Abstract
Enterococci rank as one of the leading causes of nosocomial infections, such as urinary tract infections, surgical wound infections, and endocarditis, in humans. These infections can be hard to treat because of the rising incidence of antibiotic resistance. Enterococci inhabiting nonhuman reservoirs appear to play a critical role in the acquisition and dissemination of antibiotic resistance determinants. The spread of antibiotic resistance has become a major concern in both human and veterinary medicine, especially in Southeast Asia, where many developing countries have poor legislation and regulations to control the supply and excessive use of antimicrobials. This review addresses the occurrence of antibiotic-resistant enterococci in Association of Southeast Asian Nations countries and proposes infection control measures that should be applied to limit the spread of multiple-drug-resistant enterococci.
INTRODUCTION
The enterococci are a complex and diverse group of bacteria. They are commonly found in the gastrointestinal tracts, female genital tracts, and oral cavities of humans and animals and on their skin. Enterococci are also found in soil, water, and foods. Different species of enterococci are able to grow at 10°C to 45°C in environments with a broad range of pH values (1). These characteristics present a challenge to those who wish to control the spread of the pathogenic species of these organisms, which can cause serious infections in humans and animals. In addition, enterococci have the capacity to acquire a wide variety of antimicrobial resistance factors through horizontal exchange of mobile genetic material, which presents further problems in the management of patients with enterococcal infections (2). Enterococci can be transmitted to humans by various means, including contaminated food and water (3). The presence of antibiotic-resistant enterococci in the fecal material of animals has therefore become a major global concern in both human and veterinary medicine. Most of the studies concerning the transmission of microorganisms from food animals to humans have focused on pathogens that pose a direct threat to human health (4). Given the significant importance of Enterococcus spp. to public health and the farming industry, additional information on the genetics and transmission of multidrug resistance in these species is essential.
Legislation and regulations to control the supply and excessive use of antimicrobials are very poor in many developing Southeast Asian countries (5–7), and the prevalence of antimicrobial resistance in major bacterial pathogens such as enterococci has been rapidly increasing in Asia (8–11). In particular, the rise of multidrug-resistant (MDR) enterococci is of great concern.
This review briefly summarizes the classification of enterococci and discusses the incidence and causes of MDR enterococci in nonhuman reservoirs, particularly farm animals and water supplies. Their prevalence in hospitals is also reviewed, and possible control measures are suggested, with a particular focus on the Association of Southeast Asian Nations (ASEAN).
HUMAN RESERVOIRS OF ENTEROCOCCUS SPP.
Enterococcus spp. are normal flora of the human gastrointestinal tract (12). Enterococci are minority members of the bacterial community in humans, as molecular analysis has shown that these bacteria make up no more than 1% of the intestinal microflora of an adult (1, 13). However, the medical importance of these bacteria overshadows their relative numbers in the intestinal tract. This is because Enterococcus spp. now rank among the leading causes of nosocomial infections in humans (14).
Enterococci are well adapted for living in biofilms, where adhesion to extracellular matrix proteins of the human gut is the first step in colonization and infection (15, 16). The ability to form biofilms is a critical factor in causing endodontic and urinary tract infections, as well as endocarditis. According to the National Institutes of Health, biofilms are involved in over 80% of microbial infections in the body (17). A mature biofilm can tolerate antibiotics at concentrations 10 to 1,000 times as high as those required to kill planktonic bacteria (18). A recent study in Australia determined significant clonal variation of clinical Enterococcus faecalis isolates in the capacity to form biofilms when subjected to sub-MICs of the antimicrobial compounds clindamycin and tetracycline, which are found in endodontic medicaments (19). A strong correlation between the presence of the virulence gene esp and the ability of enterococci to form biofilms in vitro has also been reported (20–22). The contribution of esp to biofilm formation was found to be most pronounced in the presence of ≥0.5% (wt/vol) glucose (20). These results suggest that whereas esp is important in biofilm formation, additional determinants in E. faecalis may also contribute to biofilm formation (20). Studies on antibiotic resistance and biofilm production of enterococci with relevance to Southeast Asia have not been focused on because of fragmented information.
Certain strains of enterococci have long been known as important causes of endocarditis and in the 1970s began to be recognized as common causes of hospital-acquired urinary tract and wound infections (14). While traditionally 90% of all enterococcal infections were caused by E. faecalis and only 10% were caused by E. faecium, the proportion of E. faecium has gradually increased over the years to 40% (1). Other enterococcal species, including E. avium, E. casseliflavus, E. cecorum, E. dispar, E. durans, E. gallinarum, E. hirae, E. malodoratus, E. mundtii, E. pseudoavium, E. raffinosus, E. saccharolyticus, E. seriolicida, and E. solitarius, are found primarily in the gastrointestinal tracts of various animals but are occasionally isolated from human infections (1).
