Highlights
-
•
Socioeconomic inequalities in survival persist for the majority of adult cancers.
-
•
Socioeconomic inequalities have not been observed for childhood cancers.
-
•
We studied the effect of age on socioeconomic differences in survival for breast, lung and colon cancer patients.
-
•
Substantial age-specific socioeconomic inequalities in survival from cancers of the breast, lung and colon.
-
•
Policy makers should ensure that young and old, from affluent and deprived areas, seek and obtain timely access to care.
Keywords: Breast cancer, Colon cancer, Lung cancer, Net survival, Deprivation, Socioeconomic inequalities, Survival
Abstract
Background
Understanding the age at which persistent socioeconomic inequalities in cancer survival become apparent may help motivate and support targeting of cancer site-specific interventions, and tailoring guidelines to patients at higher risk.
Patients and methods
We analysed data on more than 40,000 patients diagnosed in England with one of three common cancers in men and women, breast, colon and lung, 2001–2005 with follow-up to the end of 2011. We estimated net survival for each of the five deprivation categories (affluent, 2, 3, 4, deprived), cancer site, sex and age group (15–44, 45–54, 55–64, and 65–74 and 75–99 years).
Results
The magnitude and pattern of the age specific socioeconomic inequalities in survival was different for breast, colon and lung. For breast cancer the deprivation gap in 1-year survival widened with increasing age at diagnosis, whereas the opposite was true for lung cancer, with colon cancer having an intermediate pattern. The ‘deprivation gap’ in 1-year breast cancer survival widened steadily from −0.8% for women diagnosed at 15–44 years to −4.8% for women diagnosed at 75–99 years, and was the widest for women diagnosed at 65−74 years for 5- and 10-year survival. For colon cancer in men, the gap was widest in patients diagnosed aged 55–64 for 1-, 5- and 10-year survival. For lung cancer, the ‘deprivation gap’ in survival in patients diagnoses aged 15–44 years was more than 10% for 1-year survival in men and for 1- and 5-year survival in women.
Conclusion
Our findings suggest that reduction of socioeconomic inequalities in survival will require updating of current guidelines to ensure the availability of optimal treatment and appropriate management of lung cancer patients in all age groups and older patients in deprived groups with breast or colon cancer.
1. Introduction
In spite of notable improvements in cancer survival in recent decades, socioeconomic inequalities in survival persist for the great majority of common cancers in adults [1–3]. For many cancers, however, survival has improved more rapidly for patients living in more affluent areas than for those living in deprived areas [4], including cancers of the breast, colon and lung [5–7]. These trends have led to wider socioeconomic inequalities (‘deprivation gap’) in survival in the last two decades, in spite of major policy initiatives designed to improve outcomes and reduce inequality [4,8].
A different picture is apparent for childhood cancers. The survival of children with cancer has improved more rapidly than that of adult patients in recent decades, chiefly reflecting notable advances in chemotherapy for many childhood cancers [9–11]. In addition, socioeconomic inequalities have not been observed for childhood cancers [12]. This may reflect a range of factors including the availability of effective treatments for many childhood cancers, the centralisation of care in specialist hospitals, and the high proportion of children treated in clinical trials [12].
These observations pose a question about the age-specific socioeconomic inequalities in cancer survival. Understanding this may help motivate and support targeting of interventions and tailoring guidelines to patients at higher risk. The answer to this question may also provide insights into the mechanisms responsible for socioeconomic inequalities in cancer survival and the potential contribution of differences in diagnosis. Against this background, we aimed to examine, the patterns of socioeconomic inequalities in survival for three common cancers in several age groups.
2. Patients and methods
All adults aged 15–99 years diagnosed in England with a first, invasive, primary malignant neoplasm of the breast (International Classification of Diseases, tenth revision [13] (ICD-10), C50), colon (C18) or lung (C33, C34) during the 5 years from 2001 to 2005, with follow-up to 31 December 2011 were considered for analysis. These three cancer sites are characterised by high incidence (allowing for more precise survival estimates by age and deprivation group), variable prognosis and a persistent ‘deprivation gap’ in survival in recent periods [1,3].
Standard exclusion criteria were used to decide whether a patient record was eligible for inclusion [1,14]. Cases were excluded if the cancer was only registered from the death certificate (DCO) (14,853 (3.5%)), or for unknown vital status or sex, duplicate registration, synchronous tumours, or invalid dates or sequences of dates (10,178 (2.4%)). Patients who had had a previous cancer of the same organ at any time since 1971 were also excluded (Table 1). One day was added to the survival time of patients for whom the dates of diagnosis and death were the same (zero survival), enabling the inclusion of these patients in analyses. Age at diagnosis was categorised in five groups (15–44, 45–54, 55–64, 65–74, and 75–99 years).
Table 1.
Number of patients eligible for analysis, exclusions, and number (%) of eligible patients included in analyses: three cancers, England, adults(15–99 years) diagnosed 2001–2005 and followed up to 2011.
| Malignancy | ICD-10 | Eligible | Exclusions |
Included |
||
|---|---|---|---|---|---|---|
| codea | DCOb | Otherc | Number | % | ||
| Colon | C18 | 90,928 | 3,129 | 1,880 | 86,378 | 95.0 |
| Lung | C33, C34 | 155,555 | 8,991 | 1,032 | 145,532 | 93.6 |
| Breast (women) | C50 | 183,885 | 2,733 | 7,266 | 173,886 | 94.6 |
International Classifications of Diseases, tenth edition.
Registration from a death certificate only (DCO): date of diagnosis unknown.
Aged 100 years or over at diagnosis, sex or vital status unknown, sex-site error, invalid dates, missing deprivation category, or previous cancer of the same organ since 1971.
The Office for National Statistics (ONS) provides information on each patient's vital status (alive, dead, emigrated or lost to follow-up) and their postcode of residence at diagnosis, from which patients were assigned to one of five deprivation categories (from most affluent (1) to most deprived (5)). An ecological deprivation score was assigned to each patient based on the characteristics of the Lower Super-Output Area (LSOA) in which the patient was resident at the time of diagnosis, and the year of diagnosis. The LSOAs in England are small areas (mean population 1500), covering the whole of England and for which detailed data on housing, income and employment are available. These information can be used to characterise the level of the socioeconomic group of residents. These groups were defined by quintiles of the income domain score of the Indices of Multiple Deprivation (IMD) [15] of 34,378 LSOAs in England.
Net survival is the survival probability we would observe if the disease under study was the only cause of death. It may be interpreted as the survival of cancer patients after controlling for competing causes of death. This method is recommended for the estimation of cancer survival when the cause of death is either unknown or unreliable. It estimates the excess mortality due to cancer as the difference between the all-cause mortality experienced by cancer patients and the expected or ‘background’ mortality derived from life tables of all-cause death rates of the general population. We used cancer registry data to estimate all-cause mortality, and life tables to estimate the expected or background mortality in the general population. Background mortality varied between socioeconomic groups and geographic regions in England. Death records were assigned to deprivation categories using the postcode and LSOA. Abridged (5-year) life tables were completed and extended to age 99 years and smoothed using flexible parametric Poisson regression with spline functions to model the death rate. We then derived complete (single-year-of-age) life tables by sex, socioeconomic group, geographic region and calendar year for 2001–2009 (Cancer Research UK Cancer Survival Group, 2004). Life tables for 2010–2011 could not be constructed because the relevant data (death during 2010–2011) were unavailable, so life tables for 2009 were used for these years.
