ABSTRACT
Bordetella filamentous hemagglutinin (FHA), a primary component of acellular pertussis vaccines, contributes to virulence, but how it functions mechanistically is unclear. FHA is first synthesized as an ~370-kDa preproprotein called FhaB. Removal of an N-terminal signal peptide and a large C-terminal prodomain (PD) during secretion results in “mature” ~250-kDa FHA, which has been assumed to be the biologically active form of the protein. Deletion of two C-terminal subdomains of FhaB did not affect production of functional FHA, and the mutant strains were indistinguishable from wild-type bacteria for their ability to adhere to the lower respiratory tract and to suppress inflammation in the lungs of mice. However, the mutant strains, which produced altered FhaB molecules, were eliminated from the lower respiratory tract much faster than wild-type B. bronchiseptica, suggesting a defect in resistance to early immune-mediated clearance. Our results revealed, unexpectedly, that full-length FhaB plays a critical role in B. bronchiseptica persistence in the lower respiratory tract.
IMPORTANCE
The Bordetella filamentous hemagglutinin (FHA) is a primary component of the acellular pertussis vaccine and an important virulence factor. FHA is initially produced as a large protein that is processed during secretion to the bacterial surface. As with most processed proteins, the mature form of FHA has been assumed to be the functional form of the protein. However, our results indicate that the full-length form plays an essential role in virulence in vivo. Furthermore, we have found that FHA contains intramolecular regulators of processing and that this control of processing is integral to its virulence activities. This report highlights the advantage of studying protein maturation and function simultaneously, as a role for the full-length form of FHA was evident only from in vivo infection studies and not from in vitro studies on the production or maturation of FHA or even from in vitro virulence-associated activity assays.
INTRODUCTION
Two-partner secretion (TPS) is a widespread protein secretion pathway for Gram-negative bacteria in which a large exoprotein (generically called TpsA) is translocated through a cognate outer membrane β-barrel pore protein (TpsB) to the bacterial surface, where the exoprotein is then able to interact with its environment. The mechanism by which this occurs is complex, with fundamental aspects of the process, such as how unidirectional protein translocation across a membrane is achieved in the absence of chemical energy and how these massive proteins are able to fold correctly in the absence of typical quality control mechanisms, remaining unclear. Virulence functions have been attributed to most TPS systems and include adherence to host tissues (1, 2), iron acquisition (3), cytotoxicity (4, 5), and immune evasion (6), which contribute to bacterial colonization and persistence.
Whooping cough, or pertussis, is currently reemerging in the United States and other developed countries. Increased incidence in recent years has coincided with a switch from whole-cell pertussis (wP) vaccines to acellular pertussis (aP) vaccines that display reduced reactogenicity (7). Pertussis is primarily caused by the human-restricted Gram-negative pathogen Bordetella pertussis, and aP vaccines typically consist of three to five proteins that it secretes: filamentous hemagglutinin (FHA), pertussis toxin (Ptx), pertactin (Prn), and, often, fimbrial subunits (Fim2 and Fim3). While the underlying reasons are not completely understood, the recent surge in pertussis incidence is likely largely due to deficiencies in aP vaccine efficacy, including induction of relatively short-lived immunity and inability to prevent colonization and transmission (8–11). Despite decades of research, our understanding of the physiological properties of aP vaccine components remains incomplete, a critical shortfall in attempts to decide how best to prevent pertussis in the future.
In accordance with its role as a virulence factor, in vivo studies have shown that FHA is required for infection and persistence in the lower respiratory tracts of mice, rats, and pigs by Bordetella bronchiseptica (2, 6, 12–15), a close relative of B. pertussis that naturally infects a broad range of mammalian hosts. Many Bordetella virulence factors, including FHA, are highly conserved and have been shown to be functionally interchangeable between B. pertussis and B. bronchiseptica in animal models of disease (13, 16, 17). For these reasons, we use B. bronchiseptica infection of common laboratory animals to assess the contribution of Bordetella virulence factors to pathogenesis. In addition to its postulated role as an adhesin (18), FHA appears to perform immunomodulatory functions that contribute to colonization and/or persistence (6, 12), though whether these effects are a direct result of some undefined FHA activity (19–23) or an indirect result of FHA-mediated adherence to specific host cells (6, 24, 25) is unclear.
FHA serves as a paradigm for TPS. FHA (defined as the ~250-kDa protein that is both surface associated and released from the bacterial surface) is initially translated as a preproprotein called FhaB (~370 kDa), which contains an N-terminal signal peptide and a large C-terminal “prodomain” that are removed during the secretion process (a general diagram of the domain structure is shown in Fig. S1 in the supplemental material). As with most processed proteins, the “mature” molecule (~250-kDa FHA, in this case) has been assumed to be the functional form of the protein. According to the current model of FHA secretion (26), the signal peptide directs FhaB across the inner membrane via the Sec translocation machinery (27) and is then removed by leader peptidase. The ~250-amino-acid (aa) region of FhaB immediately C terminal to the signal peptide, which is referred to as the TPS domain and which is highly conserved among TpsA proteins, is bound by chaperones that maintain the protein in a nonfolded state as it transits through the periplasm (28, 29). FhaB then transits through FhaC in an N- to C-terminal direction, with the N terminus remaining anchored to FhaC at the cell membrane (14, 26). The TPS domain initiates folding of FhaB into a rigid β-helix on the surface of the bacterium (30, 31), and progressive folding results in formation of an ~50-nm-long shaft. C terminal to the β-helical shaft, an ~500-aa globular domain begins to fold into the mature C-terminal domain (MCD) at the distal end of the molecule (14, 32), which mediates adherence of the bacteria to host cells in vitro and to respiratory epithelium in vivo (13, 26). Upon translocation of the residues composing the MCD, the proximal region of the FhaB prodomain (called the prodomain N terminus [PNT]), which is conserved among FhaB-like TpsA proteins, prohibits further translocation and retains the prodomain in an intracellular compartment (26). We hypothesize that anchoring of the C terminus of the MCD near the membrane by the PNT acts as a sort of intramolecular chaperone that restricts the conformations that the MCD can sample during folding (26). Deletion of the prodomain abrogates the ability of B. bronchiseptica to adhere to host cells in vitro and to colonize the lower respiratory tracts of mice and rats in vivo (14, 26), presumably due to misfolding of the MCD.
Subsequent to translocation and folding of the MCD, proteolysis of the FhaB C-terminal prodomain occurs by the activity of an as-yet-unidentified protease(s) (14, 26). Degradation is rapid and complete (14, 26, 33), making it unlikely that the prodomain performs an independent function. In ΔPNT strains, in which the prodomain is aberrantly translocated to the surface, the prodomain is readily detected (26), indicating that the prodomain is not intrinsically unstable but is instead subject to regulated degradation inside the cell. Production of FhaB molecules lacking the C-terminal half of the MCD results in detectable, stable intracellular prodomain polypeptides (26), further supporting the hypothesis of regulated degradation and indicating a role for the extracellular MCD in this regulation. Additional processing to form the C terminus of mature FHA occurs and is dependent on the surface-localized serine protease autotransporter SphB1 (34). Although the primary function attributed to FHA is adherence to respiratory epithelium, FHA is ultimately released from the cell surface, liberating FhaC to secrete another FhaB molecule.
While adherence is the primary function attributed to FHA, our previous observations suggest that portions of the FhaB prodomain contribute to additional virulence activities mediated by FHA. For example, small deletions near the C terminus of the prodomain abrogated persistent tracheal infection of rats by B. bronchiseptica, though adherence capabilities in vitro were preserved (14). Here, we set out to determine the contribution of two C-terminal FhaB subdomains, a proline-rich region (PRR) and a conserved extreme C terminus (ECT), to FhaB/FHA function. We also investigated how prodomain degradation is controlled and what role its regulation plays in the maturation and function of FHA.
RESULTS
The FhaB prodomain localizes to the periplasm.
Previous studies demonstrated that the PNT is necessary for retention of the FhaB prodomain in an intracellular compartment, which is essential for proper folding and function of FHA (26). To determine if the C terminus of the prodomain enters the periplasm or if it remains in the cytoplasm, we created a B. bronchiseptica strain containing a fusion of phoA (lacking the codons for its natural signal peptide) to the 3′ end of fhaB. Note that all mutations described in this study were made in a derivative of B. bronchiseptica strain RB50 lacking fhaS (RBX11) to facilitate genetic manipulation of fhaB and interpretation of FhaB maturation data (35). fhaS is a gene displaying high nucleotide sequence similarity to fhaB in B. bronchiseptica. However, deletion of fhaS does not produce any detectable effects on B. bronchiseptica pathogenicity in animal models (35), and we refer to RBX11 as the wild-type (WT) strain in this study. PhoA activity, assayed by conversion of the chromogenic substrate 5-bromo-4-chloro-3-indolyl phosphate (X-P) to a blue product, is observed only when PhoA is present in the periplasm (36). Western blot analysis of whole-cell lysates (WCL) and culture supernatants of the WT strain producing the fusion protein displayed no changes in the amount of FhaB or FHA (FhaB/FHA) produced, processed, or released (Fig. 1a), indicating that fusion of PhoA to the C terminus of FhaB did not alter FhaB secretion. We also performed dot blot analysis of the strain producing FhaB with the C-terminal PhoA fusion, probing the blots with an α-MCD antibody (and with a secondary antibody with green fluorescence) and an α-PhoA antibody (and with a secondary antibody with red fluorescence). As seen previously (26), the MCD was detected on the surface of intact bacteria and in disrupted cells (Fig. 1b). In contrast, PhoA was detected only in disrupted cells, indicating that the prodomain remained intracellular. Culture of this strain on plates containing X-P resulted in growth of blue colonies (Fig. 1c), which, combined with the fact that proteins are secreted through the Sec translocon in an N-to-C-terminal fashion (37), indicates that the entire FhaB prodomain is transported to the periplasm during secretion and therefore that the periplasm is the compartment in which prodomain functions occur.
