Abstract
The role of the food delivery matrix in probiotic performance in the intestine is not well understood. Because probiotics are often provided to consumers in dairy products, we investigated the contributions of milk to the health-benefiting performance of Lactobacillus casei BL23 in a dextran sulfate sodium (DSS)-induced murine model of ulcerative colitis. L. casei BL23 protected against the development of colitis when ingested in milk but not in a nutrient-free buffer simulating consumption as a nutritional supplement. Consumption of (acidified) milk alone also provided some protection against weight loss and intestinal inflammation but was not as effective as L. casei and milk in combination. In contrast, L. casei mutants deficient in DltD (lipoteichoic acid d-alanine transfer protein) or RecA (recombinase A) were unable to protect against DSS-induced colitis, even when consumed in the presence of milk. Mice fed either L. casei or milk contained reduced quantities of colonic proinflammatory cytokines, indicating that the L. casei DltD− and RecA− mutants as well as L. casei BL23 in nutrient-free buffer were effective at modulating immune responses. However, there was not a direct correlation between colitis and quantities of these cytokines at the time of sacrifice. Identification of the cecal microbiota by 16S rRNA gene sequencing showed that L. casei in milk enriched for Comamonadaceae and Bifidobacteriaceae; however, the consumption of neither L. casei nor milk resulted in the restoration of the microbiota to resemble that of healthy animals. These findings strongly indicate that probiotic strain efficacy can be influenced by the food/supplement delivery matrix.
INTRODUCTION
Certain strains of Lactobacillus and Bifidobacterium are used extensively as probiotics intended to confer health benefits upon delivery to the digestive tract in foods, beverages, or dietary supplements. Dairy products are the most popular food matrices for probiotic strains, and numerous clinical trials examining probiotic efficacy have used yogurt or (fermented) milks as carriers of probiotics to the intestine (1–3). Comparisons of data from human studies have indicated that probiotic delivery matrices, including dairy products, can influence probiotic intestinal survival and persistence (1). Remarkably, however, only a single human study and a few animal studies have compared the impacts of different carrier matrices or food product formulations on the capacity of probiotics to influence health (1, 2). Evidence from preclinical animal studies indicates that fermented milks might augment probiotic efficacy, although this has yet to be systematically investigated.
Probiotics are useful for the prevention and treatment of a variety of acute and chronic diseases (4–6). Prevention and reduction of symptoms of inflammatory bowel diseases (IBDs) are among the most extensively evaluated benefits (7). Although the pathogenesis of IBDs is unclear, these diseases are characterized by chronic intestinal inflammation and excessive accumulation of reactive oxygen species (ROS) contributing to oxidative epithelial damage (8, 9). The treatment and prevention of IBDs are elusive because these diseases are complex, multifactorial illnesses that involve host, environmental, and bacterial components (10). Probiotics were shown in several studies to induce the remission of ulcerative colitis (UC) and prevent the relapse of pouchitis (11–14). Because probiotic bacteria can typically be consumed in high quantities without adverse effects and generally do not become permanent colonists of the digestive tract, these strains are useful within the repertoire of approaches used to manage IBD.
Lactobacillus casei strains are among the most commonly used probiotics incorporated into yogurts and fermented dairy drinks for the intention of improving health (3). In clinical studies, L. casei reduced symptoms of constipation and diarrhea (15–18). Strains of L. casei have also shown promise in the prevention or mitigation of IBDs in humans (19, 20) and preclinical rodent models (21–28). We hypothesized that the consumption of L. casei BL23 in milk would improve the efficacy of this strain in a mouse model of IBD. L. casei BL23 is highly related (sharing 99.6% gene content) to commercial L. casei strains Shirota and DN-114 001 that are incorporated into fermented milk products (29). L. casei BL23 also previously conferred anti-inflammatory effects in a dextran sulfate sodium (DSS) mouse model of UC (26), and these effects were enhanced in mutants of this strain overexpressing superoxide dismutase and catalase (26, 28). To test our hypothesis, wild-type L. casei BL23 was fed to mice in milk or a nutrient-free buffer prior to and during administration of DSS. The protection against DSS colitis provided by these cultures was compared to that in DSS-treated mice given milk alone or milk containing L. casei lipoteichoic acid (LTA) d-alanyl transfer protein (DltD)- or recombinase A (RecA)-deficient mutants. The L. casei DltD− and RecA− mutants were constructed to elucidate the importance of dairy relative to different probiotic features of L. casei. Specifically, DltD was selected because this protein is involved in the d-alanylation of LTA, a feature that was previously shown to be important for immune modulation in mouse models of colitis (30–32). RecA was targeted because this protein is important for tolerance to oxidative stress and other environmental stresses (33–35). In this study, intestinal survival of wild-type and mutant L. casei strains, disease activity, colonic cytokines and chemokine levels, and the composition of the indigenous microbiota were determined for different strain and delivery matrix combinations.
MATERIALS AND METHODS
Bacterial strains and preparation for mouse feeding.
L. casei was routinely grown at 37°C for 24 h in de Man-Rogosa-Sharpe (MRS) medium (36) containing either 2% (wt/vol) lactose for broth or 2% (wt/vol) glucose for agar plates. L. casei BL23 cells were also incubated in ultrahigh-temperature (UHT)-processed 2% reduced fat milk (milk) (Gossner Foods, Inc., Logan, UT) for 24 h and then incubated at 4°C. pH was measured on a SevenEasy pH meter with an InLab Routine Pro pH electrode (Mettler-Toledo, Inc., Columbus, OH). When appropriate, the following antibiotics were added to the culture medium: 5 μg/ml erythromycin (Erm) and 50 μg/ml rifampin (Rif).
Mutant construction.
A rifampin-resistant mutant was selected from single-colony isolates of wild-type L. casei BL23 cells grown on MRS agar containing 2% glucose and 50 μg/ml of rifampin. This mutant was fed to mice and is designated here L. casei BL23. For construction of the DltD and RecA knockout mutants, an internal fragment of ∼500 bp from each gene (LCABL_08580 [DltD] and LCABL_28180 [RecA]) targeted for inactivation was amplified by PCR (Table 1). Each PCR amplification was performed with 200 ng of genomic DNA of L. casei BL23, GoTaq DNA polymerase (Promega, Madison, WI), and 200 nM each primer (Invitrogen, Carlsbad, CA). The amplicons were purified in an agarose gel with the QIAquick gel extraction kit (Qiagen, Valencia, CA), and the purified PCR products were then ligated into pRV300 (37) digested with SacI and SalI (New England BioLabs, Ipswich, MA). The resulting plasmids were then transformed into rifampin-resistant L. casei BL23 cells by electroporation (Gene Pulser Xcell; Bio-Rad, Hercules, CA) with a voltage of 1,500 V, a capacity of 25 μF, and a resistance of 400 Ω. Single-crossover mutants were selected on MRS agar containing Erm (MRSErm), validated by using PCR (Table 1), and designated strains BL580 and BL180 for the DltD and RecA gene knockouts, respectively.
