Abstract
The progression of pulmonary fibrosis (PF) entails a complex network of interactions between multiple classes of molecules and cells, which are closely related to the vagus nerve. Stimulation of the vagus nerve increases fibrogenic cytokines in humans, therefore, activation of the nerve may promote PF. The hypothesis was tested by comparing the extent and severity of fibrosis in lungs with and without vagal innervation in unilaterally vagotomized mice. The results show that in vagotomized lungs, there were less collagen staining, less severe fibrotic foci (subpleural, peri-vascular and peri-bronchiolar lesions) and destruction of alveolar architecture; decreased collagen deposition (denervated vs intact: COL1α1, 19.1 ± 2.2 vs 22.0 ± 2.6 ng/mg protein; COL1α2, 4.5 ± 0.3 vs 5.7 ± 0.5 ng/mg protein; p < 0.01, n = 21) and protein levels of transforming growth factor beta and interleukin 4; and fewer myofibroblast infiltration (denervated vs intact: 1.2 ± 0.2 vs 3.2 ± 0.6 cells/visual field; p < 0.05, n = 6) and M2 macrophages [though the infiltration of macrophages was increased (denervated vs intact: 112 ± 8 vs 76 ± 9 cells/visual field; p < 0.01, n = 6), the percentage of M2 macrophages was decreased (denervated vs intact: 31 ± 4 vs 57 ± 9%; p < 0.05, n = 5)]. It indicated that the vagus nerve may influence PF by enhancing fibrogenic factors and fibrogenic cells.
Pulmonary fibrosis (PF) is a devastating disease. Although the role of sundry cellular and immune modulator factors has been intensely investigated, the pathogenesis of PF remains idiopathic in most cases. Previous investigations have implicated multiple cell types [fibroblasts, myofibroblasts and M2 macrophages activated alternatively by T helper-2 (Th-2) cells] and cytokines [transforming growth factor β (TGF-β) and interleukin 4 (IL-4)]. TGF-β, a regulator of cell proliferation and differentiation, is a key fibrogenic cytokine. It modulates fibroblast phenotype and function, inducing myofibroblast transdifferentiation and promoting matrix preservation1. IL-4, a multi-functional cytokine playing significant roles in immune cellular regulation including chemotaxis, is also fibrogenic, which promotes alternative activation of macrophages into M2 cells (“repair macrophage”) that leads to increased production of TGF-β, thereby leading to myofibroblast differentiation and increased fibrosis2. Both TGF-β and IL-4 increase in bleomycin-induced lung injury3,4. Myofibroblasts, identified by expression of α-smooth muscle actin (α-SMA), are the primary source of type I collagen in fibrotic diseases5. The presence of myofibroblasts in fibrotic sites in lung tissues, both from humans and animals with PF, is well documented6,7. In addition to IL-4 and TGF-β, M2 macrophages are pro-fibrogenic8,9,10. In both bleomycin-induced lung injury and TGF-β over-expressing mouse models, PF can be prevented by phenotypic modulation of macrophages11,12.
Recently, the vagus nerve has been found to play an important role in neuro-immune interaction and is involved in a variety of pulmonary disease processes. However, its relation to PF is not known. Many reports suggest that the vagus nerve may be directly or indirectly involved in fibrogenic processes. For example, stimulation of the vagus nerve in patients significantly increased plasma levels of TGF-β13. Vagotomy eliminated drug-induced activation of TGF-β in the cerebral spinal fluid14. Cholinergic denervation decreased TGF-β1 expression in CCl4-induced liver fibrosis in rats15. Also, neuropeptides may alter T-cell responses, switching a Th-1 response to a pro-fibrogenic Th-2 response. However, we are not aware of any study that directly investigates the role of the vagus nerve in the development of PF. We hypothesize that activation of the vagus nerve enhances PF (Fig. 1). Specifically, if our hypothesis is correct, vagotomy should attenuate PF. To test the hypothesis, we compared bleomycin-induced fibrosis in lungs with or without vagal innervation in the same mouse, and found that vagal denervation ameliorated PF. The protective effect of vagotomy was further confirmed by decreased production of cytokines (TGF-β and IL-4) as well as myofibroblast proliferation and M2 macrophage differentiation.
Figure 1. Hypothesis—The vagus nerve promotes fibrotic progression through several pathways.
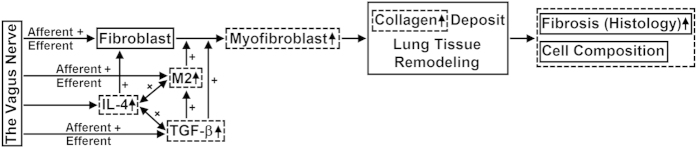
Myofibroblasts at fibrotic lesions are cells responsible for synthesis and deposition of collagen that leads to fibrosis. TGF-β transforms fibroblasts to myofibroblasts. IL-4 serves as an inducer of TGF-β. Activation of the vagal efferent nerve releases acetylcholine, which stimulates fibroblasts by acting on acetylcholine receptors (or by increasing the level of TGF-β) to synthesize collagen. Activation of vagal afferents may produce the axon reflex to release neuropeptides, which polarize macrophages toward a fibrogenic M2 phenotype. Both TGF-β and IL-4 also promote the M2 phenotype. Arrows indicate interaction direction between fibrogenic factors. The dash line squared variables were tested in the protocol to evaluate the effect of vagotomy on bleomycin-induced lung fibrosis. M2, Type 2 (alternatively activated) macrophages; TGF-β, transforming growth factor-β; IL-4, Interleukin-4; α-SMA, α-smooth muscle actin.
