ABSTRACT
The bacterial stringent response (SR) is a conserved stress tolerance mechanism that orchestrates physiological alterations to enhance cell survival. This response is mediated by the intracellular accumulation of the alarmones pppGpp and ppGpp, collectively called (p)ppGpp. In Enterococcus faecalis, (p)ppGpp metabolism is carried out by the bifunctional synthetase/hydrolase E. faecalis Rel (RelEf) and the small alarmone synthetase (SAS) RelQEf. Although Rel is the main enzyme responsible for SR activation in Firmicutes, there is emerging evidence that SASs can make important contributions to bacterial homeostasis. Here, we showed that RelQEf synthesizes ppGpp more efficiently than pppGpp without the need for ribosomes, tRNA, or mRNA. In addition to (p)ppGpp synthesis from GDP and GTP, RelQEf also efficiently utilized GMP to form GMP 3′-diphosphate (pGpp). Based on this observation, we sought to determine if pGpp exerts regulatory effects on cellular processes affected by (p)ppGpp. We found that pGpp, like (p)ppGpp, strongly inhibits the activity of E. faecalis enzymes involved in GTP biosynthesis and, to a lesser extent, transcription of rrnB by Escherichia coli RNA polymerase. Activation of E. coli RelA synthetase activity was observed in the presence of both pGpp and ppGpp, while RelQEf was activated only by ppGpp. Furthermore, enzymatic activity of RelQEf is insensitive to relacin, a (p)ppGpp analog developed as an inhibitor of “long” RelA/SpoT homolog (RSH) enzymes. We conclude that pGpp can likely function as a bacterial alarmone with target-specific regulatory effects that are similar to what has been observed for (p)ppGpp.
IMPORTANCE Accumulation of the nucleotide second messengers (p)ppGpp in bacteria is an important signal regulating genetic and physiological networks contributing to stress tolerance, antibiotic persistence, and virulence. Understanding the function and regulation of the enzymes involved in (p)ppGpp turnover is therefore critical for designing strategies to eliminate the protective effects of this molecule. While characterizing the (p)ppGpp synthetase RelQ of Enterococcus faecalis (RelQEf), we found that, in addition to (p)ppGpp, RelQEf is an efficient producer of pGpp (GMP 3′-diphosphate). In vitro analysis revealed that pGpp exerts complex, target-specific effects on processes known to be modulated by (p)ppGpp. These findings provide a new regulatory feature of RelQEf and suggest that pGpp may represent a new member of the (pp)pGpp family of alarmones.
INTRODUCTION
In order to survive under adverse environmental conditions, such as nutrient starvation, bacteria have evolved complex, interconnected regulatory networks that sense and integrate internal and external metabolic cues to activate cellular responses that enhance bacterial survival. One critical and highly conserved mechanism employed by bacteria to cope with nutritional as well as a variety of environmental and chemical stresses is the stringent response (SR). The SR is mediated by the accumulation of two guanine analogs derived by the addition of the β-γ phosphates from ATP to the ribose 3′ hydroxyl of GTP or GDP, generating guanosine pentaphosphate (pppGpp) and guanosine tetraphosphate (ppGpp) [abbreviated (p)ppGpp] (1, 2). The intracellular accumulation of (p)ppGpp coordinates the remodeling of cellular physiology as it changes from a state of rapid growth to slow growth or stasis to ensure survival. These physiological adjustments occur at the levels of transcription, translation, DNA replication, and general metabolism (3, 4). During stress, the transcription of genes required for rapid growth, such as those encoding rRNAs, is limited by (p)ppGpp accumulation along with a concomitant activation of genes involved in amino acid biosynthesis, alternate nutrient transport, and general stress responses (3, 5). In addition, (p)ppGpp leads to rapid physiological adjustments by inhibiting enzymes involved in protein synthesis, DNA replication, and nucleotide synthesis, especially GTP (6–11).
The metabolism of (p)ppGpp is orchestrated by members of the RelA/SpoT homolog (RSH) protein family; this family is divided into two categories, “long” multidomain RSHs and “short” single-domain RSHs, with the latter category comprised of small alarmone synthetases (SASs) and small alarmone hydrolases (SAHs) (12). In Gammaproteobacteria such as Escherichia coli, the SR is mediated by two “long” RSHs, RelA (1) and SpoT (13). RelA is a large monofunctional synthetase that is rapidly activated during amino acid starvation through interactions with stalled ribosomes containing deacylated tRNA bound to the A site (14). SpoT is a similar-size bifunctional RelA homolog with strong hydrolase and weak synthetase activities. The balance of SpoT activities is altered by other sources of stress (15–17). By far the most widespread “long” RSH is the bifunctional synthetase/hydrolase Rel (also known as RSH) (12). The bifunctional Rel is responsible for the rapid accumulation of (p)ppGpp during amino acid starvation through what is believed to be interaction with stalled ribosomes by extension from work on the Mycobacterium tuberculosis Rel (RelMtb) enzyme and by the ability to provoke the SR using inhibitors of tRNA aminoacylation (18, 19). Regulation of the (p)ppGpp hydrolytic activity of Rel is less well understood. In vitro, the hydrolytic activity of RelMtb seems to be insensitive to ribosomal and tRNA signals (18), which can be interpreted as meaning either that hydrolytic activity is constitutive or that the regulatory partners are yet to be discovered. In the most thoroughly characterized Rel enzyme, that from Streptococcus equisimilis (RelSeq), the hydrolase and synthetase activities are reciprocally regulated with distinct conformations corresponding to the hydrolase or synthase active form (20). In this case, hydrolase activity is neither constitutive nor regulated in isolation of synthetase but instead is linked to synthetase regulation.
Among Firmicutes, (p)ppGpp metabolism is mediated by Rel and one or two weak SASs, termed RelP and RelQ, that lack both the N-terminal hydrolase and C-terminal ribosome interaction domains of “large” RSH enzymes (12, 19, 21, 22). There is emerging evidence that SASs make important contributions to bacterial physiology despite the dominant role of Rel in SR activation (23). For example, SASs were shown to be required for fully efficient SR induction, GTP homeostasis, growth rate control, tolerance to cell wall antibiotics, and virulence (10, 21, 24–29). Moreover, the stable genomic coexistence of the bifunctional Rel with one or two SASs in a large number of bacteria indicates an evolutionary pressure that favors retention of the Rel-SAS (p)ppGpp synthetase redundancy. In principle, this might occur for a number of reasons. For example, SASs might respond to different internal or external cues than the Rel enzyme. In support, there is evidence of transcriptional induction of the Bacillus subtilis, Staphylococcus aureus, and Streptococcus mutans SASs under alkaline, cell wall, or oxygen stresses, respectively (22, 24, 30). Alternatively, they might differ from long RSH enzymes because their constitutive synthetase activity ensures persistent basal (p)ppGpp production (21, 28). A third possibility is they catalyze synthesis of other nucleotides that provide unique regulatory properties not shared by (p)ppGpp. The goal of the work presented here was to explore these possibilities using Enterococcus faecalis, in which (p)ppGpp metabolism is carried out by the bifunctional E. faecalis Rel (RelEf; previously called RSH) and the SAS RelQEf (31). To accomplish this goal, we carried out the biochemical characterization of the RelQEf enzyme and found that in addition to catalyzing the synthesis of ppGpp and pppGpp from GDP and GTP, respectively, RelQEf is able to efficiently use GMP to synthesize GMP 3′-diphosphate (pGpp). Furthermore, the availability of these compounds was exploited to demonstrate that pGpp has regulatory properties which are capable of exerting control over several processes known to be altered by (p)ppGpp.
MATERIALS AND METHODS
Bacterial strains and purification of (pp)pGpp metabolic enzymes.
Escherichia coli strains (see Table S1 in the supplemental material) were routinely grown in Bacto Luria-Bertani (LB) broth (Becton, Dickinson, and Company) supplemented with 100 μg ml−1 ampicillin at 37°C under normal atmosphere with shaking at 200 rpm. For the overexpression of Rel and RelQ from E. faecalis or of RelQ and RelP from S. mutans, coding regions were amplified by PCR using primers containing NheI and XhoI restriction sites (see Table S2 in the supplemental material) and ligated into pET21a (EMD Biosciences, Madison, WI). The ligation mix containing pET21a expressing the (pp)pGpp metabolic enzymes was transformed into chemically competent E. coli BL21(λDE3) according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). The resulting plasmids (see Table S2 in the supplemental material) remove the native stop codon, fusing the coding region to a C-terminal His tag and place the coding region under dual control of a T7 promoter and lac operator. All E. coli cultures were grown in LB broth with antibiotic selective pressure until they reached an optical density at 600 nm (OD600) of 0.5. Expression of the fusion protein was induced by treatment with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h. Cells were then harvested by centrifugation and washed once in chilled phosphate-buffered saline (PBS) (pH 7), and cell pellets were lysed using a mini-bead beater (BioSpec Products, Bartlesville, OK). Proteins were purified under native conditions using Ni-nitrilotriacetic acid (Ni-NTA)–agarose (Qiagen, Valencia, CA) and dialyzed into buffer containing 20 mM Tris base (pH 8), 500 mM NaCl, 1 mM EDTA, and 10% glycerol. The dialyzed protein was then separated into single-use aliquots and stored at −80°C. In all cases, the N-terminal affinity tag was left intact.
