.
(a, b) Survival of wild type (FV+/+; dashed line), heterozygous (FVQ/+; solid line) and homozygous (FVQ/Q; stippled line) Leiden mice after intraperitoneal inoculation with S.aureus, or E.coli. (c, d) Bacterial abundance 1 h (IP: intraperitoneal lavage fluid) or 18 h after infection with S.aureus (c) or E.coli (d) in the indicated tissues were determined by dilution plating. Values are the average ± stdev. from triplicate measurements/organ from 5 mice/group. Asterisks indicate P<0.05 by pairwise comparison to wildtype mice by 2-tailed Student’s t-test. (e,f) In situ cell death detection by TUNEL staining of liver sections from 2 different wild type animals each, prepared 18 h after infection with S.aureus (e) or E.coli (f). Images were captured at 40× original magnification; bars indicate 100 μm.
Figure 2.
Survival of wild type (FV+/+; dashed line), heterozygous (FVQ/+; solid line) and homozygous (FVQ/Q; stippled line) Leiden mice after intravenous inoculation with 102 Y.pestis KIM5 (a), after induction of polymicrobial focal peritonitis by cecal ligation and puncture (CLP) (b), intraperitoneal inoculation with 109 staphylokinase-deficient (SAK−) S.aureus LS1 (c), and intravenous infection with 103 Pla-deficient Y.pestis KIM5 (Y.pestis pla-). P-values were calculated by log-rank analysis of Kaplan-Meier survival plots.
Pathogen-derived fibrinolytic virulence factors modulate fV Leiden effects on sepsis survival
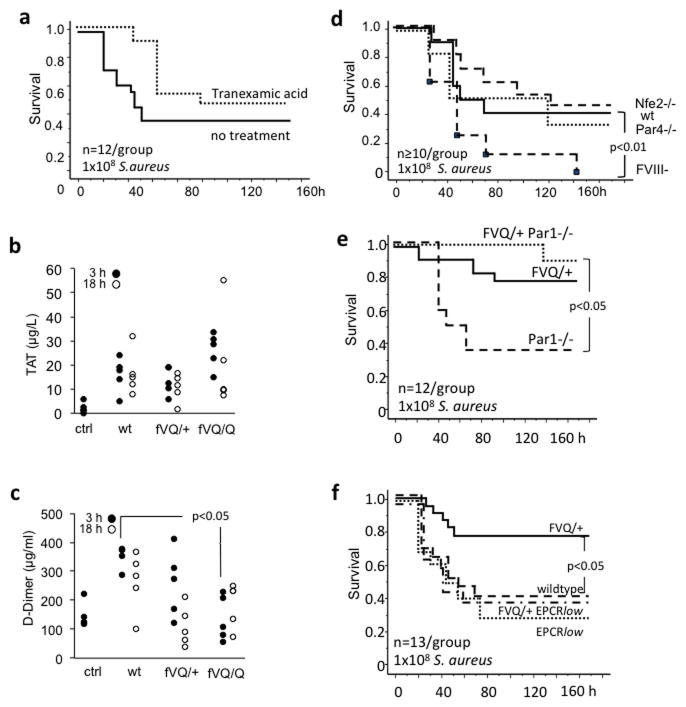
S.aureus staphylokinase and the Y.pestis Pla-gene product are virulence factors that activate the host’s fibrinolytic system and thereby attenuate bacterial killing triggered by binding of fibrin to the αMβ2 (Mac-1; CD11b) integrin on innate immune cells (30–33). Enhanced formation of thrombin in fV Leiden carriers may counteract these effects by augmenting the formation of fibrin, and by promoting the activation of the thrombin-activatable fibrinolysis inhibitor, to inhibit fibrinolysis (34). We therefore tested how fV Leiden affected the outcome of infection with strains of S.aureus and Y.pestis lacking staphylokinase or the Pla-gene, respectively. Induction of ~50% mortality required approximately 10-fold higher inoculation for both attenuated strains, as compared to the virulent parental strains. FV Leiden had no effect on survival of infection with the staphylokinase-deficient S.aureus LS1 strain, or Pla-deficient Y.pestis (Figure 2c,d). To determine whether pharmacologic attenuation of fibrinolysis altered sepsis survival after infection with virulent (staphylokinase-sufficient) S.aureus, wildtype mice were treated with tranexamic acid using the same dosing regimen shown earlier to prevent fibrinolysis-mediated in vivo placental death of thrombomodulin-deficient mice (27). However, treatment with tranexamic acid had no effect on the survival of wildtype mice infected with S.aureus (Figure 3a).