Results
Even blood supply to vagotomized and intact lungs
Prior to establishing our murine vagotomy model, we needed to rule out the possibility that vagotomy may reduce blood supply to affect lung fibrosis. Therefore, we compared the blood distribution in both lungs of unilateral vagotomized mice and found that the blood supplies per unit lung tissue were the same. The ratio of EB concentration (vagotomized over intact lungs) was 98.6 ± 1.8% (n = 8).
Vagotomy decreased collagen deposition and preserved lung architecture
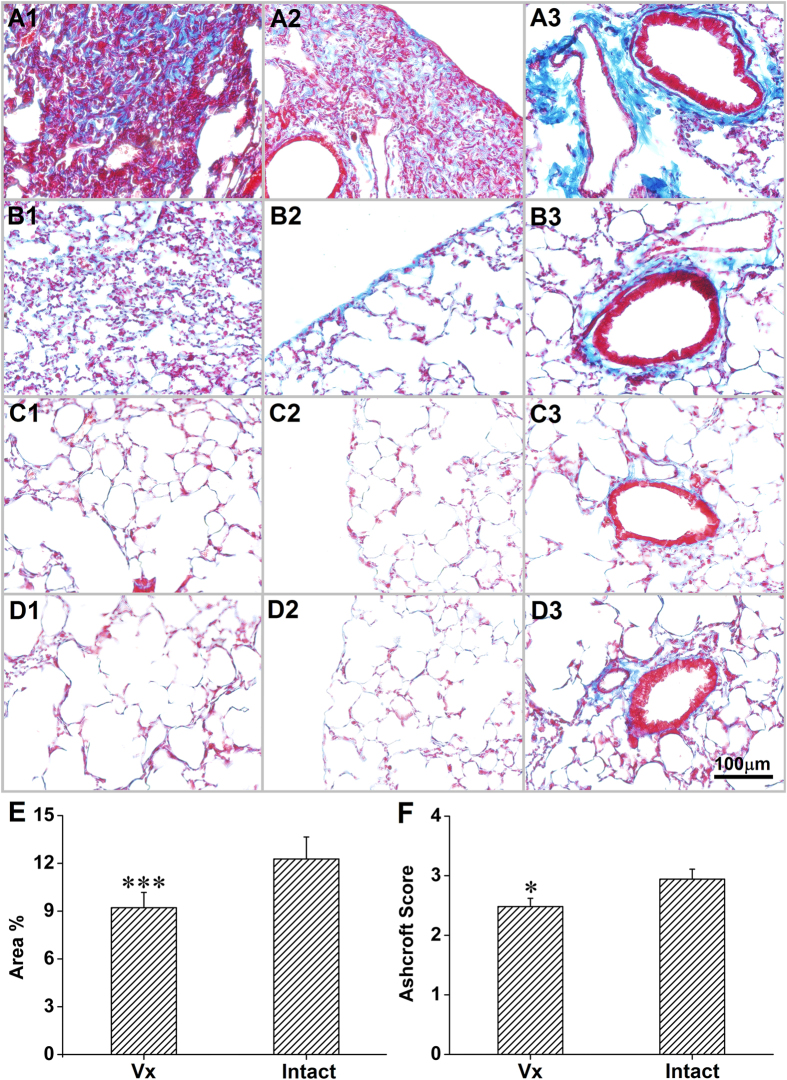
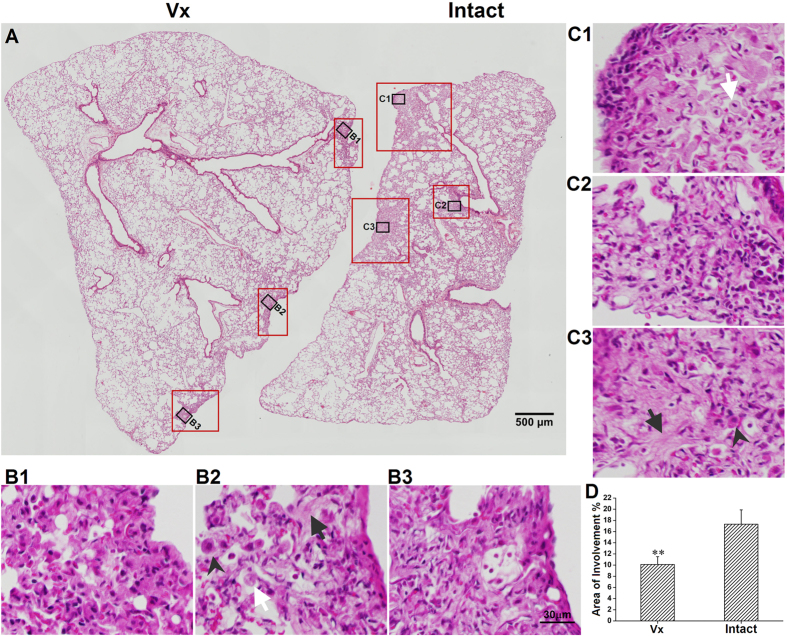
Four weeks after bleomycin treatment, collagen deposition was increased more in the intact lung than the vagotomized lung, evidenced by increased expression of COL1α1 and COL1α2 (ELISA) (Fig. 2), and by increased fibrosis in the Masson’s trichrome stain. The percentage of stained collagen area (per cross sectional area) and Ashcroft scores were significantly greater in the intact lung than the vagotomized lung (Fig. 3). In addition to fibrosis, there was increased damage to alveolar architecture. Fibrotic lesions were mainly subpleural, peri-vascular and peri-bronchial. Typical fibrous foci, distortion of lung architecture, interstitial hyperplasia and inflammatory cell infiltration were observed in the H&E stain (Fig. 4). The total area of involvement was lower in the vagotomized lung (Fig. 4D).
