Abstract
Bacterial endosymbionts have been identified as potentially useful biological control agents for a range of invertebrate vectors of disease. Previous studies of Culicoides (Diptera: Ceratopogonidae) species using conventional PCR assays have provided evidence of Wolbachia (1/33) and Cardinium (8/33) infections. Here, we screened 20 species of Culicoides for Wolbachia and Cardinium, utilizing a combination of conventional PCR and more sensitive quantitative PCR (qPCR) assays. Low levels of Cardinium DNA were detected in females of all but one of the Culicoides species screened, and low levels of Wolbachia were detected in females of 9 of the 20 Culicoides species. Sequence analysis based on partial 16S rRNA gene and gyrB sequences identified “Candidatus Cardinium hertigii” from group C, which has previously been identified in Culicoides from Japan, Israel, and the United Kingdom. Wolbachia strains detected in this study showed 98 to 99% sequence identity to Wolbachia previously detected from Culicoides based on the 16S rRNA gene, whereas a strain with a novel wsp sequence was identified in Culicoides narrabeenensis. Cardinium isolates grouped to geographical regions independent of the host Culicoides species, suggesting possible geographical barriers to Cardinium movement. Screening also identified Asaia bacteria in Culicoides. These findings point to a diversity of low-level endosymbiont infections in Culicoides, providing candidates for further characterization and highlighting the widespread occurrence of these endosymbionts in this insect group.
INTRODUCTION
Culicoides species are small hematophagous insects (Diptera: Ceratopogonidae), some of which are important vectors of viral and parasitic diseases of veterinary and medical importance (1). More than 50 different viruses have been isolated from Culicoides species, including bluetongue virus (BTV), Schmallenberg virus (SBV), and African horse sickness virus (AHSV), which cause significant impacts on livestock production through stock losses and trade restrictions (1). Capable of wind-borne displacement for several hundred kilometers, Culicoides spp. have a capacity for rapid long-distance transmission of disease and have recently been responsible for the establishment of enzootic BTV and SBV infections over vast new geographic areas (1, 2). Current control methods for Culicoides include breeding site removal and baiting of livestock and midge resting sites; however, these techniques are costly and labor-intensive and have various levels of success and permanence of control (3). Although vaccines are available for some Culicoides-transmitted viruses, such as BTV, the practicality of vaccination is limited by the large number of BTV serotypes and the potential for genome segment reassortment between live vaccines and naturally circulating virus strains (4). Inactivated vaccines are expensive and less potent but have been used effectively in Europe for control of BTV (5). Inactivated vaccines are not currently available for AHSV.
Insect vector control by use of endosymbiotic organisms has gained increasing attention in recent years (6). Bacterial endosymbionts present in arthropod species are capable of influencing host characteristics such as longevity and vector competence, as well as being involved in nutrient provisioning (7–9). The endosymbiont Wolbachia pipientis (Alphaproteobacteria) has attracted notable attention for its applicability to endosymbiont-based control of the dengue virus vector, Aedes aegypti. Wolbachia has been shown to successfully invade and be maintained in natural A. aegypti populations and to block virus transmission (8, 10–12).
Several previous studies have reported evidence of bacterial endosymbiont infection in Culicoides species. Screening studies conducted using conventional PCR assays detected Wolbachia DNA in a single Culicoides paraflavescens individual in Japan (13). “Candidatus Cardinium hertigii” (Bacteroidetes), another bacterial endosymbiont which also has a range of influences on its host insect, has been detected in four Culicoides species in Japan, two in Israel, and two in the United Kingdom (13–15). Endosymbiotic diversity in Australian Culicoides species has not been investigated previously, nor has a comparative analysis of Cardinium divergence in different Culicoides species from diverse geographical locations been reported.
Although conventional PCR has previously been used successfully to screen arthropods for endosymbionts (16–18), recent studies have demonstrated that this method can fail to detect low-level infections. More sensitive screening techniques, such as long PCR (19), nested PCR (20), or quantitative PCR (qPCR) (21), are therefore required. Low-level endosymbiont infections have been identified in a range of insects, including tsetse flies (22), Drosophila (23), cherry fruit flies (24) and planthoppers (25). Previous studies have suggested that at low levels of infection, endosymbionts are capable of influencing the host. For example, low levels of Wolbachia in Drosophila paulistorum semispecies have been shown to influence fecundity, sex ratios, and mate discrimination (23). However, other endosymbiont effects, including viral blockage and fitness effects, may depend on bacterial density (26).
In this study, a range of Culicoides species, collected predominately from southeastern Australia, were screened for evidence of Cardinium and Wolbachia infection. Global movement of Cardinium in Culicoides species was also investigated based on sequence divergence in multiple loci. Novel Cardinium and Wolbachia infections were identified in a range of Culicoides species, a high proportion of which were low-level infections. Nucleotide sequence analysis revealed that Cardinium detected in these samples was genetically similar to those previously discovered in Japan, Israel, and the United Kingdom, suggesting a global presence of a single Cardinium strain throughout a wide geographical range and in a range of Culicoides species.
MATERIALS AND METHODS
Insect collection.
Culicoides insects were collected using either Centre for Disease Control (CDC) mini-light traps (BioQuip Products, Rancho Dominguez, CA) or yellow sticky traps (YST). Mini-light traps contained either green or UV light-emitting diodes (LEDs) and were powered by a 6-V motorbike battery (27, 28). Traps were positioned approximately 2 m above the ground at dusk and collected after dawn, consistent with previous trapping methodologies (29). A downdraught fan in the traps directed insects into a beaker containing approximately 200 ml of 80% ethanol. Contents of the beakers were collected daily. Before storage at −20°C, insects were sorted, identified, and stored individually in fresh 80% ethanol. By washing insects in ethanol, the risk of contamination was reduced. YST (Trappit) were elevated approximately 10 cm above the ground by a plastic stick and illuminated by a solar garden light. Insects were individually cut from the YST and placed in a tube containing enough solvent (De-Solv-it [orange oil]) (Orange-Sol, Chandler, AZ) to cover the sample. Tubes were incubated at 60°C for 5 min with intermittent inversion until the insect floated off the YST. Insects were removed and washed twice with 80% ethanol before being stored in fresh 80% ethanol at −20°C (I. Valenzuela personal communication). Collections were made from 305 traps (81 CDC traps and 224 YST) over a period of 33 trapping nights throughout southeastern Australia from January to April in 2013 and 2014. Samples of Culicoides imicola collected using CDC light traps were also obtained from Kenya and Madagascar through the International Livestock Research Institute, Kenya. C. imicola were identified morphologically based on wing patterning and through sequence analysis of the cytochrome oxidase subunit 1 (COI) region.
Culicoides identification.
Morphological identification was performed with the aid of the pictorial atlas of Australasian Culicoides wings (Diptera: Ceratopogonidae) (30). The C. victoriae species group was separated into a series of species denoted by wing identifier number in square brackets, such as C. victoriae [241], based on the wing pictures from reference 30. One C. victoriae species not present in the pictorial atlas was collected in Geelong, Victoria, Australia, and is denoted C. victoriae [172] (GenBank accession number KT338816). Genetic confirmation of Culicoides species was based on amplification and sequencing of the COI gene, which is commonly used for species determination of Culicoides and is capable of differentiating most Culicoides species (31, 32).
DNA extractions.
Culicoides insects were removed from ethanol and air dried before extraction (33). The mouse tail protocol of the MagMax-96 DNA multisample kit (Applied Biosystems, Austin, TX) on a KingFisher Flex instrument (Thermo Scientific, Waltham, MA) was used with the following amendments. Individual Culicoides insects were incubated in 100 μl of proteinase K (PK) buffer and 10 mg/ml of proteinase K. Samples were homogenized with a FastPrep-24 instrument (MP Biomedicals, Santa Ana, CA) at 6.0 m/s for 20 s with approximately 10- by 1.0-mm zirconia-silica beads (BioSpec Products, Bartlesville, OK) before overnight incubation at 56°C. Elution was altered to 40 μl of both elution buffers 1 and 2. To assess possible contamination during extraction, every 11th well in the MagMax 96-well plate was left without a Culicoides sample but was still included in all downstream screening steps.
Conventional PCR screening.
The primers (GeneWorks, SA, Australia) utilized included those for an initial housekeeper targeting the third loop of the 28S rRNA gene (D3a and D3ba), followed by the Cardinium 16S rRNA gene (CAR-SP-F and CAR-SP-R) (13), the Alphaproteobacteria 16S rRNA gene (Alf28F and Alf684R) (34, 35), the Wolbachia 16S rRNA gene (Wol-F and Wol-R) (36), and the cytochrome oxidase subunit 1 (COI) gene (BC1culicFm and JerR2m) (32) for species confirmation. Primer sequences can be found in Table 1. PCRs with 25-μl reaction mixtures were performed using 1 unit Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA), following the manufacturer's protocol. PCR conditions were as follows: 1 cycle at 95°C for 2 min; 35 cycles of 95°C for 30 s, the annealing temperature indicated in Table 1 for 30 s, and 72°C for 30 s; and a final extension at 72°C for 5 min. Two negative controls with ultrapure water were included for all PCR screens to ensure that no contamination occurred. A single positive control was included for each PCR; DNA from C. victoriae [240] was used for 28S rRNA and COI PCRs, and DNA from Drosophila simulans was used for Wolbachia 16S rRNA and Alphaproteobacteria 16S rRNA PCRs. At the commencement of screening, no positive control was available for Cardinium; in later steps, C. victoriae [240] samples shown to be Cardinium positive were used as a control. Amplified products were separated on a 1% agarose gel containing 0.1% SYBR Safe DNA gel stain (Invitrogen, USA) and visualized with a G:BOX Syngene blue light visualization instrument.