We estimated net survival every six months and up to 10 years after diagnosis for each of the five deprivation categories, cancer site, sex and each of the five age groups using the Pohar Perme estimator [16].
The ‘deprivation’ gap was quantified as the fitted difference between survival in the ‘most affluent’ and the ‘most deprived’, using weighted least-squares regression [17] for each cancer site, sex and age group. A negative gap indicates that net survival was lower in the most deprived group than the most affluent group. This gap was quantified for each year up to 10 years after diagnosis.
All analyses were carried out in Stata 13 [18], including net survival analyses with stns [19].
3. Results
A total of 405,796 patients diagnosed between 2001 and 2005 and followed up to 2011 were included in the analyses (Table 1). The three cancer sites are more commonly diagnosed at an older age, with very few patients diagnosed with lung and colon in the youngest age group 15–55 (Table 2). While breast and colon cancer are more common among affluent patients, the percentage of lung cancer patients diagnosed late in life (75–99) are almost double those in the affluent group (Table 2). Table 3 summarises the 1-, 5, and 10-year survival for each cancer. Patterns of the ‘deprivation gap’ up to 5 years by age group are presented in Fig. 1. Net survival up to 10 years after diagnosis for the most affluent and the most deprived groups in the three age groups 15–44, 55–64 and 75–99 years, for each of the three cancers and sex in England are presented in Fig. 2. Net survival could not be estimated for colon cancer in men in the deprived youngest age group 14–55, due to the small number of patients. The detailed estimates of 1-, 5- and 10-year net survival are presented in Appendices A–C.
Table 2.
Distribution of patients eligible for survival analysis, by age group, deprivation category and sex: adults (15–99 years) diagnosed 2001–2005 and followed up to 2011 in England.
| Age group | Deprivation | Breast |
Colon |
Lung |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women |
Men |
Women |
Men |
Women |
|||||||
| N | % | N | % | N | % | N | % | N | % | ||
| 15–44 | Affluent | 4,503 | 2.6 | 230 | 0.5 | 239 | 0.6 | 125 | 0.1 | 132 | 0.2 |
| 2 | 4,189 | 2.4 | 196 | 0.4 | 201 | 0.5 | 128 | 0.1 | 154 | 0.3 | |
| 3 | 3,877 | 2.2 | 195 | 0.4 | 215 | 0.5 | 197 | 0.2 | 173 | 0.3 | |
| 4 | 3,950 | 2.3 | 223 | 0.5 | 223 | 0.5 | 227 | 0.3 | 203 | 0.3 | |
| Deprived | 3,421 | 2.0 | 252 | 0.6 | 212 | 0.5 | 309 | 0.4 | 262 | 0.4 | |
| 45–54 | Affluent | 8,594 | 4.9 | 543 | 1.2 | 561 | 1.3 | 583 | 0.7 | 534 | 0.9 |
| 2 | 7,867 | 4.5 | 538 | 1.2 | 505 | 1.2 | 761 | 0.9 | 657 | 1.1 | |
| 3 | 7,301 | 4.2 | 529 | 1.2 | 510 | 1.2 | 859 | 1.0 | 729 | 1.2 | |
| 4 | 6,553 | 3.8 | 506 | 1.1 | 440 | 1.0 | 1,125 | 1.3 | 967 | 1.6 | |
| Deprived | 5,273 | 3.0 | 457 | 1.0 | 421 | 1.0 | 1,514 | 1.8 | 1,175 | 2.0 | |
| 55–64 | Affluent | 10,410 | 6.0 | 1,772 | 4.0 | 1,331 | 3.1 | 2,196 | 2.5 | 1,342 | 2.3 |
| 2 | 10,102 | 5.8 | 1,650 | 3.7 | 1,344 | 3.2 | 2,621 | 3.0 | 1,680 | 2.8 | |
| 3 | 9,147 | 5.3 | 1,526 | 3.5 | 1,256 | 3.0 | 3,165 | 3.7 | 2,009 | 3.4 | |
| 4 | 7,825 | 4.5 | 1,497 | 3.4 | 1,124 | 2.7 | 3,856 | 4.5 | 2,488 | 4.2 | |
| Deprived | 6,046 | 3.5 | 1,262 | 2.9 | 918 | 2.2 | 4,767 | 5.5 | 3,061 | 5.2 | |
| 65–74 | Affluent | 7,401 | 4.3 | 2,944 | 6.7 | 2,164 | 5.1 | 3,898 | 4.5 | 2,167 | 3.7 |
| 2 | 7,399 | 4.3 | 2,954 | 6.7 | 2,391 | 5.7 | 4,764 | 5.5 | 2,823 | 4.8 | |
| 3 | 7,129 | 4.1 | 2,807 | 6.4 | 2,367 | 5.6 | 5,554 | 6.4 | 3,402 | 5.8 | |
| 4 | 6,612 | 3.8 | 2,762 | 6.3 | 2,193 | 5.2 | 7,014 | 8.1 | 4,363 | 7.4 | |
| Deprived | 5,219 | 3.0 | 2,481 | 5.6 | 1,884 | 4.5 | 7,950 | 9.2 | 5,290 | 9.0 | |
| 75–99 | Affluent | 7,309 | 4.2 | 3,616 | 8.2 | 3,891 | 9.2 | 5,020 | 5.8 | 3,221 | 5.5 |
| 2 | 8,626 | 5.0 | 3,997 | 9.1 | 4,547 | 10.8 | 6,273 | 7.3 | 4,302 | 7.3 | |
| 3 | 9,288 | 5.3 | 4,115 | 9.3 | 4,865 | 11.5 | 7,213 | 8.3 | 5,089 | 8.6 | |
| 4 | 9,190 | 5.3 | 4,039 | 9.2 | 4,850 | 11.5 | 8,359 | 9.7 | 6,404 | 10.8 | |
| Deprived | 6,655 | 3.8 | 3,013 | 6.8 | 3,622 | 8.6 | 8,023 | 9.3 | 6,404 | 10.8 | |
| Total | 173,886 | 100.0 | 44,104 | 100.0 | 42,274 | 100.0 | 86,501 | 100.0 | 59,031 | 100.0 | |
Table 3.
Net survival (%) at 1, 5 and 10 years and deprivation gap (with 95% CI), by age category for patients diagnosed during 2001–2005, and followed up to 2011 in England.