Figure 2. Collagen production in the lung.
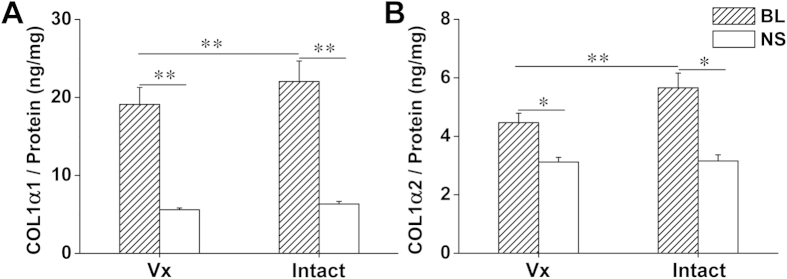
Protein levels of COL1α1 (A) and COL1α2 (B) were increased 4 weeks after bleomycin treatment with greater increases in the intact lung. BL: bleomycin (n = 21); NS: normal saline (n = 6); Vx: vagotomy. *P < 0.05, **P < 0.01.
Figure 3.
Evaluation of collagen deposit in innervated (A,C) and denervated (B,D) lungs by Masson’s trichrome stain. a and b, bleomycin-treated group; c and d, vehicle control group. Following bleomycin, there were more lesions in the intact lung, demonstrated by 1, consolidation of lung parenchyma with loss of alveolar architecture and increased cellularity; 2, subpleural lesions; 3, peri-vascular and peri-bronchial collagen deposits. (E) morphometric analysis shows that collagen deposit was higher in the intact lung. (F) the Ashcroft score was higher in the intact lung. Vx: vagotomy. *P < 0.05, ***P < 0.001, n = 27.
Figure 4. Evaluation of lung parenchyma lesions following bleomycin challenge by H&E stain.
(A), gross view. (B1,B2,B3) and (C1,C2,C3), represented high power visual field of area of involvement, showing detailed consolidation in fibrotic areas from the intact right and denervated left lungs, respectively. Arrow shows young collagen, white arrow shows macrophage, arrow head shows the type II alveolar epithelial cell. (D) morphometric analysis shows that percent consolidation area was more in the intact lung than the denervated lung. Vx: vagotomy. **P < 0.01, n = 24.
Vagotomy decreased the production of bleomycin-induced fibrogenic cytokines
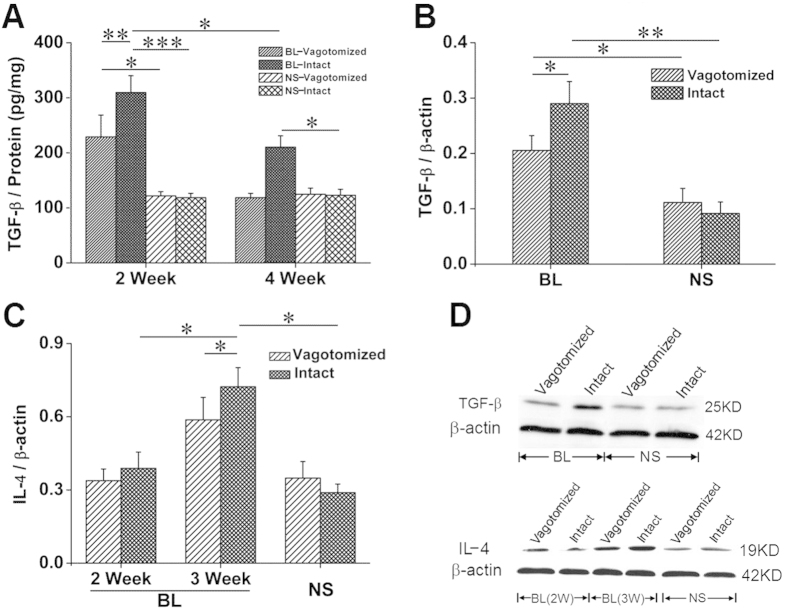
Two weeks after bleomycin treatment, levels of total (latent and activated) TGF-β1 (ELISA) as well as activated TGF-β1 (Western blot) were higher in the intact lung than in the denervated lung. Although the level of TGF-β1 decreased in both lungs, it remained significantly higher in vagus-intact lungs at 4 weeks. Similarly, IL-4 production was also increased to a greater degree in the intact lung (Fig. 5).