In vitro (pp)pGpp synthesis.
Unless otherwise indicated, in vitro synthesis of (pp)pGpp was carried out by adding 3 μM purified Rel, RelQ, or RelP to a reaction mix containing 0.05 μCi μl−1 of [γ-32P]ATP, 25 mM Bis-Tris-propane (pH 9), 150 mM NaCl, and MgCl2 at concentrations equivalent to the overall purine nucleotide concentration. See the figure legends for the concentrations of individual purine nucleotides used in each experiment. For the Mg2+ inhibition assay, reaction mixtures contained the same components described above with the addition of 0.5 mM ATP, 0.5 mM GTP, and MgCl2 at concentrations ranging from 0 to 20 mM. Reaction mixtures were incubated at 37°C for the indicated periods of time, and then (pp)pGpp synthesis was terminated by the addition of 1/4 volume 2 M formic acid (FoA). Control reactions were run under identical conditions for the duration of the experiment, including FoA acidification, but the mixtures contained no enzyme. Acidified reaction mixtures were spotted onto polyethyleneimine (PEI)-cellulose plates (J. T. Baker/Avantor Performance Materials, Center Valley, PA) and, unless otherwise noted, separated in 1.25 M KH2PO4 (pH 3.4). Reaction products were then visualized using a phosphorimager (Molecular Imager FX; Bio-Rad, Hercules, CA).
Alkali hydrolysis.
Alkali hydrolysis reactions were carried out as described previously (32). Briefly, to an aliquot of in vitro-synthesized [32P]pGpp was added a solution containing 0.5 M NaOH and 10 mM MgCl2, and incubation took place at 37°C for the indicated time period. A control reaction mixture lacking NaOH was used to monitor for inherent pGpp instability in the presence of divalent cations. Samples from the alkali hydrolysis reactions were stopped by the addition of 1/4 volume 2 M FoA. Acidified reaction products were resolved by thin-layer chromatography (TLC), visualized, and quantitated as described above.
Rel (pp)pGpp hydrolysis assays.
Labeled [32P]pGpp, ppGpp, or pppGpp was synthesized from 200-μl reaction mixtures containing 2 mM GMP, GDP, or GTP as the pyrophosphate acceptor and 10 μCi [γ-32P]ATP. An excess of pyrophosphate donor (GMP, GDP, or GTP) was used to ensure that all [32P]ATP was consumed and could not serve as a pyrophosphate donor for Rel-mediated (pp)pGpp production during degradation experiments. Synthesis reaction products were visualized and quantified by phosphorimaging to confirm [32P]ATP consumption and equalize the amount of labeled pGpp, ppGpp, and pppGpp product added to each degradation assay, respectively. The labeled (pp)pGpp was transferred into a reaction mix containing 150 nM RelEf, 25 mM Bis-Tris-propane (pH 9), 150 mM NaCl, and 1 mM MnCl2. Reaction mixtures were incubated at 37°C, and at the indicated time points, aliquots were removed and mixed with 1/4 volume 2 M FoA to halt (pp)pGpp degradation. Acidified aliquots were spotted onto PEI-cellulose TLC plates and resolved from the labeled degradation product inorganic pyrophosphate (PPi) in either 4 M (NH4)2SO4 for pGpp or 1.25 M KH2PO4 (pH 3.4) for (p)ppGpp. Reaction products were visualized and quantified using phosphorimaging as described above.
(pp)pGpp preparations.
To make pure (pp)pGpp, 5-ml RelQEf-catalyzed in vitro synthesis reaction mixtures were prepared as described above with minor modifications, including the removal of [γ-32P]ATP and the addition of 1 mg ml−1 bovine serum albumin (BSA) to enhance the long-term protein stability and activity. For ppGpp and pppGpp, reaction mixtures contained 6 mM unlabeled ATP and 4 mM GDP or GTP, respectively. For pGpp synthesis, reaction mixtures contained 4 mM ATP and 6 mM GMP. The reaction mixtures were incubated at 37°C and monitored for the complete consumption of the limiting nucleotide by TLC. Reaction mixtures were then diluted 1:3 into 10:1 (mM) Tris-EDTA at pH 7.5 and bound to DEAE-Sephadex A25. All of the following wash and elution buffers were made in 10:1 Tris-EDTA (pH 7.5). The column was washed with 5 volumes of 0.1 M LiCl, and (pp)Gpp was eluted in 0.5 M LiCl by the successive addition of 0.25-ml aliquots that were allowed to drip into 1 ml of absolute ethanol. Elutions were continued until the formation of white precipitate was no longer observed. Tubes were then placed on ice for 30 min to complete precipitation of (pp)pGpp. Purified (pp)pGpp was pelleted by centrifugation, washed 3 times with absolute ethanol to remove LiCl, and dried in a fume hood. A small aliquot of (pp)pGpp was resuspended in 0.5 M FoA, separated by TLC using 1.25 M KH2PO4, and visualized by shortwave UV light to confirm the purity of each compound.
Inhibition of HprT and Gmk enzymes.
Expression and purification of the E. faecalis HprT and GMP kinase 2 (Gmk-2) enzymes have been previously published (28). The gene encoding Gmk-1 from E. faecalis OG1RF was cloned into pLICTrPC-HA (33) using a ligation-independent cloning technique, and the recombinant plasmids were transformed into E. coli BL21(DE3). Cells were grown from a single colony at 37°C in LB supplemented with 100 μg ml−1 carbenicillin to an OD600 of ∼0.6, and IPTG was added to a final concentration of 1 mM. Cells were grown for another 3 h before harvest. Proteins were purified using Ni-NTA spin columns (Qiagen) following the manufacturer's instructions.
HprT reactions were performed at 25°C in a 100-μl reaction mix containing 100 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 1 mM 5-phosphoribosyl 1-pyrophosphate (PRPP), 50 μM guanine, 20 nM purified HprT enzyme, and various (pp)pGpp concentrations. Reactions were initiated by adding the enzyme and monitored for 10 min by measuring the change of absorbance at 257 nm in a temperature-controlled spectrophotometer (Shimadzu UV-2401PC). Gmk reactions were performed at 25°C in a 100-μl mix containing 100 mM Tris (pH 7.5), 100 mM KCl, 10 mM MgCl2, 4 mM ATP, 1.5 mM phospho(enol)pyruvic acid, 250 μM NADH, 2 U pyruvate kinase (from rabbit muscle; Sigma), 2.64 U l-lactic dehydrogenase (from bovine heart; Sigma), and 10 nM E. faecalis Gmk-1 or Gmk-2. Reactions were initiated by adding either GMP or Gmk and monitored for 5 min by measuring the A340 in a temperature-controlled spectrophotometer. For inhibition curves, GMP was used at 50 μM and the pGpp concentration was varied. Data fitting was done as previously described (10). For control experiments, pyruvate kinase was used at 0.02 U per 100-μl reaction mixture and tested at 25°C. Reactions were initiated by adding GMP and monitored for 10 min by measuring the change in absorbance at 340 nm.
Inhibition of rrnB transcription.
E. coli rrnB P1 promoter activity was measured by in vitro transcription essentially as described previously (34). Briefly, the reactions were carried out in a buffer consisting of 50 mM Tris-acetate (pH 8.0), 10 mM Mg acetate, 10 mM β-mercaptoethanol, and 10 mg ml−1 BSA, and 30 nM E. coli RNA polymerase (RNAP) (Epicentre Technologies) was preincubated with the indicated concentrations of pGpp, ppGpp, or GDP for 7 min at room temperature. Next, 10 nM linear DNA template, nucleoside triphosphates (NTPs) (100 μM ATP, CTP, and GTP, 10 μM UTP, and 2 μCi [per reaction] [α-32P]UTP [PerkinElmer]), salt (90 mM potassium glutamate), and 300 nM DksA were added. The reactions were carried out at 30°C for 8 min and were terminated by the addition of an equal volume of the stop solution (95% formamide, 20 mM EDTA, 0.05% bromphenol blue, and 0.05% xylene cyanol). Samples were analyzed on 7 M urea–6% polyacrylamide sequencing gels and quantified using a phosphorimager (GE Healthcare imaging system).