FIG 1 .
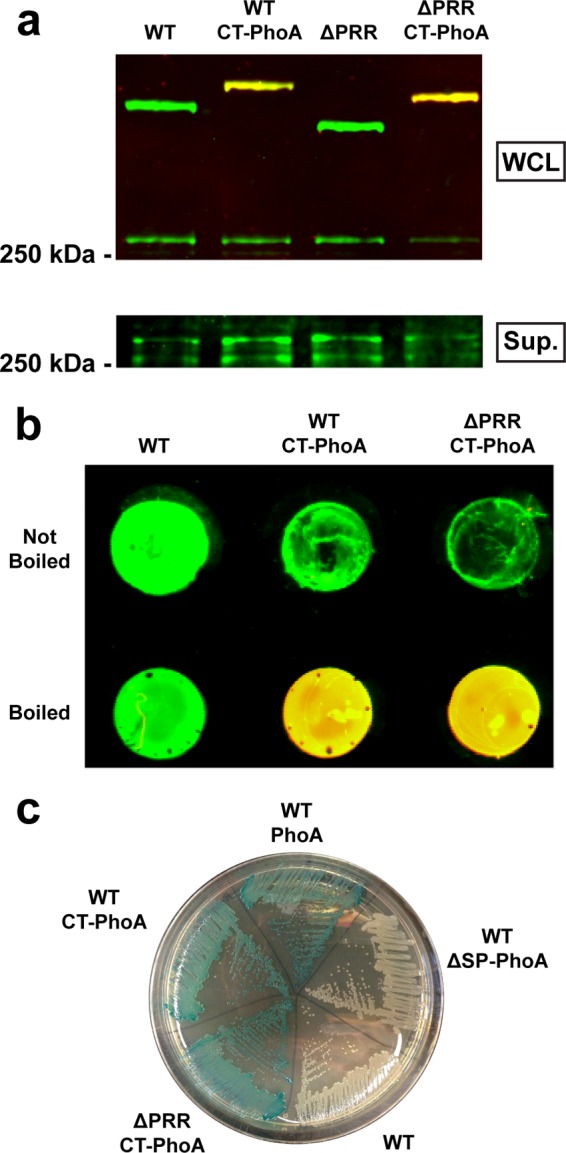
The FhaB prodomain transits to the periplasm, where it is retained during secretion. (a) Western blot analysis of whole-cell lysates (WCL) and culture supernatants (Sup.) from strains containing 3′ fusions of phoA to fhaB. C-terminal PhoA fusion (CT-PhoA strains) did not alter production or processing of FhaB or release of FHA. Membranes were probed as described for panel b. (b) Dot blot analysis of intact (not boiled) and disrupted (boiled) bacteria for strains not producing PhoA (WT) or with PhoA fused to the C terminus of FhaB (CT-PhoA strains). Membranes were probed with α-MCD (green) and α-PhoA (red) antibodies, and the green and red channels are overlaid. PhoA was accessible to antibodies only after lysis of the cells by boiling. (c) Culture of strains containing various phoA insertions on an agar plate containing a chromogenic PhoA substrate. Fusion of PhoA (lacking its native signal peptide) to the C terminus of FhaB (CT-PhoA strains) resulted in production of blue colonies similar to those produced by a strain constitutively producing PhoA (WT PhoA), while strains constitutively producing PhoA lacking its native signal peptide (WT ΔSP-PhoA) or not producing PhoA (WT) did not produce blue colonies.
The ECT of FhaB negatively regulates prodomain degradation, while the PRR plays no apparent role in FhaB/FHA processing.
Western blot analysis of WT B. bronchiseptica revealed the presence of ~370-kDa FhaB preproprotein and multiple processed FHA proteins of ~250 kDa in whole-cell lysates (WCL) and processed ~250-kDa FHA proteins only in culture supernatants (Fig. 2). The largest processed polypeptide is referred to as FHA′ in B. bronchiseptica (FHA* in B. pertussis) and is produced via degradation of the prodomain by an unidentified protease(s) (14, 34). The most abundant processed polypeptide is referred to as FHA and is produced in an SphB1-dependent manner via cleavage of FhaB or FHA′ (14, 34). Two smaller processed polypeptides, which are similar in size and often comigrate on SDS-PAGE gels, are referred to as FHA1 and FHA2 and are also produced in an SphB1-dependent manner. The SphB1-dependent FHA, FHA1, and FHA2 proteins are the predominant forms released from the bacterial surface, while FHA′/FHA* is primarily released only in strains lacking SphB1, although a small amount can sometimes be detected in culture supernatants of WT cells (14, 34).
FIG 2 .
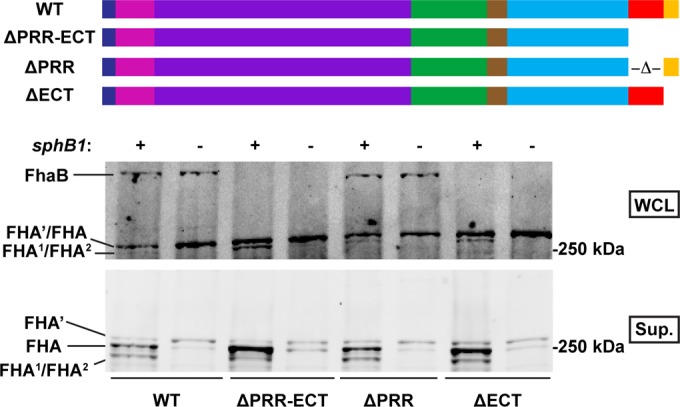
The extreme C terminus (ECT) of the FhaB prodomain inhibits prodomain degradation. Results of Western blot analysis of whole-cell lysates (WCL) and culture supernatants (Sup.) from strains lacking the FhaB PRR (ΔPRR) or the FhaB ECT (ΔECT) or both (ΔPRR-ECT) or neither (WT), as well as each strain lacking sphB1, are shown. Strains lacking the FhaB ECT did not contain any observable FhaB in the WCL or Sup. fractions. Membranes were probed with α-MCD antibodies. The FhaB molecules translated by each strain are diagrammed at the top of the figure (dark blue, signal peptide; pink, TPS domain; purple, β-helical domain; green, MCD; brown, PNT; light blue, uncharacterized prodomain; red, PRR; yellow, ECT).
The FhaB C terminus includes a proline-rich region (PRR) (26, 38) that is composed of 27% proline residues (as opposed to 2% across the remainder of FhaB) and contains two conspicuous repeat motifs (see Fig. S1b in the supplemental material). Excluding minor differences in the length or number of repeats, the predicted PRRs are 87% identical and 92% similar among the strains of Bordetella spp. that cause disease in the mammals for which genome sequence information is available. C terminal to the PRR are 98 aa (see Fig. S1c) that are 100% identical among all predicted FhaB proteins, which we refer to here as the FhaB extreme C terminus (ECT). Western blot analysis of a strain producing FhaB that lacks both subdomains (ΔPRR-ECT) revealed the absence of full-length FhaB in WCL (Fig. 2), but a lack of these subdomains had no discernible effect on the amount of FHA produced or released into the culture medium. Deletion of sphB1 in the ΔPRR-ECT strain resulted in detection of a doublet that included FHA′ and a slightly smaller polypeptide that we had not observed previously but which was present also in culture supernatants of the WT strain (Fig. 2).