TABLE 1.
PCR screening primers
| Target group | Primer | Sequence (5′→3′) | Melting temp (°C) | Size (bp) | Reference |
|---|---|---|---|---|---|
| Alphaproteobacteria | Alf28F | ARCGAACGCTGGCGGCA | 56 | 700 | 35 |
| Alf684R | TACGAATTTYACCTCTACA | ||||
| Cardinium | CAR-SP-F | CGGCTTATTAAGTCAGTTGTGAAATCCTAG | 52 | 544 | 13 |
| CAR-SP-R | TCCTTCCTCCCGCTTACACG | ||||
| COI | BC1culicFm | GTAAAACGACGGCCAGTTCWACWAAYCAYAAARWTATTGG | 50 | 692 | 32 |
| JerR2 m | CAGGAAACAGCTATGACCCAAARAATCARAAYARRTGTTG | ||||
| Housekeeper third loop of 28S rRNA gene | D3a | GACCCGTCTTGAAACACGGA | 57 | 400 | 69 |
| D3ba | TCGGAAGGAACCAGCTACTA | ||||
| Wolbachia | Wol-F | TTGTAGCCTGCTATGGTATAACT | 52 | 900 | 36 |
| Wol-R | GAATAGGTATGATTTTCATGT | ||||
| Wolbachia wsp (nested) | wsp-L-F2 | TGGTCCAATAAGTGATGAAGAAACTAGCTACTACGTTCG | 68 | 609 | 39 |
| wsp-L-R2 | AAAAATTAAACGCTACTCCAGCTTCTGCACCAAC | ||||
| wsp151F | TGGTTACAAAATGGACGACA | 50 | 422 | ||
| wsp599R | CACCAACAGTGCTGTAAAGAAC |
Multiallele typing.
Samples which tested positive for Cardinium by conventional PCR screening were typed for multiple alleles using the 16S rRNA and DNA gyrase subunit B (gyrB) genes. Four primers (16SA1F, 16SA1R, 16SA2F, and 16SA2R) (Table 2) were designed to amplify two overlapping regions of the Cardinium 16S rRNA gene, based on the Clustal W alignment of sequences from Culicoides (accession numbers AB506776 to AB506779, JN166961, and JN166962) available in the GenBank database. Primers were designed using Geneious v7.0.5 and covered 1,404 bp of the 16S rRNA gene, with a 243-bp overlap between the amplicons (37). Four primers (gyrBA1F, gyrBA1R, gyrBA2F, and gyrBA2R) targeting a 1,399-bp region of the gyrB gene with a 168-bp overlap between amplicons (Table 2) were designed using the same process as described above with sequences available in GenBank (accession numbers AB506791, AB506792, JN166963, and JN166964). PCRs were performed using the conditions previously stated; an annealing temperature of 50°C was used for the Cardinium 16S rRNA gene and gyrB primers.
TABLE 2.
Primers for 16S rRNA gene and gyrB multiallele typing
| Cardinium target | Primer | Sequence (5′→3′) | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA gene amplicon 1 | 16SA1 F | AGCGGGACACTTCGGTGTTG | 745 | This study |
| 16SA1 R | TCATCGTTTACGGCGTGGAC | |||
| 16S rRNA gene amplicon 2 | 16SA2 F | CGTAGGCGGCTTATTAAGTC | 900 | This study |
| 16SA2 R | GTCCCAGTCGCTGGTCTAAC | |||
| gyrB amplicon 1 | gyrBA1 F | CATGGCGTGGGTATTTCTT | 724 | This study |
| gyrBA1 R | CAGGTTTCTACCGCTCCTTG | |||
| gyrB amplicon 2 | gyrBA2 F | TGCGGATAAATCTGGTCTGC | 841 | This study |
| gyrBA2 R | GCTGTACATACACGGCATCAAC |
For low-level Wolbachia detection, amplification was with a combination of heminested (16S rRNA gene) and nested (wsp) PCRs. Heminested primers for the 16S rRNA gene used two sets of previously published primers from Wol-F and Wol-R (36) and Rao-F and Rao-R (38). First-round amplification was performed using the Wol-F and Wol-R primers as previously outlined, generating an 897-bp amplicon. Second-round amplification yielded two amplicons, amplicon 1 (464 bp) (Wol-F and Rao-R) and amplicon 2 (495 bp) (Rao-F and Wol-R); both rounds of amplification had an annealing temperature of 52°C. A nested PCR developed by Hughes et al. (39) (Table 1) was used to amplify a 423-bp region of wsp. The nested protocol was altered to a two-step nested PCR instead of the previously outlined single-tube nested PCR (39). Two negative controls, both involving ultrapure water as the template, were run for first-round amplification, with the product used as the template for second-round amplification.
qPCR screening.
Quantitative PCR (qPCR) was used to increase the level of sensitivity of detection of Wolbachia and Cardinium. Samples screened with the conventional assays were rescreened with the qPCR assays. A Wolbachia qPCR was obtained based on the primer and probe design of Rao et al. (38), who used this assay to detect Wolbachia in field-collected mosquitoes. The probe reporter dye at the 5′ end was changed to VIC (4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein) as opposed to FAM (6-carboxyfluorescein), but the same TAMRA (6-carboxytetramethylrhodamine) quencher dye was retained at the 3′ end. The Wolbachia primers and the probe reaction setup was modified to a 20-μl reaction mixture using 10 μl of TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA), 1 μl of both primers (0.3 μM forward primer and 0.9 μM reverse primer), 1 μl of probe (0.05 μM), 2 μl of template DNA, and 5 μl of ultrapure water.
To develop a Cardinium qPCR, published Cardinium sequences isolated from Culicoides (accession numbers AB506776 to AB506779, JN166961, and JN166962) were aligned using Geneious v7.0.5 and a consensus sequence was obtained. Primer Express software v3.0.1. (Applied Biosystems) was used to design a TaqMan probe and primer pair to amplify a 111-bp region (Table 3) to specifically target Cardinium group C. The Cardinium primers and probes were calibrated to a final reaction volume of 20 μl consisting of 10 μl of TaqMan universal PCR master mix, 2 μl of forward and reverse primers (0.3 μM), 2 μl of probe (0.05 μM), 2 μl of template DNA, and ultrapure water. Optimal primer and probe concentrations for both assays were determined as in the protocol for the TaqMan universal PCR master mix. Triplicate reactions were performed on a QuantStudio 6 real-time PCR machine (Life Technologies, Carlsbad, CA). Cycling conditions included an initial incubation at 50°C for 2 min to activate uracil-N-glycosylase, followed by denaturation for 10 min at 95°C and then 45 cycles of 95°C for 15 s and a combined annealing and primer extension phase at 60°C for 1 min. Screening was conducted in a 96-well plate format, with 6 wells each plate being left for use as negative controls.
TABLE 3.
Primers and probes for quantitative TaqMan assays
| Primer or probe | Sequence (5′→3′) | Dyea |
Reference | |
|---|---|---|---|---|
| Reporter | Quencher | |||
| Car F | ACGCCGTAAACGATGATTACTAGA | FAM | TAMRA | This study |
| Car R | TTCCTTTGAGTTTCACCCTTGC | |||
| Car probe | ATGTACAACGTAGTTGTACGTGTCCAAGC | |||
| Wol F | CCAGCAGCCGCGGTAAT | VIC | TAMRA | 38 |
| Wol R | CGCCCTTACGCCCAA T | |||
| Wol probe | CGGAGAGGGCTAGCGTTATTCGGAATT | |||
FAM, 6-carboxyfluorescein; VIC, 4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Cloned standards of Cardinium and Wolbachia were generated and used as positive controls, as follows. Conventional PCR was conducted using qPCR primers to amplify known Wolbachia-positive material from Drosophila simulans and Cardinium-positive material from Culicoides victoriae [240]. Amplicons were visualized on a 1% agarose gel and purified using a QIAquick gel extraction kit (Qiagen, Valencia, CA). Purified DNA was ligated into pGEM-T Easy vector (Promega, Madison, WI) according to the manufacturer's protocol and then electroporated into competent Escherichia coli DH5α cells (New England BioLabs, Beverly, MA). Plasmid DNA was purified from positive clones using the QIAprep Spin Miniprep kit (Qiagen, Valencia, CA) and sequenced using SP6 and T7 sequencing primers. Plasmid DNA clones were serially diluted before being used as positive controls. Wolbachia and Cardinium plasmids were also used to generate a standard regression curve based on 6 serial dilutions of the plasmid in each qPCR run, allowing relative quantification of infection. Plasmid copy numbers were calculated as per established protocols (Applied Biosystems).