Synthesis of relacin.
Relacin was prepared by a modification of the method of Wexselblatt et al. (58) (Fig. 1). First, commercially available diglycine (compound 1) was esterified with benzylalcohol by refluxing in toluene in a Dean-Stark apparatus under toluene sulfonic acid catalysis, affording benzyl ester (compound 2). Commercially available 2-N-isobutyryl-2-deoxyguanosine was reacted with carbonyldiimidazole (CDI) and subsequently with benzyl ester 2 in dimethylformamide (DMF), giving intermediate 4. Relacin was obtained by catalytic hydrogenation over palladium on charcoal in ethanol (for removal of benzyl esters) and converted to its sodium salt by passing through a column of Dowex 50 in Na+ form.
FIG 1.
Synthesis of relacin.
Regulation of RelAEc and RelQEf synthetase activity by pGpp, ppGpp, and relacin.
The experiments were performed essentially as described in reference 36. If not stated otherwise, experiments were performed with 250 nM RelQ or 30 nM RelA, 0.5 μM E. coli 70S ribosomes, 0.3 mM 3H-labeled GDP (ARC), 1 mM ATP, 100 μM ppGpp, and 0.5 mg ml−1 BSA. The reaction mixture was preincubated at 37°C for 2 min before the reaction was started by addition of ATP. Radiolabeled ppGpp product was separated from GDP by TLC and quantified using scintillation counting. All experiments were performed from three to five times.
RESULTS
RelQEf prefers GDP over GTP as a substrate and is insensitive to high Mg2+ concentrations.
We previously observed that in the absence of RelEf, constitutive (p)ppGpp synthesis by RelQEf led to an approximately 4-fold increase in basal (p)ppGpp levels (28). Interestingly, this elevated basal level consisted primarily of ppGpp, whereas pppGpp predominates in wild-type cells with both Rel and RelQEf (28). This result suggested that RelQEf prefers GDP over GTP as a substrate. However, there are other explanations for removal of the 5′ γ-phosphate from pppGpp, generating ppGpp, such as activities of nonspecific phosphohydrolases, including polyphosphate phosphatase, Nudix family hydrolases, or GTP-requiring enzymes for protein synthesis such as EF-G or EF-Tu (37–40). A preference for GDP over GTP as an in vitro substrate has been observed for SASs from Mycobacterium smegmatis and S. aureus (24, 41). Of note, the ability of RelQEf to synthesize (p)ppGpp in vitro was previously documented, but the relative efficiency of substrate utilization has not been (40).Therefore, we determined the efficiency of GTP versus GDP as a substrate for RelQEf synthesis of pppGpp and ppGpp (Table 1). The experiments were performed using various guanine nucleotide substrate concentrations in the presence of constant amounts of either 100 μM pppGpp or ppGpp. The kcat, Km, and specificity constant kcat/Km were calculated to determine substrate preferences of RelQEf (42). RelQEf has a moderate preference toward GDP as a substrate over GTP, but the strength of this preference (kcat/Km) is dependent on the nature of the alarmone present. In the presence of ppGpp, RelQEf has an approximately 2-fold higher specificity for GDP [kcat/Km(GDPppGpp) of 2.08 ± 0.82 mM−1 s−1 versus kcat/Km(GTPppGpp) of 1.19 ± 0.43 mM−1 s−1], while pppGpp resulted in an approximately 4-fold-higher specificity for GDP [kcat/Km(GDPpppGpp) of 3.90 ± 1.28 mM−1 s−1 versus kcat/Km(GTPpppGpp) of 0.87 ± 0.30 mM−1 s−1]. Direct-competition experiments, comparing (p)ppGpp production with individual substrates (see Fig. S1A in the supplemental material) to (p)ppGpp production when both GTP and GDP are present as substrates simultaneously, support the GDP preference (see Fig. S1B in the supplemental material).
TABLE 1.
Kinetic constants for pGpp, ppGpp, and pppGpp synthesis reactions catalyzed by E. faecalis SAS enzyme RelQEf in the presence of ppGpp or pppGppa
| Substrate | Cofactor | kcat (s−1) | Km (mM) | kcat/Km (mM−1 s−1) | Efficiency (relative to GMP) |
|---|---|---|---|---|---|
| GTP | ppGpp | 0.58 ± 0.07 | 0.49 ± 0.17 | 1.19 ± 0.43 | 1.14 |
| pppGpp | 0.74 ± 0.1 | 0.85 ± 0.27 | 0.87 ± 0.3 | 0.46 | |
| GDP | ppGpp | 1.34 ± 0.2 | 0.65 ± 0.24 | 2.08 ± 0.82 | 2.0 |
| pppGpp | 0.81 ± 0.07 | 0.21 ± 0.07 | 3.9 ± 1.28 | 2.08 | |
| GMP | ppGpp | 1.28 ± 0.1 | 1.23 ± 0.19 | 1.04 ± 0.18 | 1.0 |
| pppGpp | 1.6 ± 0.12 | 0.85 ± 0.15 | 1.87 ± 0.37 | 1.0 |
All experiments were performed at 37°C with 250 nM RelQEf in Polymix buffer (67) at a 5 mM Mg2+ concentration in the presence of 0.5 mg ml−1 BSA and 100 μM ppGpp or pppGpp.
Previous studies suggested that the GDP substrate preference of monofunctional synthetases is due to the presence of two charge reversals (underlined residues) in the catalytic domain of RelA (EXDD) compared to bifunctional Rel enzymes (RXKD) (43). This charge reversal also renders the monofunctional RelA from E. coli (RelAEc) insensitive to inhibition by Mg2+ ions in excess over the total purine nucleotide concentration, whereas RelMtb was inhibited at higher concentrations of Mg2+ ions (44). The RelQEf enzyme has an EERD motif in its catalytic domain carrying three negative residues, similar to RelAEc but like RelMtb also bears a positive charge at the third residue of the catalytic motif. In vitro synthesis reactions with increasing concentrations of MgCl2 revealed that RelQEf, like RelAEc, is effectively insensitive to excess Mg2+ (see Fig. S2 in the supplemental material). Only under conditions where Mg2+ ions were in 10-fold or 20-fold excess over the total nucleotide concentration was a small reduction in ppGpp synthesis (10% and 22%, respectively) observed compared to that under optimal conditions where purine and MgCl2 were in a 1:1 to 1:5 ratio. For comparison, the synthetase capacities of the bifunctional Rel enzymes of S. equisimilis and M. tuberculosis drop over 50% when Mg2+ is in 2-fold excess to the total purine concentration, and activity is nearly absent once Mg2+ is in 3-fold excess (18, 45). Inhibition of the E. faecalis Rel enzyme (RelEf) synthetase activity at high concentrations of Mg2+ was slightly stronger than that of RelQEf but not as dramatic as previously described for either RelMtb or RelSeq (see Fig. S2 in the supplemental material). The relatively low Mg2+ sensitivity of RelEf compared to other Rel enzymes (RelMtb and RelSeq) may result from biologically meaningful species differences or from differences in the precise in vitro reaction conditions.
pGpp is synthesized by RelQEf from GMP and ATP.
Previous in vitro characterization of the catalytic products of the B. subtilis SAS YwaC (RelP) revealed the production of an unknown compound from radiolabeled [γ-32P]ATP and unlabeled GTP or GDP that migrated almost identically to GTP (22). We reasoned that this GTP-like product could be GMP 3′-diphosphate (pGpp) formed from GMP contamination in the GTP or GDP substrates. Therefore, we decided to assess the ability of RelQEf to utilize GMP as a substrate to synthesize pGpp. The incubation of RelQEf with labeled ATP and unlabeled GMP resulted in the formation of a labeled product migrating as expected for a GTP, most likely pGpp (Fig. 2A). Although pGpp comigrates with GTP when 1.25 M KH2PO4 was used for TLC development (Fig. 2A), the GTP produced by RelQEf was resolved from a GTP standard using 0.75 M KH2PO4 and visualization with UV light, which excludes the possibility that the GTP product was GTP (Fig. 2B). Alternatively, the observed GTP could be an isomer of pGpp, ppGp. These isomers differ with respect to their susceptibility to hydrolysis in alkali: ppGp is stable and pGpp is alkali labile, with hydrolysis products of pGp (which comigrates with GDP) and inorganic phosphate (Pi) (46, 47). We used 0.5 M NaOH with aliquots of in vitro-synthesized GTP from GMP and [γ-32P]ATP. The labeled product is degraded in alkali, which further supports that the synthetic product is pGpp and not ppGp (Fig. 2C).