NONHUMAN RESERVOIRS OF ENTEROCOCCUS SPP.
Apart from humans, Enterococcus spp. are a natural part of the intestinal flora of most mammals and birds (23). The livestock industries of Southeast Asia, China, and Papua New Guinea play a major role globally in terms of meat production, contributing roughly 13 to 33% of the global meat production from 1979 to 2004 (24). Southeast Asia also imports livestock from China, India, Australia, and the United States. The large importers of livestock, mainly cattle and pigs, are Singapore, Malaysia, and Indonesia (24). Studies carried out in Malaysia, Thailand, Vietnam, Indonesia, and other Southeast Asian countries reported MDR enterococci isolated from livestock and animal-related products (23, 25). Many Southeast Asian nations, such as Malaysia, Myanmar, Indonesia, Thailand, and Vietnam, have flourishing poultry and livestock industries and are also major exporters around the Asian region (26). Countries that either export or import livestock or chickens could be inadvertently involved in the spread of MDR E. faecalis because of the widespread use of antimicrobials in these industries, as discussed later (27).
USE OF ANTIMICROBIALS IN SOUTHEAST ASIA
In addition to the treatment of human infections, antimicrobial agents are used on food animals, on pets, and in laboratories. In modern food animal production, antimicrobial agents are used in four different ways: (i) therapy, i.e., the treatment of infections of animals; (ii) metaphylactics, i.e., the treatment of clinically healthy animals belonging to the same flock or pen as animals with clinical signs; (iii) prophylactics, i.e., the treatment of healthy animals in a period of stress to prevent disease, such as during early weaning; and (iv) growth promotion, i.e., the inclusion of antimicrobial agents continuously in animal feed to prevent infections and improve growth (28, 29).
It is challenging to obtain reliable data on the quantities of antimicrobial agents used on food animals worldwide. In the United States, the farm animal population (consisting of approximately 5.34 million lambs and sheep, 89.3 million cattle, 113.2 million pigs, and 479 million fowls in 2012) used an estimated 13,542 metric tons of antimicrobial agents, while the usage for humans was estimated to be approximately 3,289 metric tons, in 2011 (30, 31). According to a 2013 report from the Department for Environmental Food and Rural Affairs in the United Kingdom, it was estimated that approximately 290 metric tons of antimicrobial agents were sold for administration to food animals in 2011 (32). The United Kingdom farm animal population consisted of approximately 32 million lambs and sheep, 9.7 million cattle, 4.8 million pigs, and 162 million fowls in 2012 (33). Antimicrobial consumption data are lacking in many developing countries, including ASEAN countries (34). Table 1 shows the livestock population in ASEAN countries in 2010, as well as the estimated antimicrobial consumption of cattle, chickens, and pigs (35). The estimates of antimicrobial consumption presented in Table 1 are based on antimicrobial consumption per population correction unit (PCU), as devised by Van Boeckel et al. (36) for Organization for Economic Cooperation and Development countries. The mean of the posterior for antimicrobial consumption was 45 mg/PCU for cattle, 148 mg/PCU for chickens, and 172 mg/PCU for pigs (36). PCUs are used to compare populations and production of different types of livestock across countries and correspond to 1 kg of a living or slaughtered animal (37) using an estimate of 2.5 kg per chicken (38), 100 kg per pig (39), and 600 kg per cow (40). Assuming that antimicrobial consumption by chickens, cattle, and pigs represents the majority of the antimicrobial consumption by food-producing animals, the total consumption of antimicrobials was calculated for each country by pooling the estimates collected by multiplying the per-PCU value by the total national population of each type of livestock (36). On the basis of the estimated values of antimicrobial consumption in Table 1, Indonesia, Vietnam, and Myanmar are the three leading consumers of antimicrobials for farm use on a total per-country basis.
TABLE 1.
