ABSTRACT
Clostridium difficile is an anaerobic, Gram-positive, spore-forming opportunistic pathogen and is the most common cause of hospital-acquired infectious diarrhea. Although iron acquisition in the host is a key to survival of bacterial pathogens, high levels of intracellular iron can increase oxidative damage. Therefore, expression of iron acquisition mechanisms is tightly controlled by transcriptional regulators. We identified a C. difficile homologue of the master bacterial iron regulator Fur. Using targetron mutagenesis, we generated a fur insertion mutant of C. difficile. To identify the genes regulated by Fur in C. difficile, we used microarray analysis to compare transcriptional differences between the fur mutant and the wild type when grown in high-iron medium. The fur mutant had increased expression of greater than 70 transcriptional units. Using quantitative reverse transcriptase PCR (qRT-PCR), we analyzed several of the Fur-regulated genes identified by the microarray and verified that they are both iron and Fur regulated in C. difficile. Among those Fur- and iron-repressed genes were C. difficile genes encoding 7 putative cation transport systems of different classes. We found that Fur was able to bind the DNA upstream of three Fur-repressed genes in electrophoretic mobility shift assays. We also demonstrate that expression of Fur-regulated putative iron acquisition systems was increased during C. difficile infection using the hamster model. Our data suggest that C. difficile expresses multiple iron transport mechanisms in response iron depletion in vitro and in vivo.
IMPORTANCE Clostridium difficile is the most common cause of hospital-acquired infectious diarrhea and has been recently classified as an “urgent” antibiotic resistance threat by the CDC. To survive and cause disease, most bacterial pathogens must acquire the essential enzymatic cofactor iron. While import of adequate iron is essential for most bacterial growth, excess intracellular iron can lead to extensive oxidative damage. Thus, bacteria must regulate iron import to maintain iron homeostasis. We demonstrate here that C. difficile regulates expression of several putative iron acquisition systems using the transcriptional regulator Fur. These import mechanisms are induced under iron-limiting conditions in vitro and during C. difficile infection of the host. This suggests that during a C. difficile infection, iron availability is limited in vivo.
INTRODUCTION
Almost all living organisms require iron as a cofactor for essential metabolic chemistry (1). Although iron is one of the most abundant of Earth's elements, ferric iron has very limited solubility in aqueous, nonacidic, or oxygenated environments (1). Ferric iron is most often found as iron oxides or hydroxides, which cannot be used by most organisms. Competition over bioavailable iron is fierce both among bacteria in complex microbial communities and between bacterial pathogens and their eukaryotic hosts (2).
Organisms have evolved various mechanisms of iron transport to obtain this essential cofactor. Many bacteria produce low-molecular-weight, high-affinity iron chelators known as siderophores (3). Specific ABC transporters translocate iron-bound siderophores into cells. The siderophores can be made by the organism itself or coopted from other prokaryotic or eukaryotic neighbors (3). Under anaerobic or low-pH conditions, ferrous iron predominates over the ferric form. The solubility of ferrous iron is orders of magnitude higher than that of ferric iron, and thus it can be directly transported into cells as a free metal. Bacteria are known to transport free ferrous iron using G-protein-like ferrous permeases (4).
Although iron acquisition is crucial for survival, high levels of intracellular iron can react with hydrogen peroxide to form reactive hydroxyl radicals. These reactive radicals can damage DNA as well as iron-containing enzymes (5, 6). Thus, maintenance of appropriate intracellular iron levels in bacteria is an important task. Many Gram-positive and Gram-negative bacteria utilize a transcriptional regulator called Fur (ferric uptake regulator) to control intracellular iron homeostasis (7–9). In these organisms, Fur is known to regulate expression of multiple proteins involved in survival under iron-limiting conditions, including iron acquisition systems. In most known examples, Fur binds intracellular iron and binds DNA as a homodimer (7, 10). Most commonly, the Fe-Fur dimer binds to DNA near the promoter regions of genes in the Fur regulon, thus blocking transcription (7, 11). Expression of Fur-repressed genes is lower in the presence of iron and higher under iron-depleted conditions.
In this study, we generated a Clostridium difficile fur insertion mutant. This mutant was used to identify iron-repressed genes whose expression was regulated by Fur in an iron-dependent manner. Expression of several Fur-regulated C. difficile genes was induced in infected hamsters, suggesting that iron is limiting during a C. difficile infection.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and primers.
The bacterial strains and plasmids used in this study are described in Table 1. The C. difficile strains are isogenic with the erythromycin-sensitive strain JIR8094, a derivative of the sequenced clinical isolate 630 (12). C. difficile was grown in or on TY medium (0.4% tryptone, 0.5% yeast extract) at 37°C in an atmosphere of 10% hydrogen, 5% CO2, and 85% nitrogen in an anaerobic chamber (Coy Laboratory Products). To determine low-iron medium conditions, we performed MIC experiments with dipyridyl. We found that 250 μM dipyridyl inhibited growth of wild-type C. difficile, while growth was not inhibited by up to 500 μM FeCl3. Low-iron TY medium contained dipyridyl (final concentration, 100 μM; Sigma), and high-iron TY medium was supplemented with FeCl3 (final concentration, 250 μM; RPI Corp.).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Referencea or source |
|---|---|---|
| Clostridium difficile strains | ||
| JIR8094 | Spontaneous erythromycin-sensitive derivative of 630 | 12 |
| TCD90 | JIR8094 fur300::ltrB::ermB | |
| TCD91 | JIR8094 fur300::ltrB::ermB/pRPF185 | |
| TCD93 | JIR8094 fur300::ltrB::ermB/pTHE884 | |
| Escherichia coli strains | ||
| Omnimax-2 T1R | F′ [proAB+ lacIq lacZΔM15 Tn10(Tetr) Δ(ccdAB)] mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 supE44 thi-1 gyrA96 relA1 tonA panD | Invitrogen |
| HB101/pRK24 | F− mcrB mrr hsdS20(rB− mB−) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20(Smr) glnV44 λ− pRK24 | 15 |
| Plasmids | ||
| pBL100 | Pfac ltrB::ermBRAM ltrA cat repA orfB oriT | 13 |
| pRPF185 | Ptet-gus colE1 bla cat repA orfB oriT aad9 | 17 |
| pTHE627 | Pfac ltrBfur::ermBRAM ltrA cat repA | |
| pTHE884 | Pfur-furC. difficile-gus colE1 bla cat repA orfB oriT aad9 |
This study unless otherwise noted.
C. difficile strains were grown on TY agar (2%) plates containing thiamphenicol (Thi) (10 μg/ml), erythromycin (Erm) (5 μg/ml), or kanamycin (Kan) (50 μg/ml) as needed. Optical densities (ODs) of bacterial cultures were measured using a WPA spectrophotometer (CO800 cell density meter).
Escherichia coli strains were grown in or on LB medium (1% tryptone, 0.5% yeast extract, 0.5% sodium chloride) at 37°C with ampicillin (Amp) (50 μg/ml) or chloramphenicol (Cam) (10 μg/ml) as needed. The primers used in this work are listed in Table S1 in the supplemental material. All primers were synthesized by IDT DNA Inc. (Coralville, IA).
Plasmid and bacterial strain construction.
Plasmid cloning was performed in the E. coli strain Omnimax-2 T1R (Invitrogen). To construct a fur mutant, we retargeted the intron Ll.LtrB from plasmid pBL100 (13). The primers were designed using the Clostron algorithm (14). The intron was amplified from a targetron template (Sigma) using the TE2125, TE2126, and TE2127 primers (see Table S1 in the supplemental material). This insert was digested with HindIII and BsrGI and cloned into the pBL100 plasmid digested with the same restriction enzymes. The resulting plasmid, pTHE627, was transformed into the conjugation donor HB101/pRK24 (15) to move the retargeted plasmid into C. difficile to generate the fur intron insertion mutant as previously described (16). The insertion of the intron into fur was confirmed by PCR using oligomers TE2721 and TE2280 (see Table S1 in the supplemental material).