Figure 5. Protein expression of pro-fibrogenic cytokines in the lung.
(A) two weeks after bleomycin injection, total TGF-β (ELISA) increased with more in the intact lung (BL, n = 6; NS, n = 5) and so TGF-β activity (Western blot) (B, n = 8). (C) three weeks after bleomycin injection, IL-4 (Western blot) increased with more in the intact lung (BL, n = 4; NS, n = 5). (D) representative Western blots for TGF-β and IL-4. *P < 0.05; **P < 0.01; ***P < 0.001.
Vagotomy suppressed proliferation of fibrogenic cells
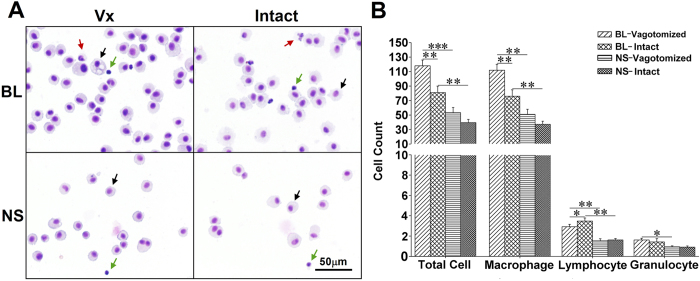
Myofibroblasts (α-SMA-positive cells) in lung sections and CD206 +/F4/80 + cells in BALF smears were counted. There were increased number of α-SMA positive myofibroblasts and M2 (CD206 +/F4/80 +) macrophages per visual field in the intact lung. Myofibroblasts were mainly located in the subpleural areas and within fibrous lesions (Fig. 6). Diff-quick stain showed mainly macrophages/monocytes in BALF. The total number and type of leukocytes (i.e., macrophage, lymphocyte and granulocyte) were analyzed. Bleomycin challenge increased cell numbers with more cells detected in the vagotomized lungs (8.4 ± 3.5 × 104/ml) than in the vagus-intact lungs (5.5 ± 3.0 × 104/ml; n = 22, p < 0.001). However, there were more lymphocytes in the intact lung (Fig. 7). Although M2 macrophages were only slightly higher, the ratio of M2/M1 macrophages was significantly higher in BALF from the intact lung (Fig. 8).
Figure 6. Myofibroblasts in the lung.
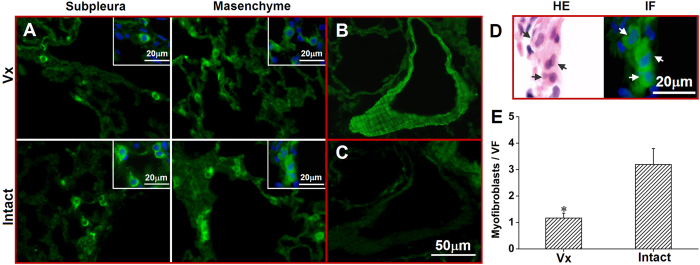
(A) there were more myofibroblasts (green α-SMA positive cells) located in subpleural area and lung interstitium in the intact lung. (B) stain of vascular smooth muscle serves as a positive control; (C) negative control (no primary antibody); (D) A representative morphology of myofibroblasts in both H&E and IF stains; (E) group data (n = 6, *P < 0.05). Vx: vagotomy.
Figure 7. Cell differentiation in BALF with the Diff-quick method.
(A) representative visual fields. Cells were primarily macrophages (black arrows) with a few lymphocytes (green arrows) and granulocytes (red arrows) 4 weeks after bleomycin treatment. (B) group data show that cell number in both lungs increased with a greater number of macrophages and lower number of lymphocytes in the vagotomized lung after bleomycin treatment. BL, bleomycin; NS, normal saline; Vx, vagotomy. *P < 0.05; **P < 0.01; ***P < 0.001; n = 6 in BL group, n = 4 in NS group.
Figure 8. Macrophages in BALF slides 4 weeks after bleomycin treatment.
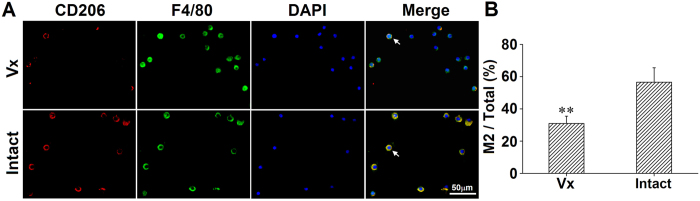
(A) visual fields show that cells were primarily macrophages (F4/80 +). The number of macrophages was greater but M2 (CD206 +/F4/80 +) was lower in the vagotomized lung. (B) group data show that percentage of M2 over the total number of cells was lower in the vagotomized lung. Vx: vagotomy. n = 5, **P < 0.01.