TABLE 1.
Primers used for L. casei BL23 mutant construction and PCR validation
| Primer | Nucleotide sequence | Predicted product size (bp) |
|---|---|---|
| dltD forward | 5′-GTCGACAATGGGAAAAAGGC-3′ | 498 |
| dltD reverse | 5′-TGAGCTCAAATTACGCTTAACC-3′ | |
| recA forward | 5′-GTCGACAACTAGAAAAAGCCC-3′ | 556 |
| recA reverse | 5′-AGAGCTCTAACCAGCGTATTGG-3′ | |
| pRV300 forward | 5′-GATTAAGTTGGGTAACGCC-3′ | |
| dltD KO reverse | 5′-TGAACATCTGATTGACTTGG-3′ | 1,225 |
| recA KO reverse | 5′-GGAATGTTATTCAGCATTGG-3′ | 1,486 |
Mouse study.
The mouse study was performed according to the animal care and use protocol approved by the Institutional Animal Care and Use Committee at the University of California, Davis (UC Davis) (protocol number 15922). Conventionally raised, female BALB/c mice (5 weeks old) were purchased from Harlan Laboratories (Livermore, CA). The mice were group housed with 3 or 4 mice to a cage with a 12-h-light/12-h-dark cycle and initially fed a Teklad global 18% protein rodent diet (Harlan Laboratories, Livermore, CA) for 5 days.
Mice were then acclimated to a Western diet (43% kcal refined sugars and 41% kcal fat) (catalog number D12079B; Research Diets, New Brunswick, NJ) for 7 days and remained on this diet during the subsequent administration of different L. casei strains and matrix combinations. Administration was performed by allowing the mice to drink the (cell) suspensions from the tip of a gavage needle. A total of 50 μl of either milk or phosphate-buffered saline (PBS) was provided to the mice each day. This amount of milk is equivalent to a human adult intake of 185 ml per 70 kg of body weight. The mice given L. casei received ∼2 × 107 Rif-resistant L. casei BL23 wild-type or mutant cells with each feeding. Wild-type L. casei (9 mice), BL580 (9 mice), and BL180 (9 mice) were provided in milk. Prior to administration, the cells were incubated in milk for 24 h at 37°C, and the cultures were then moved to a refrigerator maintained at 4°C. The same cultures were used for each feeding and remained in the refrigerator for up to 16 days. Incubation of L. casei BL23, BL580, and BL180 in UHT milk resulted in pHs of 4.95, 4.50, and 5.20, respectively, and neither the pH values nor the viabilities of these cultures changed significantly during the course of the study. There were also no significant differences in the growth rates or levels of survival between the wild-type and mutant strains in milk (data not shown). Another group of mice received wild-type L. casei BL23 cells grown in MRS broth for 24 h at 37°C and then washed twice and suspended in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4 [pH 7.4]) immediately prior to each feeding. Other mice served as controls and received either PBS (12 mice) or acidified (pH 4.95) UHT milk (9 mice). The pH of the UHT milk was reduced with 11.3 M lactic acid (Fisher Scientific, Hampton, NH) for mice receiving milk without added L. casei. This was performed in order to permit comparisons to the L. casei milk cultures.
Four days after initiation of L. casei, PBS, or milk feeding, mice received 2 to 3% DSS salt (molecular weight of 36,000 to 50,000) (MP Biomedicals, LLC, Solon, OH) in their drinking water for 8 days, followed by 3 days of regular drinking water (autoclaved distilled water) to induce acute colitis. DSS is a saturated polysaccharide that induces acute intestinal inflammation through hyperosmotic damage to epithelial cells when provided in drinking water (38). Mouse body weights were monitored daily. Because of the severity of weight loss (15%), one control mouse administered DSS was sacrificed on the last day of DSS administration, and another two were sacrificed on the following day. Two mice fed DltD− mutant strain BL580 were also sacrificed within the first 2 days after DSS administration was stopped. The remaining mice were sacrificed 3 days after cessation of DSS treatment, the planned endpoint of the study.
During and after DSS administration, mouse stools were also monitored daily to score for stool consistency and blood. Based on the size and shape (compared to those of stools from healthy controls), stool consistency was scored from 0 (normal) to 5 (watery diarrhea). A disease activity index (DAI) was calculated by percent total weight loss (before/after DSS treatment), histology score, the presence of blood in the stools, and stool consistency. The scoring scale for each DAI component is shown in Table 2.
TABLE 2.
Criteria for disease activity index (DAI) scores
| Parameter | Value for DAI score ofa: |
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Body wt loss (%)b | 0–5 | 5.1–10 | 10.1–15 | 15.1–20 | 20.1–25 | >25 |
| Histology scorec | 0–3 | 4–6 | 7–9 | 10–12 | 13–15 | 16–18 |
| Blood in stool | No | Presence of bleeding | Gross bleeding | |||
| Diarrhea scored | <0.5 | 0.5–1.4 | 1.5–2.4 | 2.5–3.4 | 3.5–4.4 | 4.5–5 |
For mice that were sacrificed due to weight loss and sickness, DAI calculations included the last measured body weight and diarrhea and blood scores prior to sacrifice. These mice were assigned the highest histology score (18).
Calculated as (mouse body weight at time of sacrifice/body weight on day 4) × 100.
Sum of Picarella scores for proximal and distal colons. Mice that were sacrificed prior to the planned endpoint were given the highest score.
A score of <0.5 indicates normal stools. The score increased on a gradient to 5 as the amounts of fecal water increased and solids decreased.
L. casei enumeration.
Freshly expelled feces were collected from the mice every other day immediately prior to L. casei BL23 administration. The feces were homogenized in PBS as previously described (39), and serial dilutions of the fecal homogenate were plated onto MRSRif or MRSRif+Erm agar for colony enumerations.
Necropsy and histological analysis.
Mice underwent euthanasia by CO2 asphyxiation and cervical dislocation. The colon from the cecocolic junction to the rectum was removed, and any gross changes related to the surrounding tissues were documented. The colon was opened longitudinally and laid out flat (mucosa side up), and tissue samples from each mouse constituting 4 cm each of both the proximal and distal colons were then coded in a blind fashion for histological processing at the UC Davis Comparative Pathology Laboratory (http://www.vetmed.ucdavis.edu/). The collected tissue sections were fixed in a 10% formalin solution; embedded in paraffin blocks, from which two sections were made 300 μm apart; and then stained with hematoxylin and eosin. The severity of colitis was determined by using Picarella criteria (40) and is shown as the sum of the scores from the proximal and distal colons of each mouse.
Cytokine and chemokine measurements.