| Survival | Age group | Breast |
Colon |
Lung |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women |
Men |
Women |
Men |
Women |
|||||||||||||||||
| Deprivation gap |
Deprivation gap |
Deprivation gap |
Deprivation gap |
Deprivation gap |
|||||||||||||||||
| Net survival | % | 95% CI | Net survival | % | 95% CI | Net survival | % | 95% CI | Net survival | % | 95% CI | Net survival | % | 95% CI | |||||||
| 1 year | 15–44 | 97.8 | −0.8 | −1.4 | −0.2 | 80.8 | −1.0 | −7.5 | 5.5 | 83.2 | −7.2 | −13.5 | −0.9 | 40.3 | −10.7 | −19.5 | −1.9 | 47.6 | −12.3 | −21.3 | −3.2 |
| 45–54 | 97.9 | −1.5 | −2.0 | −1.1 | 77.2 | −7.5 | −12.1 | −2.9 | 81.6 | −3.1 | −7.5 | 1.3 | 32.3 | −4.6 | −8.5 | −0.8 | 39.0 | −5.8 | −10.2 | −1.5 | |
| 55–64 | 97.2 | −1.7 | −2.2 | −1.2 | 77.2 | −8.5 | −11.2 | −5.7 | 78.6 | −7.5 | −10.6 | −4.4 | 31.3 | −3.9 | −5.9 | −1.8 | 36.3 | −5.7 | −8.4 | −3.0 | |
| 65–74 | 94.3 | −3.8 | −4.6 | −3.0 | 72.7 | −6.7 | −8.9 | −4.4 | 71.5 | −8.5 | −11.0 | −5.9 | 27.2 | −2.5 | −4.0 | −1.0 | 30.0 | −2.7 | −4.7 | −0.7 | |
| 75–99 | 85.1 | −4.8 | −6.1 | −3.5 | 59.6 | −6.9 | −9.2 | −4.6 | 56.2 | −7.6 | −9.8 | −5.5 | 19.4 | −1.0 | −2.3 | 0.3 | 20.4 | −2.3 | −3.8 | −0.8 | |
| 5 year | 15–44 | 83.4 | −5.2 | −6.7 | −3.7 | 57.9 | −2.1 | −10.2 | 6.0 | 60.9 | −14.8 | −22.9 | −6.7 | 19.5 | −2.1 | −9.1 | 4.9 | 23.7 | −10.0 | −17.8 | −2.2 |
| 45–54 | 88.2 | −4.6 | −5.7 | −3.6 | 53.6 | −7.1 | −12.7 | −1.4 | 55.8 | −3.0 | −8.7 | 2.7 | 10.3 | −3.1 | −5.7 | −0.5 | 13.3 | −1.6 | −4.7 | 1.4 | |
| 55–64 | 88.3 | −4.5 | −5.5 | −3.5 | 54.2 | −8.8 | −12.2 | −5.4 | 56.9 | −7.2 | −11.0 | −3.4 | 9.0 | −2.1 | −3.4 | −0.8 | 11.7 | −2.9 | −4.7 | −1.0 | |
| 65–74 | 82.3 | −8.3 | −9.8 | −6.8 | 51.9 | −6.5 | −9.3 | −3.7 | 53.2 | −7.7 | −10.7 | −4.7 | 7.2 | −0.9 | −1.9 | 0.1 | 8.9 | −1.5 | −2.8 | −0.2 | |
| 75–99 | 66.4 | −5.1 | −7.4 | −2.8 | 43.0 | −6.6 | −9.7 | −3.6 | 43.1 | −4.6 | −7.4 | −1.8 | 3.9 | −0.9 | −1.7 | −0.2 | 4.3 | −0.8 | −1.7 | 0.1 | |
| 10 year | 15–44 | 74.5 | −5.7 | −7.7 | −3.7 | 52.7 | −2.0 | −11.2 | 7.1 | 56.2 | −13.9 | −22.7 | −5.1 | 18.0 | 1.5 | −5.3 | 8.3 | 21.3 | −9.5 | −17.8 | −1.1 |
| 45–54 | 81.7 | −4.8 | −6.3 | −3.3 | 50.0 | −7.9 | −14.2 | −1.5 | 52.0 | −0.9 | −6.9 | 5.2 | 8.0 | −2.5 | −5.1 | 0.1 | 9.7 | 0.0 | −3.1 | 3.1 | |
| 55–64 | 83.0 | −5.1 | −6.7 | −3.5 | 49.7 | −8.6 | −12.8 | −4.3 | 53.2 | −8.7 | −13.2 | −4.2 | 6.6 | −1.6 | −2.9 | −0.2 | 8.5 | −3.6 | −5.5 | −1.6 | |
| 65–74 | 76.9 | −8.9 | −11.5 | −6.3 | 49.2 | −5.2 | −9.4 | −1.1 | 50.0 | −8.2 | −12.3 | −4.1 | 4.9 | −1.2 | −2.4 | 0.0 | 5.9 | −0.9 | −2.4 | 0.6 | |
| 75–99 | 57.8 | −7.4 | −13.0 | −1.8 | 37.8 | −6.1 | −12.4 | 0.3 | 47.1 | 4.5 | −2.1 | 11.0 | 2.6 | −0.2 | −1.3 | 0.9 | 3.0 | 0.0 | −1.2 | 1.2 | |
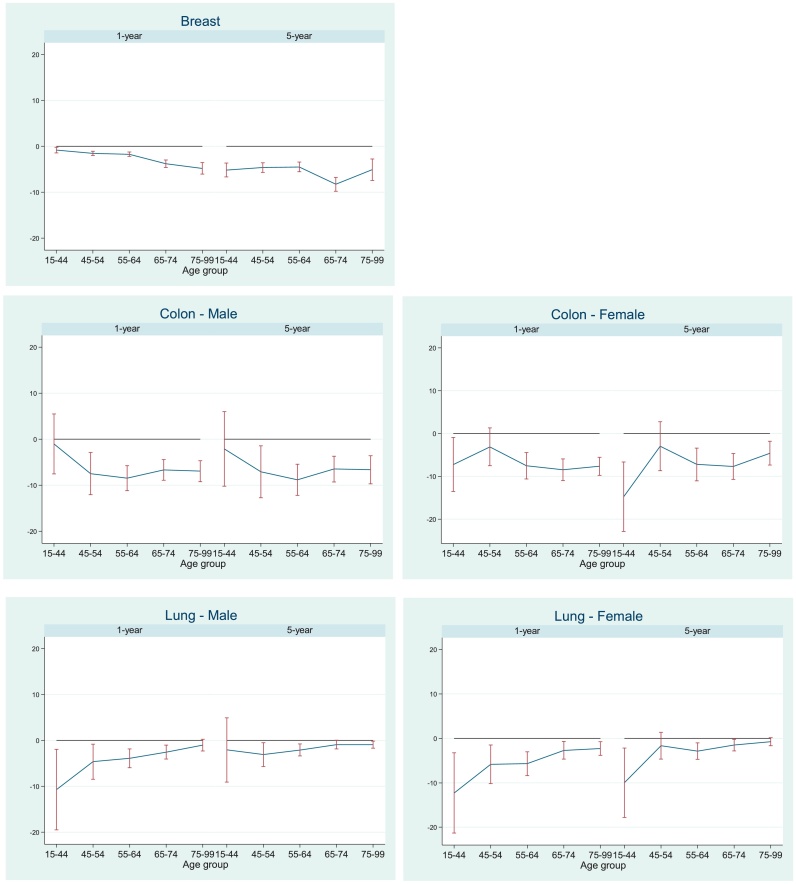
Fig. 1.
Deprivation gap (%) in 1- and 5-year survival for five age groups, patients diagnosed 2001–2005, England.