To investigate the contribution of the individual C-terminal subdomains to FhaB processing, we constructed B. bronchiseptica strains that produced FhaB proteins lacking either the PRR or the ECT. Similarly to the ΔPRR-ECT strain, no full-length FhaB was detected by Western blot analysis of the ΔECT strain (Fig. 2). Deletion of sphB1 in the strain lacking the ECT resulted in detection of only FHA′ and the slightly smaller polypeptide (Fig. 2). Western blot analysis of a strain containing an HA epitope insertion 7 aa N terminal to the FhaB C terminus also abolished detection of full-length FhaB (see Fig. S3 in the supplemental material) (26), suggesting that the reason for the strict conservation of the ECT in Bordetella cells is that this functional element is unable to tolerate mutation. Additionally, SphB1-dependent processing still occurred in the ΔECT strain (Fig. 2), indicating that FhaB reaches the surface during secretion. These results indicate that the ECT is a negative regulator of prodomain degradation; without it, the prodomain is degraded aberrantly quickly such that full-length FhaB cannot be detected. Additionally, stable prodomain fragments were not detected in WCL (see Fig. S2), indicating that prodomain removed from FhaB in the ΔECT strain is degraded rapidly, as it is in the parental strain.
Western blot analysis of WCL and culture supernatants of the ΔPRR strain, in contrast, displayed no difference in the amount of FhaB/FHA produced, processed, or released compared with WT bacteria (Fig. 2). This finding suggests that the PRR does not play a role in FhaB processing. Additionally, fusion of PhoA to the C terminus of FhaB in the strain lacking the PRR resulted in blue colonies (Fig. 1c), while Western blot analysis of WCL and culture supernatants of the ΔPRR strain producing the fusion protein displayed no changes in the amount of FhaB/FHA produced, processed, or released (Fig. 1a). These results indicate that the PRR does not influence prodomain transport to the periplasm. To test whether the PRR is involved in retention of the prodomain in the periplasm, we performed a dot blot analysis. Similar to the WT strain results, the MCD was detected on the surface of intact bacteria and in disrupted cells and PhoA was detected only in disrupted cells (Fig. 1b), indicating that the prodomain remained intracellular and that the PRR does not contribute to intracellular retention of the prodomain. Furthermore, deletion of the PRR did not result in increased proportions of full-length FhaB in WCL samples or release of full-length FhaB into culture supernatants in a ΔsphB1 strain (Fig. 2), as is seen with strains lacking the PNT (26). SphB1-dependent processing still occurs in the ΔPRR strain (Fig. 2), suggesting that FhaB reaches the surface during secretion. Together, these results suggest that the PRR does not play a role in production of mature FHA.
Mature FHA is sufficient to mediate adherence to respiratory epithelium.
Since the ΔECT strain essentially produces only mature FHA, this strain provided a tool to investigate whether mature FHA is indeed the active form of the protein in vivo. Because in vitro adherence assays are typically performed with nonciliated, nonpolarized cell lines and Bordetella cells adhere primarily to ciliated respiratory epithelium in vivo (39, 40), we developed an in vivo assay to assess the contribution of FHA to adherence to the respiratory tract (41). Using this protocol, ~1% of the inoculum of WT bacteria was recovered in bronchoalveolar lavage fluid (BALF), indicating that ~99% of the bacteria were retained in the respiratory tract. In contrast, ~27% of the inoculum was recovered for an avirulent Bvg-negative (Bvg−) phase-locked strain that is completely nonadherent in vitro (Fig. 3) (42). Thus, ~30% recovery appears to be the upper limit of detection in this assay for nonadherent strains. Inoculation with a FHA-null strain resulted in recovery of ~30% of the inoculum in the BALF (Fig. 3), indicating that FHA is an essential adhesin for B. bronchiseptica in the murine respiratory tract.
FIG 3 .
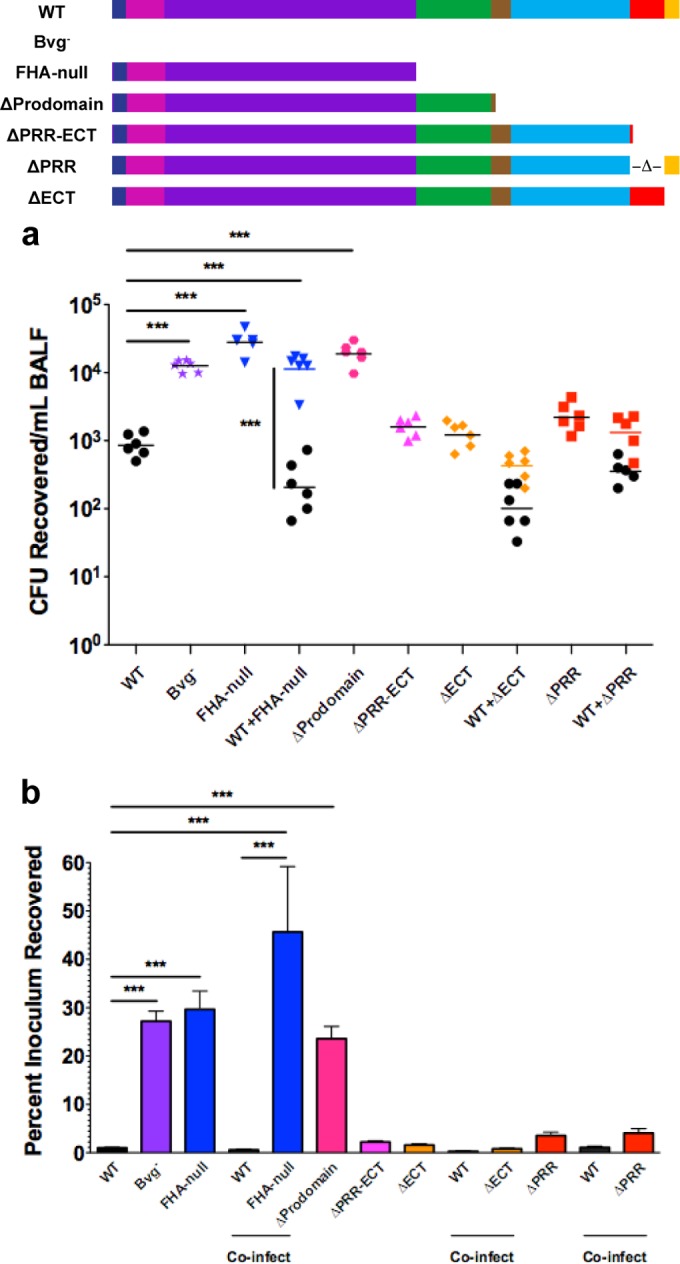
Properly folded FHA is necessary and sufficient for adherence of B. bronchiseptica to the respiratory tract. (a) CFU recovered in BALF 30 to 60 min after intranasal inoculation of mice. Each point represents the number of CFU recovered from a single animal. (b) Recovery of bacteria in BALF represented as the percentage of the inoculum for each strain. Only strains lacking a properly folded MCD displayed decreased retention in the murine respiratory tract. The FhaB molecules translated by each strain are diagrammed at the top of the figure (dark blue, signal peptide; pink, TPS domain; purple, β-helical domain; green, MCD; brown, PNT; light blue, uncharacterized prodomain; red, PRR; yellow, ECT). Data are pooled from 2 separate experiments conducted on different days. Percentages of inoculum data are represented as means ± standard errors of the means (SEM). ***, P < 0.001.
To determine whether the accelerated degradation of the prodomain in strains lacking the C-terminal subdomains of FhaB alters adherence of B. bronchiseptica in vivo, we inoculated mice with strains lacking the PRR, the ECT, or both. Approximately 2% of the inoculum of the ΔPRR-ECT strain was recovered in the BALF (Fig. 3), indicating that the C-terminal subdomains are not required for FHA-mediated adherence in vivo. Accordingly, ~2% of the inoculum of the ΔECT strain and ~4% of the inoculum of the ΔPRR strain were recovered in the BALF (Fig. 3). These findings reveal that mature FHA is sufficient to mediate adherence to the respiratory tract. Additionally, coinoculation of WT B. bronchiseptica with the ΔPRR strain, the ΔECT strain, or the FHA-null strain did not alter recovery of each strain compared to inoculation with each strain alone (Fig. 3). These results indicate that in vivo adherence is determined on a per-bacterium basis and that FHA proteins secreted by WT bacteria are unable to complement adherence defects.
Deletion of a large portion of the FhaB prodomain (including most of the PNT) was previously demonstrated to abrogate B. bronchiseptica adherence to rat lung epithelial L2 cells and B. pertussis adherence to human lung epithelial A549 cells in vitro (14, 26). This mutation also resulted in aberrant folding of the FHA MCD (26). Furthermore, α-MCD antibodies were able to abrogate adherence of B. pertussis and B. bronchiseptica to both rat lung epithelial L2 cells and mouse macrophage-like J774A.1 cells in vitro (13). Together, these findings suggest that the MCD facilitates FHA-mediated adherence and that the FhaB prodomain is required for correct folding of the MCD. In agreement with these findings, ~24% of the inoculum of a B. bronchiseptica ΔProdomain strain was recovered in the BALF (Fig. 3). Considered together with the result that deletion of the C-terminal subdomains did not abrogate FHA-mediated adherence to the respiratory tract, these findings support the requirement of an intact PNT to mediate retention of the prodomain, which facilitates folding of the MCD, and to produce a FHA molecule capable of mediating adherence to the respiratory tract. Combined, these data further suggest that FHA is folded correctly and is able to confer adherence capabilities to B. bronchiseptica in strains lacking the ECT. Since the PRR does not influence FhaB/FHA processing (Fig. 2) or FhaB prodomain localization (Fig. 1), these data also strongly suggest that the PRR does not play a role in production of functional mature FHA. The mature FHA molecules produced in the ΔPRR and ΔECT strains are thus indistinguishable from those produced by WT bacteria, and therefore the only difference between the WT and mutant strains is the full-length FhaB molecules they produce.