With the exception of the five mice sacrificed prior to the study endpoint, time of necropsy, the ileum and the mid-region from the opened colon were collected, flash-frozen in liquid N2, and then stored at −80°C. Frozen ileal and colonic tissues were homogenized in 600 μl of lysis buffer (39) and centrifuged at 15,000 × g for 15 min at 4°C to remove insoluble materials. These extracts were then applied to the Bio-Plex Pro mouse cytokine/chemokine 23-plex panel (Bio-Rad, Hercules, CA) for identification on a Magpix instrument (Bio-Rad, Hercules, CA). The target cytokines/chemokines included interleukin-1α (IL-1α), IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), keratinocyte-derived chemokine (KC), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and RANTES (regulated on activation, normal T cell expressed and secreted). The acquired results were normalized by tissue weights and then subjected to principal component analysis (PCA) with xlstat (2013; Microsoft).
Intestinal microbiota analysis.
Procedures for DNA extraction, amplification, and sequencing were described previously (41). Briefly, total genomic DNA from cecal contents was extracted by mechanical lysis and purified with the QIAamp DNA stool minikit (Qiagen, Inc., Valencia, CA). Mice used for this analysis included 12 healthy (sham) mice; 9 mice provided DSS only (controls), acidified milk, wild-type L. casei BL23 in milk or PBS, or L. casei BL180 in milk; and 7 mice fed L. casei strain BL580 in milk. Barcoded primers F515 and R806 were used for PCR amplification of the 16S rRNA gene V4 region (42). Approximately equal molar amounts of the PCR amplicons were pooled and purified with a QIAquick PCR purification kit (Qiagen, Inc., Valencia, CA). Library preparation, cluster generation, and DNA sequencing were performed according to the paired-end protocol on the Illumina MiSeq platform (Illumina, Inc., San Diego, CA) (43) at the UC Davis Genome Center (http://dnatech.genomecenter.ucdavis.edu/).
Raw FASTQ files were demultiplexed and quality filtered by using the Quantitative Insights into Microbial Ecology 1.7.0 (QIIME) software package (44). Operational taxonomic units (OTUs) sharing at least 97% nucleotide identity were clustered and assigned with an open-reference OTU picking strategy against the Greengenes database (13_5 release) (45). Beta-diversity was calculated by using the UniFrac distance between samples (46) and visualized in three-dimensional (3D) plots based on principal coordinate analysis (PCoA). The dendrogram was plotted by using the Euclidean distance calculated from the bacterial family abundance matrix in the MATLAB 7.11.0 (R2010b) environment (MathWorks, Inc., Natick, MA). Linear discriminant analysis (LDA) effect size analysis (LEfSe) was also performed (http://huttenhower.sph.harvard.edu/galaxy) to identify significantly different phylotypes between DSS-treated colitic mice and healthy controls (47).
Statistical analysis.
Statistical analyses of mouse samples were performed by using either the Mann-Whitney U test in GraphPad Prism 5 (GraphPad Software, Inc.) or the Student t test in Excel (2010; Microsoft). For gut microbiota taxonomic comparisons, nonparametric tests were performed by using SPSS 17.0 (SPSS, Inc., Chicago, IL) because not all of the taxa were normally distributed. Spearman correlations between specific taxa and animal physiological parameters (DAI and body weight loss) were calculated.
Nucleotide sequence accession number.
The DNA sequences were deposited in the Sequence Read Archive (SRA) with the accession number SRP057397.
RESULTS
Survival of L. casei in mice with DSS colitis.
Mice on a high-fat, high-sucrose, Western-style diet were fed 2 × 107 cells of wild-type L. casei BL23 in milk or PBS or the L. casei DltD− (strain BL580) or L. casei RecA− (strain BL180) mutant in milk for 15 consecutive days. From days 4 to 12, DSS was provided to the mice in their drinking water to induce colitis. Enumerations of viable L. casei cells from fecal samples confirmed that both wild-type and mutant L. casei cells survived passage through the mice prior to and during DSS administration (Fig. 1). However, the levels of viable cells recovered differed significantly between strains and delivery matrix combinations. L. casei BL23 cells fed to the animals in milk were detected in higher quantities (5.4-fold on average) than those detected with delivery in PBS, and these differences increased by the end of the study (Fig. 1). Similarly, wild-type BL23 cells fed to mice in milk were recovered in 7.8-fold-higher quantities than in mice fed DltD− mutant strain BL580 (Fig. 1). In contrast, the numbers of RecA− strain BL180 cells increased in the mouse stools over repeated feedings, reaching 20-fold-higher numbers than those of L. casei BL23 cells within 8 days after the start of the study (4 days after the initiation of DSS administration) (P = 0.023 by Student t test) (Fig. 1). After this time point, however, the numbers of culturable RecA− mutant cells declined rapidly, and cells were no longer detected in approximately half of the mice (five out of nine) at the time of sacrifice.
FIG 1.
Survival of L. casei in DSS-treated mice. Viable, rifampin-resistant L. casei cells in mouse stools were enumerated every second day of the study. The average CFU ± standard errors from fecal samples are shown for each treatment and time point (lower limit of detection, 1,000 CFU/g feces). Fecal samples were recovered from nine mice in each group, except for two BL580- and milk-fed mice on days 14 and 15 due to early termination. Because of diarrhea in some animals, stools were collected from the following numbers of mice on days 14 and 15, respectively: 7 and 6 mice fed BL23 and PBS, 6 and 8 mice fed BL23 and milk, 5 and 5 mice fed BL580 and milk, and 9 and 9 mice fed BL180 and milk.
L. casei BL23 in milk is protective against DSS colitis.
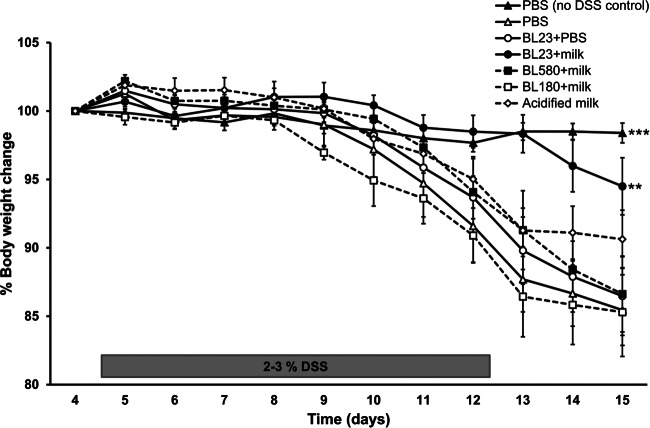
Wild-type L. casei BL23 in milk protected mice against weight loss upon DSS administration (Fig. 2). Mice receiving wild-type L. casei BL23 in milk maintained their initial body weight, with only slight (nonsignificant) reductions compared to the body weights of healthy (PBS-fed) controls throughout the course of the study. These mice also lost significantly less weight than did mice administered L. casei BL23 in PBS (P = 0.038, as determined by the Student t test) (Fig. 2). In comparison, mice fed acidified milk alone exhibited intermediate weight loss, and mice fed the dltD (BL580) or recA (BL180) gene knockout mutant in milk (at the same pH) lost as much weight as the DSS-treated controls (Fig. 2). The impact of DSS on the health of the animals was clear because the DSS-treated controls lost, on average, 15% of their total body weight within 11 days after the introduction of the intestinal irritant (P < 0.001, compared to sham-treated mice) (Fig. 2).