FIG 2.
Small alarmone synthetases but not RelEf utilize GMP to synthesize pGpp. (A) TLC of an in vitro synthesis reaction mixture containing 2.5 mM ATP, 2.5 mM GMP, and 3 μM each enzyme. Control reaction mixtures (Ct) lack enzyme. (B) TLC separation of the purified pGpp product and a GTP standard. Nucleotides were resolved using 0.75 M KH2PO4 and visualized using shortwave UV light. (C) TLC of in vitro-synthesized pGpp treated with 0.5 M NaOH and sampled at the indicated times. The control (Ct) reaction mixture was not treated with NaOH but was incubated at 37°C for the full 2 h. Ef, E. faecalis; Sm, S. mutans.
The substrate hierarchy of RelQEf is GDP > GMP ≥ GTP.
We now turn to a comparison of the RelQEf enzymatic efficiency toward GMP as a substrate in comparison to GDP and GTP. Table 1 shows that GMP is used as a substrate about as efficiently as GTP in the presence of ppGpp [kcat/Km(GMPppGpp) of 1.04 ± 0.18 mM−1 s−1 versus kcat/Km (GTPppGpp) of 1.19 ± 0.43 mM−1 s−1]. However, in the presence of pppGpp, GMP is preferred over GTP [kcat/Km(GMPpppGpp) of 1.87 ± 0.37 mM−1 s−1 versus kcat/Km(GTPpppGpp) of 0.87 ± 0.3 mM−1 s−1] but is still utilized less efficiently than GDP [kcat/Km(GDPpppGpp) of 3.9 ± 1.3 mM−1 s−1].
We next estimated the efficiency with which RelQEf was able to synthesize pGpp relative to ppGpp and pppGpp with an equimolar total ratio of Mg2+ to total purine nucleotides present, 5 mM each. Equimolar amounts (2.5 mM [each] GMP and GDP or GTP) incubated with RelQEf resulted in greater synthesis of pGpp than of pppGpp but not ppGpp (see Fig. S3A and B in the supplemental material). When incubated with an equimolar (1.7 mM each) mix of all three pyrophosphate acceptors, RelQEf produced ppGpp in the greatest abundance, followed by pGpp and, lastly, pppGpp (see Fig. S3C in the supplemental material). Collectively, these results revealed that the guanine nucleotide substrate preference of RelQEf is GDP > GMP > GTP. We will adopt the term (pp)pGpp when collectively referring to pGpp, ppGpp, and pppGpp, consistent with the commonly used (p)ppGpp for pppGpp and ppGpp.
Three SAS enzymes have different pGpp synthetase specific activities.
To assess if the ability to synthesize pGpp is conserved among other SASs, we purified RelQ and RelP from S. mutans and assayed for pGpp synthetase activity. These assays show that RelQ and RelP are active but show much weaker pGpp synthetase activities than RelQEf, judging from the incubation times required for the first appearance of pGpp (Fig. 2A). RelQEf converts more than half of the ATP to pGpp in 15 min and nearly all of the ATP to pGpp within 1 h. In contrast RelQSm and RelPSm transfer ∼5% or less, respectively, of the label to pGpp after 2 h. Figure S4 in the supplemental material shows the results obtained with equivalent reaction mixtures containing GDP and GTP. It is evident that both RelQSm and RelPSm were able to produce ppGpp and pppGpp, but again the S. mutans enzymes showed weaker synthetase activities than RelQEf. We conclude that both RelQSm and RelPSm can synthesize pGpp but that their in vitro (pp)pGpp synthetic activities are markedly lower than RelQEf.
The E. faecalis Rel enzyme has no detectable pGpp synthetase activity.
The full-length RelEf from E. faecalis was included in the comparison shown in Fig. 2 to ask if it too was able to synthesize pGpp in vitro, but as expected, pGpp synthetase activity was undetectable. Figure S5 in the supplemental material shows that RelEf is able to synthesize pppGpp and therefore is catalytically active, although the activity for the enzyme preparation is very low. It is noteworthy that this low activity was found under assay conditions lacking ribosomes, uncharged tRNA, and mRNA. In contrast, efficient synthetase activity of full-length RelMtb was found to require the presence of the so-called Rel activation complex (RAC) composed of 70S ribosomes, mRNA, and uncharged tRNA (48). Nevertheless, assays of RelEf performed in the presence of RAC containing E. faecalis 70S ribosomes failed to enhance the overall synthetase efficiency of RelEf or enable pGpp production (see Fig. S5B in the supplemental material).
RelEf hydrolyzes pGpp, ppGpp, and pppGpp in vitro with equal efficiency.
Based on previous studies in Firmicutes, Rel appears to be the primary enzyme responsible for degradation of ppGpp and pppGpp, since inactivation of only the rel gene results in either slow-growth phenotypes and elevated basal (p)ppGpp levels (21, 22, 28) or lethality unless the SASs are simultaneously inactivated (49, 50). Here, we asked whether RelEf could also hydrolyze pGpp. This is important because if RelEf lacks this activity and there is no other pGpp hydrolase, then pGpp synthesis tilts the balance in favor of accumulation, amplifying its putative biological effects. Furthermore, nonspecific or spontaneous degradation of pGpp may come into play, generating compounds such as pGp or Gpp, which might also have unique regulatory effects. Hydrolysis of pGpp by RelEf was tested by first using RelQEf to synthesize radiolabeled pGpp and, as a control, ppGpp and pppGpp under conditions that completely consumed the labeled ATP pyrophosphate donor. The resulting products as well as a labeled GTP control were then incubated with RelEf protein in the presence of Mn2+. The breakdown of labeled pGpp, ppGpp, and pppGpp showed that RelEf is able to degrade all three substrates with virtually equivalent efficiency, whereas the GTP control remained intact (Fig. 3).
FIG 3.
RelEf degrades pGpp and (p)ppGpp in vitro. (A) Time course of (pp)pGpp degradation. RelEf (150 nM) was added to pGpp, ppGpp, or pppGpp synthesized with [γ-32P]ATP to label the 3′-β phosphate on the ribose moiety of each nucleotide or to a labeled GTP standard (negative control). Reaction mixtures were incubated at 37°C and sampled at the indicated time points. Percent degradation is the proportion of (pp)pGpp present at the indicated time point relative to the initial starting input, taken as 100%, for each respective nucleotide. Error bars represent the standard deviation from three independent experiments. (B) Representative TLC images of the in vitro degradation reactions. Only the residual substrate remaining after release of PPi is shown, from three different chromatograms.
pGpp inhibits hypoxanthine phosphoribosyl transferase and guanylate kinase.
The evidence presented so far is taken to indicate that ppGp is formed and degraded by the same sorts of enzymes that are responsible for (p)ppGpp metabolism. Ideally, these features can be exploited to gather evidence that pGpp has regulatory functions in cells. However, we have been unable to observe pGpp accumulation in cellular extracts for reasons that we suspect are due to technical difficulties (see Discussion). Therefore, in order to ask if pGpp might have physiologically relevant regulatory functions, a survey has been made of a selected set of diverse regulatory systems in which (p)ppGpp does have regulatory effects, although not necessarily only in Enterococcus faecalis.
In B. subtilis, (p)ppGpp was shown to be an important regulator of GTP homeostasis when guanine is present in the growth medium by inhibiting activities of hypoxanthine-guanine phosphoribosyltransferase (HprT) and GMP kinase (Gmk), which convert guanine to GMP and GMP to GDP, respectively (10). In E. faecalis pppGpp also strongly inhibits the activity of HprT (28). When pGpp was compared to ppGpp and pppGpp, it was found that pGpp strongly inhibits the activity of HprTEf (50% inhibitory concentration [IC50] of 20.8 μM) (Fig. 4A). The in vitro activity of HprT was slightly less sensitive to ppGpp (IC50 = 26 μM) and even less to pppGpp (IC50 = 72.6 μM) (Fig. 4A).
FIG 4.
Inhibition of HprT and Gmk activities by (pp)pGpp. (A) HprT inhibition by increasing concentrations of (pp)pGpp. GTP was used as a control for nonspecific inhibition. (B and C) Inhibition of Gmk-1 (B) and Gmk-2 (C) by increasing concentrations of (pp)pGpp. The inset for panel B shows the same data as the larger graph but with a narrower y axis range (0 to 0.1) to highlight differential regulation by (pp)pGpp. Relative activity for Gmk-1 and Gmk-2 in the presence of exogenous pppGpp has been previously determined 51. Error bars represent the standard error from three independent experiments. In panel B, the differences between pGpp and ppGpp and between pGpp and pppGpp were statistically significance (P ≤ 0.05, 2-tailed t test assuming unequal variance), but the differences between ppGpp and pppGpp were not (P = 0.12). In panel C, the differences between pGpp and pppGpp were not statistically significant (P = 0.09).