Figure 3.
(a) Effect of the fibrinolysis inhibitor tranexamic acid on the survival of wild type mice after intraperitoneal inoculation with 108 S.aureus. (b, c) Thrombin-antithrombin (TAT) complex and D-dimer levels were measured in the plasma of wildtype (wt), heterozygous Leiden (fVQ/+), and homozygous Leiden mice (fVQ/Q) 3 h and 18 h after intraperitoneal inoculation with 108 S.aureus bacteria. Controls (ctrl) were unchallenged wildtype mice. Each point represents the mean of triplicate measurements in a single mouse sample. With the exception of reduced D-dimer levels in homozygous Leiden mice at the 3h time point, no statistically significant differences were observed by pairwise comparison to wildtype animals via 2-tailed Student’s t-test. (d) Survival of wild type mice (wt; solid line), Nfe2-deficient mice (Nfe2−/−; dashed line w/o marks), Par4-deficient mice (Par4−/−; stippled line), and hemizygous (male) mice lacking fVIII (FVIII−/−; dashed line with square marks) after intraperitoneal inoculation with 108 S.aureus. (e,f) Survival of wildtype and mutant mice after intraperitoneal inoculation with 108 S.aureus. Par1−/−: Par1-deficient mice, fVQ/+Par1−/−: heterozygous Leiden mice with superimposed Par1-deficiency. EPCRlow: mice with reduced (≤10%) EPCR expression. FVQ/+ EPCRlow: heterozygous Leiden mice with superimposed EPCR-deficiency. P-values were calculated by log-rank analysis of Kaplan-Meier survival plots.
Role of platelets and intrinsic coagulation activation for the survival of S.aureus infection
To determine whether the differential survival of wildtype and fV Leiden mice after S.aureus infection correlated with the extent of coagulation activation, we measured plasma levels of thrombin-antithrombin- (TAT-) complex and D-dimer. Infection was associated with increased TAT levels 3 and 18h after inoculation, as compared to non-infected wildtype mice, but differences between groups of mice did not reach significance in the sample of 5 mice analyzed for each time point (Figure 3b). Compared to septic wildtype mice, there was a trend towards reduced D-dimer at 3h and 18h in both heterozygous and homozygous fV Leiden mice, which reached significance at the 3h time point in homozygous fV Leiden mice (Figure 3c). No differences were noted with respect to platelet counts, hematocrit, or fibrinogen levels (not shown). We next examined whether the extent of coagulation activation, thrombin-dependent platelet activation, platelet-mediated thrombotic organ damage, or some other host defense function of platelets had any effect at all on the outcome measure of survival after infection with S.aureus. Severe thrombocytopenia secondary to genetic deficiency of the transcription factor Nfe2, or suppression of thrombin-mediated platelet hemostatic function due to lack of the platelet thrombin receptor PAR4 had no effect on 7-day survival (Figure 3d). In contrast, attenuation of thrombin formation via the intrinsic pathway in hemophilic mice lacking fVIII shortened the time to death and significantly diminished overall 7-day survival (Figure 3d).
Role of EPCR and PAR1 for the survival of S.aureus infection
In mouse endotoxemia models, mortality reduction by therapeutically administered recombinant aPC does not involve aPC’s anticoagulant function, but is mediated by cytoprotective and anti-inflammatory cell signaling events triggered by the EPCR- and/or CD11b/Mac-1-dependent activation of PAR1 (22, 35). In theory, even small augmentations of endogenous thrombin generation in fV Leiden mice might be associated with a subtle, but nevertheless physiologically relevant enhancement of endogenous protein C activation on the surface of endothelial cells or other cells expressing EPCR. We therefore examined the potential role of endogenous aPC signaling via PAR1 and EPCR in S.aureus infection survival. Deficiency of PAR1 did not alter S.aureus sepsis survival and did not diminish the survival of heterozygous fV Leiden mice (Figure 3e). On the other hand, reduced expression of EPCR in EPCRlow-mice selectively abolished the survival advantage of heterozygous fV Leiden carriers, without altering the survival of animals expressing normal fV (Figure 3f).