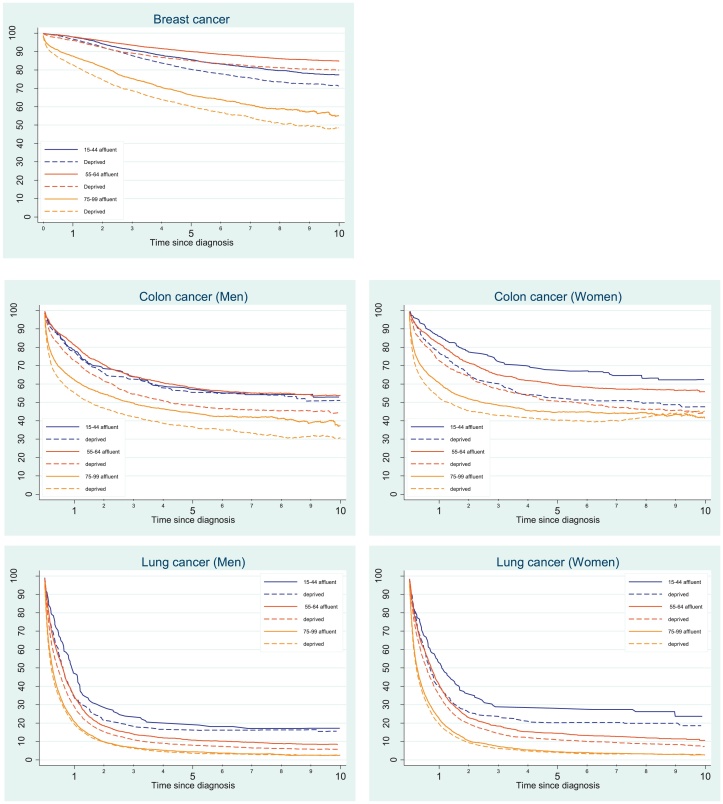
Fig. 2.
Trends in 1–10 year net survival (%) for the most deprived and the most affluent groups, by cancer and sex in the three age groups (15–44, 55–64 and 75–99), England.
The age-specific patterns of socioeconomic inequalities in survival differed between the three cancers. For breast cancer, the socioeconomic deprivation gap in 1-year survival widened with increasing age at diagnosis, whereas the opposite was true for lung cancer, with colon cancer having an intermediate pattern (Fig. 1, Table 3).
One-year survival for women with breast cancer for all ages and deprivation groups combined was high (97%). It was similar (94–98%) in the four age groups (15–44, 45–54, 55–64 and 65–74 years). For women diagnosed later in life (75–99 years), 1-year survival was still high (85.1%) but substantially lower than that of younger women. Survival of affluent breast cancer patients diagnosed in the 15–44 and 55–64 years age groups was similar up to 1 year after diagnosis. However, survival of affluent patients in the 55–64 age group was the highest of all up to 10 years after diagnosis (Fig. 2, Appendix 1–3). The ‘deprivation gap’ widened steadily from −0.8% for women diagnosed early in life (15–44 years) to −4.8% for women diagnosed late in life (75–99 years) for 1-year survival. The gap was widest for women aged 65−74 years at diagnosis (Fig. 2); −8.3% at 5 years (Fig. 1) and −8.9% at 10 years (Table 3).
For colon cancer, 1-year survival for all deprivation groups combined ranged between 80.8% and 77.2% for men and between 78.6% and 83.2% in the three age groups (15–44, 45–54 and 55–64). Survival was much lower for men (59.6%) and women (56.2%) diagnosed aged 75–99 years. Socioeconomic inequalities in survival were apparent for all age groups and both sexes up to 10 years after diagnosis. The deprivation gap in men was widest for those aged 55–64 years. The deprivation gap in 1- and 5-year survival in men ranged between −6.5% and −8.8% for those who were 45 years or older at diagnosis, with a narrower gap for men diagnosed early in life (15–44 years); −1.0% and −2.1% for 1- and 5-year survival, respectively (Fig. 1). Pattern of survival for colon cancer in women was different, with the widest deprivation gap in the youngest age group (15–44 years), however, only 2.5% of women were diagnosed at this age (Table 2).
One-year survival for lung cancer patients in all deprivation groups combined ranged between 40.3% and 31.3% in men and 47.6% and 36.3% in women in the three age groups 15–44, 45–54 and 55–64 years. Survival was much lower in the oldest age group for both men (19%) and women (20%). The ‘deprivation gap’ was more than 10% for 1-year survival in men and for 1- and 5-year survival in women aged 15–44 years. However, these groups include less than 2% of all patients, and hence a wide confidence interval is apparent. Patients diagnosed aged 75 years and older comprise more than 40% of the lung cancer population in men and women (Table 2). The ‘deprivation gap’ narrowed by age group and was less than 1% for both sexes in the oldest age groups for 5- and 10-year survival in both men and women.
4. Discussion
We studied the effect of age on socioeconomic differences in cancer survival for more than 400,000 patients diagnosed 2001–2005 with one of three common cancers (breast, colon and lung) in England. The applied methodology could be extended to understand international age-specific socioeconomic differences, and factors that have an impact on differences in magnitude and pattern of cancer survival between England and other countries if it exists. Age at diagnosis has a different influence on socioeconomic inequalities in survival for each of these cancers. Older age was associated with wider socioeconomic inequalities in short-term breast cancer survival, whilst the opposite was true for lung. No association between age at diagnosis and the deprivation gap was apparent for colon cancer.
Survival of patients in the most deprived groups was significantly lower than that of the most affluent groups for all three cancers in both sexes, extending similar findings made for patients diagnosed during 1971–1990 [1] and 1986–1999 [4]. While a ‘deprivation gap’ is common for most cancers and age groups, the overall pattern of lower survival in the most deprived groups differed between the five age groups.
Breast cancer is unique in that the deprivation gap is wider for women diagnosed at older ages. The average 1-year survival was already approaching the theoretical maximum of 100% for women diagnosed before the age of 65, suggesting a ‘ceiling effect’. Any further improvements in survival would be expected to be principally concentrated in women in the more deprived groups, whose survival has been lower. Socioeconomic inequalities in survival were still evident in all age groups up to 10 years after diagnosis. Affluent women benefit more from screening than deprived women, and are diagnosed at an earlier stage [20]. Therefore, screening could be one of the possible explanations of this survival inequality. The persistent long-term inequality, wider for older age groups, may also indicate socioeconomic disparities in the management of primary tumours and/or recurrences in old age [21].
For lung cancer, the socioeconomic differences in survival occurred among the relatively small group of patients diagnosed at a young age, and tended to diminish with time since diagnosis [22]. Lung cancer is not commonly seen under the age of 45: it typically accounts for less than 3% of all lung cancer patients under 40 years [23–25]. In our study, the difference in survival of lung cancer patients, between rich and poor was apparent at all age groups, but with different magnitude. The percentage of lung cancer patients under the age of 45 was 1.1% in men and 1.6% in women. A higher percentage of young lung cancer patients would present with advanced stage in comparison to patients over the age of 45, by the time they are referred to a specialist [26]. However, survival of young patients is much better than those diagnosed at older age [24]. Patients aged 75 years or older account for 40% of lung cancer in men and 43% of lung cancer in women. In both sexes survival in this age group was almost half that of the youngest group. Patients diagnosed at older ages are less likely to be treated by surgery, independently of their deprivation group [27]. The similarly low survival of all deprivation categories suggests that rich and poor are equally disadvantaged with respect to treatment. It would seem that socioeconomic inequalities in treatment (and particularly surgery) [27,28], are likely to be concentrated in patients in the younger age group, and this is likely to explain why the wider deprivation gaps are seen among younger lung cancer patients.
Socioeconomic inequalities in colon cancer survival have been well documented [1]. A general pattern of higher survival of affluent patients compared to deprived, with a wide and similar ‘deprivation gap’ among all high-risk age groups (older than 55 years) is clear, in men and women up to 10 years after diagnosis. In our study, more than 90% of the patients were diagnosed after the age of 55 years. Stage at diagnosis and access to optimal treatment have a major impact on survival and may thus explain at least part of the difference in survival between rich and poor in all age groups [2]. We have already shown that given equal treatment at a given stage of disease, colorectal cancer survival does not depend on socioeconomic status [29]. Although older colon cancer patients are less likely to receive recommended therapy [30], there was no evidence of socioeconomic variation in stage at diagnosis for colon cancer patients [31]. This evidence, combined with findings from our study, suggests that older, deprived patients are the most likely to be disadvantaged by lack of access to optimal treatment.