For complementation of the fur mutant, we cloned the C. difficile fur open reading frame and 200 bp of the 5′ upstream region by PCR amplifying DNA, using TE2596 and TE2597 (see Table S1 in the supplemental material). The resulting PCR product was cloned into the pRPF185 plasmid (17) which had been digested with the NheI and SacI restriction enzymes using Gibson Assembly (NEB). The P-fur construct (pTHE884) was maintained in Omnimax or HB101/pRK24 cells grown at 30°C prior to introduction into C. difficile.
Bioinformatic analysis.
Gene sequences from bacterial genomes were obtained from the BioCyc website. The multiple-sequence alignment of Fur proteins from C. difficile, Bacillus subtilis, and E. coli was generated using the default settings of Clustal Omega (18). The consensus Fur-binding sequence for C. difficile was determined by analyzing ∼250 bp upstream and ∼50 bp downstream of the predicted translational start site for the first gene each of the 8 Fur-repressed operons encoding transporters using MEME, a motif-based sequence analysis tool (19). The MEME software was used with settings to identify consensus regions between 16 and 22 bp.
Isolation of C. difficile nucleic acids.
C. difficile chromosomal DNA was purified as previously described (16). C. difficile RNA was isolated from bacteria grown in TY medium. For each sample, a single colony of C. difficile was inoculated in TY medium and grown overnight in high-iron-containing TY medium. Overnight cultures were washed in phosphate-buffered saline (PBS) and diluted 1:25 in low- or high-iron-containing TY medium grown to an OD and 600 nm (OD600) of 0.6 to 0.9. Cells were fixed by adding equal volumes of acetone-ethanol (1:1) to cells and incubated at −80°C for at least 30 min. Fixed cells were pelleted by centrifugation, washed 3 times with 0.75 ml of diethyl pyrocarbonate (DEPC)-treated water, and then resuspended in 0.6 ml of buffer RLT (Qiagen) with β-mercaptoethanol (final concentration, 10%; Sigma). Cells were disrupted by sonication (10 pulses of setting 3 for 1 s; Branson Sonifier 150). RNA was extracted using the RNeasy RNA isolation kit (Qiagen). Contaminating DNA was removed using the Turbo DNA-free kit (Ambion). Samples were tested for DNA contamination by PCR amplification (Thermo Taq polymerase; NEB) using primers TE485 and TE486 (see Table S1 in the supplemental material).
Microarray analysis.
Samples used for microarray analysis were obtained from the wild type (JIR8094) and fur mutant (TCD90) grown in high-iron-containing TY medium to an OD of 0.8. The cells were fixed with an acetone-ethanol mixture (at a 1:1 ratio). RNA was isolated from these fixed cells using Trizol reagent (Invitrogen) as previously described (16, 20). The resulting RNA was treated with DNase I (Turbo DNA-free kit; Ambion) and purified with RNeasy spin columns (Qiagen). The resulting RNA was processed by the University of Iowa Carver Center for Genomics (Iowa City, IA) with custom C. difficile Roche Nimblegen microarrays (20). RNA from three independent biological replicates grown on different days was used. Each of the biological replicates was tested in technical duplicate on the microarray slides. Data analysis workflow was performed with the Partek Genomics Suite (Partek Inc.).
qRT-PCR.
To generate cDNA from RNA samples, we used Superscript II (Invitrogen) or Moloney murine leukemia virus (MMuLV) reverse transcriptase (RT) (NEB) according to the manufacturer's protocols. The resulting reverse transcription reaction mixtures were diluted 1:5 in DEPC-treated water. For each quantitative RT-PCR (qRT-PCR), 5 μl of sample was added to 10 μl of Power Sybr green master mix (Applied Biosystems) and 5 μl gene-specific primers (2 × 2.5 μM). The list of primers used to quantitate cDNA levels of different samples is provided in Table S1 in the supplemental material. Experiments were performed in technical triplicate on three biologically independent replicates. Data were normalized to RNA levels of the C. difficile housekeeping gene rpoB (for in vitro experiments) or the C. difficile-specific mldA gene (for in vivo experiments), which was chosen due to its presence almost exclusively in C. difficile (21). Thus, mldA primers would not cross-react with commensal bacteria.
EMSA.
We attempted to express the Fur protein in E. coli cloning and protein expression strains using several different plasmid constructs. Most of the clones that we were able to obtain had point mutations in Fur which likely rendered the protein inactive or truncated. We were able to obtain and express N-terminally His-tagged Fur protein but found this protein to be inactive. Subsequent cleavage by the AcTEV protease did not generate sufficient quantities of active Fur protein for electrophoretic mobility shift assays (EMSAs). Thus, we synthesized the Fur protein using in vitro transcription and translation. For a fur-encoding template, we introduced the T7 promoter using 2 consecutive PCRs. The first Taq polymerase reaction (NEB) used oligomers CDEP3202 and CDEP3203 (see Table S1 in the supplemental material). This product was gel purified (Fermentas) and then used as the template for a subsequent PCR with oligomers CDEP3221 and CDEP3203 (see Table S1 in the supplemental material). This final product was used as the template in a PURExpress in vitro protein synthesis reaction (NEB) according to the manufacturer's instructions.
For the EMSAs, we PCR amplified putative promoter regions upstream of the cd1477 (TE2723 and TEQ130; 310 bp), cd2992 (TE2247 and TE2248; 305 bp), and fur (TE2281 and TE2722; 375 bp) genes. These DNA products were gel purified (Fermentas), and 132 ng of DNA for each probe was labeled using T4 polynucleotide kinase (NEB) with [γ-32P]ATP (PerkinElmer). Each radioactively labeled DNA probe was cleaned using a mini-spin column (Fermentas) and used in EMSA reactions.
For each EMSA reaction, 1 μl of DNA probe was incubated with 1× EMSA buffer (20 mM Tris HCl [pH 8], 50 mM KCl, 5% glycerol, 1 mM dithiothreitol [DTT], 1 mM MgCl2) with salmon sperm DNA (1 μg/ml; Invitrogen) and bovine serum albumin (BSA) (1 μg/ml; NEB) for 5 min at room temperature. One microliter of Fur in vitro synthesis reaction mixture or, as a negative control, 1 μl of mock in vitro synthesis reaction mixture (PURExpress in vitro protein synthesis with no template) was added to EMSA reaction mixtures and allowed to incubate for 30 min at room temperature under normal atmospheric oxygen conditions. Each reaction mixture was loaded on a vertical 4% acrylamide–bis-Tris-acetate-EDTA (TAE) gel and electrophoresed at 83 V for 3 h at 4°C. The gel was then immobilized on filter paper, wrapped in plastic wrap, and exposed to a phosphor screen for 1 to 2 h. The phosphor screen was then imaged using a Typhoon 8610 variable-mode imager.
C. difficile gene expression during hamster model of infection.
C. difficile spores used for infections were prepared as previously described (22). Briefly, the C. difficile wild type (0.3 ml of overnight culture) was spread on TY plates and incubated for 3 days at 37°C in an anaerobic chamber. The cells were resuspended in phosphate-buffered saline (PBS), and the vegetative cells were killed by heat inactivation at 65°C aerobically for 30 min. Spore preparations were washed extensively in PBS and stored at 4°C prior to use. Spore counts were determined by plating serial dilutions on TY plates containing 0.1% taurocholate.
Four adult Syrian gold hamsters (∼90 to 120 g; Harlan Sprague-Dawley, Inc., Indianapolis, IN) were inoculated orally with clindamycin (Sigma; 30 mg/kg) 5 days prior to infection (23). Hamsters were inoculated with 10,000 spores of wild-type C. difficile. Hamsters were monitored twice daily for signs of severe morbidity and euthanized prior to death. After euthanization, 1-cm sections of infected ceca were removed and individually fixed in 1 ml Trizol (Invitrogen). After storage at −20°C, samples were thawed and homogenized. RNA was isolated from these samples as previously described (16). The animal experiments performed in this study were approved by the University of Iowa Institutional Animal Care and Use Committee.
Microarray data accession numbers.