Mature FHA is not sufficient for B. bronchiseptica persistence in the lower respiratory tract.
Deletion of both the PRR and ECT subdomains (ΔPRR-ECT) was previously shown to reduce persistence in the tracheas of rats (14), even though this strain is capable of adhering both in vivo (Fig. 3) and in vitro (14). To determine which of the individual C-terminal subdomains is involved in FhaB/FHA-mediated virulence activities, we inoculated mice intranasally with various B. bronchiseptica strains and monitored bacterial burden in the respiratory tract over time. As has been observed previously (6, 12, 13), WT bacteria persisted at high levels in the trachea and lungs through 11 days postinoculation, and FHA-null bacteria were mostly cleared from the trachea and lungs by 11 days postinoculation (Fig. 4a). The FHA-null strain displayed an increase in burden at 1 day postinoculation compared to WT bacteria, a bimodal distribution of burden at 3 days postinoculation, and a dramatic reduction in burden by day 11 (Fig. 4a). Additionally, as has been previously shown (2, 12–14), the number of CFU recovered from the nasal cavity for the FHA-null strain was similar to that for WT bacteria (see Fig. S4 in the supplemental material), indicating that FHA is not required for colonization and persistence in the upper respiratory tract of rodents.
FIG 4 .
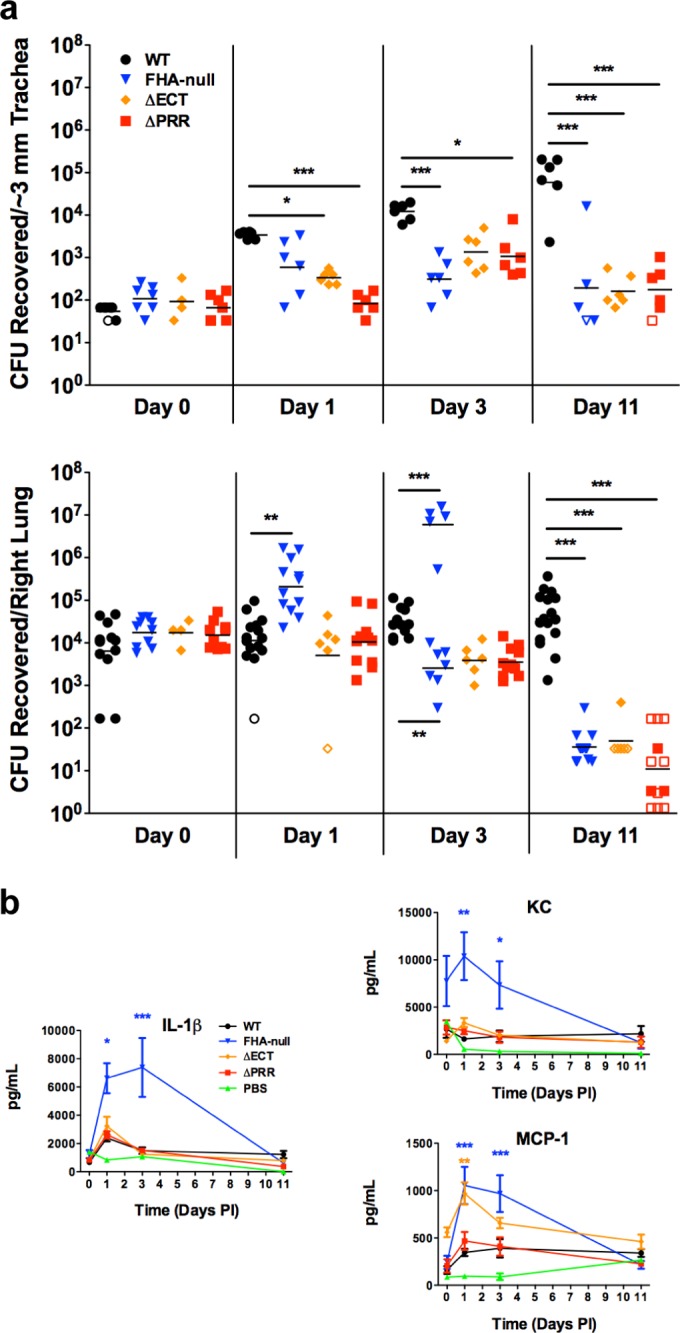
The FhaB proline-rich region and extreme C terminus are necessary for B. bronchiseptica persistence in the lower respiratory tract in a manner that is distinct from suppression of inflammation. (a) Bacterial burden in the murine lower respiratory tract after intranasal inoculation. Each point represents the number of CFU recovered from a single animal. Open symbols represent the lower limit of detection for that particular experiment, as no CFU were recovered. (b) Production of proinflammatory cytokines (IL-1β) and chemokines (KC, MCP-1) in the murine lung during infection. Cytokine and chemokine levels were determined from lung homogenates by ELISA. Data represent means ± SEM of the results determined for all samples, which were collected from all animals for which CFU are shown in Fig. 4a. Data are pooled from ≥2 separate experiments conducted on different days. PI, postinfection. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Similarly to the FHA-null strain, the ΔECT strain was unable to persist in the murine lower respiratory tract, as most mice had completely cleared this strain from their trachea and lungs by 11 days postinoculation (Fig. 4a). However, the course of infection of the ΔECT strain differed from that of the FHA-null strain; there was no increase in burden at 1 day postinoculation and no bimodal burden distribution at 3 days postinoculation in the lungs (Fig. 4a). The ΔPRR strain also displayed increased clearance from the mouse trachea and lungs compared to WT bacteria (Fig. 4a), whereas, similarly to the results seen with the FHA-null bacteria, colonization and persistence in the nasal cavity were not compromised (see Fig. S4 in the supplemental material). We also created the identical ΔPRR mutation in WT strain RB50, which contains an intact fhaS gene, and deletion of the PRR in this background resulted in the same persistence defect in the lower respiratory tract (see Fig. S5), demonstrating that the decreased persistence in the lower respiratory tract is due to the lack of the FhaB PRR rather than to the lack of fhaS (in addition to the ΔPRR mutation) or an undetected additional mutation elsewhere on the chromosome. Since the mature FHA molecules produced by the ΔPRR strain and the ΔECT strain are indistinguishable from those produced by WT bacteria, these results indicate that the presence of mature FHA is not sufficient to mediate B. bronchiseptica persistence, revealing an active role for the full-length FhaB polypeptide in vivo that is dependent on both the PRR and the ECT.
Mature FHA is sufficient for immunomodulation.
Previous studies have suggested that FHA contributes to Bordetella persistence in mice via suppression of inflammation (6, 12). To ascertain whether the increased clearance observed with our ΔPRR and ΔECT strains is due to an increased induction of inflammation compared to WT bacteria, we measured global cytokine and chemokine levels in the mouse lung during infection. As previously reported (6), the FHA-null strain induced higher production of proinflammatory cytokines, such as interleukin-1β (IL-1β), and chemokines, such as the neutrophil chemoattractant KC and monocyte chemoattractant protein-1 (MCP-1), than WT bacteria (Fig. 4b). There was no difference in global levels of gamma interferon (IFN-γ), tumor necrosis factor α (TNF-α), IL-10, IL-12p70, IL-17, IL-22, or IL-23 produced in the lungs of mice infected with WT or FHA-null bacteria (see Fig. S6 in the supplemental material), suggesting that the primary difference in the innate immune response to infection with WT or FHA-null bacteria is the intensity of the initial IL-1β-mediated inflammation.
In contrast to FHA-null bacteria, neither the ΔECT strain nor the ΔPRR strain stimulated higher inflammatory cytokine or chemokine production than the WT strain during infection (Fig. 4b). These data suggest that the increased clearance observed with these strains is not due to a lack of FHA-mediated immunosuppression and that mature FHA is sufficient to suppress the initial inflammatory response.
Full-length FhaB is required for resistance to early-immune-response-mediated clearance.