A limitation of our study is that it does not encompass information on stage at diagnosis, which is associated with the socioeconomic background of breast cancer patients [32], and may explain part of the lower survival among colon cancer patients living in deprived areas [2]. Future studies should aim to examine the proportion of the observed survival inequalities that may reflect inequalities in stage at diagnosis or treatment, and may be possible to conduct in the future. Information on stage at diagnosis is rarely available in population based datasets [33]. We therefore included all patients diagnosed with cancer in the population and these would help to provide public health prospective on age specific socioeconomic inequalities of cancer survival.
In conclusion, there are substantial age-specific socioeconomic inequalities in survival from cancers of the breast, lung and colon. Reduction of socioeconomic inequalities in survival still requires action to extend the availability of optimal treatment and appropriate management of lung cancer patients to all age groups, and in particular deprived breast and colon cancer patients diagnosed after the age of 65 and 55 years respectively, and to ensure that cancer patients, young and old, from affluent and deprived areas, seek and obtain timely access to care.
Funding
Cancer Research UK Programme Grant (C1336/A11700) to the Cancer Survival Group.
Conflict of interest
None declared.
Authorship Contribution
-
1)
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; Ula Nur, Georgios Lyratzopoulos. Michel Coleman and Bernard Rachet.
-
2)
drafting the article or revising it critically for important intellectual content; Ula Nur, Georgios Lyratzopoulos and Michel Coleman.
-
3)
final approval of the version to be published; Ula Nur, Georgios Lyratzopoulos. Michel Coleman and Bernard Rachet.
Acknowledgement
We thank the Office for National Statistics and the regional cancer registries in England for providing the data.
Appendix 1.
Net survival with 95% confidence intervals (CI) by sex and age group, for adults (15–99 years) diagnosed during 2001–2005, and followed up to 2011 in England.
| Age group | Deprivation | One-year survival |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast |
Colon |
Lung |
||||||||||||||
| Female |
Male |
Female |
Male |
Female |
||||||||||||
| Net survival |
Net survival |
Net survival |
Net survival |
Net survival |
||||||||||||
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | ||||||||||||
| 15–44 | Affluent | 98.0 | 97.6 | 98.4 | 78.8 | 73.5 | 84.0 | 86.2 | 81.9 | 90.6 | 47.2 | 38.5 | 55.9 | 53.1 | 44.6 | 61.5 |
| 2 | 98.0 | 97.6 | 98.4 | 84.8 | 79.8 | 89.8 | 83.1 | 78.0 | 88.3 | 37.5 | 29.2 | 45.9 | 50.0 | 42.2 | 57.9 | |
| 3 | 98.2 | 97.8 | 98.7 | 79.1 | 73.4 | 84.8 | 87.5 | 83.1 | 91.9 | 45.7 | 38.8 | 52.7 | 50.9 | 43.5 | 58.3 | |
| 4 | 97.8 | 97.3 | 98.2 | 84.4 | 79.7 | 89.2 | 81.2 | 76.1 | 86.4 | 42.4 | 36.0 | 48.8 | 49.3 | 42.5 | 56.2 | |
| Deprived | 96.8 | 96.2 | 97.4 | 77.6 | 72.4 | 82.7 | 77.5 | 71.8 | 83.1 | 33.8 | 28.5 | 39.0 | 39.8 | 33.8 | 45.7 | |
| 45–54 | Affluent | 98.5 | 98.2 | 98.8 | 81.4 | 78.1 | 84.7 | 82.3 | 79.2 | 85.5 | 36.7 | 32.8 | 40.6 | 41.8 | 37.7 | 46.0 |
| 2 | 98.4 | 98.1 | 98.7 | 80.0 | 76.6 | 83.4 | 83.7 | 80.5 | 87.0 | 31.9 | 28.6 | 35.2 | 40.6 | 36.8 | 44.3 | |
| 3 | 98.1 | 97.7 | 98.4 | 75.0 | 71.2 | 78.7 | 82.0 | 78.6 | 85.3 | 33.0 | 29.8 | 36.1 | 42.8 | 39.2 | 46.4 | |
| 4 | 97.7 | 97.3 | 98.1 | 73.5 | 69.6 | 77.3 | 78.2 | 74.3 | 82.1 | 32.6 | 29.9 | 35.4 | 35.9 | 32.8 | 38.9 | |
| Deprived | 96.6 | 96.1 | 97.1 | 75.8 | 71.8 | 79.8 | 81.1 | 77.3 | 84.9 | 30.2 | 27.9 | 32.5 | 37.2 | 34.4 | 40.0 | |
| 55–64 | Affluent | 98.0 | 97.7 | 98.3 | 81.2 | 79.3 | 83.0 | 82.0 | 79.9 | 84.1 | 34.3 | 32.3 | 36.3 | 40.9 | 38.3 | 43.6 |
| 2 | 97.5 | 97.2 | 97.9 | 79.0 | 76.9 | 81.0 | 79.5 | 77.3 | 81.7 | 30.8 | 29.0 | 32.6 | 38.3 | 36.0 | 40.6 | |
| 3 | 97.2 | 96.8 | 97.5 | 76.6 | 74.4 | 78.7 | 78.8 | 76.5 | 81.1 | 31.8 | 30.2 | 33.5 | 36.2 | 34.1 | 38.3 | |
| 4 | 96.8 | 96.4 | 97.3 | 75.3 | 73.0 | 77.5 | 78.0 | 75.5 | 80.4 | 32.4 | 30.9 | 33.9 | 34.0 | 32.1 | 35.9 | |
| Deprived | 96.1 | 95.5 | 96.6 | 72.4 | 69.9 | 75.0 | 72.8 | 69.9 | 75.7 | 28.9 | 27.6 | 30.2 | 35.1 | 33.4 | 36.8 | |
| 65–74 | Affluent | 96.0 | 95.4 | 96.5 | 74.6 | 73.0 | 76.2 | 75.4 | 73.6 | 77.3 | 28.2 | 26.8 | 29.7 | 32.3 | 30.3 | 34.2 |
| 2 | 95.2 | 94.7 | 95.8 | 75.4 | 73.8 | 77.1 | 74.2 | 72.4 | 76.0 | 28.1 | 26.8 | 29.4 | 30.3 | 28.6 | 32.0 | |
| 3 | 94.7 | 94.1 | 95.3 | 73.8 | 72.1 | 75.5 | 70.8 | 68.9 | 72.7 | 28.3 | 27.1 | 29.5 | 30.8 | 29.2 | 32.4 | |
| 4 | 92.7 | 92.0 | 93.4 | 70.6 | 68.8 | 72.4 | 68.5 | 66.5 | 70.5 | 26.3 | 25.3 | 27.4 | 28.7 | 27.3 | 30.1 | |
| Deprived | 92.4 | 91.5 | 93.2 | 68.4 | 66.5 | 70.3 | 67.9 | 65.7 | 70.1 | 26.2 | 25.2 | 27.1 | 29.4 | 28.1 | 30.6 | |
| 75–99 | Affluent | 87.6 | 86.6 | 88.5 | 62.3 | 60.6 | 64.1 | 60.6 | 58.9 | 62.2 | 20.7 | 19.6 | 21.9 | 22.4 | 20.9 | 23.9 |
| 2 | 86.5 | 85.6 | 87.4 | 61.8 | 60.1 | 63.5 | 57.3 | 55.8 | 58.9 | 19.2 | 18.2 | 20.3 | 20.2 | 18.9 | 21.4 | |
| 3 | 84.4 | 83.4 | 85.3 | 59.6 | 57.9 | 61.3 | 56.4 | 54.8 | 57.9 | 19.3 | 18.3 | 20.2 | 21.2 | 20.1 | 22.4 | |
| 4 | 84.2 | 83.3 | 85.2 | 58.3 | 56.6 | 60.0 | 54.3 | 52.8 | 55.8 | 18.9 | 18.0 | 19.8 | 19.7 | 18.7 | 20.8 | |
| Deprived | 82.8 | 81.6 | 83.9 | 55.2 | 53.3 | 57.2 | 52.5 | 50.7 | 54.3 | 19.4 | 18.5 | 20.3 | 19.6 | 18.6 | 20.6 | |
Appendix 2.