Sensitivity comparison of conventional PCR and qPCR.
Detection limits of conventional and quantitative PCRs were assessed by serially diluting Wolbachia-positive template DNA from Drosophila simulans and Cardinium-positive template DNA from Culicoides victoriae [240]. Ultrapure water was used to make 10-fold serial dilutions of each DNA template from neat to 10−9. Duplicate 2-μl samples of each dilution were tested using the Cardinium conventional (primers CAR-SP-F and CAR-SP-R) (13) and quantitative assays (Table 3), as well as the Wolbachia conventional (primers Wol-F and Wol-R) (36) and quantitative assays (Table 3).
Sequencing.
Nucleotide sequences of Cardinium-, Wolbachia-, and Alphaproteobacteria-positive amplicons were determined for species confirmation. DNA was purified from positive amplicons with a QIAquick gel extraction kit (Qiagen, Santa Clara, CA) according to the manufacturer's protocol. Fluorometric quantification of purified DNA was performed using a double-stranded DNA (dsDNA) HS assay on a Qubit (Invitrogen, Carlsbad, CA). Sequencing was performed using the BigDye Terminator v3.1 kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol, and sequence was generated via capillary Sanger sequencing on a 3500 Genetic Analyzer (Applied Biosystems).
A series of measures was implemented as an added precaution to reduce the risk of contamination and to ensure no false-positive detections. Clean-room working conditions were utilized, with insect identification, nucleotide extraction, master mix setup, amplification and postamplification handling all being performed in separate labs and with a nonamplified to amplified workflow being followed.
To compare the incidence of infections across sexes, contingency analyses were carried out for species where the infection was detected in at least one sex. Probabilities for detecting associations between infections and sex were based on exact tests.
Phylogenetic analysis.
Phylogenetic relationships of Cardinium were analyzed using nucleotide sequences obtained from 16S rRNA and gyrB genes. Additional group A (isolated from Ixodes scapularis [AB001518 and AB506790], Euides speciosa [AB506775 and AB506788], Tetranychus pueraricola [AB241135 and AB506784], and Oligonychus ilicis [AB241130 and AB506783]) and group B (isolated from Paenicardinium endonii [DQ314214 and DQ314215]) Cardinium sequences obtained from GenBank were included to provide phylogenetic resolution. Cardinium 16S rRNA gene and gyrB sequences were concatenated head to tail, forming a supergene alignment. Concatenating two genes together increases the number of evolutionary changes on individual branches, which in turn can generate a phylogeny more precise than when the two genes are analyzed separately (40). The concatenated alignment was screened for the presence of recombination using the RDP, GENECONV, Bootscan, MaxChi, Chimaera, and SiScan methods implemented in Recombination Detection Program v4.39 (RDP4), with default parameters (41). A Wolbachia phylogeny was determined using the 16S rRNA gene and the Wolbachia surface protein gene (wsp). The wsp gene was included in phylogenetic analysis because it has been shown to have a much higher divergence rate than the 16S rRNA gene (18, 36). Wolbachia sequences from supergroup A (Drosophila sechellia), B (Culex pipiens), and C (Dirofilaria immitis) were included to see where Culicoides Wolbachia would be placed within the wider Wolbachia phylogeny, with supergroup C being used to root the trees. Host Culicoides phylogenetic analysis was based on mitochondrial cytochrome oxidase subunit I (COI).
All sequences were aligned by using the Clustal W algorithm in Geneious v7.0.5 (Biomatters Ltd.) (37). Aligned nucleotide sequences were analyzed using jModelTest2 v2.1.3, with the topology search taking the best of Nearest Neighbor Interchange (NNI) and Subtree Pruning and Regrafting (SPR) (42). Evolutionary models implemented to construct phylogenetic trees were selected based on the Akaike information criterion output from JModelTest2 (42) (number of substitution schemes, 11; base tree for likelihood calculations maximum-likelihood [ML] optimized; base tree search best). The Hasegawa-Kishino-Yano (HKY) model was selected for the Cardinium 16S rRNA gene, Wolbachia wsp, and the concatenation of the Cardinium 16S rRNA gene and gyrB. The general time-reversible (GTR) model was selected for the Cardinium gyrB, Wolbachia 16S rRNA gene, and Culicoides COI phylogeny. Phylogenetic ML trees were constructed using the PhyML plugin in Geneious v7.0.5 with 1,000 bootstrap replicates; the proportion of invariable sites and gamma distribution were both estimated (43).
Mapping of Cardinium and Wolbachia in Culicoides.
Culicoides organisms which tested positive for Wolbachia or Cardinium based on screening with the quantitative assays were plotted against capture location. Shapefiles were obtained from the Australian Bureau of Statistics, Australian Standard Geographical Classification (ASGC) Digital Boundaries, Australia (July 2011). The distribution map was created in quantum GIS (QGIS) Wien v2.8.1 (44).
Nucleotide sequence accession numbers.
Sequences generated were deposited in GenBank. Cardinium accession numbers for the 16S rRNA gene are KR026906 to KR026918 and KR026920 to KR026923, and those for the gyrB gene are KR026924 to KR026935. Wolbachia accession numbers for the 16S rRNA gene are KR026936 to KR026941, and those for wsp are KR026942 to KR026951. Culicoides COI sequences of the C. victoriae group and C. williwilli were deposited in GenBank under accession numbers KT338814 to KT338822.
RESULTS
Endosymbiont assays.
Increased sensitivity for the Wolbachia and Cardinium quantitative PCRs in comparison to the conventional assays was obtained. Serial dilution of Wolbachia- and Cardinium-positive samples resulted in a 100-fold-lower detection limit with the qPCR assays. Detections were confirmed by sequencing; however, the Wolbachia (62 bp of the 16S rRNA gene) and Cardinium (111 bp of the 16S rRNA gene) qPCR amplicons were too small for species discrimination. Developed nested PCRs for Wolbachia (16S rRNA gene and wsp) and Cardinium (16S rRNA gene and gyrB) were used to amplify enough material of the low-level detections to allow confirmation by sequencing.
Twenty Culicoides species were identified by morphological and genetic typing of insects collected in Victoria, Tasmania, and Queensland from January to April 2013 and January to April 2014 (Fig. 1). These samples, together with samples of C. imicola from Kenya and Madagascar, were analyzed for evidence of Cardinium and Wolbachia infection through the conventional and quantitative PCR assays.
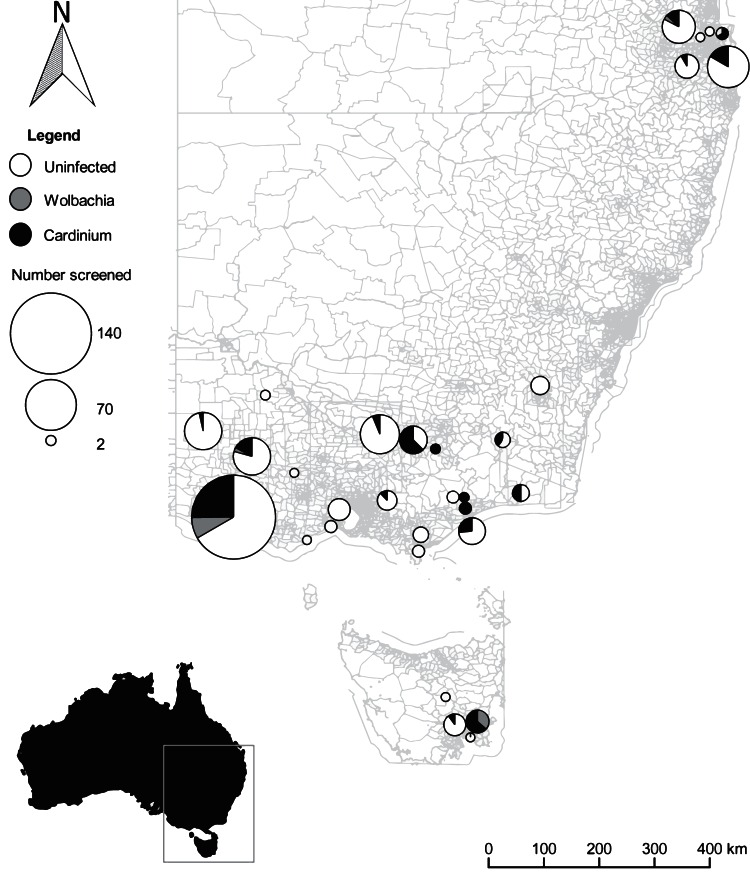
FIG 1.