We previously demonstrated that coinoculation of mice with WT and FHA-null bacteria partially “rescues” persistence of FHA-null bacteria in the lungs compared to inoculation with only the FHA-null strain, while the persistence of WT bacteria after coinoculation was unaltered compared to inoculation with only the WT strain (12). Inflammation was suppressed after coinoculation compared to inoculation with only FHA-null bacteria; however, FHA-null bacteria were still not able to persist as well as WT bacteria (12), suggesting that FhaB/FHA may mediate resistance to clearance even under less-inflammatory conditions. Similarly to those previous findings, the number of CFU of WT bacteria recovered after coinoculation with the FHA-null strain was unchanged compared to the number recovered after inoculation with only WT bacteria, while the number of CFU of the FHA-null bacteria was increased compared to the number seen after inoculation with only FHA-null bacteria at 11 days postinoculation (Fig. 4 and 5a). Additionally, production of proinflammatory IL-1β and KC after coinoculation was identical to that seen in mice inoculated with only WT bacteria (Fig. 5b). However, the number of CFU of the FHA-null strain at 11 days postinoculation in the coinoculation experiment was still lower than that of WT bacteria, supporting the hypothesis that FhaB/FHA plays another role in persistence that is distinct from its role in suppressing the intensity of the inflammatory response.
FIG 5 .
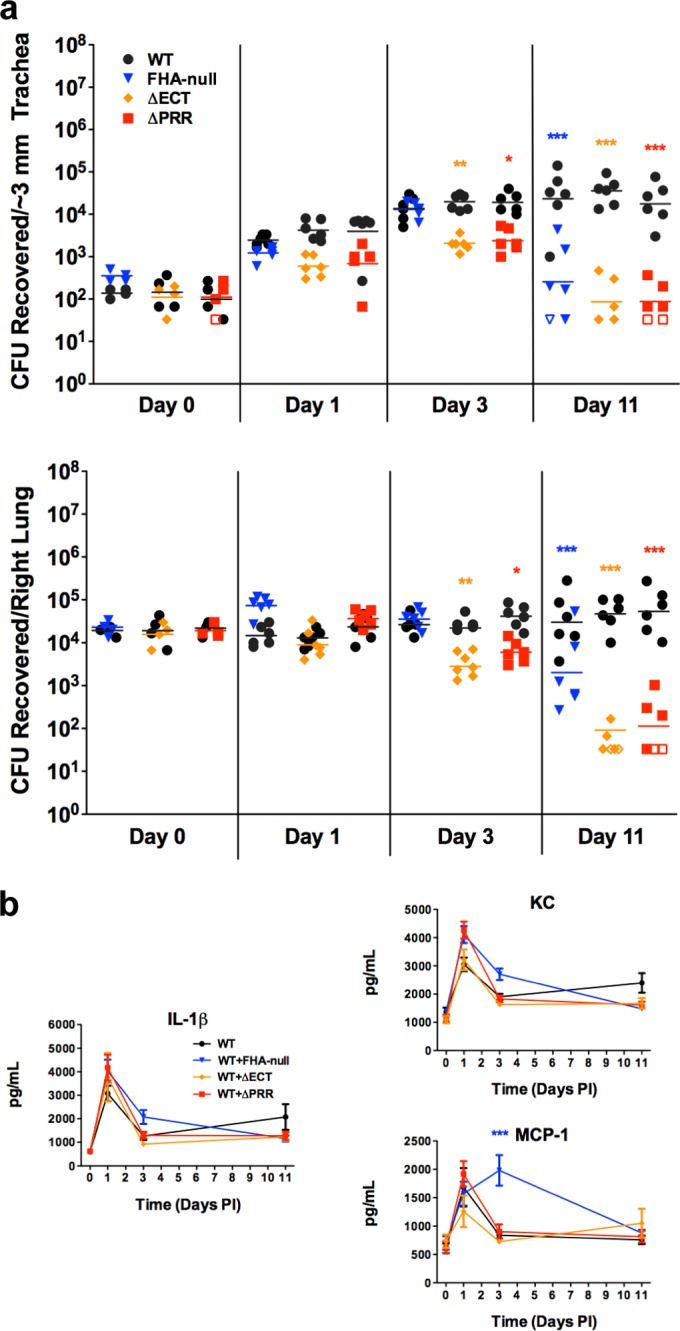
The persistence defect of the ΔPRR and ΔECT strains cannot be rescued by WT bacteria. (a) Bacterial burden in the murine lower respiratory tract after coinoculation with WT and FHA mutant strains marked with different antibiotic resistance genes. Each point represents the number of CFU recovered from a single animal. Open symbols represent the lower limit of detection for that particular experiment, as no CFU were recovered. (b) Production of proinflammatory cytokines (IL-1β) and chemokines (KC, MCP-1) in the murine lung during coinfection. Cytokine and chemokine levels were determined from lung homogenates by ELISA. Data represent means ± SEM of the results determined for all samples, which were collected from all animals for which CFU are shown in Fig. 5a. Data are pooled from the results of 2 separate experiments conducted on different days. Statistical significance is indicated for mutant bacteria compared to the WT strain from the same coinoculation group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In contrast to coinoculation of mice with WT and FHA-null bacteria, coinoculation of WT bacteria with either the ΔECT or ΔPRR strain did not alter clearance of the mutant bacteria; they were cleared as rapidly as when inoculated in the absence of WT bacteria (Fig. 5a). Production of cytokines and chemokines was similarly unaltered during coinoculation compared to inoculation with only WT bacteria (Fig. 5b). Since WT bacteria failed to “rescue” either the ΔECT or ΔPRR strain in the presence of an unaltered inflammatory response, these results support the hypothesis that the virulence defect of the ΔECT and ΔPRR strains that leads to the decreased persistence is a lack of resistance to clearance by the early immune response. Moreover, these results strongly argue that mature FHA is not sufficient for this activity, revealing a functional role for premature FhaB in resistance to innate immunity-mediated clearance and indicating that the PRR is essential for that activity.
DISCUSSION
Our previous studies revealed that FhaB secretion and maturation are highly regulated activities. The conserved N-terminal region of the prodomain is required for proper MCD folding and subsequent adherence capabilities (26). Additionally, we observed production of stable intracellular prodomain fragments in strains lacking the C-terminal portion of the MCD (26), suggesting a role for the MCD in regulating prodomain degradation. Together, these findings indicated that information is relayed bidirectionally across the outer membrane via the FhaB primary sequence (i.e., regulation of MCD folding on the bacterial surface and initiation of prodomain degradation in the periplasm). Here, we determined that the prodomain resides in the periplasm during FhaB secretion (Fig. 1) and demonstrated that deletion of the FhaB ECT resulted in the inability to detect full-length FhaB molecules (Fig. 2), indicating that the ECT is a negative regulator of prodomain degradation in the periplasm. A model of FhaB secretion taking these findings into account is shown in Fig. S7 in the supplemental material.
Taking advantage of the fact that the ΔECT strain essentially produces only mature FHA, we examined whether FHA was in fact capable of performing all virulence functions that have been attributed to this molecule. Using our recently developed in vivo adherence assay (41), we found that FHA is both necessary and sufficient to mediate adherence to the murine respiratory tract (Fig. 3). Additionally, the ΔECT strain was capable of suppressing the acute inflammatory response in the murine lower respiratory tract (Fig. 4b), indicating that mature FHA is also both necessary and sufficient to perform this function. Surprisingly, however, the ΔECT strain was unable to persist in the murine lower respiratory tract (Fig. 4a), and coinfection with WT bacteria did not rescue the persistence defect or alter the inflammatory response (Fig. 5). These data reveal three previously unappreciated aspects of FHA physiology: (i) the persistence defect of FHA-mutant strains is not due solely to lack of adherence or suppression of inflammation, (ii) mature FHA is not sufficient to facilitate all fhaB-mediated virulence activities, and (iii) full-length FhaB is therefore required for resistance to innate immune effectors. In support of these conclusions, deletion of the FhaB PRR, which did not result in any FhaB secretion abnormalities (Fig. 1 and 2) or affect production of functional mature FHA (Fig. 3 and 4b), produced phenotypes almost identical to those seen with the ΔECT strain, indicating that the defect for both these strains is the lack of functional full-length FhaB molecules and that the PRR is essential for FhaB activity.
We hypothesize that transmission of information is the basis for the activity of full-length FhaB, acting as a transmembrane sensor, and that the PRR is integral for relaying information in vivo. Due to the fact that it contains repeat motifs (see Fig. S1b in the supplemental material) and to the proclivity of polyproline peptides to adopt an extended conformation (43), we postulate that the PRR acts as a docking site for protein interactions in the periplasm and that modulation of these interactions is required for resisting the early immune response. These interactions likely direct the action of a short-range effector(s), as the defects observed with the ΔPRR and ΔECT strains appeared to operate on a per-bacterium basis (Fig. 3 and 5). Discriminating between the potential mechanisms by which this occurs will be the subject of future investigations. We expect that the defect in B. bronchiseptica lower respiratory tract persistence observed for the ΔPRR strain is due to decreased resistance to clearance by phagocytes. While decreased resistance to other aspects of the innate immune response is possible, it is unlikely that full-length FhaB is necessary for resistance to mechanical clearance, as the ΔPRR and ΔECT strains displayed normal adherence to the respiratory tract (Fig. 3) or to secreted molecular immune effectors. Additionally, while we cannot rule out a role for early adaptive immunity in clearance of the mutant strains from the lower respiratory tract between days 3 and 11 (Fig. 4), we expect that any involvement of antibody-mediated clearance would be through opsonization as a means for uptake by phagocytes. In contrast, FhaB/FHA has been reported to mediate interactions with phagocytes (44–46).