Net survival with 95% confidence intervals (CI) by sex and age group, for adults (15–99 years) diagnosed during 2001–2005, and followed up to 2011 in England.
| Age group | Deprivation | Five-year survival |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast |
Colon |
Lung |
||||||||||||||
| Female |
Male |
Female |
Male |
Female |
||||||||||||
| Net survival |
Net survival |
Net survival |
Net survival |
Net survival |
||||||||||||
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | ||||||||||||
| 15–44 | Affluent | 85.6 | 84.6 | 86.6 | 57.2 | 50.8 | 63.7 | 67.9 | 61.9 | 73.8 | 19.7 | 12.8 | 26.6 | 28.1 | 20.5 | 35.7 |
| 2 | 84.6 | 83.5 | 85.8 | 60.6 | 53.7 | 67.4 | 61.4 | 54.7 | 68.2 | 14.9 | 8.8 | 21.0 | 28.0 | 20.9 | 35.0 | |
| 3 | 83.5 | 82.3 | 84.7 | 57.9 | 50.9 | 64.9 | 66.8 | 60.5 | 73.1 | 21.9 | 16.1 | 27.7 | 27.0 | 20.4 | 33.7 | |
| 4 | 82.0 | 80.8 | 83.3 | 58.7 | 52.2 | 65.3 | 54.9 | 48.4 | 61.5 | 24.2 | 18.6 | 29.8 | 19.2 | 13.8 | 24.6 | |
| Deprived | 80.3 | 78.9 | 81.7 | – | – | – | 52.6 | 45.9 | 59.4 | 16.4 | 12.2 | 20.5 | 20.2 | 15.4 | 25.1 | |
| 45–54 | Affluent | 89.9 | 89.3 | 90.6 | 58.5 | 54.3 | 62.7 | 58.2 | 54.0 | 62.3 | 13.4 | 10.6 | 16.2 | 14.0 | 11.0 | 16.9 |
| 2 | 89.5 | 88.8 | 90.3 | 53.5 | 49.2 | 57.8 | 55.7 | 51.3 | 60.1 | 10.9 | 8.7 | 13.1 | 13.6 | 10.9 | 16.2 | |
| 3 | 88.0 | 87.2 | 88.8 | 53.7 | 49.4 | 58.0 | 56.0 | 51.6 | 60.4 | 9.5 | 7.5 | 11.4 | 13.7 | 11.2 | 16.2 | |
| 4 | 87.2 | 86.3 | 88.0 | 49.4 | 44.9 | 53.9 | 51.9 | 47.2 | 56.7 | 10.5 | 8.7 | 12.3 | 13.8 | 11.6 | 16.0 | |
| Deprived | 85.0 | 84.0 | 86.0 | 52.2 | 47.4 | 56.9 | 56.7 | 51.8 | 61.6 | 9.1 | 7.6 | 10.5 | 12.1 | 10.2 | 14.0 | |
| 55–64 | Affluent | 90.0 | 89.3 | 90.6 | 58.0 | 55.6 | 60.4 | 59.5 | 56.8 | 62.3 | 10.7 | 9.4 | 12.0 | 14.5 | 12.6 | 16.4 |
| 2 | 89.3 | 88.6 | 90.0 | 56.6 | 54.1 | 59.2 | 58.0 | 55.3 | 60.8 | 9.0 | 7.8 | 10.1 | 12.0 | 10.4 | 13.6 | |
| 3 | 88.1 | 87.4 | 88.9 | 52.8 | 50.1 | 55.4 | 58.0 | 55.2 | 60.8 | 9.1 | 8.0 | 10.1 | 11.9 | 10.5 | 13.4 | |
| 4 | 87.2 | 86.4 | 88.1 | 53.1 | 50.4 | 55.8 | 56.3 | 53.2 | 59.3 | 9.2 | 8.2 | 10.1 | 10.9 | 9.6 | 12.1 | |
| Deprived | 85.0 | 84.0 | 86.1 | 48.5 | 45.5 | 51.6 | 50.8 | 47.3 | 54.2 | 7.9 | 7.1 | 8.7 | 10.9 | 9.8 | 12.0 | |
| 65–74 | Affluent | 85.5 | 84.5 | 86.5 | 54.0 | 52.0 | 56.1 | 57.1 | 54.9 | 59.4 | 8.3 | 7.4 | 9.2 | 9.7 | 8.4 | 11.0 |
| 2 | 84.4 | 83.4 | 85.5 | 53.3 | 51.3 | 55.4 | 55.8 | 53.6 | 58.0 | 6.7 | 5.9 | 7.5 | 10.1 | 9.0 | 11.3 | |
| 3 | 82.3 | 81.2 | 83.5 | 53.8 | 51.6 | 56.0 | 51.9 | 49.6 | 54.1 | 7.7 | 6.9 | 8.5 | 8.4 | 7.4 | 9.3 | |
| 4 | 80.6 | 79.4 | 81.9 | 50.9 | 48.6 | 53.1 | 49.8 | 47.4 | 52.1 | 6.8 | 6.2 | 7.5 | 8.9 | 8.0 | 9.8 | |
| Deprived | 76.4 | 75.0 | 77.9 | 46.7 | 44.3 | 49.1 | 50.9 | 48.3 | 53.5 | 6.9 | 6.3 | 7.6 | 8.3 | 7.5 | 9.1 | |
| 75–99 | Affluent | 66.3 | 64.6 | 68.1 | 44.4 | 42.1 | 46.6 | 44.7 | 42.6 | 46.9 | 4.3 | 3.7 | 5.0 | 4.4 | 3.6 | 5.2 |
| 2 | 69.2 | 67.4 | 71.0 | 46.2 | 43.9 | 48.6 | 44.5 | 42.4 | 46.6 | 4.4 | 3.8 | 5.1 | 5.1 | 4.3 | 5.9 | |
| 3 | 67.0 | 65.2 | 68.8 | 42.6 | 40.4 | 44.9 | 43.9 | 41.8 | 46.0 | 3.8 | 3.2 | 4.3 | 4.4 | 3.7 | 5.1 | |
| 4 | 67.3 | 65.4 | 69.2 | 43.8 | 41.4 | 46.1 | 41.5 | 39.4 | 43.5 | 3.6 | 3.1 | 4.1 | 4.0 | 3.4 | 4.6 | |
| Deprived | 60.2 | 58.2 | 62.2 | 36.8 | 34.2 | 39.4 | 40.5 | 38.1 | 42.8 | 3.6 | 3.0 | 4.1 | 4.1 | 3.5 | 4.6 | |
Appendix 3.