The microarray design (GPL20243) and data (GSE69218) have been deposited at the Gene Expression Omnibus (GEO) database at NIH.
RESULTS
Construction of C. difficile Fur mutant.
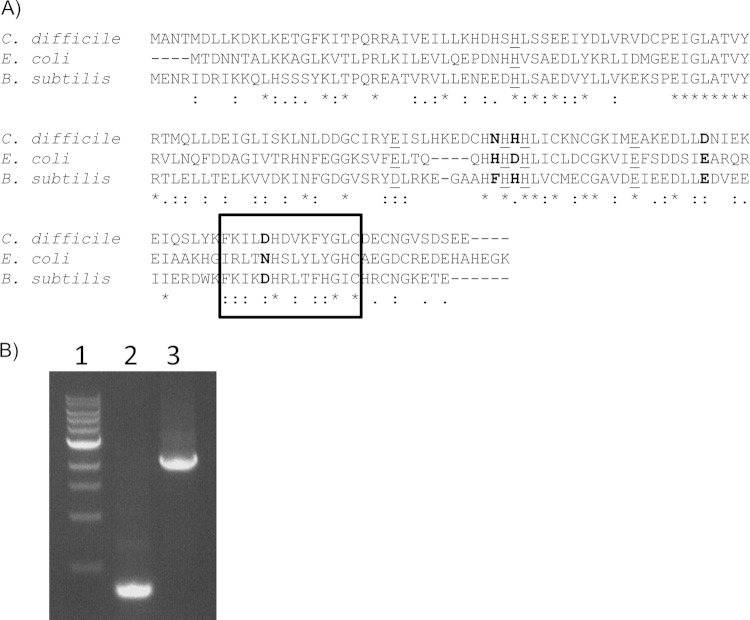
The transcriptional regulator Fur plays a major role in controlling iron homeostasis in most Gram-positive and Gram-negative bacteria (7). To study regulation of iron acquisition, we constructed a fur mutant of C. difficile. The fur gene was identified from the C. difficile 630 genome using the online bioinformatics tool BioCyc. The C. difficile Fur homologue is 62% and 70% similar to the Fur proteins of E. coli and Bacillus subtilis, respectively. Alignment of the Fur proteins from C. difficile, E. coli, and B. subtilis shows that the Zn2+-binding region, the Fe2+-binding region necessary for homodimerization, and the DNA-binding regions were conserved between the Fur proteins (Fig. 1A).
FIG 1.
C. difficile ferric uptake regulator Fur. (A) Alignment of Fur proteins from C. difficile, B. subtilis, and E. coli. The alignment was made with Clustal Omega (18) using default parameters and the following sequence accession numbers: E. coli, NP_415209.1; B. subtilis, NP_390233.2; and C. difficile, YP_001087781.1. The amino acids required for Zn2+ binding are underlined. The amino acids required for Fe2+ binding are in bold. The boxed region is the dimerization domain. (B) PCR Confirmation of wild type and fur::ltrB::erm mutant using primers TE2721 and TE2280, homologous to the 5′ and 3′ ends of the fur gene, respectively. Lane 1, 1-kb ladder; lane 2, wild-type chromosomal DNA; lane 3, fur mutant chromosomal DNA.
To construct a fur mutant of C. difficile, we used the targetron method to create an erythromycin resistance-marked insertion mutation as previously described (14, 16, 24). The insertion of the intron into the fur gene was confirmed by PCR (Fig. 1B). We then tested the ability of the C. difficile fur mutant to grow in low- and high-iron-containing media. The C. difficile fur mutant demonstrated no growth defect compared to the wild-type parent under the high- or low-iron conditions. However, during late stationary phase, the optical density of the fur mutant decreased compared to that of the wild-type parent, suggesting that a significant number of the fur mutant cells lysed at some time during stationary phase (see Fig. S1 in the supplemental material).
Identification of C. difficile genes regulated by Fur by microarray analysis.
To identify Fur-regulated genes, we isolated RNA from either the wild type or the fur mutant grown in the presence of iron. In bacteria where Fur controls gene expression in response to iron levels, Fur binds its target promoters in the presence of iron. Fur-repressed genes have lower expression in the wild type but are derepressed in the absence of Fur even in the presence of iron. Using microarray analysis, we identified greater than 70 C. difficile putative transcriptional units (single genes or putative operons) which had higher expression in the fur mutant (Fur repressed) than in the wild type (2.5-fold change cutoff and P value limit of ≤10−8). Additionally, the majority of the genes carried on the ϕC630-1 and ϕC630-2 prophages and the CTn4 and CTn6 conjugative transposons were also Fur repressed (Table 2). We found that 44 transcriptional units exhibited 2.5- to 11-fold-lower expression in the fur mutant than in the wild type, suggesting that these genes are directly or indirectly induced in the presence of Fur (Table 2).
TABLE 2.
Genes regulated in the fur mutant
| Category and gene namea | Functionb | Fold changec | P valued |
|---|---|---|---|
| Fur-repressed genes | |||
| cd1647-cd1650 (fpi) | Catecholate siderophore ABC transport | 216 to 730 | <1.03E−15 |
| cd1485 | Hypothetical protein | 449 | 4.16E−17 |
| cd0592-cd0591 | Putative P-type cation transport | 136 to 351 | <8.49E−14 |
| cd1087 (zupT) | zupT transporter | 341 | 9.29E−14 |
| cd2499 | Hypothetical protein | 269 | 7.92E−17 |
| cd1088 | Hypothetical protein | 142 | 6.84E−16 |
| cd1477-cd1480 (feo1) | feo1 ferrous iron transport operon | 36.6 to 162 | <4.68E−15 |
| cd1119 | Putative lipoprotein | 50.6 | 3.63E−15 |
| cd2992-2989 | ABC transporter | 23.6 to 38.7 | <1.03E−14 |
| cd3118 | Hypothetical protein | 36.3 | 1.63E−15 |
| cd1999 (fldX) | Flavodoxin | 31.1 | 1.71E−11 |
| cd1089-cd1090 | Two-component system | 25.4 to 30.3 | <7.82E−16 |
| cd2874-cd2878 (fhu) | Hydroxamate siderophore ABC transporter | 4.40 to 26.1 | <1.13E−11 |
| cd1887-8 (csfU-rsiU) | ECF sigma factor CsfU operon | 4.75 to 23.1 | <4.23E−10 |
| cd0593 | Hypothetical protein | 7.57 | 1.58E−16 |
| cd1085-cd1086 | Hypothetical protein, putative peptidase | 6.41 to 7.27 | <2.92E−12 |
| cd3091 (treA) | Trehalose-6-phosphate hydrolyase | 5.94 | 1.00E−12 |
| cd0762-8 (gut-srl) | Glucitol/sorbitol-specific PTS | 2.37 to 5.62 | <7.04E−08 |
| cd1287 (fur) | Ferric uptake regulator | 5.36 | 1.63E−10 |
| cd1120-2 (dhaB) | Glycerol dehydratase | 2.15 to 5.23 | <9.10E−12 |
| cd2216 | Hypothetical protein | 4.51 | 1.43E−12 |
| cd0740 | Putative aminotransferase | 4.09 | 4.75E−12 |
| cd2351 (grdB) | Glycine reductase complex B γ subunit | 3.85 | 2.20E−13 |
| cd0594-cd0595 | Hypothetical proteins | 3.35 to 3.84 | <2.64E−13 |
| cd2987-2988 | Two-component system | 3.30 to 3.80 | <7.34E−12 |
| cd3273-cd3274 | feo3 ferrous iron transport operon | 2.92 to 3.49 | <2.62E−13 |
| cd1565-6 (ilvC-ilvB) | Ketol-acid reductoisomerase | 2.98 to 3.44 | <1.20E−10 |
| cd1819 | Hypothetical protein | 3.34 | 1.79E−10 |
| cd1745A(feoA4) | feoA4 ferrous iron transport protein A | 3.30 | 1.41E−09 |
| cd0596-8 (cotJB1-cotJC1) | Putative spore coat proteins | 2.80 to 3.29 | <1.60E−10 |
| cd0797 | Hydroxymethylglutaryl-CoA lyase | 3.8 | 2.44E−12 |
| cd2418-6 (srlA-srlE) | Glucitol/sorbitol-specific PTS | 2.79 to 3.18 | <9.06E−12 |
| cd1820 (ade) | Adenine deaminase | 3.09 | 1.52E−13 |
| cd1564 | Hypothetical protein | 2.98 | 1.42E−08 |