Our results suggest that the mature form of FHA is not sufficient to mediate resistance to clearance by the early immune response. This finding perhaps elucidates a paradox in FHA physiology, namely, that FHA, a critical adhesin, is released from the bacterial surface in large quantities (Fig. 2). While our experiments were unable to determine whether released FHA plays a role in suppression of the immune response, as has been suggested (19–23), this work provides a description of an alternative role for release of FHA. FHA release may predominantly serve the purpose of liberating FhaC to secrete a new FhaB molecule, which can then mediate persistence in the lower respiratory tract. If true, this hypothesis suggests that the multiple processing events that FhaB undergoes serve several purposes. Initiation of prodomain degradation may serve as the periplasmic signal that FhaB has sensed environmental changes, while SphB1-dependent cleavage, which results in increased release of FHA from the surface (Fig. 2) (14, 34), may facilitate FhaB turnover.
Integral to understanding the physiological function of proteins is elucidation of the means by which they achieve their functional forms. For processed proteins, it is typically assumed that the final polypeptide observed in vitro is the functional form of the protein. This work suggests that precursor molecules are important to the physiological function of some processed proteins. The results presented here demonstrate the presence of multiple intramolecular determinants of FhaB prodomain degradation, in addition to multiple known intermolecular determinants, indicating that “maturation” of FhaB is a tightly regulated process. While dysregulation of prodomain degradation does not affect the ability of mature FHA to mediate adherence or immunosuppression during infection (Fig. 3 and 4b), these studies yielded the unexpected finding that full-length FhaB plays an active role in pathogenesis, specifically in resisting clearance by innate immune effectors. Our results highlight the advantage of studying protein secretion and function simultaneously, as a role for the FhaB PRR was evident only from in vivo infection studies and not from studies on the production or maturation of FHA or even from virulence-associated in vitro assays (14). Our results also provide a reminder that, while culturing bacteria in the laboratory is extremely useful and often essential for understanding the molecular mechanisms underlying microbiological processes such as protein secretion, in vitro growth conditions may not accurately mimic the conditions under which the microbiological processes under study function in nature.
MATERIALS AND METHODS
Bioinformatics.
Protein sequences were obtained from the NCBI Protein Database. Sequence alignments were conducted using Clustal Omega (47) and visualized using Jalview Version 2 (48). Searches for similar sequences were conducted with BLASTp.
Ethics statements.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH. Our protocols were approved by the University of North Carolina IACUC (10-134, 12-307, and 13-238). All animals were properly anesthetized for inoculations, monitored regularly, and euthanized when moribund, and efforts were made to minimize suffering.
Growth media and bacterial strains.
B. bronchiseptica strains were grown at 37°C in Stainer-Scholte (SS) broth or on Difco Bordet-Gengou (BG) agar (BD) supplemented with 5.5% defibrinated sheep blood (Colorado Serum Co., Denver, CO). Escherichia coli strains were grown at 37°C in Luria broth (LB) or on LB agar. A detailed description of bacterial strains, their construction, and the rationale for their use is included in Methods in Text S1 in the supplemental material. Where appropriate, media were supplemented with gentamicin (30 µg/ml), streptomycin (20 µg/ml), kanamycin (50 µg/ml), MgSO4 (50 mM), diaminopimelic acid (200 µg/ml), or 5-bromo-4-chloro-3-indolyl phosphate (XP; 40 µg/ml).
Immunoblotting.
To evaluate FHA production and processing, proteins were prepared from B. bronchiseptica cultures grown in SS broth and normalized based on optical density. For cell-associated proteins, whole-cell lysates (WCL) of bacteria were prepared by boiling in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. For released proteins, culture supernatants precipitated with 10% trichloroacetic acid (TCA; Fisher Scientific) were mixed with SDS-PAGE sample buffer and boiled. Proteins were separated by SDS-PAGE on 5% polyacrylamide gels, transferred to nitrocellulose (GE Healthcare), and probed with a rabbit polyclonal antibody generated against the FHA mature C-terminal domain (MCD) (13), a mouse monoclonal antibody generated against PhoA (Covance), or a mouse monoclonal antibody generated against an HA epitope (Covance). Corresponding α-rabbit and α-mouse IRDye secondary antibodies were used to detect proteins (Li-COR Biosciences). Membranes were imaged on a Li-COR Odyssey Classic infrared Imager (Li-COR Biosciences).
To assess the surface accessibility of proteins, B. bronchiseptica cells grown in SS broth were washed and resuspended in phosphate-buffered saline (PBS; Life Technologies), and half of each sample was boiled to disrupt the cells. Intact and disrupted cells were then spotted on nitrocellulose and probed with a rabbit polyclonal α-MCD antibody and a mouse monoclonal α-PhoA antibody. Corresponding α-rabbit and α-mouse IRDye secondary antibodies were used to detect proteins, and membranes were imaged on an Li-COR Odyssey Classic infrared Imager.
Bacterial adherence to the respiratory tract.
Bacterial adherence was measured using a recently developed in vivo adherence assay (41). Briefly, 6-week-old female BALB/cJ mice from Jackson Laboratories (Bar Harbor, ME) were anesthetized with isoflurane and inoculated intranasally with 7.5 × 104 CFU (CFU) of B. bronchiseptica–50 µl PBS. For coinoculation experiments, WT and mutant strains were marked with different antibiotic resistance genes as described in Methods in Text S1 in the supplemental material. Mice were euthanized 30 to 60 min after inoculation. Bronchoalveolar lavage fluid (BALF) was collected by inserting a cannula into the trachea, ligating with a suture, and injecting and retracting 1 ml PBS. Serial dilutions of BALF and the inoculum were plated on BG agar to quantitate recovery of bacterial CFU.
Bacterial persistence and characterization of the immune response during infection of mice.
Six-week-old female BALB/cJ mice were inoculated and euthanized as described above. For coinoculation experiments, WT and mutant strains were marked with different antibiotic resistance genes as described in Methods in Text S1 in the supplemental material. Lungs, tracheas, and nasal cavities were harvested and homogenized in PBS. Serial dilutions of homogenized tissues were plated on BG agar (with appropriate antibiotics) to quantitate recovery of bacterial CFU. Cytokine production and chemokine production were analyzed by conducting enzyme-linked immunosorbent assays (ELISA) on lung homogenates diluted in PBS (R&D Systems).
Statistical analysis.
Data were plotted and statistical analyses were performed using Prism version 5.0 software (GraphPad Software, Inc.). For all conditions analyzed, at least six experiments (two or more separate experiments on different days with n ≥ 3) were performed, and statistical significance was determined using analysis of variance (ANOVA).