Culicoides trapping and endosymbiont distribution. A map depicting collection sites across Queensland, New South Wales, Victoria, and Tasmania is shown (shapefiles were obtained from the Australian Bureau of Statistics, ASGC Digital Boundaries, Australia, July 2011; the distribution map was created in QGIS Wien v2.8.1 [44]). At all sites an equal number of Culicoides insects were screened with both the Cardinium and Wolbachia quantitative assays. The size of the pie chart represents the number of individuals screened, with the proportion of the Wolbachia (gray) or Cardinium (black) wedge representing the number of Culicoides insects which tested positive for each endosymbiont. White circles indicate Culicoides insects which were screened but in which no Wolbachia or Cardinium was detected.
Cardinium screening.
The conventional PCR assay identified evidence of Cardinium infection in females of three species, C. victoriae [240] and C. brevitarsis from Australia and C. imicola from Kenya (Table 4). Rescreening the samples using the qPCR assay indicated a substantially higher prevalence of Cardinium infection, with positive samples detected in all Culicoides species except C. victoriae [172] (Table 4). The percentage of positive samples detected in females increased from 4% (14/360; confidence interval [CI], 2.23 to 6.28%) (conventional PCR) to 26% (92/360; CI, 21.25 to 30.25%) (qPCR), and detections in males increased from 0% (0/161; CI, 0 to 1.84%) (conventional PCR) to 14% (22/161; CI, 8.98 to 19.64%) (qPCR). A lower number of male Culicoides insects were screened due to their lower representation in collections. However, in those Culicoides species in which Cardinium was detected in individuals of both sexes, there was no significant difference between prevalence in males and females for C. brevitarsis (contingency analysis, P = 0.63 and df = 1), C. bundyensis (contingency analysis, P = 0.33 and df = 1), and C. victoriae [245] (contingency analysis, P = 0.24 and df = 1), although differences were marginally nonsignificant in the case of C. parvimaculatus (contingency analysis, P = 0.065 and df = 1) and C. austropalpalis (contingency analysis, P = 0.067 and df = 1) due to a higher prevalence in female Culicoides. For C. marksi, no infection was identified in males, but it was present in some females (contingency analysis, P = 0.035).
TABLE 4.
Prevalence of Cardinium and Wolbachia detected in samples from Culicoides species based on conventional and quantitative PCR screening
| Species | No. positive/total (% positive)a by: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Conventional PCR |
Quantitative PCR |
|||||||
|
Cardinium |
Wolbachia |
Cardinium |
Wolbachia |
|||||
| Female | Male | Female | Male | Female | Male | Female | Male | |
| C. austropalpalis | 0/63 | 0/32 | 0/63 | 0/32 | 7/63 (11) | 3/32 (9) | 1/63 (2) | 0/32 |
| C. narrabeenensis | 0/14 | 0/14 | 1/14 (7) | 3/14 (21) | ||||
| C. marksi | 0/50 | 0/24 | 0/50 | 0/24 | 8/50 (16) | 0/24 | 1/50 (2) | 0/24 |
| C. parvimaculatus | 0/20 | 0/20 | 0/20 | 0/20 | 8/20 (40) | 2/20 (10) | 4/20 (20) | 0/20 |
| C. dycei | 0/5 | 0/5 | 2/5 (40) | 0/5 | ||||
| C. williwilli | 1/4 (25) | 0/1 | 0/4 | 0/1 | 4/4 (100) | 1/1 (100) | 0/4 | 0/1 |
| C. henryi | 0/11 | 0/11 | 7/11 (64) | 1/11 (9) | ||||
| C. brevitarsis | 1/22 (5) | 0/9 | 0/22 | 0/9 | 6/22 (27) | 1/9 (11) | 5/22 (23) | 0/9 |
| C. imicola (Madagascar) | 0/9 | 0/2 | 0/9 | 0/2 | 3/9 (33) | 0/2 | 2/9 (22) | 0/2 |
| C. imicola (Kenya) | 1/1 (100) | 1/1 (100) | ||||||
| C. wadai | 0/2 | 0/1 | 0/2 | 0/1 | 1/2 (50) | 0/1 | 0/2 | 0/1 |
| C. bundyensis | 0/39 | 0/23 | 0/39 | 0/23 | 6/39 (15) | 6/23 (26) | 1/39 (3) | 2/23 (9) |
| C. victoriae [172] | 0/15 | 0/13 | 0/15 | 0/13 | 0/15 | 0/13 | 0/15 | 0/13 |
| C. victoriae [245] | 1/34 (3) | 0/11 | 0/34 | 0/11 | 10/34 (29) | 1/11 (9) | 3/34 (9) | 0/11 |
| C. victoriae [241] | 0/18 | 0/9 | 0/18 | 0/9 | 1/18 (5) | 0/9 | 1/18 (6) | 0/9 |
| C. victoriae [240] | 11/18 (61) | 0/1 | 0/18 | 0/1 | 13/18 (72) | 0/1 | 0/18 | 0/1 |
| C. victoriae [true] | 0/8 | 0/2 | 0/8 | 0/2 | 1/8 (12) | 0/2 | 0/8 | 0/2 |
| C. antennalis | 0/12 | 0/12 | 8/12 (67) | 6/12 (50) | ||||
| Culicoides (Molestus group) species no. 2 | 0/2 | 0/2 | 1/2 (50) | 0/2 | ||||
| C. marmoratus | 0/6 | 0/6 | 6/6 (100) | 1/6 (17) | ||||
| C. multimaculatus | 0/19 | 0/19 | 6/19 (31) | 0/19 | ||||
| Total | 14/360 (4) | 0/160 | 0/359 | 0/160 | 92/360 (26) | 22/160 (14) | 23/359 (7) | 8/160 (5) |
Boldface indicates endosymbiont-positive results.
Detections of Cardinium by conventional PCR were seen at the lowest quantification cycle (Cq) value of 26.58 in C. williwilli (equating to 23,000,000 copies) and the highest Cq value of 34.06 (equating to 24,000 copies) in C. victoriae [240] (see Table S1 in the supplemental material). However, the Cardinium qPCR assay provided detections at the lowest Cq value of 42.68 in C. parvimaculatus, which equates to approximately 290 copies of the Cardinium target region. In Culicoides species which had detections of Cardinium in both sexes, there was no significant difference (based on t tests) in Cq values and hence Cardinium copy number between sexes in C. bundyensis (P = 0.22, df = 10) and C. austropalpalis (P = 0.29, df = 8), although there was a difference for C. parvimaculatus (P = 0.018, df = 8).
Wolbachia screening.
No evidence of Wolbachia infection was detected in any of the Culicoides samples tested using the conventional PCR. In contrast, evidence of low-level Wolbachia infections was detected in 10 species of Culicoides when individual samples were screened using the qPCR assay (Table 4). Across all species tested, the percentages of detected positive females (7%, 23/353; CI = 4.27 to 9.46) and males (5%, 8/160; CI = 2.34 to 9.27) were similar (contingency test, P = 0.55 and df = 1). Culicoides bundyensis was the only species in which evidence of Wolbachia infection was detected in both sexes, with similar proportions of positive males and females (contingency test, P = 0.54 and df = 1).
Detections of Wolbachia by qPCR were seen to range from the lowest Cq value of 30.53 in C. antennalis (equating to 11,600 copies) to the highest Cq value of 41.30 (equating to 22.4 copies) in C. brevitarsis (see Table S1 in the supplemental material). Detections of Wolbachia were rarely seen in both sexes of the same Culicoides species; hence, no comparison of Cq value and sex was performed.
There was evidence of low-level dual infections with Wolbachia and Cardinium in a number of Culicoides species. These included five individuals of C. antennalis, four individuals of C. brevitarsis and C. parvimaculatus, three individuals of C. bundyensis and C. victoriae [245], and single individuals of C. imicola (Madagascar), C. marksi, C. austropalpalis, and C. marmoratus (Table 5). The dual infections in single individuals occurred at the expected frequency on the basis of a random association between the infections.
TABLE 5.