Livestock populations and total antimicrobial consumption by chickens, cattle, and pigs in ASEAN countriesa
| Country | Livestock population (103) |
No. of PCUs (103) |
Total antimicrobial consumptionb (106 mg/PCU) | ||||
|---|---|---|---|---|---|---|---|
| Chickens | Cattle | Pigs | Chickens | Cattle | Pigs | ||
| Brunei | 16,000 | 1 | 1.3 | 40,000 | 600 | 130 | 5.9 |
| Cambodia | 17,448 | 3,484 | 2,057 | 43,620 | 2,090,400 | 205,700 | 135.9 |
| Indonesia | 1,622,750 | 1,363 | 7,212 | 4,056,875 | 817,800 | 721,200 | 761.2 |
| Lao PDRc | 23,000 | 1,400 | 3,400 | 57,500 | 840,000 | 340,000 | 104.7 |
| Malaysia | 225,790 | 909 | 1,711 | 564,475 | 545,400 | 171,100 | 137.5 |
| Myanmar | 125,000 | 13,000 | 7,900 | 312,500 | 7,800,000 | 790,000 | 533.1 |
| Philippines | 158,984 | 2,570 | 13,398 | 397,460 | 1,542,000 | 1,339,800 | 358.6 |
| Singapore | 3,300 | 0.2 | 270 | 8,250 | 120 | 27,000 | 5.8 |
| Thailand | 231,918 | 6,498 | 7,623 | 579,795 | 3,898,800 | 762,300 | 392.3 |
| Vietnam | 218,201 | 5,916 | 27,373 | 545502.5 | 3,549,600 | 2,737,300 | 711.2 |
The data shown are from FAOSTAT, the FAO Statistics Division, 2010.
By chickens, cattle, and pigs.
PDR, People's Democratic Republic.
Although there is a large amount of data about the emergence of antimicrobial-resistant enterococci in Southeast Asian countries, most of this information is fragmented since it has been published in different papers in different countries over several decades (41–44). However, several studies show the extent of unregulated and inappropriate use of antimicrobials in food animals in developing Southeast Asian countries such as Vietnam and Malaysia (23, 25, 45). Usui et al. (25) obtained results that demonstrate the use of antimicrobials in chickens in Southeast Asian countries, especially Vietnam, to be higher than in developed countries (45). In Vietnam, colistin was reported as an antibiotic commonly used on poultry, representing 4 to 7% of the total antibiotic use in quantitative terms, compared with the 1.6% reported in nine European countries (46). The use of antimicrobials in Vietnamese aquaculture has also been reported to be high, at 700 g/metric ton of production compared to 1 to 200 g/metric ton in three European countries, Canada, and Chile (47). In Malaysia, there are currently 97 antimicrobials registered for use according to the National Pharmaceutical Control Bureau of the Ministry of Health, Malaysia. Most of these registered drugs are used on poultry and pig farms. Unfortunately, more than half of the antibiotics registered with the Ministry of Health for food animal use are not recommended for veterinary use by the World Health Organization (WHO). These antibiotics include ampicillin, amoxicillin, cefadroxil, chlortetracycline, oxytetracycline, doxycycline, sulfadiazine, sulfadimethoxine, erythromycin, spiramycin, neomycin, gentamicin, and flumequine (48). Macrolides, trimethoprim, sulfonamides, fluoroquinolones, and tetracyclines are classes of antibiotics that are commonly used in animal husbandry and human medicine in the Southeast Asian region (6, 7, 49).
To summarize, in comparison with western countries, geographic variations in the use of antimicrobials for poultry and livestock are notable in Southeast Asia because of different standards and fragmented policies on antimicrobial use in different countries (41). Countries such as Indonesia have a well-designed and established system for the control of residues of veterinary drugs; however, issues relating to facilities, human resources, and law enforcement need to be controlled (50). The department of livestock and fisheries in Laos lacks consistent methods for evaluating and addressing antimicrobial resistance issues (51). Myanmar also has a major existing problem of inappropriate use of antimicrobials, and most farmers use antimicrobials without any consultation by veterinarians (52). Much work is needed to elucidate the levels of antimicrobial resistance in these countries, entailing cost, manpower resources, and policy reviews (6, 7).
Monitoring systems in developed countries, such as the Danish Integrated Antimicrobial Resistance Monitoring and Research Programme established in Denmark in 1995, are used to assess antimicrobial resistance in bacteria, including enterococci, from healthy food-producing animals (42). Control measures set by the World Organization for Animal Health and the Food and Agricultural Organization (FAO) in 2010 include published guidelines for national antimicrobial surveillance programs for animals and the responsible administration of antimicrobials to them (43). The Danish Integrated Antimicrobial Resistance Monitoring and Research Programme reported a decrease in MDR E. faecalis in pigs from 40% in 2011 to 34% in 2012. The prevalence of MDR E. faecalis in broilers also decreased from 13% in 2009 to 5% in 2013 (42).
In December 1998, the European Commission decided to ban the use of bacitracin, spiramycin, tylosin, and virginiamycin for growth promotion beginning on 1 July 1999 (53). These initiatives follow the recommendations of the WHO and have had significant effects on the types and amounts of antimicrobial agents used. In comparison to the legislation and policies in most ASEAN nations, the European Union has stronger control over the regulation of nontherapeutic uses of antibiotics in animals. The European Union leads the world in reducing antibiotic use in healthy animals. Sweden, Denmark, and Switzerland were the first countries to unilaterally ban all nontherapeutic antibiotic growth promoters in animal feed (48). A more organized system for antimicrobial resistance monitoring in both agricultural and clinical settings and restriction of antimicrobial use are essential for preserving the therapeutic value of antibiotics in Southeast Asia.