Discussion
We established an animal model to assess the role of the vagus nerve in the pathophysiological process of PF by comparing bleomycin-induced fibrosis in lungs of unilaterally vagotomized mice. Difficulties are encountered when interpreting data generated to assess the roles of the vagus nerve in lung diseases by comparing vagotomized with vagus intact animals, mainly because the nerve not only innervates the lung, but also other organs such as the heart and digestive system. It also transmits vital information to the brain, which involves neuroimmune interaction16,17. Clearly, vagotomy affects the function of multiple organs and interpretation can be difficult. However, our newly established, unilaterally vagotomized mouse model enables us to directly compare innervated vs denervated lungs in the same animal. Thus, the effects of the vagus nerve on PF can be investigated. In models where bleomycin is administered using the intra-tracheal route, we cannot compare the left with the right lungs in the same animal because the amount of bleomycin reaching each lung is unpredictable. In order to solve the problem, we used an intravenous injection model, in which bleomycin is evenly distributed. In this scenario, the severity of lung involvement in right and left lungs can be compared in the same animal. However, it can be argued that vagotomy may leave sympathetic vasoconstriction unopposed, which might negate drug delivery by an intravenous injection. To rule out this possibility, we performed experiments to compare the blood distribution in both lungs of unilaterally vagotomized mice and found that the blood supply per unit lung tissue was the same for both lungs.
Having characterized the mouse model, we engaged in studies evaluating the role of the vagus nerve in bleomycin-induced PF. Our data support the hypothesis that the vagus nerve influences bleomycin-induced PF by demonstrating that vagotomy: 1) decreased collagen deposition; 2) attenuated structural destruction; 3) reduced production of pro-fibrogenic cytokines; and 4) suppressed pro-fibrogenic cells.
In the lung, type I collagen, the main fibril collagen, consists of two α1(I) and one α2(I) chains18. Both COL1α1 and COL1α2 subunits are increased in the bleomycin-induced PF mouse model19. Although different in methodology, our results parallel prior findings of increased COL1α1 and COL1α2 subunits (Fig. 2). Interestingly, vagotomy reduced the increased COL1α1 and COL1α2 by approximately 30%. Masson’s trichrome stain shows a similar decrease in collagen stain in the vagotomized lung (Fig. 3).
Histologically, PF is characterized by an increase in inflammatory cells, fibroblasts, and collagen deposition, and by alterations in alveolar architecture with marked regeneration and remodeling20. In our studies, mice developed classic PF with structural alteration of the lung following intravenous bleomycin treatment (Figs 3 and 4). Lung lesions were apparent but not severe, as indicated by the low Ashcroft scores. Vagotomy attenuated structural damage and alleviated fibrosis. This is supported by significantly lower Ashcroft scores in the vagotomized lung.
Among various fibrogenic cytokines, TGF-β and IL-4 are the most critical. TGF-β1 production increases in patients with idiopathic pulmonary fibrosis (IPF) and in animal models of PF. TGF-β promotes epithelial-mesenchymal transition and fibroblast transdifferentiation (transforming resident fibroblasts into myofibroblasts)21. TGF-β and IL-4 act in concert to play a crucial role by promoting macrophage polarization towards the M2 phenotype. IL-4 regulates fibroblast function as well as myofibroblast differentiation22. In human studies, progression of IPF is associated with augmented IL-4 production23. IL-4-/- mice develop significantly less PF after treatment with bleomycin24. Moreover, IL-4 stimulates TGF-β production in a mouse model of skin fibrosis25. In our studies, levels of TGF-β and IL-4 were higher in innervated lungs than denervated lungs (Fig. 5).
The fibrogenic process engages both resident and recruited myofibroblasts and M2 macrophages. Myofibroblasts are characterized by the formation of a contractile apparatus (α-SMA microfilaments) and secretion of extracellular matrix components (type I collagen). Induction and maintenance of the M2 macrophages, which express CD206 (macrophage mannose receptor), is a characteristic feature of PF26. Our results show that the number of myofibroblasts and the percentages of CD206 + cells in macrophages (F4/80 + cells) were higher in the vagus-intact lung. Furthermore, lymphocytes, a key source of IL-4, were more numerous in the intact lung than the vagotomized lung.
The role of inflammation in IPF is controversial. Fibrosis may occur without inflammation and anti-inflammatory or immunosuppressive therapy has not been effective for IPF27. However, inflammation is part of the pathological process in bleomycin-induced PF animal models. This is evidenced by a significant increase in the number and percentage of lymphocytes in BALF following intra-tracheal instillation of bleomycin28. In the present study, we also found that inflammatory cells increased after intravenous treatment with bleomycin. However, cellular alterations in BALF appeared to be inversely related to severity of PF (i.e. the vagotomized lung had more inflammatory cells but less fibrosis). It needs to be emphasized that the percentage of lymphocytes was lower in vagotomized than intact lungs. This is consistent with a human study showing that T lymphocytes are increased in the areas of interstitial fibrosis and worsen PF29.