Morphological grouping of Culicoides species with Cardinium and Wolbachia detections by qPCR
| Subgenus | Complex or group | Species | No. positive/total (% positive)a |
|||||
|---|---|---|---|---|---|---|---|---|
|
Cardinium |
Wolbachia |
Dual infectedb |
||||||
| Female | Male | Female | Male | Female | Male | |||
| Avaritia | Boophagus complex | C. wadai | 1/2 (50) | 0/1 | 0/2 | 0/1 | ||
| Imicola complex | C. brevitarsis | 6/22 (27) | 1/9 (11) | 5/22 (23) | 0/9 | 4/22 (18) | ||
| C. imicola (Madagascar) | 3/9 (33) | 0/2 | 2/9 (22) | 0/2 | 1/9 (11) | |||
| C. imicola (Kenya) | 1/1 (100) | |||||||
| Marksomyia | Marksi group | C. dycei | 2/5 (40) | 0/5 | ||||
| C. marksi | 8/50 (16) | 0/24 | 1/50 (2) | 0/24 | 1/50 (2) | |||
| C. parvimaculatus | 8/20 (40) | 2/20 (10) | 4/20 (20) | 0/20 | 4/20 (20) | |||
| Unplaced | Antennalis group | C. antennalis | 8/12 (67) | 6/12 (50) | 5/12 (42) | |||
| Ornatus group | C. marmoratus | 6/6 (100) | 1/6 (17) | 1/6 (16) | ||||
| Williwilli group | C. austropalpalis | 7/63 (11) | 3/32 (9) | 1/63 (2) | 0/32 | 1/63 (2) | ||
| C. narrabeenensis | 1/14 (7) | 3/14 (21) | 0/14 | |||||
| C. williwilli | 4/4 (100) | 1/1 (100) | 0/4 | 0/1 | ||||
| Victoriae group | C. bundyensis | 6/39 (15) | 6/23 (26) | 1/39 (3) | 2/23 (9) | 1/39 (3) | 2/23 (9) | |
| C. henryi | 7/11 (64) | 1/11 (9) | 0/11 | |||||
| C. multimaculatus | 6/19 (31) | 0/19 | ||||||
| C. victoriae [172] | 0/15 | 0/13 | 0/15 | 0/13 | ||||
| C. victoriae [241] | 1/18 (5) | 0/9 | 1/18 (6) | 0/9 | 0/18 | |||
| C. victoriae [240] | 13/18 (72) | 0/1 | 0/18 | 0/1 | ||||
| C. victoriae [245] | 10/34 (29) | 1/11 (9) | 3/34 (9) | 0/11 | 3/34 (9) | |||
| C. victoriae [true] | 1/8 (12) | 0/3 | 0/8 | 0/3 | ||||
Boldface indicates endosymbiont-positive results.
Culicoides species which tested positive for both Wolbachia and Cardinium by qPCR.
Alphaproteobacteria screening.
Screening by conventional PCR was also performed using the broad-range Alphaproteobacteria primer set. The assay provided evidence of infection in individuals representing four Culicoides species: C. austropalpalis, in which 30% (13/43; CI = 17.96 to 45.09%) of females and 11% (3/28; CI = 2.79 to 26.45%) of males were positive; C. marksi, in which 7% (2/29; CI = 1.17 to 20.97%) of females and 5% (1/19; CI = 0.26 to 23.33%) of males were positive; C. brevitarsis, in which 22% (2/9; CI = 3.90 to 56.21%) of females and 50% (1/2; CI = 2.5 to 97.5%) of males were positive; and C. bundyensis, in which 18% (5/27; CI = 7.11 to 36.38%) of females were positive. The amplicons generated in the assay were sequenced. BLAST analysis indicated that amplicons obtained from C. marksi and C. brevitarsis shared a high percentage of nucleotide sequence identity (99%) with the 16S rRNA gene of an Asaia sp. bacterium isolated from Aedes albopictus mosquitoes (GenBank accession number JX445140.1).
Cardinium phylogenetic analysis.
Partial 16S rRNA and DNA gyrase subunit B gene sequences were used to construct the Cardinium phylogeny. Based on the 505-bp 16S rRNA gene amplicon, all Australian Cardinium isolates grouped with the previously described sequences of Culicoides group C Cardinium hertigii from Israel (C. oxystoma and C. imicola), Japan (C. arakawae, C. lungchiensis, C. peregrinus, and C. ohmorii), and the United Kingdom (C. punctatus and C. pulicaris) (see Fig. S1a in the supplemental material). A low level of nucleotide sequence divergence was observed, ranging from 100% identity to 98.94% identity (5 nucleotide substitutions), between C. brevitarsis collected in Brisbane, Australia, and C. oxystoma (GenBank accession number JN166962) collected in Israel.
Phylogenetic analysis of the 1,088-bp region of the gyrB gene also indicated that the Australian Cardinium isolates cluster with group C Cardinium hertigii (see Fig. S1b in the supplemental material). There was generally a higher level of sequence divergence in gyrB than in the 16S rRNA gene, but there was 100% identity observed between some Cardinium gyrB sequences. The highest divergence (95.87% identity or 45 nucleotide substitutions) occurred between Cardinium sequences from C. multimaculatus from Australia and C. imicola from Israel (GenBank accession number JN166963) and Kenya. In this analysis, the Cardinium sequences grouped predominately to the geographical region from which the host Culicoides had been collected, with the exception of C. williwilli from Brookfield in Queensland, which was most similar to Cardinium detected in C. arakawae from Japan (Fig. 2; see Fig. S1b in the supplemental material). Translation of the gyrB gene sequences indicated that the majority (90%) of amino acid substitutions in the gyrase B protein were synonymous.
FIG 2.
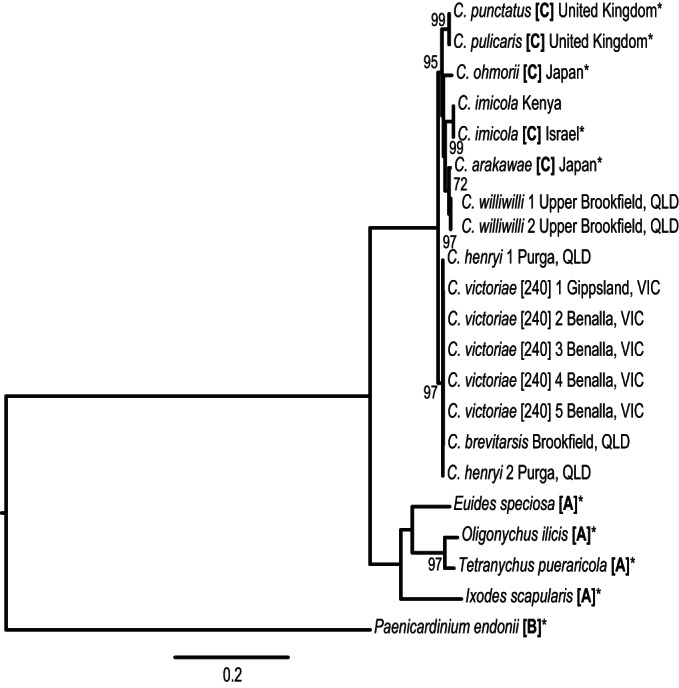
Maximum-likelihood concatenated Cardinium tree. The phylogenetic tree was constructed from the 1,563-bp concatenated 16S rRNA gene and gyrB Cardinium amplicon sequences. The sequences were aligned using the Clustal W algorithm employing the HKY substitution model based on JModelTest2 analysis with 1,000 bootstrap replicates. Bootstrap proportions of ≥70% are indicated beside nodes. Three Cardinium groups were included: group A, found in arthropods; group B, found in plant parasitic nematodes; and group C, found in Culicoides. The capture location is indicated beside the species name. Asterisks denote sequences from GenBank; all other sequences were generated in this study.
A 1,563-bp amplicon assembled by concatenation of the 16S rRNA and gyrB Cardinium genes (Fig. 2) generated a phylogeny consistent with the phylogenetic trees constructed using the individual genes (see Fig. S1a and b in the supplemental material). The highest divergence of the concatenated sequences (97.05% identity or 47 nucleotide substitutions) was between C. multimaculatus from Australia and C. ohmorii from Japan, C. imicola from Israel, and C. imicola Kenya. The concatenated tree was assessed for the presence of recombination using the program RDP v.4.39, but no putative recombination events were detected.
Wolbachia phylogenetic analysis.
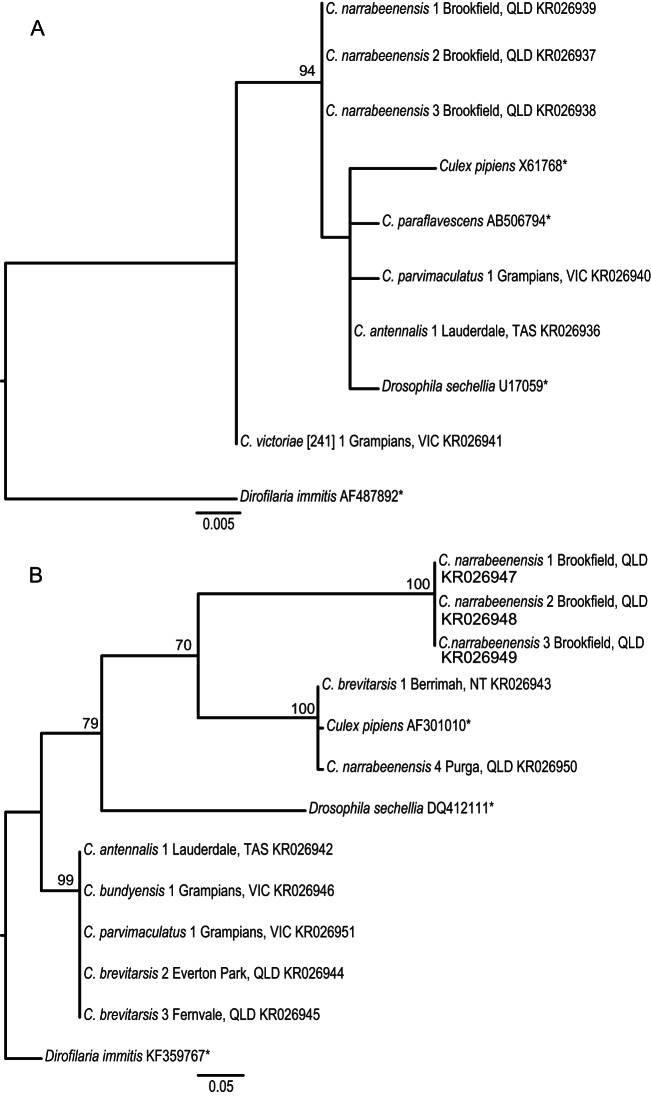
Phylogenetic analysis of Wolbachia (Fig. 3) based on partial sequences of the 16S rRNA gene (390-bp amplicon) and the wsp gene (351-bp amplicon) was done through nested and heminested PCRs. The success of amplifying sequences from low-level Wolbachia infections was lower than for Cardinium, and only 16S rRNA gene or wsp amplicons were obtained for many samples. The 16S rRNA gene sequences displayed a high level of homology to the only other Wolbachia sequence reported previously from Culicoides, which was from C. paraflavescens collected in Japan (accession number AB506794). The highest level of divergence from this sequence (98.72% identity) was with Wolbachia from C. victoriae [241] collected in Australia. The wsp gene sequences displayed generally lower levels of sequence identity, with greatest divergence (79.5% identity) observed in a group of Culicoides narrabeenensis isolates which were collected in Brisbane, Australia. BLAST analysis of C. narrabeenensis samples showed the closest sequence identity of 98% to Wolbachia isolated from Phengaris teleius (accession number JX470438), which is placed in Wolbachia supergroup B (45).