SUPPLEMENTAL MATERIAL
Supplementary Methods, including a detailed description of bacterial strains applied in this study and their construction. Download
The C terminus of the FhaB prodomain contains two distinct domains. (a) Domain structure of FhaB. The putative SphB1 cleavage site (PLFETRIK) that leads to production of mature FHA is indicated. SP, signal peptide; TPS, two-partner secretion domain; MCD, mature C-terminal domain; PNT, prodomain N terminus; PRR, proline-rich region; ECT, extreme C terminus. (b) Primary sequence of the FhaB PRR, which contains two conspicuous repeat motifs. For the N-terminal repeat motif, positions that contain the same amino acid in each of the three repeats are highlighted in black whereas positions that have the same amino acid in two of the three repeats are highlighted in gray. For the C-terminal repeat motif, a series of PA/PK repeats is highlighted in black. (c) Primary sequence of the FhaB ECT, which is 100% identical in all sequenced strains of Bordetella spp. that infect mammals. Amino acid sequences and N- and C-terminal amino acid numbers are given for FhaB from B. bronchiseptica strain RB50. Download
The ECT is intolerant to insertions. Results of Western blot analysis of whole-cell lysates (WCL) or culture supernatants (Sup.) from strains lacking the FhaB ECT or containing an HA epitope inserted in the FhaB ECT as well as each strain containing or lacking sphB1 are shown. Strains with mutations in the FhaB ECT do not contain any observable FhaB in WCL or Sup. The FhaB molecules translated by each strain are diagrammed at the top. Membranes were probed with α-MCD antibodies. Download
Deletion of the ECT does not increase prodomain stability. Results of Western blot analysis of whole-cell lysates (WCL) from strains lacking the FhaB ECT (either by deletion of the region, ΔECT, or by insertion of a premature stop codon 5′ of the region, tECT) and containing an HA epitope inserted N-terminal to the FhaB PRR as well as each strain containing or lacking sphB1 are shown. There were no stable prodomain fragments detected in the WCL. The FhaB molecules translated by each strain are diagrammed at the top. Membranes were probed with α-MCD (green) and α-HA (red) antibodies. The red channel was slightly overexposed to reveal any potential prodomain-containing fragments. Stable bands representing prodomain-containing fragments have not been observed in WT or strains lacking C-terminal subdomains and have been observed only in strains lacking portions of the MCD (26). Download
The FhaB proline-rich region is not required for B. bronchiseptica persistence in the upper respiratory tract. Data represent the bacterial burden in the murine upper respiratory tract (nasal cavity) after inoculation. Each point represents the number of CFU recovered from a single animal. Data are pooled from the results of 2 separate experiments conducted on different days. Download
The FhaB proline-rich region is required for B. bronchiseptica persistence in the lower respiratory tract regardless of the presence or absence of fhaS. Data represent the bacterial burden in the murine lower respiratory tract (trachea and lungs) after inoculation. Each point represents the number of CFU recovered from a single animal. Open symbols represent the lower limit of detection for that particular experiment, as no CFU were recovered. Data are pooled from the results of 2 separate experiments conducted on different days. ***, P < 0.001 Download
FhaB/FHA does not influence global levels of some cytokines in the lung. Pro- and anti-inflammatory cytokines levels were determined from lung homogenates by ELISA. Data represent means ± SEM of the results determined for all samples, which were collected from all animals for which CFU are shown in Fig. 4a. Data are pooled from the results of ≥2 separate experiments conducted on different days. Download
Current model of FhaB secretion. Model of secretion of FhaB subsequent to transport across the cytoplasmic membrane (cm) and translocation and folding of the β-helical domain (purple) are shown. The ECT (gold) inhibits degradation of the prodomain by a periplasmic protease(s) (green “Pac-Man”) during secretion. As the MCD (green) begins to fold, the PNT (brown) restricts further translocation of the protein to the surface. Once the MCD is folded, it may interact with the environment, potentially acting as a sensory domain. An unidentified signal (lightning bolt) is transmitted to the periplasm that instructs the ECT to relieve repression of the periplasmic protease(s) or to alter PRR-mediated interactions or both. This signal(s) leads to rapid and complete prodomain degradation and/or alteration of virulence functions that contribute to Bordetella persistence. Further SphB1 (gray)-dependent processing occurs, and mature FHA mediates adherence to epithelial cell receptors. FhaC is represented by a cylinder in the outer membrane (om) with POTRA domains (ovals) on the periplasmic side of the om and loop 6 on the extracellular side of the om. FhaC is shaded red for the first two steps and then green for subsequent steps to indicate a shift from a release-incompetent form to a release-competent form (26). The colors of the FhaB subdomains are the same as those diagrammed in Fig. 2. The periplasm is not drawn to scale to aid in visualizing periplasmic events. Download
Strains applied in this study.
Plasmids applied in this study.
ACKNOWLEDGMENTS
We thank members of our laboratory for many insightful discussions.
This work was supported by NIH awards AI094991 (P.A.C.) and AI007151 (J.A.M.).
Footnotes
Citation Melvin JA, Scheller EV, Noël CR, Cotter PA. 2015. New insight into filamentous hemagglutinin secretion reveals a role for full-length FhaB in Bordetella virulence. mBio 6(4):e01189-15. doi:10.1128/mBio.01189-15.
REFERENCES
- 1.Buscher AZ, Burmeister K, Barenkamp SJ, St Geme JW III. 2004. Evolutionary and functional relationships among the nontypeable Haemophilus influenzae HMW family of adhesins. J Bacteriol 186:4209–4217. doi: 10.1128/JB.186.13.4209-4217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, Relman DA, Miller JF. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun 66:5921–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fournier C, Smith A, Delepelaire P. 2011. Haem release from haemopexin by HxuA allows Haemophilus influenzae to escape host nutritional immunity. Mol Microbiol 80:133–148. doi: 10.1111/j.1365-2958.2011.07562.x. [DOI] [PubMed] [Google Scholar]
- 4.Hertle R, Hilger M, Weingardt-Kocher S, Walev I. 1999. Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect Immun 67:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swihart KG, Welch RA. 1990. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun 58:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson MW, Inatsuka CS, Sheets AJ, Williams CL, Benaron DJ, Donato GM, Gray MC, Hewlett EL, Cotter PA. 2012. Contribution of Bordetella filamentous hemagglutinin and adenylate cyclase toxin to suppression and evasion of interleukin-17-mediated inflammation. Infect Immun 80:2061–2075. doi: 10.1128/IAI.00148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark TA. 2014. Changing pertussis epidemiology: everything old is new again. J Infect Dis 209:978–981. doi: 10.1093/infdis/jiu001. [DOI] [PubMed] [Google Scholar]
- 8.Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA, Skoff TH. 2013. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 131:e1047–e1052. doi: 10.1542/peds.2012-1928. [DOI] [PubMed] [Google Scholar]
- 9.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witt MA, Katz PH, Witt DJ. 2012. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis 54:1730–1735. doi: 10.1093/cid/cis287. [DOI] [PubMed] [Google Scholar]
- 11.Althouse BM, Scarpino SV. 2015. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med 13:146. doi: 10.1186/s12916-015-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inatsuka CS, Julio SM, Cotter PA. 2005. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc Natl Acad Sci U S A 102:18578–18583. doi: 10.1073/pnas.0507910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Julio SM, Inatsuka CS, Mazar J, Dieterich C, Relman DA, Cotter PA. 2009. Natural-host animal models indicate functional interchangeability between the filamentous haemagglutinins of Bordetella pertussis and Bordetella bronchiseptica and reveal a role for the mature C-terminal domain, but not the RGD motif, during infection. Mol Microbiol 71:1574–1590. doi: 10.1111/j.1365-2958.2009.06623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazar J, Cotter PA. 2006. Topology and maturation of filamentous haemagglutinin suggest a new model for two-partner secretion. Mol Microbiol 62:641–654. doi: 10.1111/j.1365-2958.2006.05392.x. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson TL, Brockmeier SL, Loving CL. 2009. Contribution of Bordetella bronchiseptica filamentous hemagglutinin and pertactin to respiratory disease in swine. Infect Immun 77:2136–2146. doi: 10.1128/IAI.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inatsuka CS, Xu Q, Vujkovic-Cvijin I, Wong S, Stibitz S, Miller JF, Cotter PA. 2010. Pertactin is required for Bordetella species to resist neutrophil-mediated clearance. Infect Immun 78:2901–2909. doi: 10.1128/IAI.00188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez de Tejada G, Miller JF, Cotter PA. 1996. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol 22:895–908. doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- 18.Melvin JA, Scheller EV, Miller JF, Cotter PA. 2014. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol 12:274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramson T, Kedem H, Relman DA. 2001. Proinflammatory and proapoptotic activities associated with Bordetella pertussis filamentous hemagglutinin. Infect Immun 69:2650–2658. doi: 10.1128/IAI.69.4.2650-2658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieterich C, Relman DA. 2011. Modulation of the host interferon response and ISGylation pathway by B. pertussis filamentous hemagglutinin. PLoS One 6:e27535. doi: 10.1371/journal.pone.0027535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuirk P, McCann C, Mills KH. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med 195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuirk P, Mills KH. 2000. Direct anti-inflammatory effect of a bacterial virulence factor: il-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur J Immunol 30:415–422. doi:. [DOI] [PubMed] [Google Scholar]