FIG 2.
Changes in mouse body weight during and after DSS administration. The percent change in body weight was calculated by using the weight on day 4 (the day of DSS initiation) as the reference. The averages ± standard deviations of data from 12 mice (healthy sham control and DSS treated) and 9 mice (L. casei or acidified milk fed) are shown. **, P < 0.01; ***, P < 0.001 according to the Student t test compared to the DSS control group.
DSS treatment resulted in diarrhea, whereas healthy control animals had normal stools (Fig. 3A). Among the DSS-treated groups, mice fed wild-type L. casei BL23 in milk exhibited significantly lower levels of diarrhea than the DSS controls, and their stool consistency was most similar to that of stools from healthy mice (Fig. 3A). Mice fed L. casei BL23 in PBS, milk alone, or RecA− strain BL180 had reduced levels of diarrhea but still with a higher severity than that in mice fed L. casei BL23 in milk (Fig. 3A). These differences in intestinal health were also observable in measurements of neutrophil infiltration and tissue damage. Histological examination confirmed that intestinal inflammation was significantly lower in mice fed L. casei BL23 in milk than in DSS-treated control mice (Fig. 3B; see also Fig. S1 in the supplemental material). There was no significant difference between mice administered DSS alone and mice fed milk, L. casei BL23 in PBS, or the L. casei RecA− and DltD− mutants (Fig. 3B; see also Fig. S1 in the supplemental material).
FIG 3.

L. casei in milk protects against DSS colitis. Stool consistency (diarrhea) (A), histological scores (B), and disease activity indices (C) were calculated according to the criteria shown in Table 2. A higher score means greater severity. The averages ± standard deviations of data from 12 mice (healthy sham control and DSS treated) and 9 mice (L. casei or acidified milk fed) are shown. *, P < 0.05; ***, P < 0.001 according to the Student t test compared to the DSS control group.
To obtain a global measure of mouse health upon DSS administration, a disease activity index (DAI) was calculated by incorporating data on animal weights, stool consistency, colonic bleeding, and histological scores (Table 2). Healthy sham-treated mice showed no signs of colitis (DAI of 0), and the DAI of mice administered DSS was 13 (out of 19) (Fig. 3C). The average DAI of mice fed L. casei BL23 in milk was 6, and this score was significantly lower than that of the DSS-treated controls and most similar to that of the healthy (PBS-fed) mice (Fig. 3C). Mice fed milk exhibited a modestly reduced DAI, whereas L. casei BL23 in PBS or the L. casei RecA− or DltD− mutant in milk resulted in a DAI equivalent to that of the DSS-treated control mice (Fig. 3C).
L. casei and milk reduce proinflammatory cytokine production in the colon.
Cytokine levels in colonic and ileal tissues were measured to establish the intestinal immune responses to L. casei and milk during DSS-induced colitis. Levels of 19 and 15 out of the 23 cytokines tested were within the range of detection for colon and ileal tissues, respectively (see Table S1 in the supplemental material). Principal component analysis (PCA) of the compiled cytokine quantities showed that the immune responses differed between colonic and ileal tissues (Fig. 4A). Cytokine levels also differed between healthy (PBS-fed) and DSS-treated control mice, and these differences were greater in the colon (Fig. 4B) than in the ileum (Fig. 4C). These results are consistent with the localization of inflammation to the large and not the small intestine in DSS-induced colitis in mice (48) and UC in humans (49). PCA of the compiled colonic cytokine quantities showed that approximately half of the mice administered L. casei BL23 in milk exhibited an immune response identical to that of healthy mice (PBS-fed sham controls), while the other half of the mice in this group clustered with the DSS-treated controls and mice fed milk alone (Fig. 4D). Collectively, cytokine levels in mice fed L. casei BL23 in PBS (Fig. 4E) or either of the two mutants (Fig. 4F) were indistinguishable from those in mice given DSS alone.
FIG 4.
Intestinal cytokine production differs depending on intestinal location, DSS-induced colitis, and consumption of L. casei or milk. (A to C) PCA of cytokine amounts in colonic and ileal tissues (A) and the colon (B) and ileum (C) of healthy (sham) and DSS-treated mice. (D to F) PCA comparisons of cytokine quantities in colonic tissues of healthy (sham) and DSS-treated control mice and DSS-treated mice fed milk with or without BL23 (D), L. casei BL23 in PBS (E), or the L. casei DltD− (BL580) or L. casei RecA− (BL180) strain in milk (F). The numbers of mice and cytokines used for this analysis are listed in Table S1 in the supplemental material.
DSS-induced colitis is associated with increased levels of proinflammatory cytokines, including IL-6, IL-17, and KC (50). Consistent with the levels of colitis observed in this study, the levels of these cytokines were also increased in the colons of DSS-treated mice (Fig. 5). In general, the levels of these cytokines were reduced in mice fed L. casei or milk; however, the extent to which the quantities were reduced differed among the treatment groups. The levels of IL-6 and KC were significantly lower in all mice that received L. casei, except for mice fed BL180 (RecA−) (Fig. 5B and D). Quantities of IL-1α, G-CSF, and MCP-1 were also reduced in mice fed L. casei BL23 in PBS (Fig. 5A, E, and F), and the IL-17 level was lower in mice fed the L. casei dltD mutant (Fig. 5C) than in the DSS-treated controls. Remarkably, even though the mice fed L. casei BL23 in milk exhibited the lowest DAI, their colonic tissues did not contain the lowest levels of proinflammatory cytokines, on average, compared to the other L. casei-fed animals (Fig. 5). Examination of individual mice fed L. casei BL23 in milk revealed that the colons of four of the nine mice contained significantly reduced quantities of all six cytokines (IL-1α, IL-6, IL-17, KC, G-CSF, and MCP-1) (see Fig. S2 in the supplemental material). These were the same mice that grouped together with the PBS-fed healthy controls according to PCA of the total immune profiles (Fig. 4D).
FIG 5.
L. casei regulates cytokine production in colon during DSS-induced colitis. The quantities of IL-1α (A), IL-6 (B), IL-17 (C), KC (D), G-CSF (E), and MCP-1 (F) in colonic tissue were measured. Shown are averages ± standard deviations of data from 12 healthy sham-treated mice; 9 mice treated with DSS alone (control), acidified milk, L. casei BL23 in milk, L. casei in PBS, or L. casei BL180 in milk; and 7 mice fed L. casei BL580 in milk. *, P < 0.05; **, P < 0.01; ***, P < 0.001 according to the Mann-Whitney U test compared to the DSS-treated control group.
DSS and L. casei enrich for specific bacteria in the intestine.