| cd0798 | Hypothetical protein | 2.88 | 7.59E−12 |
| cd0995-6 (serA) | d-3-Phosphoglycerase dehydrogenase | 2.39 to 2.77 | <5.59E−11 |
| cd2000 (ispD) | Major intracellular serine protease | 2.73 | 3.33E−09 |
| cd2324-3 (gatD) | Galactitol-1-phosphate 5-dehydrogenase | 2.15 to 2.58 | <2.55E−08 |
| ϕC630-1 | Phage proteins | 2.15 to 4.33 | <2.55E−08 |
| ϕC630-2 | Phage proteins | 2.70 to 4.78 | <4.60E−10 |
| CTn4 | Conjugative transposon genes | 3.62 to 4.02 | <2.20E−07 |
| CTn6 | Conjugative transposon genes | 2.90 to 3.73 | <1.12E−06 |
| Fur-induced genes | |||
| cd2214-5 | Putative regulatory proteins | −6.83 to −11.1 | <1.86E−11 |
| cd2822-20 | Hypothetical proteins | −5.49 to −9.58 | <1.07E−14 |
| cd2169-8 | Hydroxylamine reductase | −3.13 to −7.93 | 6.70E−12 |
| cd2003 (effD) | Efflux pump | −7.01 | 1.85E−09 |
| cd2241-39 (nanE-nanA) | N-Acetyl-neuraminate, mannosamine degradation | −5.21 to −6.43 | <3.26E−12 |
| cd2276 | Putative Na:solute symporter | −6.30 | 8.05E−14 |
| cd2738-2737 | Cytosine permease, C-N hydrolase | −5.86 to −5.57 | <1.13E−14 |
| cd0902 | Putative cation efflux protein | −5.38 | 4.54E−14 |
| cd3096-3095 | 6-Phospho-beta-glucosidase | −4.49 to −5.33 | <4.92E−11 |
| cd3097 | PTS, IIABC component | −4.98 | 2.27E−11 |
| cd0899 (dinB) | DNA polymerase IV | −4.66 | 1.77E−14 |
| cd2593 | ABC transporter, ATP-binding component | −4.55 | 1.47E−12 |
| cd0332 (bclA1) | Putative exosporium glycoprotein | −4.49 | 4.91E−11 |
| cd2230 (nirC) | Putative nitrite transporter | −4.43 | 4.07E−12 |
| cd2629 (spoIVA) | Stage IV sporulation protein A | −4.23 | 2.93E−15 |
| cd2055 | Hypothetical protein | −4.01 | 1.49E−09 |
| cd0587-0588 | Hypothetical proteins | −3.55 to −4.00 | <9.89E−12 |
| cd2479 | Hypothetical protein | −3.88 | 1.58E−11 |
| cd1753-1755 | Putative ABC transporter | −2.05 to −3.71 | <2.11E−08 |
| cd0126 (spoIIID) | Stage III sporulation protein D | −3.68 | 1.27E−09 |
| cd3264 | Putative membrane protein | −3.55 | 2.02E−09 |
| cd2097 | Putative membrane protein | −3.50 | 2.82E−10 |
| cd0892 (cspA) | Cold shock protein | −3.50 | 2.74E−09 |
| cd3417-3416 | Putative ABC transporter | −2.02 to −3.49 | <2.08E−08 |
| cd1752 | Putative transcriptional regulator | −3.43 | 1.51E−11 |
| cd0581-2 | Transcriptional regulator, PEP kinase | −2.83 to −3.33 | <2.40E−10 |
| cd1993 | Putative carboxylase | −3.20 | 9.49E−10 |
| cd2566-7 | PTS, IIA IIB component | −2.12 to −3.09 | <9.49E−11 |
| cd2206 (aldH) | Aldehyde dehydrogenase | −2.97 | 1.07E−12 |
| cd2121 | Hypothetical protein | −2.96 | 2.07E−07 |
| cd0773 (spoVAC) | Stage V sporulation AC | −2.93 | 1.49E−08 |
| cd3515 | Pilin | −2.90 | 8.95E−09 |
| cd0739 | Putative exported protein | −2.89 | 1.91E−11 |
| cd0488 (sugE) | Quaternary ammonium resistance protein | −2.80 | 1.14E−06 |
| cd1192-4 (spoIIIAA,AB,AC) | Stage III sporulation protein AA, AB, AC | −2.59 to −2.76 | <2.08E−07 |
| cd2231-3 (asrABC) | Anaerobic sulfite reductase | −2.19 to −2.76 | <1.67E−08 |
| cd0106 (cwlD) | Germination specific N-acetylmuramoyl–l-alanine amidase | −2.73 | 1.69E−09 |
| cd3520 | Putative cation efflux protein | −2.69 | 3.27E−13 |
| cd0589-590 | Hypothetical proteins | −2.21 to −2.66 | <3.43E−10 |
| cd0670 | Regulatory protein | −2.65 | 5.85E−11 |
| cd1063B-1063C | Hypothetical proteins | −2.56 to −2.63 | <2.08E−05 |
| cd1291 (dacF) | d-Alanyl-d-alanine carboxypeptidase | −2.63 | 2.28E−11 |
| cd2310 (cspD) | Cold shock protein | −2.60 | 8.71E−08 |
| cd2749A-50 (agrBD) | Auotinducer peptide, regulator | −2.51 to −2.57 | <2.41E−08 |
From GenBank.
Putative functions as determined by current annotation of the C. difficile genome. PTS, phosphotransferase system; CoA, coenzyme A; PEP, phosphoenolpyruvate.
The fold changes listed are averages from three biological replicates, with each done in technical replicates. Fold changes signify expression that is increased in the fur mutant compared to the wild-type C. difficile when both strains were grown in high-iron-containing medium. For genes in a putative operon, the range of fold change is reported.
The P values listed are averages from three biological replicates, with each done in technical replicates. For genes in a putative operon, the highest P value is reported.
Iron regulation of Fur-regulated genes.
To verify the effect of Fur on expression of genes identified in our microarray analysis, we performed quantitative reverse transcriptase PCR (qRT-PCR) on several genes of interest. As in the microarray analysis, we isolated RNA from the fur mutant and wild-type cells grown in the presence of high iron concentrations. The RNA from each sample was converted to cDNA and quantified using qRT-PCR. For 14 of the 15 genes which were identified as Fur repressed by microarray analysis, mRNA levels were significantly higher in the fur mutant than in the wild type (Table 3). Furthermore, the levels of repression in the qRT-PCR experiments were comparable to the levels from the microarray analysis.
TABLE 3.
Fur- and iron-dependent regulation of C. difficile genes
| Gene namea | Fur repression in qRT-PCRb | Iron repression in qRT-PCRc | Primersd |
|---|---|---|---|
| cd1647 (yclO) | 9,600 | 920 | TEQ081, TEQ082 |
| cd1485 | 930 | 180 | TEQ089, TEQ090 |
| cd0591 | 81.3 | 1,200 | TEQ085, TEQ086 |
| cd1087 (zupT) | 679 | 327 | TEQ083, TEQ084 |
| cd2499 | 270 | 189 | TEQ091, TEQ092 |
| cd1477 | 118 | 38.5 | TEQ129, TEQ130 |
| cd1489 (feoB1) | 41.1 | 92 | TEQ061, TEQ062 |
| cd2992 | 71.7 | 95.7 | TEQ077, TEQ078 |
| cd3118 | 12.3 | 3.54 | TEQ093, TEQ094 |
| cd1999 (fldX) | 154 | 162 | TEQ087, TEQ088 |
| cd2878 (fhuD) | 800 | 867 | TEQ065, TEQ066 |
| cd1887 (csfU) | 0.46 | 0.455 | TEQ005, TEQ006 |
| cd1889 | 4.58 | 0.792 | TEQ079, TEQ080 |
| cd1287 (fur) | 17.0 | 3.62 | TEQ099, TEQ100 |
| cd1517 (feoB2) | 0.500 | 1.17 | TEQ057, TEQ058 |
| cd3273 (feoA3) | 4.51 | 5.61 | TEQ059, TEQ060 |
| cd1745A (feoA4) | 12.7 | 3.55 | TEQ097, TEQ098 |
| cd0627A (ferredoxin) | −4.9 | NDe | TEQ105, TEQ106 |
| cd2214 | −2.90 | ND | TEQ109, TEQ110 |
From GenBank.