Discussion
The current study shows, for the first time, that heterozygous carriers of the fV Leiden allele benefit from a selective survival advantage over homozygous fV Leiden carriers and carriers of the normal fV allele in two distinct modes of infection with human bacterial pathogens, i.e. gram-positive S.aureus and gram-negative Y.pestis KIM5. This finding extends earlier observations documenting a similar selective survival benefit of heterozygous fV Leiden mice in endotoxemia models. In mice, fV Leiden therefore emerges as a naturally occurring human polymorphism that modulates not only the survival of sterile inflammation, but also the evolutionary selectable outcome measure of infection survival.
The effect of fV Leiden on sepsis survival of heterozygous carriers was pathogen-specific and only evident after infection with fully virulent strains of S.aureus and Y.pestis, but not with attenuated strains lacking the ability to activate the host fibrinolytic system, after infection with E.coli, or after induction of polymicrobial, focal peritonitis in the CLP sepsis model. The latter observations corroborate and extend findings by others documenting that fV Leiden had no effect in mouse models of pneumococcal pneumonia (14), septic E.coli peritonitis (29), or lethal H1N1 influenza (36). One rationale for testing the effect of fV Leiden on the outcomes of infection S.aureus and Y.pestis was that both pathogens express potent virulence factors (staphylokinase and the Pla gene product, respectively) that subvert fibrin-mediated antibacterial host defenses by activating the principal fibrinolytic effector, plasminogen (32). We hypothesized that the known anti-fibrinolytic effect of fV Leiden might counteract the effects of these bacterial virulence factors and thereby sustain effective bacterial killing. On the one hand, we observe indeed more effective elimination of S.aureus from the site of infection, as well as approximately 10-fold diminished bacterial loads 18h after incoluation in the liver and spleen, and –in homozygous carriers- also in blood (Figure 3c), and fV Leiden had no effect on infection outcome with attenuated strains lacking these fibrinolytic virulence factors. While these data are consistent with the notion that the anti-fibrinolytic effects of fV Leiden may alter fibrin- and/or plasmin-mediated host-pathogen interactions, this mechanism is not sufficient to explain the differential survival hetero- and homozygous fV Leiden mice: first, bacterial killing and dissemination into solid organs were (with the exception of blood) comparable in heterozygous and homozygous fV Leiden mice. Second, the formation of D-dimer fibrin degradation products at the 3h time point was significantly reduced only in homozygous fV Leiden carriers, which show unaltered survival; and at the 18h time point were (non-significantly) reduced to a comparable extent in homo- and heterozygous carriers. If the anti-fibrinolytic effect of fV Leiden was solely responsible for improved survival, homozygous carriers should exhibit an even more robust survival benefit, similar to the improved survival of homozygous carriers in mouse models of pneumococcal pneumonia with concomitant antibiotic treatment (14). Third, in spite of the only modest effect of fV Leiden on D-dimer levels, pharmacologic inhibition of fibrinolysis with tranexamic acid did not alter the survival of wildtype mice infection. Fourth, given that enhanced capacity for killing of bacteria is unlikely to modify survival of sterile inflammation, such fibrin-mediated mechanisms also cannot explain the selective survival advantage of heterozygous fV Leiden carriers in endotoxemia. The mechanisms underlying the pathogen-specifity of the fV Leiden effect on sepsis survival thus remain unknown, and might involve complex differences in pathology (as seen with respect to liver necrosis), model-specific differences in disease etiology (focal and slow versus systemic and acute), and potential differences in the action of pathogen-specific virulence factors. Interestingly, fVIII-deficiency has also reported to have no effect on survival of endotoxemia (37) or E.coli peritonitis (38), but was associated with diminished survival in the current model of S.aureus infection. This suggests that coagulation activation via the intrinsic pathway –possibly triggered by pathogen-derived factors- also exhibits pathogen- or model-specific effects that positively impact S.aureus peritonitis survival. Such a function of fVIII also argues against the hypothesis that the lack of a survival benefit in homozygous fV Leiden mice may be attributed to compromised anticoagulant fV cofactor function for inactivation of fVIIIa by aPC.