Comparing the relative biological effects of (p)ppGpp on E. faecalis Gmk activity requires the consideration of two enzymes, since its genome encodes two Gmk paralogs, unlike the case for other Firmicutes. The Gmk-1 enzyme (EF2595) was extremely sensitive to pGpp inhibition, with an IC50 of 0.90 μM (Fig. 4B). Gmk-1 was also strongly inhibited by pppGpp (IC50 = 8.9 ± 0.3 μM). Previously, we showed that the Gmk-2 (EF3127) enzyme is only modestly, and nonspecifically, inhibited by pppGpp, with an IC50 of 461.9 ± 106.4 μM (51). With pGpp, the inhibition is 3.3-fold more severe than with pppGpp (IC50 of 138.5 μM) (Fig. 4C) but not nearly as potent as with Gmk-1 (28).
pGpp inhibits E. coli RNA polymerase transcription of the rrnB P1 promoter for rRNA.
Many regulatory elements of SR in E coli and several other Gammaproteobacteria are associated with accumulation of (p)ppGpp. Among them is a reduction in transcription initiating at rRNA promoters, such as rrnB P1. Many studies of this phenomenon indicate that direct interactions of (p)ppGpp and DksA with RNA polymerase limit promoter activity (34, 52, 53). Comparisons of individual regulatory contributions reveal ppGpp to be a more potent inhibitor than pppGpp with respect to promoter regulation in vitro, stable RNA accumulation in vivo, cellular growth rate control, RpoS induction, and other phenomena (54). Although pGpp has not been associated with Gammaproteobacteria, existing evidence of regulatory specificity for ppGpp versus pppGpp makes comparisons with pGpp a meaningful example of analog behavior using an additional member of a series of incremental 5′-phosphate additions (pGpp, ppGpp, and pppGpp). Therefore, E. coli-based in vitro transcription assays of rrnBP1 promoter initiation were performed. The assays revealed that pGpp is a specific inhibitor (relative to GDP controls) but with far less potency than ppGpp and, by extrapolation with previous work (54), less than pppGpp (Fig. 5).
FIG 5.
Inhibition of rrnB1 transcription from the P1 promoter by pGpp and ppGpp. RNAP was preincubated with increasing concentration of pGpp, ppGpp, and GDP. In vitro transcription was started by the addition of DksA, DNA template, and NTP substrates. All reaction mixtures were incubated at 37°C for 8 min prior to transcript analysis by PAGE and quantitation by phosphorimaging. Error bars represent the standard deviation of three independent replicates. Compared to the GDP control, the differences observed with pGpp or ppGpp were statistically significance (P ≤ 0.05, 2-tailed t test assuming unequal variance).
RelAEc and RelQEf autocatalytic activities are differentially regulated by pGpp and ppGpp.
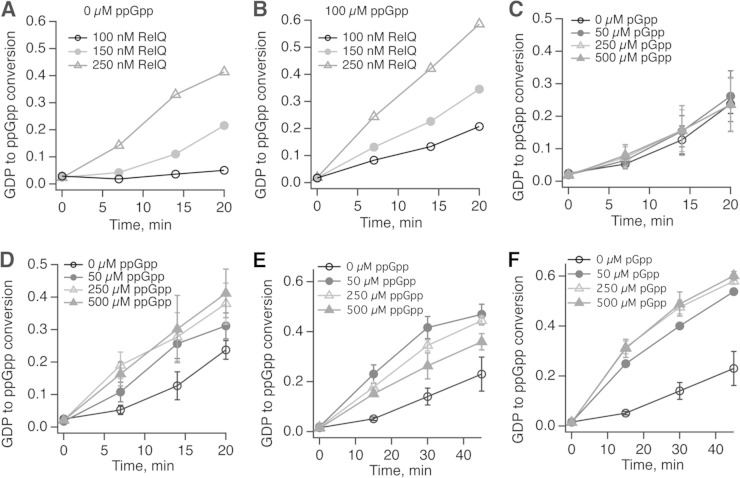
Recently, ppGpp was shown to stimulate the synthetase activity of E. coli RelAEc (36). Importantly, when high concentrations of RelAEc were used in the system, this product-mediated activation was masked by the in situ-produced ppGpp. We have followed the kinetics of ppGpp synthesis by increasing concentrations of RelQEf, both in the absence and in the presence of addition of 100 μM ppGpp. In the absence of exogenous ppGpp, endogenous ppGpp production was nearly absent with 100 nM RelQ. At 150 nM RelQEf, endogenous production was detected but with a pronounced lag. Both of these effects were absent at higher (250 nM) concentrations of the enzyme, and activity became linear with time (Fig. 6A). Addition of ppGpp rendered all the three time courses linear, and the kinetics of ppGpp production was proportional to concentration of RelQEf (Fig. 6B). These results strongly indicate that ppGpp activates RelQEf, in a manner similar to that for RelAEc.
FIG 6.
Effects of (p)ppGpp on the synthase activity of RelQEf and RelAEc. (A to D) Time course of ppGpp synthesis by RelQEf in the absence (A) or presence (B) of ppGpp, in the presence of increasing concentrations of pGpp (C), or with increasing concentrations of ppGpp (D). (E and F) Time course of ppGpp synthesis by RelAEc (30 nM) with increasing concentrations of ppGpp (E) and pGpp (F). Synthesis reaction mixtures involving RelAEc contain 0.5 μM E. coli 70S ribosomes, model mRNA, and deacylated tRNA. All reaction mixtures were preincubated at 37°C, and reactions were initiated by the addition of ATP. Panels A and B represent individual kinetic time series. For panels C to F, error bars represent the standard deviation from at least three independent experiments. GDP-to-ppGpp conversion of 0 corresponds to 100% GDP and that of 1.0 to complete conversion of GDP into ppGpp.
We then followed the kinetics of ppGpp production by RelQEf as well as RelAEc in the presence of increasing concentrations of pGpp or ppGpp. The experimental system was set up in such a way that in the absence of externally added guanosine alarmone, ppGpp production was detectable but showed a significant time lag. In the case of RelQEf, addition of pGpp (up to 500 μM) had no effect on enzyme activity (Fig. 6C). On the other hand, ppGpp induced activation of RelQEf with as little as 50 μM and had no inhibitory effects at concentrations up to 500 μM (Fig. 6D). As shown previously (36), ppGpp had a strong activating effect on RelAEc synthetase activity in a reconstituted translation system containing 70S ribosomes, model mRNA, and deacylated tRNA. Interestingly, pGpp also had a strong activating effect on RelAEc activity and, in contrast to ppGpp, did not inhibit the enzyme at higher concentrations (Fig. 6E and F). Activation of RelQEf by ribosomes was also tested, and, as expected for a single-domain SAS, ribosome-dependent activation was not observed (data not shown).
Relacin does not inhibit RelQEf activity.
The SR has become a target for therapeutic intervention due to its established importance in pathogenesis and persister cell formation (3, 55–57). The RelA/Rel inhibitor and ppGpp mimic relacin (58) represents an important stepping-stone toward the development of chemotherapeutics to block (p)ppGpp synthesis, thereby interfering with long-term bacterial survival strategies. Relacin appears to block synthesis activity of long RSH enzymes (Rel/RelA) by inhibiting release from ribosomes as well as through a ribosome-independent mechanism (58). Importantly, SASs have significant structural differences from larger RSH proteins apart from synthesis specificity, since they lack the (p)ppGpp hydrolase domain as well as the regulatory C-terminal region necessary for ribosome interaction. To assess the ability of relacin to inhibit the action of RelQEf, increasing concentrations of relacin were added to in vitro ppGpp synthesis reaction mixtures containing RelQEf and, as a positive control, E. coli RelAEc. The addition of relacin to reaction mixtures containing RelAEc produced the expected dose-dependent reduction in enzyme activity (Fig. 7), agreeing well with the original report (58). However, relacin had no significant inhibitory effects on RelQEf activity even at 5 mM, which completely abolished the synthesis activity of RelAEc (Fig. 7).
FIG 7.

RelQEf synthase activity is not inhibited by relacin. In vitro ppGpp synthesis activities of E. faecalis RelQEf (500 nM) (red) or E. coli RelAEc (100 nM) (black) with increasing concentrations of the (p)ppGpp analog relacin are shown. Reaction conditions were the same as those described for Fig. 6. Total ppGpp accumulation in the absence of relacin is set to 1 and used to calculate the relative enzyme activity. Error bars represent the standard deviation from at least three independent experiments.