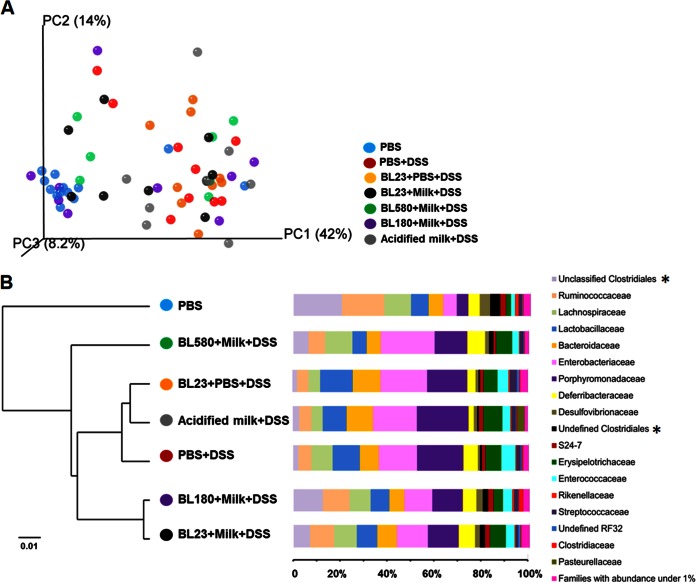
Bacterial diversity in the mouse cecal contents at the time of necropsy was assessed. An average of 64,191 high-quality 16S rRNA gene (V4 region) reads were obtained for each mouse (DNA sequence length of 221.97 ± 1.24 bp), with an average of 405 operational taxonomic units (OTUs) (97% identity) per animal. Both principal coordinate analysis (PCoA) and clustering analysis of UniFrac distances indicated that the cecal microbiota structure of mice consuming DSS was significantly different from that of healthy (PBS-fed, sham-treated) mice (Fig. 6A; see also Fig. S3 in the supplemental material). The ceca of DSS-treated mice were populated by significantly higher proportions of Parabacteroides, Enterococcus, Sutterella, and Anaerotruncus (see Fig. S3D in the supplemental material). PBS-fed, sham-treated mice contained higher proportions of Oscillospira, Bilophila, Coprococcus, Desulfovibrio, Lactococcus, Ruminococcus, Dorea, Odoribacter, Bifidobacterium, Comamonas, and Clostridium (see Fig. S3D in the supplemental material). Proportions of these and other taxa from DSS-treated and healthy mice were significantly correlated with changes in body weight and DAI (see Table S2 in the supplemental material).
FIG 6.
Mouse cecal microbiota is altered by DSS, milk, and L. casei. (A) Weighted UniFrac PCoA of the cecal microbiota structure. (B) Taxonomy analysis at the family level. In panel B, the dendrogram on the left shows the Euclidean distance calculated from the bacterial family abundance in each treatment group. The bars on the right indicate the relative abundances of different families with representation of at least 1% in the total DNA sequence reads examined. Asterisks indicate “ unclassified” DNA sequences that are similar to more than one sequence from the reference database and “undefined” sequences that are similar to an unnamed reference sequence.
The intestinal microbiota of DSS-treated mice fed L. casei or milk resembled those of the other mice fed DSS but with significant differences in the proportions of certain bacterial families (Fig. 6B). Remarkably, Euclidian distances between bacterial taxa showed that mice that had ingested the DltD− mutant (BL580) harbored a cecal microbiota that was different from those of all other mice. These mice were distinguished by their significantly reduced proportions of Clostridiaceae (P = 0.04). In comparison, mice fed milk contained a bacterial community composition similar to that of mice fed L. casei BL23 in PBS or DSS alone (Fig. 6B). Mice given wild-type BL23 in milk and the RecA− mutant (BL180) shared similar cecal microbial compositions (Fig. 6B). One distinction, however, was that mice fed L. casei BL23 in milk contained increased proportions of Bifidobacteriaceae (P = 0.049) compared to those in all other DSS-treated mice. Comamonadaceae were also significantly enriched in mice fed L. casei BL23 when consumed in either milk (P = 0.007) or PBS (P = 0.028).
DISCUSSION
We showed in a preclinical (rodent) model of IBD that the delivery matrix can have a significant effect on probiotic efficacy. Mice fed L. casei BL23 in milk but not a nutrient-free buffer maintained their normal weights throughout the course of 8 days of DSS administration, exhibited significantly less diarrhea and rectal bleeding, and suffered from fewer necrotic lesions, resulting in a lower DAI. These variables are also important clinical features of IBD patients (49). Our results therefore strongly indicate that dairy products might be the preferred delivery matrix for at least certain probiotic strains for benefiting human health.
L. casei BL23 fed to mice in PBS was not effective in attenuating DSS-induced colitis. This result differs from results of previous studies with this strain (26, 28, 51). The opposing findings might be the result of our use of oral rather than intragastric feeding, female rather than male mice, or a high-fat/high-sucrose mouse diet rather than the low-fat/plant polysaccharide-rich chow diets applied previously. Notably, we recently showed that host diet can alter the extent to which probiotic Lactobacillus attenuates intestinal inflammation (39). Therefore, dietary differences might have been a major factor influencing the outcomes of L. casei protection against DSS-induced colitis in these different studies. Because mouse studies typically rely on feeding the probiotic cultures in pH-neutral, nutrient-free buffer, as we provided in PBS here (2, 27, 52), the outcomes of such studies should be viewed with caution when the probiotic is intended for use in food or nutrient-containing supplement matrices in humans.
Milk also resulted in an attenuation of DSS-induced colitis, although the reduction was modest compared to that with the addition of L. casei BL23. Anti-inflammatory effects and improvements in IBD symptoms in humans have been reported for fermented milks as well as milk components (53, 54). In rodent models, a cheese-containing diet attenuated DSS-induced colitis in mice through a mechanism mediated by decreased production of proinflammatory cytokines (IL-17 and IL-6) and increased production of the anti-inflammatory cytokine transforming growth factor β1 (55). Cheese whey protein also decreased the clinical symptoms of diarrhea and fecal bleeding in DSS-treated rats (56). Because we acidified milk from pH 6.62 to pH 4.95 with lactic acid to enable comparisons to the L. casei-containing milks, it is possible that the benefits of milk were possibly due to the addition of the organic acid. Additional studies are needed to identify the specific components of fluid milk that mitigate the effects of DSS.