We considered the possibility that homozygous fV Leiden mice engage some beneficial survival mechanism to the same or even greater extent than heterozygous carriers, but that such benefits would be countered in homozygous, but not heterozygous carriers by deleterious effects of increased thrombotic organ damage, or thrombin signaling. However, TAT levels were only marginally elevated in homozygous carriers, as compared to wildtype mice or fV Leiden heterozygotes, and deficiency of the thrombin receptors PAR1 and PAR4, or severe thrombocytopenia did not improve survival, arguing against a significant impact of thrombin signaling or platelet-mediated thrombotic organ damage in the S.aureus peritonitis model. The unaltered infection outcome in PAR1-deficient fV Leiden heterozygous mice also eliminates cytoprotective and anti-inflammatory effects secondary to CD11b- or EPCR-mediated activation of PAR1 by endogenous aPC as the mechanism underlying the selective survival advantage of heterozygous fV Leiden carriers.
In contrast to the thrombin receptors PAR1 and PAR4, genetic deficiency of EPCR selectively abrogated the survival advantage of heterozygous fV Leiden mice, without affecting the survival of mice carrying the normal fV alleles. EPCR augments several-fold the activation of protein C by the thrombomodulin-thrombin complex, sequesters a pool of aPC to the vascular endothelial surface, is required for effective activation of PAR’s by aPC, and may fulfill additional functions mediated by interaction with fXa and fVIIa, with the αMβ2-proteinase 3 complex, or the T cell receptor complex on γδ T cells (reviewed in (39)). The above data strongly mitigate against the importance of cytoprotective and anti-inflammatory EPCR-dependent aPC signaling via PAR1, but are not informative about the role of other EPCR functions. Given that the survival advantage of heterozygous fV Leiden carriers is observable in models of sterile endotoxemia as well as bacterial sepsis, the beneficial effects of the Leiden mutation in fV are unlikely to involve the regulation of antibacterial defense mechanisms. Rather, we speculate that it modulates an as yet unknown EPCR-dependent survival pathway that renders the host more resistant to a component of immunopathology that is common to sterile inflammation and bacterial infection.
In summary, the current study documents a remarkable link between heterozygous fV Leiden carriership and resistance to infection. While we were as yet unable to identify the mechanism underlying this phenomenon, it provides a plausible potential explanation how this mutation may have been established in the human gene pool.
Acknowledgments
We thank Drs. S. Coughlin, D. Ginsburg, and F. Castellino for providing mutant mouse strains. This work was supported by the National Institutes of Health grants HL44612-14, AI080557, HL093388, Bridge Funding from the American Society of Hematology and the Ziegler Family Research Chair Foundation (H.W.).
Footnotes
Addendum
J. Kerschen and H. Weiler designed and performed the experiments, analyzed the data, and wrote the manuscript. M. Maas performed CLP experiments, M. Zogg and I. Hernandez assisted in the conduct of experiments, mouse husbandry, and data preparation. M. Maas is currently affiliated with the Universitätsklinikum Münster, 48149 Münster, Germany.
Disclosure of conflict of interest
The authors state that they have no conflicts of interest.