Net survival with 95% confidence intervals (CI) by sex and age group, for adults (15–99 years) diagnosed during 2001–2005, and followed up to 2011 in England.
| Age group | Deprivation | Ten-year survival |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast |
Colon |
Lung |
||||||||||||||
| Female |
Male |
Female |
Male |
Female |
||||||||||||
| Net survival |
Net survival |
Net survival |
Net survival |
Net survival |
||||||||||||
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | ||||||||||||
| 15–44 | Affluent | 77.2 | 75.8 | 78.7 | 52.9 | 45.9 | 59.9 | 62.4 | 55.8 | 69.0 | 17.2 | 10.5 | 23.8 | 23.7 | 15.4 | 31.9 |
| 2 | 75.5 | 74.0 | 77.0 | 53.8 | 46.4 | 61.2 | 56.1 | 48.6 | 63.6 | 12.6 | 6.9 | 18.4 | 28.2 | 21.1 | 35.3 | |
| 3 | 74.8 | 73.2 | 76.5 | 53.6 | 45.8 | 61.4 | 63.2 | 56.5 | 69.9 | 19.9 | 14.3 | 25.5 | 24.1 | 17.3 | 30.9 | |
| 4 | 72.8 | 71.1 | 74.5 | 52.2 | 44.4 | 60.1 | 50.8 | 43.8 | 57.8 | 23.1 | 17.5 | 28.7 | 14.0 | 7.0 | 21.1 | |
| Deprived | 71.4 | 69.6 | 73.1 | 51.1 | 43.9 | 58.3 | 47.6 | 40.3 | 55.0 | 15.5 | 11.3 | 19.8 | 18.6 | 13.5 | 23.8 | |
| 45–54 | Affluent | 83.6 | 82.7 | 84.6 | 54.1 | 49.4 | 58.8 | 52.9 | 48.5 | 57.3 | 10.3 | 7.6 | 13.1 | 9.6 | 6.8 | 12.4 |
| 2 | 82.6 | 81.6 | 83.7 | 52.0 | 47.6 | 56.5 | 52.2 | 47.4 | 56.9 | 8.8 | 6.6 | 10.9 | 10.07 | 2 12 | 12.7 | |
| 3 | 81.7 | 80.5 | 82.8 | 50.1 | 45.3 | 54.9 | 51.9 | 47.2 | 56.7 | 7.3 | 4.9 | 9.7 | 9.6 | 7.2 | 12.0 | |
| 4 | 81.0 | 79.8 | 82.2 | 44.6 | 39.7 | 49.5 | 49.3 | 44.3 | 54.3 | 7.9 | 6.2 | 9.7 | 9.4 | 7.2 | 11.5 | |
| Deprived | 78.1 | 76.7 | 79.6 | 48.7 | 43.2 | 54.2 | 53.6 | 48.4 | 58.7 | 7.2 | 5.7 | 8.7 | 9.9 | 7.7 | 12.0 | |
| 55–64 | Affluent | 84.9 | 83.9 | 85.9 | 53.8 | 50.9 | 56.7 | 55.9 | 52.7 | 59.0 | 8.2 | 6.8 | 9.6 | 10.7 | 8.6 | 12.8 |
| 2 | 84.4 | 83.4 | 85.5 | 51.4 | 48.2 | 54.5 | 54.9 | 51.8 | 58.0 | 6.3 | 5.1 | 7.4 | 10.0 | 8.5 | 11.6 | |
| 3 | 83.0 | 81.8 | 84.1 | 48.5 | 45.3 | 51.7 | 55.2 | 52.0 | 58.5 | 6.9 | 5.8 | 8.0 | 8.6 | 7.1 | 10.2 | |
| 4 | 81.2 | 79.9 | 82.6 | 48.6 | 45.0 | 52.1 | 53.0 | 49.5 | 56.6 | 6.8 | 5.8 | 7.8 | 7.5 | 6.2 | 8.8 | |
| Deprived | 79.9 | 78.3 | 81.5 | 44.5 | 40.6 | 48.4 | 44.3 | 40.1 | 48.5 | 5.8 | 5.0 | 6.7 | 7.3 | 6.1 | 8.5 | |
| 65–74 | Affluent | 80.2 | 78.5 | 81.8 | 50.5 | 47.6 | 53.4 | 54.2 | 51.2 | 57.1 | 5.7 | 4.6 | 6.8 | 5.8 | 4.3 | 7.3 |
| 2 | 79.4 | 77.5 | 81.2 | 49.9 | 46.8 | 52.9 | 53.4 | 50.4 | 56.5 | 4.8 | 4.0 | 5.7 | 6.8 | 5.4 | 8.1 | |
| 3 | 77.0 | 75.0 | 79.0 | 53.3 | 50.1 | 56.5 | 46.6 | 43.4 | 49.9 | 5.6 | 4.7 | 6.5 | 6.1 | 5.0 | 7.2 | |
| 4 | 76.5 | 74.4 | 78.6 | 47.6 | 44.2 | 51.1 | 48.6 | 45.4 | 51.7 | 4.2 | 3.4 | 5.0 | 5.9 | 4.9 | 7.0 | |
| Deprived | 69.1 | 66.6 | 71.7 | 44.1 | 40.3 | 47.8 | 46.7 | 43.0 | 50.4 | 4.5 | 3.7 | 5.4 | 5.3 | 4.4 | 6.3 | |
| 75–99 | Affluent | 55.3 | 51.0 | 59.6 | 37.4 | 32.4 | 42.4 | 41.6 | 36.9 | 46.3 | 2.4 | 1.6 | 3.3 | 2.5 | 1.5 | 3.6 |
| 2 | 63.2 | 58.3 | 68.1 | 39.1 | 33.9 | 44.4 | 49.1 | 42.9 | 55.4 | 3.1 | 2.0 | 4.1 | 3.8 | 2.6 | 5.0 | |
| 3 | 58.1 | 52.6 | 63.6 | 40.8 | 36.0 | 45.6 | 47.1 | 41.2 | 53.0 | 2.6 | 1.7 | 3.5 | 3.3 | 2.3 | 4.3 | |
| 4 | 60.4 | 54.6 | 66.2 | 38.5 | 32.7 | 44.3 | 50.1 | 44.6 | 55.6 | 2.3 | 1.4 | 3.1 | 2.4 | 1.5 | 3.3 | |
| Deprived | 48.5 | 44.3 | 52.7 | 30.8 | 26.0 | 35.6 | 45.5 | 40.1 | 50.9 | 2.5 | 1.7 | 3.3 | 3.1 | 2.3 | 3.9 | |
References
- 1.Coleman M.P., Babb P., Damiecki P., Grosclaude P.C., Honjo S., Jones J. The Stationery Office; London: 1999. Cancer Survival Trends in England and Wales 1971–1995: Deprivation and NHS Region. (Studies on Medical and Population Subjects No. 61) [Google Scholar]
- 2.Woods L.M., Rachet B., Coleman M.P. Origins of socio-economic inequalities in cancer survival: A review. Ann. Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 3.Rachet B., Ellis L., Maringe C., Chu T., Nur U., Quaresma M. Socioeconomic inequalities in cancer survival in England after the NHS cancer plan. Br. J. Cancer. 2010;103(4):446–453. doi: 10.1038/sj.bjc.6605752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman M.P., Rachet B., Woods L.M., Mitry E., Riga M., Cooper N. Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br. J. Cancer. 2004;90(7):1367–1373. doi: 10.1038/sj.bjc.6601696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn M.J., Cooper N., Rachet B., Mitry E., Coleman M.P. Survival from cancer of the breast in women in England and Wales up to 2001. Br. J. Cancer. 2008;99(Suppl. 1):53–55. doi: 10.1038/sj.bjc.6604587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitry E., Rachet B., Quinn M.J., Cooper N., Coleman M.P. Survival from cancer of the colon in England and Wales up to 2001. Br. J. Cancer. 2008;99(Suppl. 1):S26–S29. doi: 10.1038/sj.bjc.6604578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rachet B., Quinn M.J., Cooper N., Coleman M.P. Survival from cancer of the lung in England and Wales up to 2001. Br. J. Cancer. 2008;99(Suppl. 1):40–42. doi: 10.1038/sj.bjc.6604583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Department of Health . Department of Health; London: 2000. The NHS Cancer Plan: A Plan for Investment, A Plan for Reform. [Google Scholar]