DISCUSSION
In this study, the RelQEf SAS of E. faecalis has been shown to synthesize pGpp, eliminating the otherwise likely possibility that this elusive nucleotide is but an intermediate in (p)ppGpp degradation. In vitro pGpp synthetase activity is appreciable when comparing the relative efficiency of (p)ppGpp production, more efficient than that of pppGpp but less efficient than that of ppGpp. This and other evidence that pGpp might qualify as a member of the (pp)pGpp alarmone family comes from a series of in vitro assays, documented here, comparing the regulatory effects of pGpp with those of (p)ppGpp. Previous observations that the cellular SR mediated by RelEf and RelQEf together is not as robust as that when RelQEf is deleted (25) also suggest the possibility of unique regulatory roles for pGpp, since the contribution of (p)ppGpp synthetase activity by RelQ is minor compared to that by RelEf in vivo. Clearly, cellular assays of pGpp abundance are needed for further study of these activities.
pGpp may represent a new member of the (p)ppGpp regulatory family.
During the SR, (p)ppGpp was estimated to exist at 1 to 2 mM levels in E. coli (59). A report examining the nucleotide pools of B. subtilis starved for isoleucine by treatment with O-methylthreonine found that pGpp accumulated to ∼10% of the level of (p)ppGpp (46). However, until now, the biological effects of pGpp were unknown. Given that pGpp has IC50s of 20 μM and 0.9 μM for HprT and Gmk-1, respectively, pGpp would be predicted to have physiologically significant effects even if its peak levels were 10% or less of levels achieved by (p)ppGpp during a fully developed SR. To this end, we attempted both two-dimensional TLC (2D-TLC) and high-pressure liquid chromatography (HPLC) approaches to measure pGpp abundance in vivo in order to establish it as a meaningful biological entity. This task has been historically complicated by the similar migration patterns and identical mass values of pGpp, ppGp, and GTP and equivocal assignments of enzymatic sources in natural systems. Despite exhaustive efforts to modify our 2D-TLC solvent system, including the same conditions used to resolve pGpp in B. subtilis (46), they proved insufficient to separate a pGpp standard from complex radiolabeled cell extracts. The separation of GTP from pGpp shown in Fig. 2B was possible because it involves separation of pure substrates and products from a defined synthesis reaction. The use of an HPLC system employing a column and mobile-phase combination similar to those described by Ooga et al. (38) overcame the hurdle of reliably separating pGpp standards from cell lysates. However, the selective loss of highly phosphorylated nucleotides (pGpp, ppGpp, and pppGpp) during sample processing and column separation, coupled with the reduced sensitivity of UV detection, greatly reduced our power to detect (pp)pGpp in E. faecalis. Work is under way to optimize sample processing and separation protocols to overcome the technical barriers preventing in vivo pGpp quantification.
Despite our unsuccessful attempts to detect pGpp in vivo, a recent study by Liu and colleagues (51) strongly suggests that pGpp might reach biologically relevant concentrations, because high GMP levels were measured during the SR in Firmicutes. Specifically, in B. subtilis, GMP pools were shown to dramatically increase from 10 μM during exponential growth to 0.3 to 0.5 mM, levels comparable or even exceeding those of GTP, which precipitously drop during amino acid downshift (51). Our preliminary HPLC analysis also indicated that GMP pools rise to the millimolar level in starved cells of E. faecalis (A. O. Gaca et al., unpublished data). As GMP is used with an efficiency approximately equal to that for GTP by RelQEf (Table 1), it is a plausible that after the GTP and GDP substrates are depleted, GMP could become the most abundant guanosine nucleotide, and pGpp might then become the primary alarmone responsible for extending the duration of the SR. However, it is important to note that E. faecalis is unique in that two Gmk enzymes can be found. The two Gmk orthologs of E. faecalis show a large difference in sensitivity to (pp)pGpp analogs. Gmk-2 is not strongly inhibited by pppGpp or pGpp, and this creates an interesting scenario. High Gmk-2 activity despite (pp)pGpp should abolish, or limit, (p)ppGpp-mediated inhibition of conversion of GMP to GDP and, by extension, relieve inhibition of de novo GTP biosynthesis. However, our previous results showed that a slight 4-fold increase in basal (p)ppGpp resulted in a disproportionately large reduction of intracellular GTP pools (∼40%), which are typically several orders of magnitude greater than levels estimated for basal (p)ppGpp (5, 28). Therefore, the ability of elevated (p)ppGpp to repress de novo GTP synthesis suggests that Gmk-2 inhibition by (p)ppGpp is not critical for regulating GTP levels in E. faecalis, for unknown reasons. The relative intracellular abundance or relative activities of Gmk-1 and Gmk-2 could also impact the contribution of each enzyme to de novo GTP biosynthesis and (p)ppGpp-mediated control over GTP homeostasis.
Feedforward or autocatalytic stimulation of RelQ synthetase activity by ppGpp raises the possibility that RelQ or Rel products serve as a signal amplifier for (pp)pGpp production in a manner homologous to the direct activation of RelAEc by (p)ppGpp (36). Multiple attempts to test the activating effects of (pp)pGpp on RelEf activity were unsuccessful, due in part to the enzyme's poor in vitro activity, even in the presence of ribosomes. Still, it is interesting that positive allosteric feedback regulation by ppGpp is present in a SAS, which lacks numerous sites conserved across the larger synthetases suggested to function in inter- and intramolecular interactions, such as those that might contact ppGpp to enhance synthetase efficiency in RelAEc (60). The role of RelQ is further complicated because its synthetase activity shows direct activation by ppGpp but not by pGpp (Fig. 6). Presently, it is unclear if activation occurs by separate mechanisms in RelAEc and RelQEf or by the same catalytic center that binds ppGpp but not pGpp.
RelQEf appears to be a uniquely efficient source of pGpp synthesis.
Over the past 3 to 4 decades there have been reports of several bacterial nucleotide analogs related to (p)ppGpp as potential candidates for involvement in stringent control, such as pGp, pGpp, and ppGp (35, 46, 61, 62). Although the source of many of these compounds was then unknown, there is conflicting evidence as to the involvement of RSH family enzymes. In B. subtilis, it was noted that pGpp accumulation was eliminated in a probable RelBs deletion mutant (46), suggesting Rel as a source of pGpp other than a SAS. The caveat is that Rel deletions may be viable only if there are suppressor mutations inactivating “sister” SAS synthetases (50). Synthesis of pGpp in vitro by a mutated RelMtb enzyme has been observed to generate pGpp, but the route was not through use of GMP as a substrate but rather through hydrolysis of the β phosphate of ppGpp to yield pGpp (43). Although it has been shown that RelAEc produced only trace amounts of pGpp in ribosome-dependent assays (63), we also cannot rule out the potential importance of pGpp in Proteobacteria due to the presence of SAS enzymes in Vibrio species or as a metabolic intermediate of (p)ppGpp degradation due to the presence of nonspecific hydrolases, such as Nudix (38).
The large discrepancy in the relative ability of the SASs from S. mutans to synthesize pGpp from GMP compared to RelQEf is intriguing considering the relatively high degrees of similarity of RelQEf with RelQSm (∼80%) and RelPSm (∼60%). However, this appears to reflect overall catalysis, regardless of the guanine nucleotide substrate, because in vitro activity of both S. mutans enzymes was not as robust as that of RelQEf using either GDP or GTP as a substrate (see Fig. S4 in the supplemental material). As discussed, during characterization of the (p)ppGpp synthetase activities of the B. subtilis RelPBs (YjbM) and RelQBs (YwaC) enzymes, an extra major spot with a migration pattern identical to that of GTP was identified in reaction mixtures containing RelQBs and either GDP or GTP as a substrate (22). As pGpp is expected to comigrate with GTP under the conditions used in this study, it is tempting to speculate that the GTP and GDP preparations might have been contaminated with GMP, which may have resulted in the synthesis of pGpp by RelQBs. Clearly, additional studies are required to determine the degree of conservation and efficiency of pGpp synthesis among different SASs.
SAS enzymes may alter the efficiency of relacin treatment.