FIG 3.
Maximum-likelihood phylogenetic trees of Wolbachia. Phylogenetic trees based on a 390-bp region of the 16S rRNA gene (A) and a 351-bp region of the wsp gene (B) are shown. Sequences were aligned using the Clustal W algorithm employing the GTR substitution model (16S rRNA gene) and the HKY substitution model (wsp) based on JModelTest2 analysis with 1,000 bootstrap replicates. Bootstrap proportions of ≥70% are indicated beside nodes. Three supergroups were also included for reference; Drosophila sechellia (Wolbachia supergroup A), Culex pipiens (Wolbachia supergroup B), and Dirofilaria immitis (Wolbachia supergroup C). The Wolbachia sequence from Dirofilaria immitis was used to root the trees. The capture location is indicated beside the species name. Asterisks denote sequences obtained from GenBank; all other sequences were generated in this study.
Occurrence of endosymbionts within the genus Culicoides and location.
Wolbachia and Cardinium detections were investigated across the Culicoides genus to determine if there was a specific association with a particular subgenus. This was done based on the morphological classification of Culicoides species, largely based on wing classification (Table 5) (30). Wolbachia was detected sporadically across different subgenera, with no obvious pattern identified (Table 5). Cardinium was seen to infect all species except C. victoriae [172], with a low prevalence in other C. victoriae species. This was further investigated by constructing a phylogenetic tree based on COI of the C. victoriae group, with the absence of Cardinium not restricted to a particular lineage (see Fig. S2 in the supplemental material). As expected, Cardinium detections were more common across Culicoides species than across Wolbachia. Finally, to determine if there was an association with the presence of Wolbachia, Cardinium, and geographical location, infection prevalence was plotted against capture site (Fig. 1), with no obvious association apparent.
DISCUSSION
The genus Culicoides is one of the least studied of the major dipteran vector groups, with limited information known about their endosymbionts. Recent studies have identified Wolbachia and Cardinium in Culicoides species collected in Japan, Israel, and the United Kingdom. These studies used conventional PCR assays to reveal a relatively low prevalence of both Wolbachia (1/34) and Cardinium (8/34) (13–15). In the present study, 20 species of Culicoides, collected predominately in southeastern Australia, were screened for the presence of these endosymbionts. Previously established screening methodologies (13) were utilized to identify Cardinium in samples of C. victoriae [240], C. victoriae [245], C. williwilli, C. imicola (Kenya), and C. brevitarsis; however, these methods failed to detect Wolbachia in individuals of any of the species tested. Bacteria of the species Asaia were identified in C. marksi and C. brevitarsis samples by conventional PCR screening utilizing Alphaproteobacteria primers. Asaia species have previously not been identified in Culicoides. Asaia has been detected in Anopheles mosquitoes, colonizing the salivary glands and midgut of the insect (46). Unlike Wolbachia and Cardinium, Asaia species can be cultured on medium and easily colonize insects. Hence, these bacteria have been suggested as potential paratransgenesis control agents for insect vectors (46, 47).
Through the use of qPCR assays, previously undetected low-level Cardinium and Wolbachia infections were found in Culicoides species. Low-level detections were due to an improvement in detection sensitivity of approximately 100-fold gained through utilizing qPCR assays, compared to conventional PCR screening methodologies. The qPCR screening identified a significant number of low-level Wolbachia and Cardinium infections in Culicoides, with the improved sensitivity increasing detection prevalence. Using conventional PCR, Nakamura et al. (13) reported a Cardinium infection prevalence of 16% in Culicoides, but our analysis indicates that this is likely to be an underestimate of the true prevalence. Following a similar pattern, Wolbachia has previously been detected in only a single C. paraflavescens individual (13), whereas we have detected Wolbachia in 7% of female and 5% of male Culicoides insects that were screened.
This study also detected evidence of a range of low- and high-level infections in Culicoides, with no discernible pattern identified to explain this variability. High-density Cardinium infections detected by conventional PCR were found in C. brevitarsis, C. victoriae [240], C. victoriae [245], C. williwilli, and C. imicola Kenya, with target copy numbers ranging from ≈120,000 to 47,140,000 based on relative quantification. Low-level infections detected only by qPCR and confirmed by nested PCR were found in a range from ≈125 to 60,000 target copies per Culicoides insect. Changes in endosymbiont density can be a result of a range of factors, such as the bacterial strain (48, 49), host age (49, 50), sex (50), and temperature (51, 52). Endosymbiont density based on Cq values was investigated with Culicoides species that had Cardinium infections in both sexes. No difference was seen between sexes in C. bundyensis, C. austropalpalis, and C. victoriae [240], whereas C. parvimaculatus females had a higher density, but this result is dependent on low numbers (only 2 males and 8 females). Morag et al. (12) provided evidence that temperature could affect endosymbiont density, based on a lower prevalence of Cardinium in Culicoides in arid regions than in Mediterranean regions. Low-level endosymbiont infections can be highly localized within the insect host, such as in some Drosophila species (53), highlighting the importance of screening the whole insect instead of using only abdomens (54). Although these infections occur at a low level, they may still have a significant impact on their host insect, as has been shown for Wolbachia in Drosophila paulistorum semispecies, which can influence fecundity, sex ratio, and mate discrimination (23). Studies on the effect of Cardinium in C. imicola have found no infection impact on Culicoides survival under optimal, starvation, heat, and antibiotic treatments and no effect on wing size (55). However, detection in that study involved conventional PCR screening, and perhaps an effect on Cardinium was overlooked because low-level endosymbiont infections were not identified or other effects of Cardinium were not characterized.
Based on 16S rRNA gene and gyrB sequences, Cardinium hertigii was the only Cardinium strain identified in this study. Specific to Culicoides, group C Cardinium has previously been reported with <3.1% sequence divergence for 16S rRNA gene and <19% for gyrB (13). This pattern of similar Cardinium and Wolbachia endosymbiont strains in closely related hosts has been seen in other infected insects (56–59).
The phylogeny generated by analysis of gyrB sequences showed Cardinium strains clustering primarily geographically rather than by host Culicoides species. For example, strains from species in the Avaritia subgenus (C. imicola and C. brevitarsis) were not positioned together, but similar Cardinium strains were detected in C. brevitarsis and several species in the C. victoriae species group (C. henryi, C. multimaculatus, and C. victoriae) from Australia (see Fig. S1b in the supplemental material). A similar pattern has been seen in Wolbachia infecting ants from a range of geographical locations, with closely related Wolbachia strains typically confined to the geographical location of the host insects (60), indicating that Wolbachia populations can be isolated by geographic barriers (60). There were only two exceptions to this general trend: Cardinium strains from Australian C. williwilli did not cluster with strains from other Australian Culicoides species, and strains from C. arakawae and C. ohmorii in Japan did not cocluster in the phylogeny (Fig. 2; see Fig. S1b in the supplemental material). Also, there was no evidence of Wolbachia or Cardinium infections being restricted to a particular geographical region (Fig. 1). A higher number of individuals infected with Cardinium from different geographical regions as well as analysis of other, more rapidly evolving genes will be required to examine this association more definitively.