We should highlight the fact that our results show that the vagus nerve does not drive the development of PF, but modifies it, because PF develops with or without vagus innervation. The degree of fibrosis, as measured by the ratio of area of fibrosis to total lung area, is 25% higher in vagus intact lungs than vagotomized lungs. Vagotomy reduced bleomycin-induced collagen deposition by 30%. This effect is not trivial and is comparable with the effect of blocking TGF-β, one of the most important fibrogenic molecules. For example, injection of anti-TGF-β antibody into the mouse was able to neutralize the fibrogenic effect of TGF-β by virtue of a 40% reduction in collagen accumulation30. Neutralizing TGF-β with a chimeric soluble receptor decreased lung hydroxyproline by 33%31. Epithelium-specific deletion of TGF-β receptor type II attenuated collagen production by 50%32. Compared to these studies, it appears that the vagus nerve is a powerful modulator in the development of PF.
Bleomycin, an antibiotic, is a cytotoxic agent used to treat a variety of neoplasms. Its cytotoxicity occurs mainly in the lung. Thus, it is commonly used for studying human PF in C57BL/6. Following bleomycin treatment the lung undergoes significant biochemical, histological and physiological changes, which are similar to those of humans, leading to PF. Thus, this model can provide useful insights into the mechanisms of lung injury, repair, and fibrosis. However, the fibrosis in the mouse resolves. Thus, direct extrapolation of mouse data into humans is dangerous. Furthermore, our current intervention only substantiates a potentially effective preventative strategy, however, whether it can be used as a treatment procedure awaits verification.
One concern is that vagotomy lacks clinical application. However, we use vagotomy as a tool to examine the neural mechanisms in PF. Our data demonstrate that removing the nerve actually ameliorates bleomycin-induced fibrosis. Therefore, further investigation to delineate effects of vagal afferent and efferent nerves is warranted. More questions need to be addressed regarding specific neural transmitters and peptides involved in the observed effects.
In summary, we have established a mouse model to assess the role of the vagus nerve in the development of PF. Our data demonstrate that the vagus nerve is a modulator for PF development. Since vagotomy decreases deposition of collagen, we conclude that activation of the vagus nerve promotes PF. This is further confirmed by the decreased numbers of fibrogenic cells (myofibroblasts and M2 macrophages) and decreased production of fibrogenic cytokines (TGF-β and IL-4) in the vagotomized lung.
Methods
Animals
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, 20 weeks, 30–40 g) were housed 4 in one acrylic cages with shredded corn cob bedding in an acclimatized room (12/12 h light/dark cycle; 22 ± 3°C) and provided with water and mouse breeder chow ad libitum, according to standard protocols for animal care. The procedures used were approved by the Institutional Animal Care and Use Committees of the University of Louisville and the Robley Rex VA Medical Center, in compliance with the United States Public Health Service Standards and National Institutes of Health guidelines.
Mid-cervical vagotomy
To eliminate potential difference between the two lungs we randomly divided 50 mice into left vagotomy (LV) and right vagotomy (RV) groups. After anesthesia with 3% isoflurane using a veterinary anesthesia system, left or right vagus nerve (a minimum of 5 mm) was isolated and sectioned at the mid-cervical level33. Analgesic was provided for two days. Two weeks were allowed for recovery before bleomycin treatment.
Bleomycin treatment
Mice were anesthetized with 3% isoflurane and injected with bleomycin via the tail vein (80 mg/kg body weight, dissolved in 200 μl of 0.9% NaCl solution)34. For control mice, 200 μl of 0.9% NaCl solution was injected. Body weight was measured every other day. Mice were sacrificed in 2, 3 and 4 weeks.
Blood distribution assessment
Unilaterally vagotomized mice were anesthetized and injected with Evans Blue (EB, 50 mg/ml at 50 mg/kg) via the tail vein. Lung tissues were harvested 10 minutes after injection and oven-dried. EB in the tissue was extracted in formamide (50 ml/g tissue). EB concentrations were measured with a spectrophotometer at a wavelength of 620 nm.
Morphological examination
The lung was fixed by intratracheal infusion of 4% paraformaldehyde and then immersed in fixative overnight. Transverse sections from each side of the lung were embedded in paraffin and 5 μm sections were prepared and stained with haematoxylin-eosin (H&E) and Masson’s trichrome. The intact image of total lung transverse slice was scanned with an Olympus microscope. Fibrotic lung injury was assessed by Ashcroft scoring and quantitative analysis by two investigators blinded to tissue samples.
Ashcroft scoring
Each side of the lung was individually assessed for severity of interstitial fibrosis and given a score between 0–8 with the Ashcroft scoring system as described: grade 0-normal lung; grade 1-minimal fibrous thickening of alveolar or bronchiolar walls; grade 3-moderate thickening of the walls without obvious damage to lung architecture; grade 5-increased fibrosis with definite damage to lung structure and formation of fibrosis or small fibrous masses; grade 7-severe distortion of lung structure and large fibrous areas; grade 8-total fibrous obliteration of the field35. Difficulty in deciding between odd-numbered categories was resolved by giving the intervening even-numbered grade.