References
- 1.Cramer TJ, Gale AJ. The anticoagulant function of coagulation factor V. Thromb Haemost. 2012;107:15–21. doi: 10.1160/TH11-06-0431. [DOI] [PubMed] [Google Scholar]
- 2.Castoldi E, Rosing J. APC resistance: biological basis and acquired influences. J Thromb Haemost. 2010;8:445–53. doi: 10.1111/j.1538-7836.2009.03711.x. [DOI] [PubMed] [Google Scholar]
- 3.Dahlback B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005;25:1311–20. doi: 10.1161/01.ATV.0000168421.13467.82. [DOI] [PubMed] [Google Scholar]
- 4.Kalafatis M, Mann KG. Factor V: Dr. Jeckyll and Mr. Hyde. Adv Exp Med Biol. 489:31–43. doi: 10.1007/978-1-4615-1277-6_3. [DOI] [PubMed] [Google Scholar]
- 5.Cox MJ, Rees DC, Martinson JJ, Clegg JB. 1996 Evidence for a single origin of factor V Leiden. Br J Haematol. 2001;92:1022–5. doi: 10.1046/j.1365-2141.1996.4961037.x. [DOI] [PubMed] [Google Scholar]
- 6.Zivelin A, Griffin JH, Xu X, Pabinger I, Samama M, Conard J, Brenner B, Eldor A, Seligsohn U. A single genetic origin for a common Caucasian risk factor for venous thrombosis. Blood. 1997;89:397–402. [PubMed] [Google Scholar]
- 7.Lindqvist PG, Dahlback B. Carriership of Factor V Leiden and evolutionary selection advantage. Curr Med Chem. 2008;15:1541–4. doi: 10.2174/092986708784638852. [DOI] [PubMed] [Google Scholar]
- 8.van Mens TE, Levi M, Middeldorp S. Evolution of Factor V Leiden. Thromb Haemost. 2013;110:23–30. doi: 10.1160/TH13-02-0115. [DOI] [PubMed] [Google Scholar]
- 9.Kerlin BA, Yan SB, Isermann BH, Brandt JT, Sood R, Basson BR, Joyce DE, Weiler H, Dhainaut JF. Survival advantage associated with heterozygous factor V Leiden mutation in patients with severe sepsis and in mouse endotoxemia. Blood. 2003;102:3085–92. doi: 10.1182/blood-2003-06-1789. [DOI] [PubMed] [Google Scholar]
- 10.Adamzik M, Frey UH, Riemann K, Sixt S, Lehmann N, Siffert W, Peters J. Factor V Leiden mutation is associated with improved 30-day survival in patients with acute respiratory distress syndrome. Crit Care Med. 2008;36:1776–9. doi: 10.1097/CCM.0b013e318174373d. [DOI] [PubMed] [Google Scholar]
- 11.Peter A, Fritsche A, Machicao F, Nawroth PP, Haring HU, Isermann B. Lower plasma creatinine and urine albumin in individuals at increased risk of type 2 diabetes with factor v leiden mutation. ISRN Endocrinol. 2014:530830. doi: 10.1155/2014/530830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Madhusudhan T, He T, Hummel B, Schmidt S, Vinnikov IA, Shahzad K, Kashif M, Muller-Krebs S, Schwenger V, Bierhaus A, Rudofsky G, Nawroth PP, Isermann B. Low but sustained coagulation activation ameliorates glucose-induced podocyte apoptosis: protective effect of factor V Leiden in diabetic nephropathy. Blood. 2011;117:5231–42. doi: 10.1182/blood-2010-10-314773. [DOI] [PubMed] [Google Scholar]
- 13.Schouten M, van‘t Veer C, van der Poll T, Levi M. Effect of the factor V Leiden mutation on the incidence and outcome of severe infection and sepsis. Neth J Med. 2012;70:306–10. [PubMed] [Google Scholar]
- 14.Schouten M, van’t Veer C, Roelofs JJ, Levi M, van der Poll T. Impact of the factor V Leiden mutation on the outcome of pneumococcal pneumonia: a controlled laboratory study. Crit Care. 2010;14:R145. doi: 10.1186/cc9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–21. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 16.Castellino FJ, Liang Z, Volkir SP, Haalboom E, Martin JA, Sandoval-Cooper MJ, Rosen ED. Mice with a severe deficiency of the endothelial protein C receptor gene develop, survive, and reproduce normally, and do not present with enhanced arterial thrombosis after challenge. Thromb Haemost. 2002;88:462–72. [PubMed] [Google Scholar]