ENTEROCOCCUS SPP. IN THE ENVIRONMENT AND WATER
Environmental and water samples often contain enterococci (54). Large amounts of human and animal waste are distributed into the environment through sewage or nonsewage systems. For almost a century, enterococci have been used as indicators of fecal contamination of water and food for human consumption (1). Pathogenic bacteria in environmental surface waters originate mainly from the final effluent discharge from sewage wastewater treatment plants. Treated sludge, a by-product from treated sewage wastewater containing the fecal contents of animals and humans, can be used as fertilizer on agricultural land, which could potentially pass MDR strains on to the food supply (55). Challenges for effective wastewater management differ in Southeast Asian countries as well. These include poor sanitation levels, especially in rural areas, inadequate sewerage network coverage, and lack of sewage treatment facilities (56). Many countries in Southeast Asia still depend on septic tanks and other low-cost onsite sanitation facilities. However, most of these countries do not have specific policies or a legal and institutional framework for appropriate septage management. Unfortunately, septic tanks are poorly designed and not accurately constructed, operated, and maintained in many cases. In Vietnam (57), a low treatment performance efficacy of only 20 to 30% biochemical oxygen demand removal was observed. According to AECOM and the Department of Water and Sanitation in Developing Countries (SANDEC) in 2010, the amount of generated septage that has been treated varies among different Southeast Asian countries, amounting to 4% in Indonesia, 5% in Metro Manila in the Philippines, less than 4% in Vietnam, and 30% in Thailand (58). In environmental water such as agricultural wells on animal farms, coastal waters, rivers, and canals, the species considered fecal contaminants are mainly E. faecalis and E. faecium, but other species can also be recovered (1). The water cycle has been suggested as a transmission route for resistance to antibiotics (55), and this may be particularly true if incentives for water quality monitoring are lacking and the possibility of direct discharge of poorly treated sewage into seawater and rivers is present. Two studies (59, 60) have isolated MDR enterococci from coastal bathing waters and storm waters that lead to recreational beaches around Malaysia. The findings suggest that these recreational beaches may contribute to the dissemination of MDR enterococci and virulence characteristics. Another study carried out in Thailand found a high prevalence of MDR enterococci, 10.3% of which were vancomycin-resistant Enterococcus (VRE) isolates, in environmental water, including agricultural wells on animal farms, rivers, and canals (55). This again suggests a potential route for the transfer of MDR enterococci and resistance genes into the human food chain and environment that could potentially pose a threat to public health. Table 2 summarizes studies carried out in Southeast Asian countries investigating incidences of antibiotic-resistant Enterococcus species in the environment, namely, water sources and farm animals.
TABLE 2.
Summary of key studies investigating incidences of antibiotic-resistant Enterococcus species in the environment
| Country | Source | Resistance rates | Reference |
|---|---|---|---|
| Thailand | Environmental watera | 48.4% resistant to ciprofloxacin, 46.8% resistant to tetracycline | 55 |
| Malaysia | Feces of live broiler chickens | VRE (48% E. faecalis, 25.7% E. faecium, 12.1% E. gallinarum, 1.4% E. casseliflavus, 12.8% other Enterococcus spp.) | 80 |
| Malaysia | Coastal bathing waters | 76.63% resistant to kanamycin, 10.87% resistant to novobiocin, 8.38% resistant to chloramphenicol | 60 |
| Malaysia | Sewage treated effluent | 71.4% resistant to ampicillin, 4.7% resistant to ciprofloxacin, 95.2% resistant to cefuroxime | 59 |
| Vietnam | Feces of live chicken | E. faecalis 86.3% resistant to chloramphenicol, 90.9% resistant to erythromycin and lincomycin, 100% resistant to oxytetracycline; E. faecium 97.8% resistant to oxytetracycline, 88,8% resistant to lincomycin, 86.5% resistant to enrofloxacin | 25 |
| Indonesia | Feces of live chicken | E. faecalis 79.3% resistant to lincomycin, 77.6% resistant to erythromycin, 65.5% resistant to oxytetracycline; E. faecium 81% resistant to oxytetracycline, 69% resistant to enrofloxacin, lincomycin, and kanamycin | 25 |
| Thailand | Feces of live chicken | E. faecalis 56.8% resistant to oxytetracycline, 54% resistant to lincomycin, 48.5% resistant to erythromycin; E. faecium 92.2% resistant to oxytetracycline, 83.9% resistant to lincomycin, 82.8% resistant to enrofloxacin | 25 |
| Vietnam | Pig manure | 100% resistant to tetracycline, 32% resistant to enrofloxacin | 11 |
| Vietnam | Water sediment from pond | 90% resistant to tetracycline, 45% resistant to enrofloxacin | 11 |
Agricultural wells on animal farms, rivers, and canals.