- 23.Dirix V, Mielcarek N, Debrie AS, Willery E, Alonso S, Versheure V, Mascart F, Locht C. 2014. Human dendritic cell maturation and cytokine secretion upon stimulation with Bordetella pertussis filamentous haemagglutinin. Microbes Infect 16:562–570. doi: 10.1016/j.micinf.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Gray MC, Donato GM, Jones FR, Kim T, Hewlett EL. 2004. Newly secreted adenylate cyclase toxin is responsible for intoxication of target cells by Bordetella pertussis. Mol Microbiol 53:1709–1719. doi: 10.1111/j.1365-2958.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- 25.Zaretzky FR, Gray MC, Hewlett EL. 2002. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: a role for toxin-filamentous haemagglutinin interaction. Mol Microbiol 45:1589–1598. doi: 10.1046/j.1365-2958.2002.03107.x. [DOI] [PubMed] [Google Scholar]
- 26.Noël CR, Mazar J, Melvin JA, Sexton JA, Cotter PA. 2012. The prodomain of the Bordetella two-partner secretion pathway protein FhaB remains intracellular yet affects the conformation of the mature C-terminal domain. Mol Microbiol 86:988–1006. doi: 10.1111/mmi.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevalier N, Moser M, Koch HG, Schimz KL, Willery E, Locht C, Jacob-Dubuisson F, Müller M. 2004. Membrane targeting of a bacterial virulence factor harbouring an extended signal peptide. J Mol Microbiol Biotechnol 8:7–18. doi: 10.1159/000082076. [DOI] [PubMed] [Google Scholar]
- 28.Baud C, Hodak H, Willery E, Drobecq H, Locht C, Jamin M, Jacob-Dubuisson F. 2009. Role of DegP for two-partner secretion in Bordetella. Mol Microbiol 74:315–329. doi: 10.1111/j.1365-2958.2009.06860.x. [DOI] [PubMed] [Google Scholar]
- 29.Hodak H, Wohlkönig A, Smet-Nocca C, Drobecq H, Wieruszeski JM, Sénéchal M, Landrieu I, Locht C, Jamin M, Jacob-Dubuisson F. 2008. The peptidyl-prolyl isomerase and chaperone Par27 of Bordetella pertussis as the prototype for a new group of parvulins. J Mol Biol 376:414–426. doi: 10.1016/j.jmb.2007.10.088. [DOI] [PubMed] [Google Scholar]
- 30.Clantin B, Hodak H, Willery E, Locht C, Jacob-Dubuisson F, Villeret V. 2004. The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway. Proc Natl Acad Sci U S A 101:6194–6199. doi: 10.1073/pnas.0400291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kajava AV, Cheng N, Cleaver R, Kessel M, Simon MN, Willery E, Jacob-Dubuisson F, Locht C, Steven AC. 2001. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol Microbiol 42:279–292. doi: 10.1046/j.1365-2958.2001.02598.x. [DOI] [PubMed] [Google Scholar]
- 32.Makhov AM, Hannah JH, Brennan MJ, Trus BL, Kocsis E, Conway JF, Wingfield PT, Simon MN, Steven AC. 1994. Filamentous hemagglutinin of Bordetella pertussis. A bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol 241:110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 33.Delisse-Gathoye AM, Locht C, Jacob F, Raaschou-Nielsen M, Heron I, Ruelle JL, de Wilde M, Cabezon T. 1990. Cloning, partial sequence, expression, and antigenic analysis of the filamentous hemagglutinin gene of Bordetella pertussis. Infect Immun 58:2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coutte L, Antoine R, Drobecq H, Locht C, Jacob-Dubuisson F. 2001. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J 20:5040–5048. doi: 10.1093/emboj/20.18.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Julio SM, Cotter PA. 2005. Characterization of the filamentous hemagglutinin-like protein FhaS in Bordetella bronchiseptica. Infect Immun 73:4960–4971. doi: 10.1128/IAI.73.8.4960-4971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman CS, Wright A. 1985. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci U S A 82:5107–5111. doi: 10.1073/pnas.82.15.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori H, Ito K. 2001. The Sec protein-translocation pathway. Trends Microbiol 9:494–500. doi: 10.1016/S0966-842X(01)02174-6. [DOI] [PubMed] [Google Scholar]
- 38.Locht C, Geoffroy MC, Renauld G. 1992. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J 11:3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards JA, Groathouse NA, Boitano S. 2005. Bordetella bronchiseptica adherence to cilia is mediated by multiple adhesin factors and blocked by surfactant protein A. Infect Immun 73:3618–3626. doi: 10.1128/IAI.73.6.3618-3626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paddock CD, Sanden GN, Cherry JD, Gal AA, Langston C, Tatti KM, Wu KH, Goldsmith CS, Greer PW, Montague JL, Eliason MT, Holman RC, Guarner J, Shieh WJ, Zaki SR. 2008. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis 47:328–338. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 41.Scheller EV, Melvin JA, Sheets AJ, Cotter PA. 2015. Cooperative roles for fimbria and filamentous hemagglutinin in Bordetella adherence and immune modulation. mBio 6:e00500-15. doi: 10.1128/mBio.00500-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akerley BJ, Cotter PA, Miller JF. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 43.Adzhubei AA, Sternberg MJ, Makarov AA. 2013. Polyproline-II helix in proteins: structure and function. J Mol Biol 425:2100–2132. doi: 10.1016/j.jmb.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Mobberley-Schuman PS, Weiss AA. 2005. Influence of CR3 (CD11b/CD18) expression on phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun 73:7317–7323. doi: 10.1128/IAI.73.11.7317-7323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weingart CL, Keitel WA, Edwards KM, Weiss AA. 2000. Characterization of bactericidal immune responses following vaccination with acellular pertussis vaccines in adults. Infect Immun 68:7175–7179. doi: 10.1128/IAI.68.12.7175-7179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weingart CL, Weiss AA. 2000. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect Immun 68:1735–1739. doi: 10.1128/IAI.68.3.1735-1739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. 2009. Jalview, version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods, including a detailed description of bacterial strains applied in this study and their construction. Download
The C terminus of the FhaB prodomain contains two distinct domains. (a) Domain structure of FhaB. The putative SphB1 cleavage site (PLFETRIK) that leads to production of mature FHA is indicated. SP, signal peptide; TPS, two-partner secretion domain; MCD, mature C-terminal domain; PNT, prodomain N terminus; PRR, proline-rich region; ECT, extreme C terminus. (b) Primary sequence of the FhaB PRR, which contains two conspicuous repeat motifs. For the N-terminal repeat motif, positions that contain the same amino acid in each of the three repeats are highlighted in black whereas positions that have the same amino acid in two of the three repeats are highlighted in gray. For the C-terminal repeat motif, a series of PA/PK repeats is highlighted in black. (c) Primary sequence of the FhaB ECT, which is 100% identical in all sequenced strains of Bordetella spp. that infect mammals. Amino acid sequences and N- and C-terminal amino acid numbers are given for FhaB from B. bronchiseptica strain RB50. Download
The ECT is intolerant to insertions. Results of Western blot analysis of whole-cell lysates (WCL) or culture supernatants (Sup.) from strains lacking the FhaB ECT or containing an HA epitope inserted in the FhaB ECT as well as each strain containing or lacking sphB1 are shown. Strains with mutations in the FhaB ECT do not contain any observable FhaB in WCL or Sup. The FhaB molecules translated by each strain are diagrammed at the top. Membranes were probed with α-MCD antibodies. Download
Deletion of the ECT does not increase prodomain stability. Results of Western blot analysis of whole-cell lysates (WCL) from strains lacking the FhaB ECT (either by deletion of the region, ΔECT, or by insertion of a premature stop codon 5′ of the region, tECT) and containing an HA epitope inserted N-terminal to the FhaB PRR as well as each strain containing or lacking sphB1 are shown. There were no stable prodomain fragments detected in the WCL. The FhaB molecules translated by each strain are diagrammed at the top. Membranes were probed with α-MCD (green) and α-HA (red) antibodies. The red channel was slightly overexposed to reveal any potential prodomain-containing fragments. Stable bands representing prodomain-containing fragments have not been observed in WT or strains lacking C-terminal subdomains and have been observed only in strains lacking portions of the MCD (26). Download
The FhaB proline-rich region is not required for B. bronchiseptica persistence in the upper respiratory tract. Data represent the bacterial burden in the murine upper respiratory tract (nasal cavity) after inoculation. Each point represents the number of CFU recovered from a single animal. Data are pooled from the results of 2 separate experiments conducted on different days. Download
The FhaB proline-rich region is required for B. bronchiseptica persistence in the lower respiratory tract regardless of the presence or absence of fhaS. Data represent the bacterial burden in the murine lower respiratory tract (trachea and lungs) after inoculation. Each point represents the number of CFU recovered from a single animal. Open symbols represent the lower limit of detection for that particular experiment, as no CFU were recovered. Data are pooled from the results of 2 separate experiments conducted on different days. ***, P < 0.001 Download
FhaB/FHA does not influence global levels of some cytokines in the lung. Pro- and anti-inflammatory cytokines levels were determined from lung homogenates by ELISA. Data represent means ± SEM of the results determined for all samples, which were collected from all animals for which CFU are shown in Fig. 4a. Data are pooled from the results of ≥2 separate experiments conducted on different days. Download
Current model of FhaB secretion. Model of secretion of FhaB subsequent to transport across the cytoplasmic membrane (cm) and translocation and folding of the β-helical domain (purple) are shown. The ECT (gold) inhibits degradation of the prodomain by a periplasmic protease(s) (green “Pac-Man”) during secretion. As the MCD (green) begins to fold, the PNT (brown) restricts further translocation of the protein to the surface. Once the MCD is folded, it may interact with the environment, potentially acting as a sensory domain. An unidentified signal (lightning bolt) is transmitted to the periplasm that instructs the ECT to relieve repression of the periplasmic protease(s) or to alter PRR-mediated interactions or both. This signal(s) leads to rapid and complete prodomain degradation and/or alteration of virulence functions that contribute to Bordetella persistence. Further SphB1 (gray)-dependent processing occurs, and mature FHA mediates adherence to epithelial cell receptors. FhaC is represented by a cylinder in the outer membrane (om) with POTRA domains (ovals) on the periplasmic side of the om and loop 6 on the extracellular side of the om. FhaC is shaded red for the first two steps and then green for subsequent steps to indicate a shift from a release-incompetent form to a release-competent form (26). The colors of the FhaB subdomains are the same as those diagrammed in Fig. 2. The periplasm is not drawn to scale to aid in visualizing periplasmic events. Download
Strains applied in this study.
Plasmids applied in this study.