- 9.Birch J.M., Marsden H.B., Jones P.H., Pearson D., Blair V. Improvements in survival from childhood cancer: results of a population based survey over 30 years. Br. Med. J. (Clin. Res. Ed.) 1988;296(6633):1372–1376. doi: 10.1136/bmj.296.6633.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan R., Kowalczyk J.R., Agarwal B., Ladenstein R., Fitzgerald E., Barr R. New policies to address the global burden of childhood cancers. Lancet Oncol. 2013;14(3):e125–e135. doi: 10.1016/S1470-2045(13)70007-X. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard-Jones K., Pieters R., Reaman G.H., Hjorth L., Downie P., Calaminus G. Sustaining innovation and improvement in the treatment of childhood cancer: Lessons from high-income countries. Lancet Oncol. 2013;14(3):e95–e103. doi: 10.1016/S1470-2045(13)70010-X. [DOI] [PubMed] [Google Scholar]
- 12.Coleman M.P., Babb P., Sloggett A., Quinn M., De Stavola B. Socioeconomic inequalities in cancer survival in England and Wales. Cancer. 2001;91(1):208–216. doi: 10.1002/1097-0142(20010101)91:1+<208::aid-cncr6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Tenth revision. WHO; Geneva: 1994. International Statistical Classification of Diseases and Related Health Problems. [PubMed] [Google Scholar]
- 14.Li R., Abela L., Moore J., Woods L.M., Nur U., Rachet B. Control of data quality for population-based cancer survival analysis. Cancer Epidemiol. 2014;38(3):314–320. doi: 10.1016/j.canep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Office of the Deputy Prime Minister . 2004. The English Indices of Deprivation 2004 – Summary (Revised) http://www.communities.gov.uk/archived/publications/communities/indicesdeprivation. [Google Scholar]
- 16.Pohar Perme M., Stare J., Estève J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 17.Grizzle J.E., Starmer C.F., Koch G.G. Analysis of categorical data by linear models. Biometrics. 1969;25:489–504. [PubMed] [Google Scholar]
- 18.Statacorp . 13.0 ed. Stata Corporation; College Station, TX: 2013. STATA Statistical Software. [Google Scholar]
- 19.Clerc-Urmès I., Grzebyk M., Hédelin G. Net survival estimation with stns. Stata J. 2014;14:87–102. [Google Scholar]
- 20.Jack R.H., Robson T., Davies E.A. The varying influence of socioeconomic deprivation on breast cancer screening uptake in London. J. Public Health (Oxf) 2015 doi: 10.1093/pubmed/fdv038. [DOI] [PubMed] [Google Scholar]
- 21.Halmin M., Bellocco R., Lagerlund M., Karlsson P., Tejler G., Lambe M. Long-term inequalities in breast cancer survival – A ten year follow-up study of patients managed within a National Health Care System (Sweden) Acta Oncol. 2008;47(2):216–224. doi: 10.1080/02841860701769768. [DOI] [PubMed] [Google Scholar]
- 22.Rachet B., Woods L.M., Mitry E., Riga M., Cooper C., Quinn M.J. Cancer survival in England and Wales at the end of the 20th century. Br. J. Cancer. 2008;99(Suppl. 1):2–10. doi: 10.1038/sj.bjc.6604571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liam C.K., Lim K.H., Wong C.M. Lung cancer in patients younger than 40 years in a multiracial Asian country. Respirology. 2000;5(4):355–361. [PubMed] [Google Scholar]
- 24.Subramanian J., Morgensztern D., Goodgame B., Baggstrom M.Q., Gao F., Piccirillo J. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J. Thorac. Oncol. 2010;5(1):23–28. doi: 10.1097/JTO.0b013e3181c41e8d. [DOI] [PubMed] [Google Scholar]
- 25.Icard P., Regnard J.F., de Napoli S., Rojas-Miranda A., Dartevelle P., Levasseur P. Primary lung cancer in young patients: a study of 82 surgically treated patients. Ann. Thorac. Surg. 1992;54(1):99–103. doi: 10.1016/0003-4975(92)91150-8. [DOI] [PubMed] [Google Scholar]
- 26.Lyratzopoulos G., Abel G.A., Brown C.H., Rous B.A., Vernon S.A., Roland M. Socio-demographic inequalities in stage of cancer diagnosis: evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann. Oncol. 2013;24(3):843–850. doi: 10.1093/annonc/mds526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riaz S.P., Luchtenborg M., Jack R.H., Coupland V.H., Linklater K.M., Peake M.D. Variation in surgical resection for lung cancer in relation to survival: population-based study in England 2004–2006. Eur. J. Cancer. 2012;48(1):54–60. doi: 10.1016/j.ejca.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Forrest L.F., Adams J., Wareham H., Rubin G., White M. Socioeconomic inequalities in lung cancer treatment: systematic review and meta-analysis. PLoS Med. 2013;10(2):e1001376. doi: 10.1371/journal.pmed.1001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nur U., Rachet B., Coleman M.P., Sydes M., Cooper N., Lepage C. No socioeconomic inequalities in colorectal cancer survival within a randomised clinical trial. Br. J. Cancer. 2008;99:1923–1928. doi: 10.1038/sj.bjc.6604743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsley C., Pond G.R., Peloza B., Kok J., Naidoo K., Dale D. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J. Clin. Oncol. 2005;23(16):3802–3810. doi: 10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 31.Wrigley H., Roderick P., George S., Smith J., Mullee M., Goddard J. Inequalities in survival from colorectal cancer: a comparison of the impact of deprivation, treatment, and host factors on observed and cause specific survival. J. Epidemiol. Commun. Health. 2003;57(4):301–309. doi: 10.1136/jech.57.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macleod U., Ross S., Gillis C., McConnachie A., Twelves C., Watt G.C. Socio-economic deprivation and stage of disease at presentation in women with breast cancer. Ann. Oncol. 2000;11(1):105–107. doi: 10.1023/a:1008385321476. [DOI] [PubMed] [Google Scholar]
- 33.Walters S., Maringe C., Butler J., Brierley J.D., Rachet B., Coleman M.P. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int. J. Cancer. 2013;132(3):676–685. doi: 10.1002/ijc.27651. [DOI] [PubMed] [Google Scholar]