TRANSFER OF RESISTANCE BETWEEN NONHUMAN AND HUMAN RESERVOIRS
Infections of animals with enterococci are rarely specifically targeted with antimicrobial agents. However, as normal inhabitants of the intestinal tract, enterococci are exposed to antimicrobial selection every time animals are subjected to antimicrobial therapy or given antimicrobial agents for growth promotion (61).
Enterococci are one of the traditional bacterial markers of fecal contamination of food and water for human consumption, and it has been accepted for several decades that enterococci from nonhuman sources could contaminate food intended for human consumption (55). Clearly, enterococci with resistance genes may reach humans in several ways, including direct contact with farm personnel (23, 62), via wastewater and surface water (55, 59, 60), or by contact with or consumption of food animals and food of animal origin (23, 25). Although the hygienic standards of meat production are high in most developed countries, fecal contamination of meat products cannot be completely eliminated (63). Figure 1 shows the complex epidemiology of enterococci and the ecological relationships among different reservoirs (64). The interaction between the different reservoirs contributes to the wide spread of MDR enterococci. Transmission of resistance can take place through food animals or directly through contact between animals and humans. Studies have suggested the potential for zoonotic transmission of enterococci. Research in Vietnam documented the isolation of the same clone of E. faecalis in a patient's urine and poultry from the same household in which patient had close contact with poultry. In 23% of urinary tract infection cases, pulsed-field gel electrophoresis patterns identical or closely related to those found in poultry were detected (65). In another study carried out in Malaysia, one vancomycin-resistant E. faecium strain isolated from a chicken was found to be clonal to that of humans (23). Treated sewage sludge, a by-product from treated sewage wastewater containing the fecal contents of animals and humans, can be used as fertilizers, which potentially pass MDR strains on to the food supply. A study conducted in Vietnam found similar relative occurrences of E. faecium, E. faecalis, and other Enterococcus spp. in the water sediment of ponds and manure samples of pigs, suggesting that Enterococcus spp. isolated from the ponds originated mainly from pig manure (11). Insufficient data on the interaction between different reservoirs concerns the wide spread of MDR enterococci in Southeast Asian countries.
FIG 1.
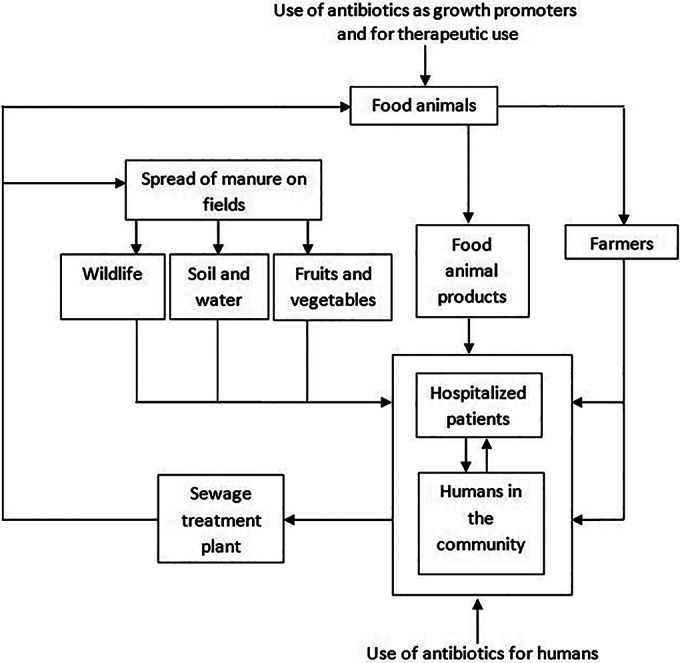
Ecological relationships among different reservoirs of Enterococcus spp. (Adapted from reference 64.)
USE OF ANTIMICROBIALS IN HOSPITALS AND ANTIMICROBIAL RESISTANCE
Generally, the antibiotic of choice for the treatment of enterococcal infections in humans is ampicillin, and vancomycin is an alternative agent (66). Prudent antibiotic use is an essential component for control of the spread of VRE. The Healthcare Infection Control Practices Advisory Committee (HICPAC) guidelines insist on curtailing the use of antibiotics for routine surgical prophylaxis and empirical therapy (67).