To determine the possible synergistic interactions between milk and L. casei in the DSS model, mice were also fed an L. casei dltD (BL580) or recA (BL180) gene knockout mutant in the presence of milk. Neither strain was protective, even in the presence of milk, and instead appeared to counteract any of the benefits of consuming the fermented milk alone. dltD is a member of the dltABCD operon required for d-alanylation of cell wall-associated LTA of Gram-positive bacteria (57, 58). In other lactobacilli, mutants deficient in LTA d-alanylation and LTA biosynthetic pathways were more protective against chemically induced murine colitis than the wild-type strains (30, 31, 52). Because the opposite result was found here, it is possible that L. casei DltD gene knockout mutant strain BL580 is altered for cellular functions other than LTA d-alanylation. However, this result is unlikely because the DltD gene is the last gene in the dlt operon, and the gene located downstream has the opposite orientation. Strain BL580 also exhibited the same phenotypes in vitro as those found for a DltD− mutant of Lactobacillus rhamnosus GG, including a decreased growth rate in MRS broth, altered cell morphology, increased cell length, and altered resistance to anionic detergents (59) (data not shown). However, LTA compositions can vary between Lactobacillus species and strains in ways that result in different immunomodulatory effects (32). The lack of benefit of the DltD− mutant in this study might have been due to the beneficial immunoreactivity of L. casei BL23 LTA. Alternatively, this difference might be the result of interactions of the L. casei DltD− mutant with the milk matrix or Western diet-induced changes to the intestinal environment and microbiota that differed from those in previous studies.
The RecA− mutant was also similarly ineffective against DSS-induced colitis. One possibility for the impairment in the L. casei RecA− mutant efficacy is that DSS results in the production of ROS and increased oxidative stress in the intestine (60). Because RecA is important for DNA damage repair under conditions of oxidative stress in lactic acid bacteria and other species (33, 34), it is likely that the L. casei RecA− strain was more susceptible to killing in the presence of increased intestinal ROS concentrations. In this regard, L. casei BL23 mutants overexpressing heterologous catalase and superoxide dismutase enzymes involved in detoxifying ROS were more efficacious than wild-type L. casei in DSS-treated mice (26, 28, 51).
Reductions in L. casei RecA− cell viability coincided with significant declines in mouse body weights and ultimately a lack of effect against DSS-induced colitis. Similarly, in mice, the numbers of viable DltD− mutant cells as well as wild-type L. casei BL23 cells in PBS were also reduced compared to those of L. casei BL23 cells consumed in milk. Overall, the numbers of viable L. casei cells in mouse stools were inversely correlated with weight loss after the onset of DSS administration (R2 = 0.33) (see Fig. S4 in the supplemental material). Mice for which at least 106 viable L. casei cells were detected per g feces also retained at least 95% of their body weights. Hence, delivery matrices should ultimately be designed to retain the highest levels of probiotic Lactobacillus cell viability in vivo.
The localization of changes in immune responses by DSS to the colon as opposed to the small intestine is in agreement with the definition of UC as a relapsing nontransmural inflammatory disease that is restricted to the colon (49). The increased inflammatory status of the colon was indicated by the significantly larger quantities of IL-1α, IL-6, IL-17, KC, G-CSF, and MCP-1 in the DSS-treated mice. Overall, the colons of mice given L. casei contained reduced quantities of those cytokines, even though these reductions were not always significant. Although cytokine amounts were not at levels detected in healthy mice, the results show the significant potential of L. casei to influence immune function. These findings are in agreement with the previously reported anti-inflammatory properties of other L. casei strains (19, 61–63).
The findings here indicate that immune responses in whole tissues do not necessarily reflect disease pathology. For example, the levels of the majority of the proinflammatory cytokines were significantly reduced in the colons of mice fed wild-type BL23 in PBS or the BL580 (DltD−) mutant; however, neither of these strains conferred protection against the inflammatory agent DSS. In comparison, the colonic tissues of mice administered wild-type L. casei BL23 in milk showed, on average, significantly lower levels of IL-6 and KC but nonsignificant reductions in the levels of the other cytokines tested. Because approximately half of these mice expressed all proinflammatory cytokines at levels equivalent to those in healthy animals, future studies should examine specific immune cell types upon feeding wild-type and mutant strains in milk during acute, chronic, and recovery phases in the DSS mouse model. Such an approach might resolve the variations in immune responses over time, rather the relying on single measurements at the time of sacrifice.
Gut microbiota dysbiosis is commonly found in IBD patients, and aberrant microbial interactions with the immune system are suggested to be a possible cause of IBD (64, 65). Microbial dysbiosis in IBD is usually characterized by global reductions in bacterial diversity and changes in the abundances of certain species, including higher proportions of aerotolerant bacteria such as Enterococcaceae and Enterobacteriaceae species (66–68). Notably, a similar change in the intestinal microbiota was found in mice fed normal (low-fat, polysaccharide-enriched) chow (69–71).
Previously, we showed that consumption of milk or milk with L. casei BL23 alters the proportions of certain bacterial species in the ceca of healthy mice (41). Consistent with our findings for healthy mice, ingestion of L. casei BL23, with and without milk, resulted in increased proportions of Comamonadaceae. This result is significant because it indicates the presence of cooperative interactions between L. casei and the Comamonadaceae family, potentially through microbial cross-feeding or other, currently unknown, microbe-microbe interactions. Aside from this similarity, however, there was a general lack of correspondence between the indigenous intestinal microbiota structure and the health of the mice after L. casei or milk administration. Hence, these results strongly indicate that the effects of L. casei against intestinal inflammation were most likely the result of direct interactions of this strain or its cell products with the host epithelium rather than global alterations of the indigenous intestinal microbiota.
The concise mechanisms by which probiotic bacteria benefit human health are now starting to be understood (72, 73). Host-probiotic interactions appear to be guided largely by specific cell surface and secreted compounds produced by probiotic cells (74–77). Although our findings here should be validated in human studies, an understanding of the conditions of food products and the digestive tract that influence the expression of these probiotic effectors is pivotal to maximizing the health-altering outcomes of probiotic consumption. Carrier matrices for probiotic bacteria should therefore be carefully selected and optimized to ensure the highest levels of probiotic survival and effector production in the digestive tract.
Supplementary Material
ACKNOWLEDGMENTS
We thank Vicente Monedero, IATA-CSIC, Spain, for providing L. casei strain BL23. We also appreciate Jose zaragoza, Carolyn Smith, and Saleda Braggs for their technical assistance with the mouse study.
Funds for this study were provided by the National Dairy Council (Rosemont, IL) and administered by the Dairy Research Institute through the California Dairy Research Foundation (Davis, CA).
We report that we have no conflicts of interest or biases related to the findings of the work presented here.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01360-15.