Quantitative analysis
(a) Collagen fraction: the degree of fibrosis was quantified with the photoshop image program as previously described and by adjusting the threshold of color settings28. The image analysis program was configured to detect areas of blue-stained collagen within each lung transverse section stained by Masson’s trichrome. By reverse selection of the background, the software calculated the area of lung parenchyma. The fractional area of collagen was expressed as a percentage of the total area of lung parenchyma. (b) Lung involvement fraction: Lung involvement was defined as definite distortion of lung architecture36. Using the cellsens dimension program, areas of lung involvement in the total lung section were circled and calculated by the polygon method. Lung involvement fraction was expressed as a percentage of the total area of lung sections.
Bronchoalveolar lavage
The left and right lungs were separated and lavaged37. A tracheal cannula attached to a 1 ml syringe was used to lavage three times [1.2 ml (0.4 × 3) aliquots of 1 × HBSS without Ca2 + or Mg2 + for the right lung and 0.6 ml (0.2 × 3) for the left lung]. Lavage cells were collected, and centrifuged at 200 g for 5 minutes at 4 °C. The cells from the right and left lungs were suspended in 1.2 ml and 0.6 ml fresh HBSS, respectively and centrifuged onto glass slides, and stained with a Diff-Quick Staining kit (Fisher Scientific, MA, USA ). The total number and classification of cells from both sides of the lung were counted and analyzed separately.
Immunofluorescence staining
Tissue sections were deparaffinized and rehydrated using sequential xylene and ethanol rinses. After antigen retrieval in 0.01 M citrate buffer, pH6.0, 98 °C for 15 min (bronchoalveolar lavage fluid (BALF) was smeared and fixed in methanol), the sections were pre-incubated with 5% normal goat serum to block nonspecific binding and then incubated with primary antibodies (rabbit anti-mouse α-SMA, goat anti-CD206 antibody and FITC conjugated rat anti F4/80 antibody) at 4 °C overnight. Extensive washing was followed by FITC-conjugated goat anti-rabbit IgG or cy3-donkey anti goat IgG (1:200) for 1 h at room temperature. The numbers of α-SMA-positive cells in lung sections and CD206 +/F4/80 + cells in BALF smears were counted at 200 × magnification in lung specimens obtained from each lung side (n = 3 in each group). Six fields from each side were counted. Positive cell counts and proportions were expressed as the average number of cellular profiles per field.
Western Blotting
Lung tissue samples containing 20 μg protein were electrophoresed upon polyacrylamide gel and electro-blotted onto nitrocellulose membranes. Then, membranes were incubated with rabbit anti-TGF-β, IL-4 and β-actin antibodies. After reacting with peroxidase-conjugated secondary antibodies, blots were developed using Hybond-Electrochemiluminescence.
ELISA
The concentrations of collagens COL1α1and COL1α2 in lung tissue supernatants were determined by ELISA (Uscn Life Science Inc., Hubei, PRC) in duplicate in accordance with manufacturer’s recommendations. The concentrations were corrected for protein concentration. The lowest limit of detection was 0.156 ng/ml.
Statistical Analysis
Data were presented in Means ± SEM and analyzed using SPSS software. Statistical analyses including paired t-test (between vagotomized and intact lungs) and independence t-test (between different groups) were regarded as significant when p < 0.05.
Additional Information
How to cite this article: Song, N. et al. Vagotomy attenuates bleomycin-induced pulmonary fibrosis in mice. Sci. Rep. 5, 13419; doi: 10.1038/srep13419 (2015).
Acknowledgments
This work was funded by V.A. Merit Review (PULM-029-10S) and University of Louisville (RIG 50766).
Footnotes
Author Contributions N.S. and J.L. were involved in data collection and analysis, and writing the manuscript. N.S., J.L., S.S., D.L., M.P., J.R. and J.Y. participated in the discussion of the research progress at different stages, involved in data interpretation and reversion of the article and approved the final version to be published.