Although the full extent of MDR Enterococcus spp. in Southeast Asia remains undiscovered, data are available from some countries. A linezolid-resistant E. faecalis strain was isolated in July 2010 from a diabetic patient in Thailand who received linezolid for at least 3 months prior to the isolation of the resistant strain (68). From 1999 to 2009, 1.9% of the enterococcal isolates recovered from patients at Rajavithi Hospital in Thailand were VRE. In this 1.9%, there was a significantly higher prevalence of VRE isolates in the inpatient department than in the outpatient department (10). In Indonesia, antibiotics can easily be obtained without a prescription from medical retailers despite existing regulations (69). According to the National Surveillance of Antimicrobial Resistance in Malaysia, antibiotic susceptibility testing was carried out on bacterial isolates from hospitalized patients whereby analysis was based on one isolate per patient (70). This analysis revealed that roughly 1.2% of the E. faecalis isolates were vancomycin resistant in 2012 and the proportion was 1.4% in 2013; a greater time frame is required to determine if the rate is increasing over time. There was also an increase in the number of patients with ciprofloxacin-resistant E. faecalis from 248 (20.6%) in 2012 to 437 (21.1%) in 2013 and in the number of patients with penicillin-resistant E. faecium from 309 (84.4%) in 2012 to 415 (89.6%) in 2013 (70). A study in Malaysia isolated tazobactam-piperacillin-, ampicillin-, and penicillin-resistant and high-level gentamicin-resistant enterococcal strains from hospitalized patients (66). Another case study in 2008 discovered vancomycin-, teicoplanin-, ampicillin-, and gentamicin-resistant E. faecium strains in two patients with chronic diabetes mellitus and urinary tract infection undergoing a 3- to 12-day course of treatment with cloxacillin, ceftriaxone, erythromycin, and vancomycin (71). The first VRE strain isolated in Singapore was obtained in 1994 from a patient at the Singapore National Burns Centre (72). Two consecutive outbreaks followed later, in 2004 (73) and 2005 (74). According to the Network for Antimicrobial Resistance Surveillance in Singapore in 2006, VRE constitutes 0.8% of all enterococcal isolates in Singapore public hospitals (75). An epidemiology study in Singapore documenting VRE in public hospitals from 2006 to 2010 reported a percentage of VRE clinical isolates of 24.4% (9). While the prevalence of VRE clinical isolates remains low in Singapore public hospitals, the need for continued vigilance is necessary to prevent any further increase in VRE prevalence. Documented cases of antibiotic-resistant Enterococcus species from hospitalized patients were reported in Myanmar during 2009 to 2013, of which 30.8% were resistant to ampicillin and 68.8% were resistant to erythromycin (76). In 2012, a case study in Vietnam reported vancomycin-resistant E. faecium in a patient with liver cirrhosis undergoing antimicrobial therapy consisting imipenem and vancomycin for 1 week (77). Thus, not only regulation of antibiotic use but also diligent prescribing of other broad-spectrum antimicrobials should be carried out in hospitals around the region in an attempt to decrease colonization with MDR E. faecalis.
INFECTION SOURCE CONTROL
In past years, the source of infection for most patients was thought to be their own endogenous enterococci (1). However, with the increase of sophisticated molecular typing techniques and the rise in nosocomial acquisition of antibiotic-resistant enterococci in the 1980s and 1990s, studies have clearly demonstrated the transmission of enterococci among patients in acute-care hospital settings (2). A recent study in Malaysia discovered clinical strains of MDR E. faecium that were presumably spread from patient to patient via the hands of health care workers (23). Transient carriage of E. faecalis on the hands of health care workers has also been documented in another study (72). Transmission of enterococci from a transiently colonized health care worker's hand to a patient may involve direct contact with hands, environmental surfaces, or medical equipment, but it is more likely that transmission results in colonization of the patient's gut (72). The acquired antibiotic-resistant strain is able to survive in the human gastrointestinal tract with the aid of selective pressure from broad-spectrum antibiotics, which are used frequently in hospitalized patients (72). Infections consequently arise from these newly acquired enterococcal strains.