- 17.Connolly AJ, Ishihara H, Kahn ML, Farese RV, Jr, Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–9. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 18.Cui J, Eitzman DT, Westrick RJ, Christie PD, Xu ZJ, Yang AY, Purkayastha AA, Yang TL, Metz AL, Gallagher KP, Tyson JA, Rosenberg RD, Ginsburg D. Spontaneous thrombosis in mice carrying the factor V Leiden mutation. Blood. 2000;96:4222–6. [PubMed] [Google Scholar]
- 19.Darrow AL, Fung-Leung WP, Ye RD, Santulli RJ, Cheung WM, Derian CK, Burns CL, Damiano BP, Zhou L, Keenan CM, Peterson PA, Andrade-Gordon P. Biological consequences of thrombin receptor deficiency in mice. Thromb Haemost. 1996;76:860–6. [PubMed] [Google Scholar]
- 20.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–8. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 21.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 22.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–48. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremell T, Lange S, Svensson L, Jennische E, Grondahl K, Carlsten H, Tarkowski A. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990;33:1739–44. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- 24.Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol. 2004;172:1169–76. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 25.Une T, Brubaker RR. In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maier S, Traeger T, Entleutner M, Westerholt A, Kleist B, Huser N, Holzmann B, Stier A, Pfeffer K, Heidecke CD. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock. 2004;21:505–11. doi: 10.1097/01.shk.0000126906.52367.dd. [DOI] [PubMed] [Google Scholar]
- 27.Isermann B, Sood R, Pawlinski R, Zogg M, Kalloway S, Degen JL, Mackman N, Weiler H. The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nat Med. 2003;9:331–7. doi: 10.1038/nm825. [DOI] [PubMed] [Google Scholar]
- 28.Kerlin B, Cooley BC, Isermann BH, Hernandez I, Sood R, Zogg M, Hendrickson SB, Mosesson MW, Lord S, Weiler H. Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood. 2004;103:1728–34. doi: 10.1182/blood-2003-08-2886. [DOI] [PubMed] [Google Scholar]
- 29.Bruggemann LW, Schoenmakers SH, Groot AP, Reitsma PH, Spek CA. Role of the factor V Leiden mutation in septic peritonitis assessed in factor V Leiden transgenic mice. Crit Care Med. 2006;34:2201–6. doi: 10.1097/01.CCM.0000228918.30931.E8. [DOI] [PubMed] [Google Scholar]
- 30.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–6. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- 32.Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007;5 (Suppl 1):24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 33.Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb Haemost. 2007;98:512–20. [PubMed] [Google Scholar]
- 34.Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arterioscler Thromb Vasc Biol. 2006;26:2445–53. doi: 10.1161/01.ATV.0000244680.14653.9a. [DOI] [PubMed] [Google Scholar]
- 35.Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120:1971–80. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schouten M, van der Sluijs KF, Roelofs JJ, Levi M, Van’t Veer C, van der Poll T. Factor V Leiden mutation does not affect coagulopathy or outcome in lethal H1N1 influenza. Eur Respir J. 2010;36:1346–54. doi: 10.1183/09031936.00204909. [DOI] [PubMed] [Google Scholar]
- 37.Vancine SM, Picoli-Quaino SK, Costa DS, Montalvao SA, Ozelo MC, Annichino-Bizzacchi JM, de Paula EV. Evaluation of the host response to endotoxemia of FVIII and FIX deficient mice. Haemophilia. 2011;17:800–7. doi: 10.1111/j.1365-2516.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 38.Schoenmakers SH, Bruggemann LW, Groot AP, Maijs S, Reitsma PH, Spek CA. Role of coagulation FVIII in septic peritonitis assessed in hemophilic mice. J Thromb Haemost. 2005;3:2738–44. doi: 10.1111/j.1538-7836.2005.01649.x. [DOI] [PubMed] [Google Scholar]
- 39.Mohan Rao LV, Esmon CT, Pendurthi UR. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood. 2014;124:1553–62. doi: 10.1182/blood-2014-05-578328. [DOI] [PMC free article] [PubMed] [Google Scholar]