Fur repression is the level of gene expression in the fur mutant divided by that in the wild-type C. difficile strain when both strains were grown in high-iron-containing medium. The reported values are the arithmetic averages from three biological replicates, with each done in technical replicates.
Iron repression is the level of gene expression in wild-type C. difficile grown in low-iron-containing medium divided by that in wild-type C. difficile grown in high-iron-containing medium. The reported values are the arithmetic averages from three biological replicates, with each done in technical replicates.
Sequences for the DNA primers used in qRT-PCRs are listed in Table S1 in the supplemental material.
ND, not determined.
To further examine the iron regulation of our genes of interest, we compared RNA levels of the Fur-repressed genes from wild-type cells grown under low- or high-iron conditions. Fourteen genes which were found to be Fur repressed were also iron repressed to an extent similar to their Fur regulation (Table 3). Interestingly, two genes (cd3118 and cd1889) were regulated by Fur but not repressed in the presence of iron. Similar Fur-repressed iron-independent regulation has been observed with the AmiF foramidase in Helicobacter pylori (25), and this suggests that regulation of cd3118 and cd1889 may be more complicated.
We identified 8 Fur-repressed putative transport systems (Table 2). Of these, 3 (cd1647-cd1650, cd2992-cd2989, cd2874-cd2878, and cd1891-cd1892) were homologous to ABC transporters. The cd1647-cd1650 and cd2874-cd2878 operons are highly similar to the genes encoding the Fpi (catecholate) and Fhu (hydroxamate) siderophore transport systems, respectively. Two of the Fur- and iron-repressed operons (feo1 and feo3) were homologous to the Feo ferrous transport system. The remaining two operons (cd0592-1 and cd1087) encoded proteins similar to P-type cation transporters and the low-affinity zinc transporter ZupT, respectively.
To further demonstrate that the fur mutation was responsible for the regulation of these putative transport systems, we complemented the fur mutant with Fur expressed from a low-copy-number plasmid (26, 27). When grown in the presence of iron, the fur mutant containing an empty vector exhibited between 120- and 350-fold higher expression of the feo1, fpi, and zupT operons than did the wild type (Fig. 2). We observed no significant differences in feo1, fpi, and zupT RNA levels between the wild type and the fur mutant carrying the Fur-expressing plasmid (Fig. 2). This suggests that the regulation defect of the fur mutant can be complemented in trans by expressing fur from a plasmid.
FIG 2.
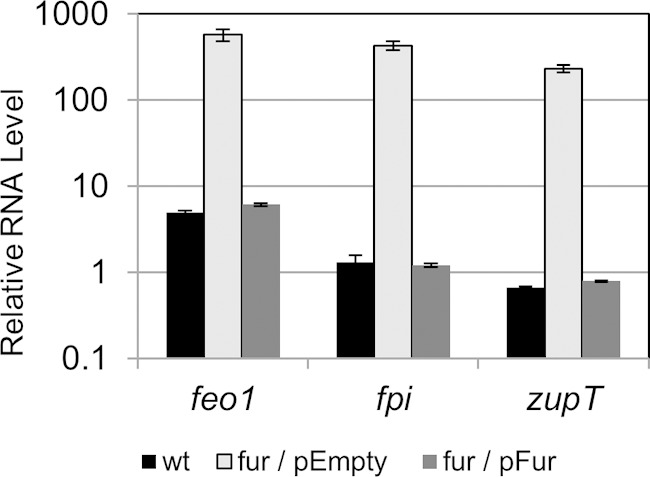
Complementation of Fur-regulated genes in C. difficile. The wild type (wt), the fur mutant containing empty vector (fur/pEmpty), and the fur mutant containing the Pfur-fur+ plasmid (fur/pFur) were grown to mid-log phase (OD, 0.8) in TY medium with a high iron concentration. The mRNA levels of feo1, fpi, and zupT were normalized to the level of rpoB transcript in each sample using the primers listed in Table S1 in the supplemental material. The data are graphed as the arithmetic mean and standard deviation of three biological replicates.
Fur also regulated expression of other genes which are not predicted to transport ions. We found that expression of 2 putative two-component systems operons was induced by iron depletion and was Fur repressed. These regulators (cd1089-1090 and cd2987-2988) (Table 3) may be involved in a regulatory cascade in which Fur indirectly controls expression of genes in its regulon. Additionally, expression of one flavodoxin gene, fldX, was strongly repressed by Fur and by high iron, while one ferredoxin gene (cd0627A) had lower expression in the fur mutant or under low-iron conditions.
Fur binding to Fur-regulated promoters.
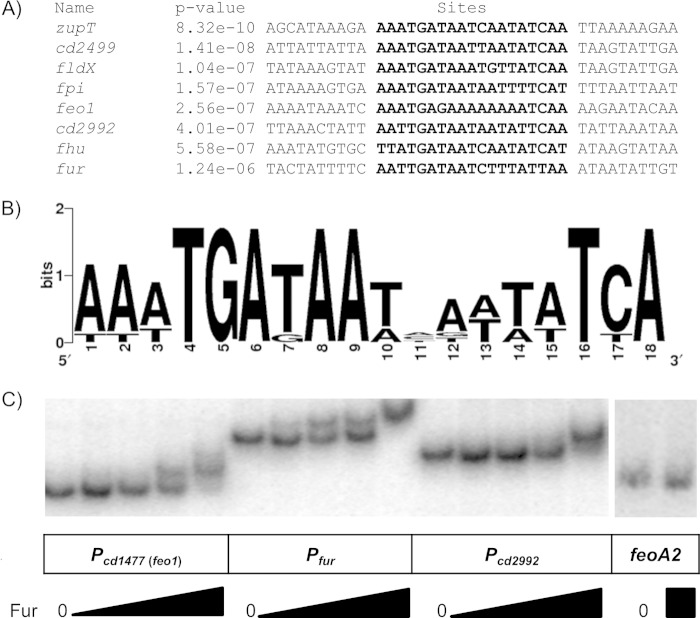
We used the bioinformatics software MEME (19) to identify a putative Fur-binding site in the promoter regions of the Fur-regulated genes identified as encoding putative transporters. We compared 250 bp upstream through 50 bp downstream of the translational start sites of 8 highly Fur-repressed C. difficile genes (Fig. 3A). We were able to identify a highly homologous 18-bp region in these sequences (Fig. 3A and B). The putative Fur-binding consensus sequence of C. difficile is similar to the consensus Fur-binding sites from the 19-bp consensus sequence identified in B. subtilis (28).
FIG 3.
Fur binding of iron-regulated promoters. (A) Alignment of the Fur-binding regions of iron-regulated promoters. The consensus sequence was determined using MEME software with settings to identify consensus regions between 16 and 22 bp and using ∼250 bp upstream and 50 bp downstream of the predicted start of translation for each of the Fur-repressed genes. In bold is the predicted Fur-binding site. (B) Consensus sequence logo of the C. difficile Fur-binding region. (C) Fur electrophoretic mobility shift assays. A [γ-32P]ATP-labeled DNA probe was incubated with either the in vitro transcription-translation reaction mixture of a mock control (0) or increasing amounts of in vitro transcription-translation Fur protein for 30 min. Protein was diluted 1:16, 1:8, 1:4, and 1:2 in 1× EMSA binding buffer prior to addition to the DNA probe.