Various guidelines have been set up by countries in Southeast Asia to provide infection control information for hospitals, health care facilities, and livestock/animal health to prevent the spread of MDR enterococci. Indonesia aims to strengthen the implementation of regulations for the production, distribution, sale, and prescription of antibiotics, as well as establish the Antimicrobial Resistance Control Program as a national program. This program will aid in developing regulations for antibiotic use in veterinary practices, as well as guidelines for community-acquired infection and public access to antibiotics (48). Myanmar is currently establishing a national multisectoral steering committee for antimicrobial resistance and is in the process of constituting a national policy for antibiotic use in humans and animals. Data collection is ongoing in Thailand to understand trends in antimicrobial resistance and develop an antibiotic policy on MDR bacteria (48). Treatment options for infections with antibiotic-resistant Enterococcus spp., especially VRE, are limited. Therefore, measures to minimize the spread of these resistant organisms within a facility are essential. Each facility should establish a comprehensive infection control program aimed at decreasing the transmission of VRE among patients (78). Specific policies should be based on the rates of resistance within the facility and should be appropriate for the specific health care setting. In 1995, the Centers for Disease Control and Prevention HICPAC published recommendations aimed at controlling the nosocomial transmission of VRE (67). These recommendations provide a base on which specific policies can be developed for individual facilities. The major HICPAC recommendations focus on (i) prudent use of vancomycin to decrease the selective pressure for the emergence of VRE, (ii) education of health care personnel about the importance of VRE and its mode of transmission, (iii) use of the microbiology lab to quickly identify patients with VRE, and (iv) infection control measures that minimize transmission to other patients. The emergence and severity of VRE have also been reported in other regions of Southeast Asia (5, 41). These findings suggest that early detection of VRE is necessary to prevent further spread in health care settings.
Conclusion.
Enterococci inhabiting nonhuman reservoirs appear to play a critical role in the acquisition and distribution of antibiotic resistance determinants (61, 79). The introduction of antimicrobial agents into clinical medicine and animal husbandry has been one of the most important medical achievements; however, surveillance and enforcement of the use of antibiotics in hospital settings and on farms is often lax in most Southeast Asian countries. In addition, the Southeast Asian region lacks systemic studies to understand the epidemiology of MDR enterococci. The most effective way to limit the spread of antimicrobial resistance and thereby extend the usefulness of antimicrobials is to restrict their use (48). As a consequence, it has been recommended that antimicrobial agents that select for resistance to antibiotics used for human therapy should not be used for growth promotion in animal husbandry. Growth promoters should be limited to agents that are of no value for therapeutic use (48). To limit the emergence of antimicrobial resistance and the consequences for human and animal health, it is necessary to collect data on factors affecting the occurrence, emergence, and spread of resistance. At present, the knowledge of antimicrobial resistance among food animals in Southeast Asia is fragmentary. This review highlights the need for health care settings, industries, and governments in Southeast Asian countries to strictly regulate the use of antibiotics to curb the emerging threat of MDR enterococci.
REFERENCES
- 1.Gilmore MS. 2002. The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC. [Google Scholar]
- 2.Handwerger S, Raucher B, Altarac D, Monka J, Marchione S, Sing KV, Murray BE, Wolff J, Walters B. 1993. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin and gentamicin. Clin Infect Dis 16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 3.Leclercq R. 2009. Epidemiological and resistance issues in multidrug-resistant staphylococci and enterococci. Clin Microbiol Infect 15:224–231. doi: 10.1111/j.1469-0691.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- 4.Marshall BM, Levy SB. 2011. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jean SS, Hsueh PR. 2011. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents 37:291–295. doi: 10.1016/j.ijantimicag.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Food and Agriculture Organization of the United Nations. 2012. Proceedings of the International Workshop on the Use of Antimicrobials in Livestock Production and Antimicrobial Resistance in the Asia-Pacific Region 2012. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- 7.World Health Organization. 2012. Report of regional workshop on antimicrobial resistance 2012. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 8.Kang CI, Song JH. 2013. Antimicrobial resistance in Asia: current epidemiology and clinical implications. Infect Chemother 45:22–31. doi: 10.3947/ic.2013.45.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Y, Chan JP, Fisher DA, Hsu LY, Koh TH, Krishnan P, Kwa AL, Tan TY, Tee NW. 2012. Vancomycin-resistant enterococci in Singaporean hospitals: 5 year results of a multi-centre surveillance programme. Ann Acad Med Singapore 41:77–81. [PubMed] [Google Scholar]