References
- Biernacka A., Dobaczewski M. & Frangogiannis N. G. TGF-beta signaling in fibrosis. Growth Factors 29, 196–202 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempowski G. D., Beckmann M. P., Derdak S. & Phipps R. P. Subsets of murine lung fibroblasts express membrane-bound and soluble IL-4 receptors. Role of IL-4 in enhancing fibroblast proliferation and collagen synthesis. The Journal of Immunology 152, 3606–3614 (1994). [PubMed] [Google Scholar]
- Khalil N., Whitman C., Zuo L., Danielpour D. & Greenberg A. Regulation of alveolar macrophage transforming growth factor-beta secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest 92, 1812–1818 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaee-Kermani M., Nozaki Y., Hatano K. & Phan S. H. Lung interleukin-4 gene expression in a murine model of bleomycin-induced pulmonary fibrosis. Cytokine 15, 138–147 (2001). [DOI] [PubMed] [Google Scholar]
- Phan S. H. The myofibroblast in pulmonary fibrosis. Chest Journal 122, 286S–289S (2002). [DOI] [PubMed] [Google Scholar]
- Kuhn C. & McDonald J. A. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 138, 1257–1265 (1991). [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. et al. Alpha-smooth muscle actin in parenchymal cells of bleomycin-injured rat lung. Lab Invest. 60, 643–650 (1989). [PubMed] [Google Scholar]
- Goerdt S. & Orfanos C. E. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 10, 137–142 (1999). [DOI] [PubMed] [Google Scholar]
- Komohara Y., Ohnishi K., Kuratsu J. & Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol. 216, 15–24 (2008). [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nature Reviews Immunology 3, 23–35 (2003). [DOI] [PubMed] [Google Scholar]
- Murray L. A. et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS. One. 5, e9683 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L. A. et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int. J. Biochem. Cell Biol. 43, 154–162 (2011). [DOI] [PubMed] [Google Scholar]
- Corcoran C., Connor T. J., O’ Keane V. & Garland M. R. The effects of vagus nerve stimulation on pro- and anti-inflammatory cytokines in humans: a preliminary report. Neuroimmunomodulation 12, 307–309 (2005). [DOI] [PubMed] [Google Scholar]
- Fujikawa T. et al. Inhibition of fatty acid oxidation activates transforming growth factor-beta in cerebrospinal fluid and decreases spontaneous motor activity. Physiology & Behavior 101, 370–375 (2010). [DOI] [PubMed] [Google Scholar]
- Lam H. B., Yeh C. H., Cheng K. C., Hsu C. T. & Cheng J. T. Effect of cholinergic denervation on hepatic fibrosis induced by carbon tetrachloride in rats. Neuroscience Letters 438, 90–95 (2008). [DOI] [PubMed] [Google Scholar]
- Song N. et al. The vagus nerve and pulmonary disease. Fudan University Journal of Medical Science 39, 117–122 (2012). [Google Scholar]
- Ohira H. et al. Vagal nerve activity as a moderator of brain-immune relationships. Journal of Neuroimmunology 260, 28–36 (2013). [DOI] [PubMed] [Google Scholar]
- Lee J. et al. Mechanisms of carvacrol-induced expression of type I collagen gene. J Dermatol Sci 52, 160–169 (2008). [DOI] [PubMed] [Google Scholar]
- Huang L. S. et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. The FASEB Journal 27, 1749–1760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. B. & Hogaboam C. M. Murine models of pulmonary fibrosis. American Journal of Physiology—Lung Cellular and Molecular Physiology 294, L152–L160 (2008). [DOI] [PubMed] [Google Scholar]
- Ueno M. et al. Hypoxia-inducible factor-1α mediates TGF-β-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. American Journal of Physiology—Lung Cellular and Molecular Physiology 300, L740–L752 (2011). [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Holness M. A., Katai H. & Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest 90, 1479–1485 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Miyazaki E., Fukami T., Kumamoto T. & Tsuda T. Interleukin-4-producing cells in idiopathic pulmonary fibrosis: An immunohistochemical study. Respirology 4, 383–391 (1999). [DOI] [PubMed] [Google Scholar]
- Huaux F. O., Liu T., McGarry B., Ullenbruch M. & Phan S. H. Dual roles of IL-4 in lung injury and fibrosis. The Journal of Immunology 170, 2083–2092 (2003). [DOI] [PubMed] [Google Scholar]
- Kodera T., McGaha T. L., Phelps R., Paul W. E. & Bona C. A. Disrupting the IL-4 gene rescues mice homozygous for the tight-skin mutation from embryonic death and diminishes TGF-production by fibroblasts. Proceedings of the National Academy of Sciences 99, 3800–3805 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechkovsky D. V. et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clinical Immunology 137, 89–101 (2010). [DOI] [PubMed] [Google Scholar]
- Luzina I. G., Todd N. W., Iacono A. T. & Atamas S. P. Roles of T lymphocytes in pulmonary fibrosis. Journal of Leukocyte Biology 83, 237–244 (2008). [DOI] [PubMed] [Google Scholar]
- Izbicki G., Segel M. J., Christensen T. G., Conner M. W. & Breuer R. Time course of bleomycin-induced lung fibrosis. International Journal of Experimental Pathology 83, 111–119 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra E. R., Kairalla R. A., Ribeiro de Carvalho C. R., Eher E. & Capelozzi V. L. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration 74, 159–169 (2007). [DOI] [PubMed] [Google Scholar]
- Giri S. N., Hyde D. M. & Hollinger M. A. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax 48, 959–966 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. et al. Reduction of bleomycin induced lung fibrosis by transforming growth factor β soluble receptor in hamsters. Thorax 54, 805–812 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest 121, 277–287 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M., Danielsson, Axelsson H. & Qian B.-F. Neuroendocrine peptide levels in the gastrointestinal tract of mice after unilateral cervical vagotomy. Regulatory Peptides 88, 15–20 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang X., Shimura S., Masuda T., Saitoh H. & Shirato K. Antisense oligonucleotides to nf-kappa b improve survival in bleomycin-induced pneumopathy of the mouse. Am. J. Respir. Crit. Care Med. 162, 1561–1568 (2000). [DOI] [PubMed] [Google Scholar]
- Ashcroft T., Simpson J. M. & Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. Journal of Clinical Pathology 41, 467–470 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib S. A. et al. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. Journal of Leukocyte Biology 95, 9–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimark B. D. et al. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. The Journal of Immunology 160, 4580–4586 (1998). [PubMed] [Google Scholar]