We performed electrophoretic mobility shift assays (EMSAs) to demonstrate the binding of Fur protein to putative Fur-regulated promoters. In these assays, Fur protein was synthesized using in vitro transcription and translation. When the promoter regions of the cd1477 (feo1 operon), fur, and cd2992 genes were incubated with the product of the Fur in vitro transcription-translation reaction, we observed a shift of each of these promoter fragments (Fig. 3C). Importantly, we did not observe a shift the mobility of the DNA when incubation was with the mock transcription-translation reaction mixture (Fig. 3C). In addition, we did not observe a Fur-induced shift when we tested a probe including the feoA2 open reading frame, which is not predicted to contain a Fur-binding site and was not regulated by Fur (Fig. 3C). Taken together, these findings suggest that Fur likely acts to directly repress expression of at least some of these genes.
Expression of Fur-regulated genes during C. difficile infection of hamsters.
Eukaryotic hosts are thought to sequester available iron to limit the growth of bacterial pathogens. Under these iron-limiting conditions, it is advantageous for the bacteria to induce expression of iron uptake mechanisms. To determine whether expression of Fur-regulated genes was increased in vivo, we measured the expression levels of Fur-regulated putative cation acquisition genes during wild-type C. difficile infection of the hamster cecum. Hamsters were infected with wild-type C. difficile spores and exhibited at least 2 days of diarrhea before animals were sacrificed. The most distal and most proximal 1-cm cecal sections of each infected cecum (4 animals) were removed and immediately fixed in Trizol for RNA isolation. In these qRT-PCR experiments, we compared RNA levels of our genes of interest to the levels of mldA. Previous work has shown that the C. difficile-specific cell division gene mldA must be expressed at a low, constitutive level in vitro and in vivo (21).
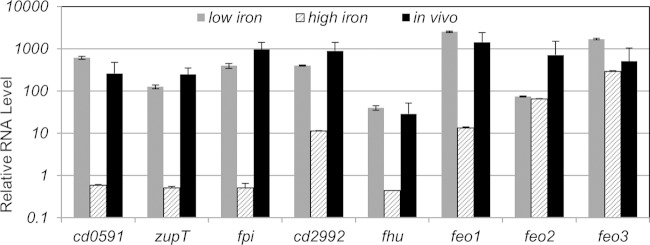
We measured expression of 7 Fur-regulated putative ion transport systems during C. difficile infection. Each cation transport operon demonstrated higher expression in the infected cecum than when the wild type was grown under iron-replete conditions, but to different extents (Fig. 4). The cd0591 and fpi operons exhibited the highest induction under both low-iron and in vivo conditions (Fig. 4). Expression of the feo1, zupT, cd2992, and fhu operons also showed substantial in vivo induction, although the overall RNA levels of fhu were considerably lower than those of other Fur-regulated genes (Fig. 4).
FIG 4.
Expression of putative cation transporter genes during infection of hamster model. To determine the expression of Fur-repressed genes during a C. difficile infection, hamsters were infected with 10,000 wild-type spores 5 days after receiving a single dose of clindamycin. Two days after the initial onset of diarrhea, infected hamsters were sacrificed. The ceca of infected hamsters were removed, and RNA was extracted from the cecal samples. The mRNA levels of cd0591, zupT, fpi, cd2992, fhu, feo1, feo2, and feo3 were normalized to the level of mldA transcript in each sample using the primers listed in Table S1 in the supplemental material. The mRNA levels were compared to those in wild-type cells grown in high-iron (250 μM FeCl3) TY and low-iron (100 μM dipyridyl) TY. Experiments were performed in technical and biological triplicate. The data are graphed as the arithmetic mean with standard deviation.
Interestingly, expression profiles of the 3 C. difficile FeoB permeases were significantly different. Although feo2 expression was not responsive to iron levels (Table 2; Fig. 4), it may be induced ∼10-fold during infection (Fig. 4). This would suggest that feo2 expression may be regulated in a Fur- and iron-independent manner. Our data suggest that feo3 expression is significantly less repressed by Fur (Table 2) and iron (Table 2; Fig. 4) than is feo1 expression. In accordance with these observations, there appears to be less induction of feo3 during in vivo infection as well (Fig. 4). In comparison, the feo1 operon is highly induced in vivo and under low iron conditions (Fig. 4). Taken together, our data suggest that Fur-regulated, iron-regulated genes are induced during C. difficile infection of the hamster cecum, thus suggesting that iron is limited during a C. difficile infection.
DISCUSSION
We have been investigating the role of the transcriptional repressor Fur in C. difficile. Here we have identified and confirmed the C. difficile Fur regulon. We have demonstrated Fur and iron regulation of several classes of ion transporters in C. difficile in vitro and in vivo.
It is well established that many bacteria encode ABC transporters which import iron-bound siderophores (29). Siderophores can be classified into 3 major groups: hydroxamates, catecholates, or mixed-ligand siderophores (30). We have found that C. difficile encodes a putative Fhu (ferric hydroxamate uptake) ABC transporter system which is expressed under iron-limiting conditions and is repressed by Fur. Fhu transporters have been shown to function in iron acquisition in many Gram-positive as well as Gram-negative bacteria (31–34). C. difficile also encodes a system homologous to the catecholate siderophore petrobactin ABC transporter Fpi/Ycl from B. subtilis (35). Like that of fhu, fpi expression is both iron and Fur repressed. In B. subtilis, Fpi imports petrobactin, the primary siderophore produced by Bacillus anthracis strains but not synthesized by B. subtilis itself (35).
In addition to these putative siderophore ABC transporters, we have identified two other Fur- and iron-regulated ABC transporters (CD2992-2989 and CD1891-1892). At this time, the substrates for CD2992-2989 and CD1891-1892 have not yet been determined. So far, bioinformatics has not provided any clues as to the identity of the substrate for CD1891-2. However, bioinformatic analysis indicates that C. difficile CD2992-2989 is structurally similar to the ABC transporter systems SsuCBA, which likely imports aliphatic sulfonates. Unlike ssuCBA which is not regulated by Fur or iron (data not shown), we found that cd2992-2989 is in the Fur regulon. To our knowledge, ABC transporters of sulfonated siderophores have not been characterized. However, members of the Marinobacter genus have been shown to synthesize a sulfonated siderophore (36, 37). It is possible that CD2992-2989 has specificity for a sulfonated derivative of a siderophore.
Often siderophore biosynthesis genes are also regulated by Fur (3). However, we have not found evidence for siderophore biosynthesis genes repressed by Fur, nor has bioinformatics revealed any obvious homologues to known siderophore biosynthesis genes in the C. difficile genome. It is possible that C. difficile synthesizes its own siderophores via an uncharacterized mechanism. It is also possible that C. difficile coopts siderophores produced by neighboring bacteria such as the resident microflora C. difficile encounters during infection. Under anaerobic conditions, iron is found in the ferrous form. As a strict anaerobe, C. difficile may be more dependent upon transport of free ferrous iron rather than ferric iron.
In many bacteria, transport of ferrous iron is dependent upon a class of transporters known as Feo transporters (4). In the canonical example, these transporters require a membrane protein, FeoB, and a cytoplasmic protein, FeoA. Some bacteria encode single Feo transporter systems, while others harbor multiple Feo paralogues. For example, Porphyromonas gingivalis encodes 2 Feo transport systems, one of which transports ferrous iron while the other imports Mn2+ (38). The genomes of all sequenced C. difficile strains available include genes encoding 3 paralogous Feo transport systems, which we have termed feo1, feo2, and feo3. In our work, we have found that expression of the Feo2 system is not repressed by Fur or iron levels. It is possible that this Feo system transports a cation other than iron, as is the case for P. gingivalis.