To determine Wolbachia's placement within the currently recognized 10 Wolbachia supergroups, 16S rRNA gene and wsp sequences were analyzed (61). Wolbachia sequences from Culicoides were seen to place in either Wolbachia supergroup A or B, which is consistent with the single Wolbachia isolate from C. paraflavescens (13). To provide further Wolbachia species discrimination, additional genes should be screened, such as that for cell division protein FtsZ (62). Nested primers for ftsZ were tested in this study with no success. A high sequence similarity was also seen between some Wolbachia isolates infecting Culicoides and those infecting Culex pipiens obtained from GenBank (Fig. 3A and B). This is not surprising, as highly related, and even genetically identical, Wolbachia strains are occasionally found in distantly related hosts (60). The cooccurrence of Wolbachia and Cardinium was detected in a number of individual Culicoides insects. Wolbachia and Cardinium are commonly seen to coinfect the same insect host species (57, 63, 64), but the nature of the interaction of these endosymbionts within the host insects can be unclear (65).
The Culicoides genus is divided into a number of subgenera based on morphology. Wolbachia was seen to occur sporadically throughout the Culicoides genus. On the other hand, Cardinium was seen to occur in almost every species. This sporadic occurrence of endosymbionts, such as Wolbachia, has been seen before in other insect species and could be a consequence of a weak cytoplasmic incompatibility (CI) phenotype (66). It was also noted that Cardinium was not detected in samples of C. victoriae [172] (see Fig. S2 in the supplemental material), but this could also be due to the relatively low numbers of individuals screened (Table 4). The reported higher prevalence of Cardinium than of Wolbachia across the Culicoides genus is consistent with previous observations (13). Taxonomical grouping based on wing morphology was used to construct a Culicoides phylogeny; a COI sequence-based phylogeny was also investigated but proved to be inadequate for separating species and clades, consistent with previous studies (31). However, COI was informative for resolving taxa at a finer level within the C. victoriae group (see Fig. S2 in the supplemental material).
Conventional PCR has previously been shown to be an inadequate method for accurately profiling endosymbiont distribution in insect populations (19). The detection of low-level endosymbiont infections requires more sensitive screening assays, such as nested, long, or quantitative PCR (19, 67). However, techniques such as nested and long PCR increase the risk of obtaining false positives through contamination (68). For this study, qPCR was the preferred method, as it has been shown previously that increased sensitivity can be achieved with a lower risk of contamination. Regardless of the technique used when working with low-level infections, additional precautions must be implemented; in this study we used a nonamplified to amplified workflow in separate labs and multiple negative controls within each procedure.
This study identified evidence of low-level endosymbiont infections of both Cardinium and Wolbachia in Culicoides species. Low-level endosymbionts were detected infrequently and sporadically within Culicoides populations, as has been observed in other insect species, such as Drosophila equinoxialis, Drosophila paulistorum, Pityogenes chalcographus, Perkinsiella saccharicida, and Perkinsiella vitiensis (20, 23, 25). This sporadic occurrence could be a consequence of a weak cytoplasmic incompatibility (CI) phenotype. Unfortunately, due to difficulties in maintaining Culicoides colonies, there is limited knowledge yet available on the effect that Cardinium or Wolbachia may have on the host.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maria Onyango for providing C. imicola samples from Kenya and Stacey Lynch for C. multimaculatus samples from Australia. We thank Lee Trinidad for assistance with laboratory techniques.
Funding of this research was partly provided by Meat and Livestock Australia through project number B.AHE.0210.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01239-15.
REFERENCES
- 1.Mellor PS, Boorman J, Baylis M. 2000. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol 45:307–340. doi: 10.1146/annurev.ento.45.1.307. [DOI] [PubMed] [Google Scholar]
- 2.Sellers RF, Pedgley DE, Tucker MR. 1977. Possible spread of African horse sickness on the wind. J Hyg Camb 79:279–298. doi: 10.1017/S0022172400053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter S, Mellor PS, Torr SJ. 2008. Control techniques for Culicoides biting midges and their application in the U.K. and Northwestern Palaearctic. Med Vet Entomol 22:175–187. doi: 10.1111/j.1365-2915.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- 4.Maan S, Maan NS, Nomikou K, Veronesi E, Bachanek-Bankowska K, Belaganahalli MN, Attoui H, Mertens PPC. 2011. Complete genome characterisation of a novel 26th bluetongue virus serotype from Kuwait. PLoS One 6:e26147. doi: 10.1371/journal.pone.0026147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zientara S, Sánchez-Vizcaína JM. 2013. Control of bluetongue in Europe. Vet Microbiol 165:33–37. doi: 10.1016/j.vetmic.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Brelsfoard CL, Dobson SL. 2009. Wolbachia-based strategies to control insect pests and disease vectors. AsPac J Mol Biol Biotechnol 17:55–63. [Google Scholar]
- 7.O'Neill SL, Hoffmann AA, Werren JH. 1997. Influential passengers: inherited microogranisms and arthropod reproductions. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 8.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 9.Douglas AE, Prosser WA. 1992. Synthesis of the essential amino acid Tryptophan in the pea aphid (Acyrthosiphon pisum) symbiosis. J Insect Physiol 38:565–568. doi: 10.1016/0022-1910(92)90107-O. [DOI] [Google Scholar]
- 10.Laven H. 1951. Crossing experiments with Culex strains. Evolution 5:370–375. doi: 10.2307/2405682. [DOI] [Google Scholar]
- 11.Riegler M, Sidhu M, Miller WJ, O'Neill SL. 2005. Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biol 15:1428–1433. doi: 10.1016/j.cub.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Kawai S, Yukuhiro F, Ito S, Gotoh T, Kisimoto R, Yanase T, Matsumoto Y, Kageyama D, Noda H. 2009. Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Appl Environ Microbiol 75:6757–6763. doi: 10.1128/AEM.01583-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morag N, Klement E, Saroya Y, Lensky I, Gottlieb Y. 2012. Prevalence of the symbiont Cardinium in Culicoides (Diptera: Ceratopogonidae) vector species in associated with land surface temperature. FASEB J 26:4025–4034. doi: 10.1096/fj.12-210419. [DOI] [PubMed] [Google Scholar]
- 15.Lewis SE, Rice A, Hurst GDD, Baylis M. 2014. First detection of endosymbiotic bacteria in biting midges Culicoides pulicaris and Culicoides punctatus, important Palaearctic vectors of bluetongue virus. Med Vet Entomol 28:453–456. doi: 10.1111/mve.12055. [DOI] [PubMed] [Google Scholar]
- 16.Werren JH, Windsor D, Guo L. 1995. Distribution of Wolbachia among neotropical arthropods. Proc Biol Sci 262:197–204. doi: 10.1098/rspb.1995.0196. [DOI] [Google Scholar]
- 17.Johanowicz DL, Hoy MA. 1996. Wolbachia in a predator-prey system: 16S ribosomal DNA analysis of two Phytoseiids (Acari: Phytoseiidae) and their prey (Acari: Tetranychidae). Ann Entomol Soc Am 89:435–441. doi: 10.1093/aesa/89.3.435. [DOI] [Google Scholar]
- 18.Zhou W, Rousset F, O'Neill S. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci 265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeyaprakash A, Hoy MA. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol 9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- 20.Arthofer W, Riegler M, Avtzis DN, Stauffer C. 2009. Evidence for low-titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, Scolytinae). Environ Microbiol 11:1923–1933. doi: 10.1111/j.1462-2920.2009.01914.x. [DOI] [PubMed] [Google Scholar]
- 21.Simoncini L, Casiraghi M, Bazzocchi C, Sacchi L, Bandi C, Genchi C. 2001. Real-time PCR for quantification of the bacterial endosymbionts (Wolbachia) of filarial nematodes. Parassitologia 43:173–178. [PubMed] [Google Scholar]
- 22.Schneider DI, Garschall KI, Parker AG, Abd-Alla AMM, Miller WJ. 2013. Global Wolbachia prevalence, titre fluctuations and their potential of causing cytoplasmic incompatibilities in tsetse flies and hybrids of Glossina morsitans subgroup species. J Invertebr Pathol 112:S104–S115. doi: 10.1016/j.jip.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller WJ, Ehrman L, Schneider D. 2010. Infectious speciation revisited: impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLoS Pathog 6:e1001214. doi: 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arthofer W, Riegler M, Schneider D, Krammer M, Miller WJ, Stauffer C. 2009. Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae). Mol Ecol 18:3816–3830. doi: 10.1111/j.1365-294X.2009.04321.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes GL, Allsopp PG, Brumbley SM, Woolfit M, McGraw EA, O'Neill SL. 2011. Variable infection frequency and high diversity of multiple strains of Wolbachia pipientis in Perkinsiella planthoppers. Appl Environ Microbiol 77:2165–2168. doi: 10.1128/AEM.02878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann AA, Ross PA, Rašić G. 20 July 2015 Wolbachia strains for disease control: ecological and evolutionary considerations. Evol Appl. doi: 10.1111/eva.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bishop AL, Bellis GA, McKenzie HJ, Spohr LJ, Worrall RJ, Harris AM, Melville L. 2006. Light trapping of biting midges Culicoides spp. (Diptera: Ceratopogonidae) with green light-emitting diodes. Aust J Entomol 45:202–205. doi: 10.1111/j.1440-6055.2006.00538.x. [DOI] [Google Scholar]