REFERENCES
- 1.Sanders ME, Klaenhammer TR, Ouwehand AC, Pot B, Johansen E, Heimbach JT, Marco ML, Tennila J, Ross RP, Franz C, Page N, Pridmore RD, Leyer G, Salminen S, Charbonneau D, Call E, Lenoir-Wijnkoop I. 2014. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann N Y Acad Sci 1309:1–18. doi: 10.1111/nyas.12363. [DOI] [PubMed] [Google Scholar]
- 2.Sanders ME, Marco ML. 2010. Food formats for effective delivery of probiotics. Annu Rev Food Sci Technol 1:65–85. doi: 10.1146/annurev.food.080708.100743. [DOI] [PubMed] [Google Scholar]
- 3.Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM. 2005. Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol 16:204–211. doi: 10.1016/j.copbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EMM, Sartor RB, Sherman PM, Mayer EA. 2013. An update on the use and investigation of probiotics in health and disease. Gut 62:787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 6.Floch MH. 2014. Recommendations for probiotic use in humans—a 2014 update. Pharmaceuticals (Basel) 7:999–1007. doi: 10.3390/ph7100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. 2014. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol 7:473–487. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB. 2002. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med 33:311–322. doi: 10.1016/S0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 9.Rezaie A, Parker RD, Abdollahi M. 2007. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci 52:2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaser A, zeissig S, Blumberg RS. 2010. Inflammatory bowel disease. Annu Rev Immunol 28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer BJ, Dieleman LA. 2011. Probiotics in the treatment of human inflammatory bowel diseases. Update 2011. J Clin Gastroenterol 45:S139–S144. [DOI] [PubMed] [Google Scholar]
- 12.Veerappan GR, Betteridge J, Young PE. 2012. Probiotics for the treatment of inflammatory bowel disease. Curr Gastroenterol Rep 14:324–333. doi: 10.1007/s11894-012-0265-5. [DOI] [PubMed] [Google Scholar]
- 13.Whelan K, Quigley EM. 2013. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol 29:184–189. doi: 10.1097/MOG.0b013e32835d7bba. [DOI] [PubMed] [Google Scholar]
- 14.Shen J, zuo zX, Mao AP. 2014. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn's disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis 20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 15.Pedone CA, Bernabeu AO, Postaire ER, Bouley CF, Reinert P. 1999. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int J Clin Pract 53:179–184. [PubMed] [Google Scholar]
- 16.Pedone CA, Arnaud CC, Postaire ER, Bouley CF, Reinert P. 2000. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int J Clin Pract 54:568–571. [PubMed] [Google Scholar]
- 17.Mazlyn MM, Nagarajah LHL, Fatimah A, Norimah AK, Goh KL. 2013. Effects of a probiotic fermented milk on functional constipation: a randomized, double-blind, placebo-controlled study. J Gastroenterol Hepatol 28:1584–1584. doi: 10.1111/jgh.12353. [DOI] [PubMed] [Google Scholar]
- 18.Aoki T, Asahara T, Matsumoto K, Takada T, Chonan O, Nakamori K, Nonaka C, Yamaji I, Hisamoto T, Sato M, Matsuda T, Nomoto K. 2014. Effects of the continuous intake of a milk drink containing Lactobacillus casei strain Shirota on abdominal symptoms, fecal microbiota, and metabolites in gastrectomized subjects. Scand J Gastroenterol 49:552–563. doi: 10.3109/00365521.2013.848469. [DOI] [PubMed] [Google Scholar]
- 19.Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espin-Basany E, Guarner F, Malagelada JR. 2009. Lactobacillus casei downregulates commensals' inflammatory signals in Crohn's disease mucosa. Inflamm Bowel Dis 15:275–283. doi: 10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- 20.Peluso I, Fina D, Caruso R, Stolfi C, Caprioli F, Fantini MC, Caspani G, Grossi E, Di Iorio L, Paone FM, Pallone F, Monteleone G. 2007. Lactobacillus paracasei subsp. paracasei B21060 suppresses human T-cell proliferation. Infect Immun 75:1730–1737. doi: 10.1128/IAI.01172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingrassia I, Leplingard A, Darfeuille-Michaud A. 2005. Lactobacillus casei DN-114 001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn's disease patients to adhere to and to invade intestinal epithelial cells. Appl Environ Microbiol 71:2880–2887. doi: 10.1128/AEM.71.6.2880-2887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, zarzuelo A, Galvez J. 2007. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol 103:836–844. doi: 10.1111/j.1365-2672.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 23.Chung YW, Choi JH, Oh TY, Eun CS, Han DS. 2008. Lactobacillus casei prevents the development of dextran sulphate sodium-induced colitis in Toll-like receptor 4 mutant mice. Clin Exp Immunol 151:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herias MV, Koninkx JFJG, Vos JG, Huis in't Veld JHJ, van Dijk JE. 2005. Probiotic effects of Lactobacillus casei on DSS-induced ulcerative colitis in mice. Int J Food Microbiol 103:143–155. doi: 10.1016/j.ijfoodmicro.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Kokesova A, Frolova L, Kverka M, Sokol D, Rossmann P, Bartova J, Tlaskalova-Hogenova H. 2006. Oral administration of probiotic bacteria (E. coli Nissle, E. coli O83, Lactobacillus casei) influences the severity of dextran sodium sulfate-induced colitis in BALB/c mice. Folia Microbiol (Praha) 51:478–484. doi: 10.1007/BF02931595. [DOI] [PubMed] [Google Scholar]
- 26.Rochat T, Bermudez-Humaran L, Gratadoux JJ, Fourage C, Hoebler C, Corthier G, Langella P. 2007. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb Cell Fact 6:22. doi: 10.1186/1475-2859-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Schillde MA, Hormannsperger G, Weiher M, Alpert CA, Hahne H, Bauerl C, van Huynegem K, Steidler L, Hrncir T, Perez-Martinez G, Kuster B, Haller D. 2012. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 11:387–396. doi: 10.1016/j.chom.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Watterlot L, Rochat T, Sokol H, Cherbuy C, Bouloufa I, Lefevre F, Gratadoux JJ, Honvo-Hueto E, Chilmonczyk S, Blugeon S, Corthier G, Langella P, Bermudez-Humaran LG. 2010. Intragastric administration of a superoxide dismutase-producing recombinant Lactobacillus casei BL23 strain attenuates DSS colitis in mice. Int J Food Microbiol 144:35–41. doi: 10.1016/j.ijfoodmicro.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Douillard FP, Kant R, Ritari J, Paulin L, Palva A, de Vos WM. 2013. Comparative genome analysis of Lactobacillus casei strains isolated from Actimel and Yakult products reveals marked similarities and points to a common origin. Microb Biotechnol 6:576–587. doi: 10.1111/1751-7915.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claes IJ, Lebeer S, Shen C, Verhoeven TL, Dilissen E, De Hertogh G, Bullens DM, Ceuppens JL, Van Assche G, Vermeire S, Rutgeerts P, Vanderleyden J, De Keersmaecker SC. 2010. Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin Exp Immunol 162:306–314. doi: 10.1111/j.1365-2249.2010.04228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, Mercenier A. 2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A 102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebeer S, Claes IJJ, Vanderleyden J. 2012. Anti-inflammatory potential of probiotics: lipoteichoic acid makes a difference. Trends Microbiol 20:5–10. doi: 10.1016/j.tim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 33.van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E. 2002. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187–216. doi: 10.1023/A:1020631532202. [DOI] [PubMed] [Google Scholar]
- 34.Duwat P, Ehrlich SD, Gruss A. 1995. The recA gene of Lactococcus lactis: characterization and involvement in oxidative and thermal stress. Mol Microbiol 17:1121–1131. doi: 10.1111/j.1365-2958.1995.mmi_17061121.x. [DOI] [PubMed] [Google Scholar]