The remaining 2 Feo systems of C. difficile are Fur and iron regulated. Of these two Feo systems, the Feo1 system is more highly expressed and more strongly Fur regulated. Unlike the other two C. difficile feo operons, the feo1 operon includes 2 additional small open reading frames flanking the annotated genes encoding the cytoplasmic protein FeoA and the membrane-bound FeoB permease. In Yersinia pestis, FeoC, encoded by the third gene in the feo operon, is not required for Feo transport activity but is involved in regulation of the system (39). In V. cholerae, the FeoC protein does not affect expression of the feo operon but is essential for Feo transport, although its role in Feo transport is not known (40). In C. difficile, the role of these small proteins in the Feo1 system is not known.
In addition to the Feo systems, we also identified two putative ion transporters belonging to different families of divalent cations transporters whose expression was repressed in the presence of Fur and iron. CD0591 is homologous to the P-type family of cation transporters (41). CD1087 is similar to ZupT, a broad-specificity divalent metal cation transporter (42). Members of both of these transporter families can be found in prokaryotes and eukaryotes (42–44). Homologues of CD0591 and CD1087 in other bacteria have been shown to transport multiple divalent cations, including Mn2+, Zn2+, Cd2+, and Fe2+ (43, 45, 46). While several of these cation transporters are capable of importing ferrous iron, they have higher affinity for other divalent cations (46). It is possible that CD0591 and CD1087 are important for transporting multiple metal ions.
Although C. difficile and B. subtilis both utilize Fur as a regulator of iron homeostasis, many other ion-sensing transcriptional regulators found in B. subtilis (MntR, Zur, and PerR) are not identifiable by homology in C. difficile (47, 48). For example unlike B. subtilis, C. difficile does not encode a zinc-sensing Zur or a manganese-responsive MntR homologue. Presently, it is unclear how C. difficile may sense levels of zinc or manganese and control their homeostasis. However, expression of ion transporters can be affected by the levels of metal ions which they do not transport. For example, E. coli MntH imports Mn2+ but is repressed by both the iron-sensing Fur and the manganese-responsive MntR regulators (49). Manganese can serve as a substitute for iron in many metalloenzymes under iron-depleted conditions (50). Thus, one hypothesis is that expression of cd0591 and cd1087 may be repressed by Fur because the CD0591 and CD1087 transporters may be importing Mn2+ as an alternative enzyme cofactor when iron is scarce.
In addition to iron transport, we demonstrate Fur regulation of ferredoxin and flavodoxin. Ferredoxin and flavodoxin are isofunctional electron transfer proteins involved in numerous metabolic reactions (51). Ferredoxins require iron-sulfur clusters to coordinate electron transfer, while flavodoxins use flavin mononucleotide (FMN)/flavins and do not require iron. Interestingly, C. difficile encodes 4 putative ferredoxins and 6 potential flavodoxins. We have found that the flavodoxin gene fldX is induced under iron-limiting conditions, while cd0627A is repressed. This regulation is dependent upon Fur, suggesting that it may play a role in the balance of ferredoxin-flavodoxin expression.
We also found that expression of several mobile elements was higher in the fur mutant than in wild-type C. difficile. While it is possible that Fur directly represses expression of key regulators of these elements, Fur repression of mobile element induction may be more indirect. In the absence of Fur, C. difficile presumably imports higher levels of iron, which can catalyze oxidative radical production from the Fenton reaction, ultimately leading to increased DNA damage. Many phages and conjugative transposons use DNA damage as a signal for induction of mobile element excision from the chromosome (52). Phage induction can lead to lysis of the fur mutant and may account for the drop in optical density that we observed in the fur mutant during stationary growth phase. As an obligate anaerobe, C. difficile is extremely sensitive to oxidative stress. Iron potentiates the damaging effects of oxidative stress. Thus, Fur may play an important role in obtaining an adequate iron level for iron-dependent metabolism while avoiding excessive intracellular iron levels that could cause damage to DNA.
Recently it has been appreciated that Fur not only represses but can directly act as a transcriptional activator in Helicobacter pylori (53), Campylobacter jejuni (54), and Neisseria meningitidis (8). Our microarray analyses suggest that a class of C. difficile genes have lower expression in the fur mutant. These genes may be directly activated by Fur or may be indirectly controlled by a Fur-dependent regulator. Future experiments on C. difficile Fur binding to Fur-activated promoters may distinguish the role of Fur in activation of C. difficile gene expression.
Having determined iron repression of several putative ion transport systems in vitro, we investigated their expression during a C. difficile infection. We tested several Fur-regulated genes for in vivo expression in the hamster model of C. difficile infection, which mimics the pathology of a C. difficile infection in humans (23). In this work, we demonstrate substantial increases in expression of all the Fur-regulated genes that we tested. Our data strongly suggest that C. difficile induces expression of the Fur regulon during infection of the hamster cecum. They further suggest that the hamster cecum may be an iron-limiting environment. A previous study of in vivo C. difficile gene transcription suggested that none of the Fur-regulated genes were induced during the first 38 h of colonization of the gnotobiotic mouse (55). This would imply that early colonization of the gnotobiotic mouse model iron is not limiting. This difference in iron levels between the gnotobiotic mouse and hamster models of infection may be due to differences in the host sequestration of iron or the lack of resident microflora competing for available iron in the gnotobiotic mouse. Our data suggest that Fur plays an important role in regulating a large class of proteins induced during hamster infection. Dysregulation of Fur-regulated genes may have significant impact on C. difficile survival during infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant R01AI087834 from the National Institute of Allergy and Infectious Diseases to C.D.E.
We thank Linda McCarter and members of the Ellermeier lab for helpful comments.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00098-15.
REFERENCES
- 1.Andrews SC, Robinson AK, Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 2.Skaar EP. 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. 2006. Feo–transport of ferrous iron into bacteria. Biometals 19:143–157. doi: 10.1007/s10534-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 5.Imlay JA, Linn S. 1988. DNA damage and oxygen radical toxicity. Science 240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 6.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 7.Troxell B, Hassan HM. 2013. Transcriptional regulation by ferric uptake regulator (Fur) in pathogenic bacteria. Front Cell Infect Microbiol 3:59. doi: 10.3389/fcimb.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifantini R, Sebastian S, Frigimelica E, Draghi M, Bartolini E, Muzzi A, Rappuoli R, Grandi G, Genco CA. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc Natl Acad Sci U S A 100:9542–9547. doi: 10.1073/pnas.1033001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porcheron G, Garénaux A, Proulx J, Sabri M, Dozois CM. 2013. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol 3:90. doi: 10.3389/fcimb.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojiljkovic I, Hantke K. 1995. Functional domains of the Escherichia coli ferric uptake regulator protein (Fur). Mol Gen Genet 247:199–205. doi: 10.1007/BF00705650. [DOI] [PubMed] [Google Scholar]
- 11.Baichoo N, Wang T, Ye R, Helmann JD. 2002. Global analysis of the Bacillus subtilis fur regulon and the iron starvation stimulon. Mol Microbiol 45:1613–1629. doi: 10.1046/j.1365-2958.2002.03113.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor JR, Lyras D, Farrow KA, Adams V, Powell DR, Hinds J, Cheung JK, Rood JI. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol Microbiol 61:1335–1351. doi: 10.1111/j.1365-2958.2006.05315.x. [DOI] [PubMed] [Google Scholar]