- 28.Bishop AL, Worrall RJ, Spohr LJ, McKenzie HJ, Barchia IM. 2004. Improving light-trap efficiency for Culicoides spp. with light-emitting diodes. Vet Ital 40:266–269. [PubMed] [Google Scholar]
- 29.Murray MD. 1987. Local dispersal of the biting-midge Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae) in south-eastern Australia. Aust J Zool 35:559–573. doi: 10.1071/ZO9870559. [DOI] [Google Scholar]
- 30.Dyce AL, Bellis GA, Muller MJ. 2007. Pictorial atlas of Australasian Culicoides wings (Diptera: Ceratopogonidae). Australian Biological Resources Study, Canberra, Australia. [Google Scholar]
- 31.Ander M, Troell K, Chirico J. 2013. Barcoding of biting midges in the genus Culicoides: a tool for species determination. Med Vet Entomol 27:323–331. doi: 10.1111/j.1365-2915.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 32.Bellis GA, Dyce AL, Gopurenko D, Mitchell A. 2013. Revision of the Immaculatus group of Culicoides Latreille (Diptera: Ceratopogonidae) from the Australiasian region with description of two new species. Zootaxa 3680:15–37. doi: 10.11646/zootaxa.3680.1.4. [DOI] [Google Scholar]
- 33.Dittrich-Schröder G, Wingfield MJ, Klein H, Slippers B. 2012. DNA extraction techniques for DNA barcoding of minute gall-inhabiting wasps. Mol Ecol Resour 12:109–115. doi: 10.1111/j.1755-0998.2011.03074.x. [DOI] [PubMed] [Google Scholar]
- 34.Ashelford KE, Weightman AJ, Fry JC. 2002. PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res 30:3481–3489. doi: 10.1093/nar/gkf450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mühling M, Wollven-Allen J, Murrell JC, Joint I. 2008. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J 2:379–392. doi: 10.1038/ismej.2007.97. [DOI] [PubMed] [Google Scholar]
- 36.O'Neill SL, Giordano R, Colbert AME, Karr TL, Robertson HM. 1992. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci U S A 89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious basic: an intergrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao RU, Atkinson LJ, Ramzy RMR, Helmy H, Farid HA, Bockarie MJ, Susapu M, Laney SJ, Williams SA, Weil GJ. 2006. A real-time PCR-based assay for detection of Wuchereria bancrofti in blood and mosquitoes. Am J Trop Med Hyg 74:826–832. [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes GL, Arthofer W, Pike AD, Coghlin PC, Floate KD, Rasgon JL. 2012. Single tube nested-PCR (STN-PCR): a sensitivity detection technique for Wolbachia that is less prone to contamination, poster. Abstr 7th Int Wolbachia Conf, Olėron, France. [Google Scholar]
- 40.Gadagkar SR, Rosenberg MS, Kumar S. 2005. Inferring species phylogenies from multiple genes: concatenated sequence tree versus consensus gene tree. J Exp Zool 304B:64–74. doi: 10.1002/jez.b.21026. [DOI] [PubMed] [Google Scholar]
- 41.Martin DP, Williamson C, Posada D. 2005. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics 21:260–262. doi: 10.1093/bioinformatics/bth490. [DOI] [PubMed] [Google Scholar]
- 42.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 44.Development Team QGIS. 2012. QGIS Geographica Information System, 2.8.1 ed. Open Source Geospatial Foundation, Beaverton, OR. [Google Scholar]
- 45.Ritter S, Michalski SG, Settele J, Wiemers M, Fric ZF, Sielezniew M, MŠašić Rozier Y, Durka W. 2013. Wolbachia infections mimic cryptic speciation in two parasitic butterfly species, Phengaris teleius and P. nausithous (Lepidoptera: Lycaenidae). PLoS One 8:e78107. doi: 10.1371/journal.pone.0078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Capone A, Ricci I, Damiani C, Mosca M, Rossi P, Scuppa P, Crotti E, Epis S, Angeletti M, Valzano M, Sacchi L, Bandi C, Daffonchio D, Mandrioli M, Favia G. 2013. Interactions between Asaia, Plasmodium and Anopheles: new insights into mosquito symbiosis and implications in Malaria symbiotic control. Parasit Vectors 6:182–195. doi: 10.1186/1756-3305-6-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, Rizzi A, Urso R, Brusetti L, Borin S, Mora D, Scuppa P, Pasqualini L, Clementi E, Genchi M, Corona S, Negri I, Grandi G, Alma A, Kramer L, Esposito F, Bandi C, Sacchi L, Daffonchio D. 2007. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci U S A 104:9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutton TJ, Sinkins SP. 2004. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol Biol 13:317–322. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 49.Duron O, Fort P, Weill M. 2007. Influence of aging on cytoplasmic incompatibility, sperm modification and Wolbachia density in Culex pipiens mosquitoes. Heredity 98:368–374. doi: 10.1038/sj.hdy.6800948. [DOI] [PubMed] [Google Scholar]
- 50.Tortosa P, Charlat S, Labbé P, Dehecq J-S, Barré H, Weill M. 2010. Wolbachia age-sex-specific density in Aedes albopictus: a host evolutionary response to cytoplasmic incompatibility. PLoS One 5:e9700. doi: 10.1371/journal.pone.0009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurst GD, Johnson AP, Schulenburg JH, Fuyama Y. 2000. Male-killing Wolbachia in Drosphila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mouton L, Henri H, Bouletreau M, Vavre F. 2006. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 132:49–56. [DOI] [PubMed] [Google Scholar]
- 53.Miller WJ, Riegler M. 2006. Evolutionary dynamics of wAu-like Wolbachia variants in neotropical Drosophila spp. Appl Environ Microbiol 72:826–835. doi: 10.1128/AEM.72.1.826-835.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urban JM, Cryan JR. 2012. Two ancient bacterial endosymbionts have coevolved with the planthoppers (Insecta: Hemiptera: Fulgoroidea). BMC Evol Biol 12:87–87. doi: 10.1186/1471-2148-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morag N, Mullens BA, Gottlieb Y. 2013. Assessment of survival and body size variation of Culicoides imicola (Diptera: Ceratopogonidae) as funcations of “Candidatus Cardinium” (Bacteroidetes) infection status. Appl Environ Microbiol 79:6260–6263. doi: 10.1128/AEM.01793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouchon D, Rigaud T, Juchault P. 1998. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc Biol Sci 265:1081–1090. doi: 10.1098/rspb.1998.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zchori-Fein E, Perlman SJ. 2004. Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol 13:2009–2016. doi: 10.1111/j.1365-294X.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 58.Tagami Y, Miura K. 2004. Distribution and prevalence of Wolbachia in Japanese populations of Lepidoptera. Insect Mol Biol 13:359–364. doi: 10.1111/j.0962-1075.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- 59.Dittmar K, Whiting MF. 2004. New Wolbachia endosymbionts from Nearctic and Neotropical fleas (Siphonaptera). J Parasitol 90:953–957. doi: 10.1645/GE-186R. [DOI] [PubMed] [Google Scholar]
- 60.Russell JA, Goldman-Huertas B, Moreau CS, Baldo L, Stahlhut JK, Werren JH, Pierce NE. 2009. Specialization and geographic isolation among Wolbachia symbionts from ants and lycaenid butterflies. Evolution 63:624–640. doi: 10.1111/j.1558-5646.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 61.Ros VID, Fleming VM, Feil EJ, Breeuwer JAJ. 2009. How diverse is the genus Wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl Environ Microbiol 75:1036–1043. doi: 10.1128/AEM.01109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baldo L, Hotopp JCD, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH. 2006. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weeks AR, Velten R, Stouthamer R. 2003. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc Biol Sci 270:1857–1865. doi: 10.1098/rspb.2003.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotoh T, Noda H, Ito S. 2007. Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98:13–20. doi: 10.1038/sj.hdy.6800881. [DOI] [PubMed] [Google Scholar]
- 65.White JA, Kelly SE, Cockburn SN, Perlman SJ, Hunter MS. 2011. Endosymbiont costs and benefits in a parasitoid infected with both Wolbachia and Cardinium. Heredity 106:585–591. doi: 10.1038/hdy.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyle L, O'Neill SL, Robertson HM, Karr TL. 1993. Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 67.Vega FE, Benavides P, Stuart JA, O'Neill SL. 2002. Wolbachia infection in the coffee berry borer (Coleoptera: Scolytidae). Ann Entomol Soc Am 95:374–378. doi: 10.1603/0013-8746(2002)095[0374:WIITCB]2.0.CO;2. [DOI] [Google Scholar]
- 68.Gosiewski T, Jurkiewicz-Badacz D, Sroka A, Brzychczy-Wloch M, Bulanda M. 2014. A novel, nested, multiplex, real-time PCR for detection of bacterial and fungi in blood. BMC Microbiol 14:144. doi: 10.1186/1471-2180-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharpe RG, Hims MM, Harbach RE, Butlin RK. 1999. PCR-based methods for identification of species of the Anopheles minimus group: allele-specific amplification and single-strand conformation polymorphism. Med Vet Entomol 13:265–273. doi: 10.1046/j.1365-2915.1999.00178.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.