Relacin and its analogs are (p)ppGpp mimics designed to inhibit the function of RelA/Rel “long” RSH enzymes (58, 64). If other SASs show insensitivity to relacin similar to that found here for RelQEf (Fig. 7), it could be argued that relacin-resistant sources of (p)ppGpp may represent a major hurdle to the treatment of infections caused by organisms that encode SASs, such as Firmicutes, Actinobacteria, and Vibrio species (12, 65, 66). More specifically, using E. faecalis as a model organism, we showed that (p)ppGpp exerts a regulatory role in antibiotic persistence and virulence at concentrations that are much lower than those required to trigger the SR (28). In other words, low basal (pp)pGpp levels produced by RelQEf are sufficient for persistence and full virulence potential. On the other hand, relacin was shown to reduce in vitro survival of wild-type B. subtilis and Streptococcus pyogenes, which harbor RelP and RelQ enzymes (58). It is conceivable that relacin, by virtue of its ability to block ribosome dissociation, could also inhibit (p)ppGpp accumulation by tipping the balance of synthesis and hydrolysis to strongly favor hydrolysis, thereby reducing (p)ppGpp accumulation from SAS enzymes. Further in vitro and in vivo testing of the effects of relacin on stress tolerance, persistence, and virulence are necessary before making conclusions about the effectiveness of relacin toward SAS-encoding bacteria.
Concluding remarks.
The discovery and characterization of SASs are relatively recent developments compared to the longstanding and still ongoing research to understand the enzymatic and regulatory intricacies of “long” RSHs. The results presented here reveal that RelQEf is directly activated by ppGpp and is able to synthesize pGpp. These biochemical properties may turn out to impart important biological functions to RelQEf and more generally to SAS enzymes. To our knowledge, this is the first study demonstrating regulatory effects of pGpp in vitro with specific regulatory targets shared by ppGpp and pppGpp. The inhibitory effects on Gmk, HprT, and RNAP activities and the stimulatory effect on RelAEc activity seem to qualify pGpp as a new member of the (pp)pGpp family of nucleotide second messengers. Judging from the history of (p)ppGpp, it will likely take considerable effort to pinpoint the targets of pGpp in vivo. We hope that others will be motivated by our findings to pursue the development of novel analytical tools that can be applied to study pGpp and possibly other related analogs that may be involved in bacterial regulatory circuits in addition to ppGpp and pppGpp.
Supplementary Material
ACKNOWLEDGMENTS
A.O.G. was supported by the NIDCR training program in oral sciences, grant T90 DE021985. J.D.W. was supported by NIH grant R01GM084003. M.C. was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural program of the National Institutes of Health. K.P. was supported by grant DEC-2013/10/E/NZ1/00657 from the Polish National Science Center. D.R. was supported by funds from the Czech Science Foundation, grant 15-11711S. V.H. was supported by funds from the European Regional Development Fund through the Centre of Excellence in Chemical Biology, Estonian Science Foundation grants (ETF9012 and PUT37), the Ragnar Söderberg Foundation, the Swedish Research Council, the Kempe Foundation, and Umeå University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00324-15.
REFERENCES
- 1.Cashel M, Gallant J. 1969. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 221:838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- 2.Sy J, Lipmann F. 1973. Identification of the synthesis of guanosine tetraphosphate (MS I) as insertion of a pyrophosphoryl group into the 3′-position in guanosine 5′-diphosphate. Proc Natl Acad Sci U S A 70:306–309. doi: 10.1073/pnas.70.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 4.Dalebroux ZD, Swanson MS. 2012. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol 10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- 5.Cashel M, Gentry DR, Hernandez VJ, Vinella D. 1996. The stringent response, p 1458–1496. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger AE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington DC. [Google Scholar]
- 6.Gallant J, Irr J, Cashel M. 1971. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem 246:5812–5816. [PubMed] [Google Scholar]
- 7.Rojas AM, Ehrenberg M, Andersson SG, Kurland CG. 1984. ppGpp inhibition of elongation factors Tu, G and Ts during polypeptide synthesis. Mol Gen Genet 197:36–45. doi: 10.1007/BF00327920. [DOI] [PubMed] [Google Scholar]
- 8.Maciag M, Kochanowska M, Lyzen R, Wegrzyn G, Szalewska-Palasz A. 2010. ppGpp inhibits the activity of Escherichia coli DnaG primase. Plasmid 63:61–67. doi: 10.1016/j.plasmid.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang JD, Sanders GM, Grossman AD. 2007. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 128:865–875. doi: 10.1016/j.cell.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kriel A, Bittner AN, Kim SH, Liu K, Tehranchi AK, Zou WY, Rendon S, Chen R, Tu BP, Wang JD. 2012. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol Cell 48:231–241. doi: 10.1016/j.molcel.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou Z, Cashel M, Fromm HJ, Honzatko RB. 1999. Effectors of the stringent response target the active site of Escherichia coli adenylosuccinate synthetase. J Biol Chem 274:17505–17510. doi: 10.1074/jbc.274.25.17505. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson GC, Tenson T, Hauryliuk V. 2011. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laffler T, Gallant J. 1974. spoT, a new genetic locus involved in the stringent response in E. coli. Cell 1:27–30. doi: 10.1016/0092-8674(74)90151-2. [DOI] [Google Scholar]
- 14.Haseltine WA, Block R. 1973. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A 70:1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinella D, Albrecht C, Cashel M, D'Ari R. 2005. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol Microbiol 56:958–970. doi: 10.1111/j.1365-2958.2005.04601.x. [DOI] [PubMed] [Google Scholar]
- 16.Battesti A, Bouveret E. 2006. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol Microbiol 62:1048–1063. doi: 10.1111/j.1365-2958.2006.05442.x. [DOI] [PubMed] [Google Scholar]
- 17.Bougdour A, Gottesman S. 2007. ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc Natl Acad Sci U S A 104:12896–12901. doi: 10.1073/pnas.0705561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avarbock D, Avarbock A, Rubin H. 2000. Differential regulation of opposing RelMtb activities by the aminoacylation state of a tRNA.ribosome.mRNA. RelMtb complex. Biochemistry 39:11640–11648. [DOI] [PubMed] [Google Scholar]
- 19.Wolz C, Geiger T, Goerke C. 2010. The synthesis and function of the alarmone (p)ppGpp in firmicutes. Int J Med Microbiol 300:142–147. doi: 10.1016/j.ijmm.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Hogg T, Mechold U, Malke H, Cashel M, Hilgenfeld R. 2004. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p)ppGpp metabolism during the stringent response [corrected]. Cell 117:57–68. doi: 10.1016/S0092-8674(04)00260-0. [DOI] [PubMed] [Google Scholar]
- 21.Lemos JA, Lin VK, Nascimento MM, Abranches J, Burne RA. 2007. Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol Microbiol 65:1568–1581. doi: 10.1111/j.1365-2958.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- 22.Nanamiya H, Kasai K, Nozawa A, Yun CS, Narisawa T, Murakami K, Natori Y, Kawamura F, Tozawa Y. 2008. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol Microbiol 67:291–304. [DOI] [PubMed] [Google Scholar]