- 13.Bouillaut L, Self WT, Sonenshein AL. 2013. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J Bacteriol 195:844–854. doi: 10.1128/JB.01492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heap JT, Kuehne Sa, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP. 2010. The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55. doi: 10.1016/j.mimet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Trieu-Cuot P, Carlier C, Poyart-salmeron C, Courvalin P. 1991. Shuttle vectors containing a multiple cloning site and a lacZa gene for conjugal transfer of DNA from Escherichia coli to Gram-positive bacteria. Gene 102:99–104. doi: 10.1016/0378-1119(91)90546-N. [DOI] [PubMed] [Google Scholar]
- 16.Ho TD, Ellermeier CD. 2011. PrsW is required for colonization, resistance to antimicrobial peptides, and expression of extracytoplasmic function σ factors in Clostridium difficile. Infect Immun 79:3229–3238. doi: 10.1128/IAI.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagan RP, Fairweather NF. 2011. Clostridium difficile has two parallel and essential Sec secretion systems. J Biol Chem 286:27483–27493. doi: 10.1074/jbc.M111.263889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey TL, Boden M, Buske Fa, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho TD, Williams KB, Chen Y, Helm RF, Popham DL, Ellermeier CD. 2014. Clostridium difficile extracytoplasmic function σ factor σV regulates lysozyme resistance and is necessary for pathogenesis in the hamster model of infection. Infect Immun 82:2345–2355. doi: 10.1128/IAI.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ransom EM, Williams KB, Weiss DS, Ellermeier CD. 2014. Identification and characterization of a gene cluster required for proper rod shape, cell division, and pathogenesis in Clostridium difficile. J Bacteriol 196:2290–2300. doi: 10.1128/JB.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambol SP, Tang JK, Merrigan MM, Johnson S, Gerding DN. 2001. Infection of hamsters with epidemiologically important strains of Clostridium difficile. J Infect Dis 183:1760–1766. doi: 10.1086/320736. [DOI] [PubMed] [Google Scholar]
- 23.Goulding D, Thompson H, Emerson J, Fairweather NF, Dougan G, Douce GR. 2009. Distinctive profiles of infection and pathology in hamsters infected with Clostridium difficile strains 630 and B1. Infect Immun 77:5478–5485. doi: 10.1128/IAI.00551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464. doi: 10.1016/j.mimet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Van Vliet AHM, Stoof J, Poppelaars SW, Bereswill S, Homuth G, Kist M, Kuipers EJ, Kusters JG. 2003. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori fur repressor. J Biol Chem 278:9052–9057. doi: 10.1074/jbc.M207542200. [DOI] [PubMed] [Google Scholar]
- 26.Heap JT, Pennington OJ, Cartman ST, Minton NP. 2009. A modular system for Clostridium shuttle plasmids. J Microbiol Methods 78:79–85. doi: 10.1016/j.mimet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Ransom EM, Ellermeier CD, Weiss DS. 2015. Use of mCherry red fluorescent protein for studies of protein localization and gene expression in Clostridium difficile. Appl Environ Microbiol 81:1652–1660. doi: 10.1128/AEM.03446-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baichoo N, Helmann JD. 2002. Recognition of DNA by Fur: A reinterpretation of the Fur box consensus sequence. J Bacteriol 184:5826–5832. doi: 10.1128/JB.184.21.5826-5832.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köster W. 2001. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res Microbiol 152:291–301. doi: 10.1016/S0923-2508(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 30.Höfte M. 1992. Classes of microbial siderophores, p 3–26. In Bart L, Hemming B (ed), Iron chelation in plants and soil microorganisms. Academic Press, New York, NY. [Google Scholar]
- 31.Jin B, Newton SMC, Shao Y, Jiang X, Charbit A, Klebba PE. 2006. Iron acquisition systems for ferric hydroxamates, haemin and haemoglobin in Listeria monocytogenes. Mol Microbiol 59:1185–1198. doi: 10.1111/j.1365-2958.2005.05015.x. [DOI] [PubMed] [Google Scholar]
- 32.Sebulsky MT, Hohnstein D, Hunter MD, Heinrichs DE. 2000. Identification and characterization of a membrane permease involved in iron-hydroxamate transport in Staphylococcus aureus. J Bacteriol 182:4394–4400. doi: 10.1128/JB.182.16.4394-4400.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fecker L, Braun V. 1983. Cloning and expression of the fhu genes involved in iron (III)-hydroxamate uptake by Escherichia coli. J Bacteriol 156:1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forman S, Nagiec MJ, Abney J, Perry RD, Fetherston JD. 2007. Analysis of the aerobactin and ferric hydroxamate uptake systems of Yersinia pestis. Microbiology 153:2332–2341. doi: 10.1099/mic.0.2006/004275-0. [DOI] [PubMed] [Google Scholar]
- 35.Zawadzka AM, Kim Y, Maltseva N, Nichiporuk R, Fan Y, Joachimiak A, Raymond KN. 2009. Characterization of a Bacillus subtilis transporter for petrobactin, an anthrax stealth siderophore. Proc Natl Acad Sci U S A 106:21854–21859. doi: 10.1073/pnas.0904793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hickford SJH, Küpper FC, Zhang G, Carrano CJ, Blunt JW, Butler A. 2004. Petrobactin sulfonate, a new siderophore produced by the marine bacterium Marinobacter hydrocarbonoclasticus. J Nat Prod 67:1897–1899. doi: 10.1021/np049823i. [DOI] [PubMed] [Google Scholar]
- 37.Homann VV, Edwards KJ, Webb EA, Butler A. 2009. Siderophores of Marinobacter aquaeolei: petrobactin and its sulfonated derivatives. BioMetals 22:565–571. doi: 10.1007/s10534-009-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He J, Miyazaki H, Anaya C, Yu F, Yeudall WA, Lewis JP. 2006. Role of Porphyromonas gingivalis FeoB2 in metal uptake and oxidative stress protection. Infect Immun 74:4214–4223. doi: 10.1128/IAI.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fetherston JD, Mier I, Truszczynska H, Perry RD. 2012. The Yfe and Feo transporters are involved in microaerobic growth and virulence of Yersinia pestis in bubonic plague. Infect Immun 80:3880–3891. doi: 10.1128/IAI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver EA, Wyckoff EE, Mey AR, Morrison R, Payne SM. 2013. FeoA and FeoC are essential components of the vibrio cholerae ferrous iron uptake system, and FeoC interacts with FeoB. J Bacteriol 195:4826–4835. doi: 10.1128/JB.00738-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Møller JV, Juul B, le Maire M. 1996. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta 1286:1–51. doi: 10.1016/0304-4157(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 42.Liuzzi JP, Cousins RJ. 2004. Mammalian zinc transporters. Annu Rev Nutr 24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 43.Papp-Wallace KM, Maguire ME. 2006. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol 60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 44.Hantke K. 2001. Iron and metal regulation in bacteria. Curr Opin Microbiol 4:172–177. doi: 10.1016/S1369-5274(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 45.Rensing C, Mitra B, Rosen BP. 1997. The zntA gene of Escherichia coli encodes a Zn (II)-translocating P-type ATPase. Proc Natl Acad Sci U S A 94:14326–14331. doi: 10.1073/pnas.94.26.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. 2005. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol 187:1604–1611. doi: 10.1128/JB.187.5.1604-1611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore CM, Helmann JD. 2005. Metal ion homeostasis in Bacillus subtilis. Curr Opin Microbiol 8:188–195. doi: 10.1016/j.mib.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Fuangthong M, Helmann JD. 2003. Recognition of DNA by three ferric uptake regulator (Fur) homologs in Bacillus subtilis. J Bacteriol 185:6348–6357. doi: 10.1128/JB.185.21.6348-6357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patzer SI, Hantke K. 2001. Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. J Bacteriol 183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anjem A, Varghese S, Imlay JA. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoch DC, Valentine RC. 1972. Ferredoxins and flavodoxins of bacteria. Annu Rev Microbiol 26:139–162. doi: 10.1146/annurev.mi.26.100172.001035. [DOI] [PubMed] [Google Scholar]
- 52.Witkin E. 1976. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev 40:869–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilbreath JJ, West AL, Pich OQ, Carpenter BM, Michel S, Scott Merrell D. 2012. Fur activates expression of the 2-oxoglutarate oxidoreductase genes (oorDABC) in Helicobacter pylori. J Bacteriol 194:6490–6497. doi: 10.1128/JB.01226-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butcher J, Stintzi A. 2013. The transcriptional landscape of Campylobacter jejuni under iron replete and iron limited growth conditions. PLoS One 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janoir C, Denève C, Bouttier S, Barbut F, Hoys S, Caleechum L, Chapetón-Montes D, Pereira FC, Henriques AO, Collignon A, Monot M, Dupuy B. 2013. Adaptive strategies and pathogenesis of Clostridium difficile from in vivo transcriptomics. Infect Immun 81:3757–3769. doi: 10.1128/IAI.00515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.