- 35.Erill I, Campoy S, Barbe J. 2007. Aeons of distress: an evolutionary perspective on the bacterial SOS response. FEMS Microbiol Rev 31:637–656. doi: 10.1111/j.1574-6976.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Maniatis T, Fritsch EF. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 37.Leloup L, Ehrlich SD, zagorec M, Morel-Deville F. 1997. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl Environ Microbiol 63:2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizoguchi A. 2012. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci 105:263–320. doi: 10.1016/B978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
- 39.Tachon S, Lee B, Marco ML. 2014. Diet alters probiotic Lactobacillus persistence and function in the intestine. Environ Microbiol 16:2915–2926. doi: 10.1111/1462-2920.12297. [DOI] [PubMed] [Google Scholar]
- 40.Picarella D, Hurlbut P, Rottman J, Shi XJ, Butcher E, Ringler DJ. 1997. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RB(high) CD4+ T cells. J Immunol 158:2099–2106. [PubMed] [Google Scholar]
- 41.Yin X, Yan Y, Kim EB, Lee B, Marco ML. 2014. Short communication: effect of milk and milk containing Lactobacillus casei on the intestinal microbiota of mice. J Dairy Sci 97:2049–2055. doi: 10.3168/jds.2013-7477. [DOI] [PubMed] [Google Scholar]
- 42.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bokulich NA, Joseph CML, Allen G, Benson AK, Mills DA. 2012. Next-generation sequencing reveals significant bacterial diversity of botrytized wine. PLoS One 7:e36357. doi: 10.1371/journal.pone.0036357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeSantis Tz, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. 2014. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104:Unit 15.25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumgart DC, Sandborn WJ. 2007. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 50.Alex P, zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li XH. 2009. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol 13:236–243. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohamadzadeh M, Pfeiler EA, Brown JB, zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC, Ansari MJ, O'Flaherty S, Barrett T, Klaenhammer TR. 2011. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A 108(Suppl 1):4623–4630. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russ A, Barnett M, McNabb W, Anderson R, Reynolds G, Roy N. 2010. Post-weaning effects of milk and milk components on the intestinal mucosa in inflammation. Mutat Res 690:64–70. doi: 10.1016/j.mrfmmm.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sandler RS, Lewis JD. 2013. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci 58:1322–1328. doi: 10.1007/s10620-012-2373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosoya T, Ogawa A, Sakai F, Kadooka Y. 2012. A cheese-containing diet modulates immune responses and alleviates dextran sodium sulfate-induced colitis in mice. J Dairy Sci 95:2810–2818. doi: 10.3168/jds.2011-4763. [DOI] [PubMed] [Google Scholar]
- 56.Sprong RC, Schonewille AJ, van der Meer R. 2010. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: role of mucin and microbiota. J Dairy Sci 93:1364–1371. doi: 10.3168/jds.2009-2397. [DOI] [PubMed] [Google Scholar]
- 57.Debabov DV, Kiriukhin MY, Neuhaus FC. 2000. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J Bacteriol 182:2855–2864. doi: 10.1128/JB.182.10.2855-2864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palumbo E, Deghorain M, Cocconcelli PS, Kleerebezem M, Geyer A, Hartung T, Morath S, Hols P. 2006. d-Alanyl ester depletion of teichoic acids in Lactobacillus plantarum results in a major modification of lipoteichoic acid composition and cell wall perforations at the septum mediated by the Acm2 autolysin. J Bacteriol 188:3709–3715. doi: 10.1128/JB.188.10.3709-3715.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perea Velez M, Verhoeven TL, Draing C, Von Aulock S, Pfitzenmaier M, Geyer A, Lambrichts I, Grangette C, Pot B, Vanderleyden J, De Keersmaecker SC. 2007. Functional analysis of d-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 73:3595–3604. doi: 10.1128/AEM.02083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tun X, Yasukawa K, Yamada K. 2014. Involvement of nitric oxide with activation of Toll-like receptor 4 signaling in mice with dextran sodium sulfate-induced colitis. Free Radic Biol Med 74:108–117. doi: 10.1016/j.freeradbiomed.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, Shimizu T, Yamashiro Y, Kiyoshima-Shibata J, Nanno M, Nomoto K. 2010. Well-controlled proinflammatory cytokine responses of Peyer's patch cells to probiotic Lactobacillus casei. Immunology 130:352–362. doi: 10.1111/j.1365-2567.2009.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noto Llana M, Sarnacki SH, Aya Castaneda MR, Bernal MI, Giacomodonato MN, Cerquetti MC. 2013. Consumption of Lactobacillus casei fermented milk prevents Salmonella reactive arthritis by modulating IL-23/IL-17 expression. PLoS One 8:e82588. doi: 10.1371/journal.pone.0082588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schiffer C, Lalanne AI, Cassard L, Mancardi DA, Malbec O, Bruhns P, Dif F, Daeron M. 2011. A strain of Lactobacillus casei inhibits the effector phase of immune inflammation. J Immunol 187:2646–2655. doi: 10.4049/jimmunol.1002415. [DOI] [PubMed] [Google Scholar]
- 64.Kostic AD, Xavier RJ, Gevers D. 2014. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manichanh C, Borruel N, Casellas F, Guarner F. 2012. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 66.Rigottier-Gois L. 2013. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J 7:1256–1261. doi: 10.1038/ismej.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fava F, Danese S. 2011. Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol 17:557–566. doi: 10.3748/wjg.v17.i5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, Gonzalez A, McDonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ. 2014. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe 15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berry D, Schwab C, Milinovich G, Reichert J, Mahfoudh KB, Decker T, Engel M, Hai B, Hainzl E, Heider S. 2012. Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J 6:2091–2106. doi: 10.1038/ismej.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Fazio L, Cavazza E, Spisni E, Strillacci A, Centanni M, Candela M, Pratico C, Campieri M, Ricci C, Valerii MC. 2014. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J Gastroenterol 20:2051–2061. doi: 10.3748/wjg.v20.i8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagalingam NA, Kao JY, Young VB. 2011. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis 17:917–926. doi: 10.1002/ibd.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bron PA, van Baarlen P, Kleerebezem M. 2011. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10:66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 73.Hemarajata P, Versalovic J. 2013. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sengupta R, Altermann E, Anderson RC, McNabb WC, Moughan PJ, Roy NC. 2013. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediators Inflamm 2013:237921. doi: 10.1155/2013/237921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez B, Urdaci MC, Margolles A. 2010. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa-bacteria interactions. Microbiology 156:3232–3242. doi: 10.1099/mic.0.044057-0. [DOI] [PubMed] [Google Scholar]
- 76.Marco ML, Tachon S. 2013. Environmental factors influencing the efficacy of probiotic bacteria. Curr Opin Biotechnol 24:207–213. doi: 10.1016/j.copbio.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.