- 23.Gaca AO, Colomer-Winter C, Lemos JA. 2015. Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J Bacteriol 197:1146–1156. doi: 10.1128/JB.02577-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger T, Kastle B, Gratani FL, Goerke C, Wolz C. 2014. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J Bacteriol 196:894–902. doi: 10.1128/JB.01201-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaca AO, Abranches J, Kajfasz JK, Lemos JA. 2012. Global transcriptional analysis of the stringent response in Enterococcus faecalis. Microbiology 158:1994–2004. doi: 10.1099/mic.0.060236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natori Y, Tagami K, Murakami K, Yoshida S, Tanigawa O, Moh Y, Masuda K, Wada T, Suzuki S, Nanamiya H, Tozawa Y, Kawamura F. 2009. Transcription activity of individual rrn operons in Bacillus subtilis mutants deficient in (p)ppGpp synthetase genes, relA, yjbM, and ywaC. J Bacteriol 191:4555–4561. doi: 10.1128/JB.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JN, Ahn SJ, Seaton K, Garrett S, Burne RA. 2012. Transcriptional organization and physiological contributions of the relQ operon of Streptococcus mutans. J Bacteriol 194:1968–1978. doi: 10.1128/JB.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaca AO, Kajfasz JK, Miller JH, Liu K, Wang JD, Abranches J, Lemos JA. 2013. Basal levels of (p)ppGpp in Enterococcus faecalis: the magic beyond the stringent response. mBio 4(5):e00646-13. doi: 10.1128/mBio.00646-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank KL, Colomer-Winter C, Grindle SM, Lemos JA, Schlievert PM, Dunny GM. 2014. Transcriptome analysis of Enterococcus faecalis during mammalian infection shows cells undergo adaptation and exist in a stringent response state. PLoS One 9:e115839. doi: 10.1371/journal.pone.0115839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaton K, Ahn SJ, Sagstetter AM, Burne RA. 2011. A transcriptional regulator and ABC transporters link stress tolerance, (p)ppGpp, and genetic competence in Streptococcus mutans. J Bacteriol 193:862–874. doi: 10.1128/JB.01257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abranches J, Martinez AR, Kajfasz JK, Chavez V, Garsin DA, Lemos JA. 2009. The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J Bacteriol 191:2248–2256. doi: 10.1128/JB.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mechold U, Cashel M, Steiner K, Gentry D, Malke H. 1996. Functional analysis of a relA/spoT gene homolog from Streptococcus equisimilis. J Bacteriol 178:1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stols L, Gu M, Dieckman L, Raffen R, Collart FR, Donnelly MI. 2002. A new vector for high-throughput, ligation-independent cloning encoding a tobacco etch virus protease cleavage site. Protein Expr Purif 25:8–15. doi: 10.1006/prep.2001.1603. [DOI] [PubMed] [Google Scholar]
- 34.Potrykus K, Vinella D, Murphy H, Szalewska-Palasz A, D'Ari R, Cashel M. 2006. Antagonistic regulation of Escherichia coli ribosomal RNA rrnB P1 promoter activity by GreA and DksA. J Biol Chem 281:15238–15248. doi: 10.1074/jbc.M601531200. [DOI] [PubMed] [Google Scholar]
- 35.Pao CC, Gallant J. 1979. A new nucleotide involved in the stringent response in Escherichia coli. Guanosine 5′-diphosphate-3′-monophosphate. J Biol Chem 254:688–692. [PubMed] [Google Scholar]
- 36.Shyp V, Tankov S, Ermakov A, Kudrin P, English BP, Ehrenberg M, Tenson T, Elf J, Hauryliuk V. 2012. Positive allosteric feedback regulation of the stringent response enzyme RelA by its product. EMBO Rep 13:835–839. doi: 10.1038/embor.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamel E, Cashel M. 1973. Role of guanine nucleotides in protein synthesis. Elongation factor G and guanosine 5′-triphosphate,3′-diphosphate. Proc Natl Acad Sci U S A 70:3250–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ooga T, Ohashi Y, Kuramitsu S, Koyama Y, Tomita M, Soga T, Masui R. 2009. Degradation of ppGpp by nudix pyrophosphatase modulates the transition of growth phase in the bacterium Thermus thermophilus. J Biol Chem 284:15549–15556. doi: 10.1074/jbc.M900582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keasling JD, Bertsch L, Kornberg A. 1993. Guanosine pentaphosphate phosphohydrolase of Escherichia coli is a long-chain exopolyphosphatase. Proc Natl Acad Sci U S A 90:7029–7033. doi: 10.1073/pnas.90.15.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi MY, Wang Y, Wong LL, Lu BT, Chen WY, Huang JD, Tanner JA, Watt RM. 2012. The two PPX-GppA homologues from Mycobacterium tuberculosis have distinct biochemical activities. PLoS One 7:e42561. doi: 10.1371/journal.pone.0042561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murdeshwar MS, Chatterji D. 2012. MS_RHII-RSD, a dual-function RNase HII-(p)ppGpp synthetase from Mycobacterium smegmatis. J Bacteriol 194:4003–4014. doi: 10.1128/JB.00258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herries DG. 1985. Enzyme structure and mechanism (2nd ed). Biochem Ed 13:146. [Google Scholar]
- 43.Sajish M, Kalayil S, Verma SK, Nandicoori VK, Prakash B. 2009. The significance of EXDD and RXKD motif conservation in Rel proteins. J Biol Chem 284:9115–9123. doi: 10.1074/jbc.M807187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajish M, Tiwari D, Rananaware D, Nandicoori VK, Prakash B. 2007. A charge reversal differentiates (p)ppGpp synthesis by monofunctional and bifunctional Rel proteins. J Biol Chem 282:34977–34983. doi: 10.1074/jbc.M704828200. [DOI] [PubMed] [Google Scholar]
- 45.Mechold U, Murphy H, Brown L, Cashel M. 2002. Intramolecular regulation of the opposing (p)ppGpp catalytic activities of Rel(Seq), the Rel/Spo enzyme from Streptococcus equisimilis. J Bacteriol 184:2878–2888. doi: 10.1128/JB.184.11.2878-2888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishino T, Gallant J, Shalit P, Palmer L, Wehr T. 1979. Regulatory nucleotides involved in the Rel function of Bacillus subtilis. J Bacteriol 140:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cashel M, Kalbacher B. 1970. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem 245:2309–2318. [PubMed] [Google Scholar]
- 48.Avarbock A, Avarbock D, Teh JS, Buckstein M, Wang ZM, Rubin H. 2005. Functional regulation of the opposing (p)ppGpp synthetase/hydrolase activities of RelMtb from Mycobacterium tuberculosis. Biochemistry 44:9913–9923. doi: 10.1021/bi0505316. [DOI] [PubMed] [Google Scholar]
- 49.Gentry D, Li T, Rosenberg M, McDevitt D. 2000. The rel gene is essential for in vitro growth of Staphylococcus aureus. J Bacteriol 182:4995–4997. doi: 10.1128/JB.182.17.4995-4997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivatsan A, Han Y, Peng J, Tehranchi AK, Gibbs R, Wang JD, Chen R. 2008. High-precision, whole-genome sequencing of laboratory strains facilitates genetic studies. PLoS Genet 4:e1000139. doi: 10.1371/journal.pgen.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu K, Myers AR, Pisithkul T, Claas KR, Satyshur KA, Amador-Noguez D, Keck JL, Wang JD. 2015. Molecular mechanism and evolution of guanylate kinase regulation by (p)ppGpp. Mol Cell 57:735–749. doi: 10.1016/j.molcel.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL. 2004. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Lemke JJ, Sanchez-Vazquez P, Burgos HL, Hedberg G, Ross W, Gourse RL. 2011. Direct regulation of Escherichia coli ribosomal protein promoters by the transcription factors ppGpp and DksA. Proc Natl Acad Sci U S A 108:5712–5717. doi: 10.1073/pnas.1019383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mechold U, Potrykus K, Murphy H, Murakami KS, Cashel M. 2013. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res 41:6175–6189. doi: 10.1093/nar/gkt302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. 2010. ppGpp conjures bacterial virulence. Microbiol Mol Biol. Rev 74:171–199. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maisonneuve E, Castro-Camargo M, Gerdes K. 2013. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 57.Amato SM, Orman MA, Brynildsen MP. 2013. Metabolic control of persister formation in Escherichia coli. Mol Cell 50:475–487. doi: 10.1016/j.molcel.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Wexselblatt E, Oppenheimer-Shaanan Y, Kaspy I, London N, Schueler-Furman O, Yavin E, Glaser G, Katzhendler J, Ben-Yehuda S. 2012. Relacin, a novel antibacterial agent targeting the stringent response. PLoS Pathog 8:e1002925. doi: 10.1371/journal.ppat.1002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cashel M. 1975. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol 29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- 60.Atkinson GC, Hauryliuk V. 2012. Evolution and function of the RelA/SpoT homologue (RSH) proteins. John Wiley & Sons, Ltd., New York, NY. [Google Scholar]
- 61.Pao CC, Dyess BT. 1981. Effect of unusual guanosine nucleotides on the activities of some Escherichia coli cellular enzymes. Biochim Biophys Acta 677:358–362. doi: 10.1016/0304-4165(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 62.Crosse AM, Greenway DL, England RR. 2000. Accumulation of ppGpp and ppGp in Staphylococcus aureus 8325-4 following nutrient starvation. Lett Appl Microbiol 31:332–337. doi: 10.1046/j.1472-765x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- 63.Cochran JW, Byrne RW. 1974. Isolation and properties of a ribosome-bound factor required for ppGpp and ppGpp synthesis in Escherichia coli. J Biol Chem 249:353–360. [PubMed] [Google Scholar]
- 64.Wexselblatt E, Kaspy I, Glaser G, Katzhendler J, Yavin E. 2013. Design, synthesis and structure-activity relationship of novel Relacin analogs as inhibitors of Rel proteins. Eur J Med Chem 70:497–504. doi: 10.1016/j.ejmech.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 65.Das B, Pal RR, Bag S, Bhadra RK. 2009. Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol Microbiol 72:380–398. doi: 10.1111/j.1365-2958.2009.06653.x. [DOI] [PubMed] [Google Scholar]
- 66.Dasgupta S, Basu P, Pal RR, Bag S, Bhadra RK. 2014. Genetic and mutational characterization of the small alarmone synthetase gene relV of Vibrio cholerae. Microbiology 160:1855–1866. doi: 10.1099/mic.0.079319-0. [DOI] [PubMed] [Google Scholar]
- 67.Jelenc PC, Kurland CG. 1979. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A 76:3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.