- 10.Thongkoom P, Kanjanahareutai S, Chantrakooptungool S, Rahule S. 2012. Vancomycin resistant enterococci (VRE) isolates isolated in Rajavithi Hospital between 1999 and 2009. J Med Assoc Thai 95(Suppl 3):S7–S15. [PubMed] [Google Scholar]
- 11.Dang ST, Petersen A, Van Truong D, Chu HT, Dalsgaard A. 2011. Impact of medicated feed on the development of antimicrobial resistance in bacteria at integrated pig-fish farms in Vietnam. Appl Environ Microbiol 77:4494–4498. doi: 10.1128/AEM.02975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee G. 2013. Ciprofloxacin resistance in Enterococcus faecalis strains isolated from male patients with complicated urinary tract infection. Korean J Urol 54:388–393. doi: 10.4111/kju.2013.54.6.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sghir A, Gramet G, Suau A, Rochet V, Pochart P, Dore J. 2000. Quantification of bacterial groups within the human faecal flora by oligonucleotide probe hybridization. Appl Environ Microbiol 66:2263–2266. doi: 10.1128/AEM.66.5.2263-2266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed JA, Huang DB. 2007. Biofilm formation by enterococci. J Med Microbiol 56:1581–1588. doi: 10.1099/jmm.0.47331-0. [DOI] [PubMed] [Google Scholar]
- 16.Mika KB, Imamura G, Chang C, Conway V, Fernandez G, Griffith JF, Kampalath RA, Lee CM, Lin CC, Moreno R, Thompson S, Whitman RL, Jay JA. 2009. Pilot- and bench-scale testing of faecal indicator bacteria survival in marine beach sand near point sources. J Appl Microbiol 107:72–84. doi: 10.1111/j.1365-2672.2009.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Römling U, Balsalobre C. 2012. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 18.Lewis K. 2001. Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson CE, Cathro PC, Rogers AH, Briggs N, Zilm PS. 2015. Clonal diversity in biofilm formation by Enterococcus faecalis in response to environmental stress associated with endodontic irrigants and medicaments. Int Endod J 48:210–219. doi: 10.1111/iej.12301. [DOI] [PubMed] [Google Scholar]
- 20.Toledo-Arana A, Valle J, Solano C, Arrizubieta MJ, Cucarella C, Lamata M, Amorena B, Leiva J, Penades JR, Lasa I. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl Environ Microbiol 67:4538–4545. doi: 10.1128/AEM.67.10.4538-4545.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. 2004. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect Immun 72:6032–6039. doi: 10.1128/IAI.72.10.6032-6039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upadhyaya GP, Lingadevaru UB, Lingegowda RK. 2011. Comparative study among clinical and commensal isolates of Enterococcus faecalis for presence of esp gene and biofilm production. J Infect Dev Ctries 5:365–369. [DOI] [PubMed] [Google Scholar]
- 23.Getachew Y, Hassan L, Zakaria Z, Saleha AA. 2013. Genetic variability of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis isolates from human, chickens, and pigs in Malaysia. Appl Environ Microbiol 79:4528. doi: 10.1128/AEM.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Rosario BP, Aquino AP, Tidon AG, Gerpacio RV. 2008. Livestock sector training needs assessment report for Southeast Asia, China and Papua New Guinea. International Livestock Research Institute, Nairobi, Kenya. [Google Scholar]
- 25.Usui M, Ozawa S, Onozato H, Kuge R, Obata Y, Uemae T, Ngoc PT, Heriyanto A, Chalemchaikit T, Makita K, Muramatsu Y, Tamura Y. 2014. Antimicrobial susceptibility of indicator bacteria isolated from chickens in Southeast Asian countries (Vietnam, Indonesia and Thailand). J Vet Med Sci 76:685–692. doi: 10.1292/jvms.13-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anonymous. 2007. Subregional report on animal genetic resources: Southeast Asia. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- 27.Hammerum AM. 2012. Enterococci of animal origin and their significance for public health. Clin Microbiol Infect 18:619–625. doi: 10.1111/j.1469-0691.2012.03829.x. [DOI] [PubMed] [Google Scholar]
- 28.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. 2000. Effect of antibiotic therapy on the density of vancomycin resistant enterococci in the stool of colonized patients. N Engl J Med 343:1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aarestrup FM. 2002. Veterinary drug use in farm animal production and the antibiotic resistance problem, p 153–170. In Smulders FJM, Collins JD (ed), Food safety assurance and veterinary public health, volume 1: food safety assurance in the pre-harvest phase Academic Publishers, Wageningen, The Netherlands. [Google Scholar]
- 30.Center for Science in the Public Interest. 2013. Antibiotic resistance in foodborne pathogens. Center for Science in the Public Interest, Washington, DC. [Google Scholar]
- 31.Anonymous. 2012. Overview of U.S. livestock, poultry, and aquaculture production in 2012. National animal health monitoring system. U.S. Department of Agriculture, Washington, DC. [Google Scholar]
- 32.Veterinary Medicines Directorate. 2014. United Kingdom veterinary antibiotic resistance and sales surveillance 2013. Government Department for the Environment, Food and Rural Affairs, New Haw, Addlestone, Surrey, United Kingdom: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/440744/VARSS.pdf. [Google Scholar]
- 33.Anonymous. 2012. Agriculture in the United Kingdom 2012. Department for Environment, Food and Rural Affairs, London, United Kingdom: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/208436/auk-2012-25jun13.pdf. [Google Scholar]
- 34.Huttner A, Harbath S, Carlet J, Cosgrove S, Goossens H, Holmes A, Jarlier V, Voss A, Pittet D, World Healthcare-Associated Infections Forum participants. 2013. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2:31. doi: 10.1186/2047-2994-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anonymous. 2010. FAOSTAT on livestock population in ASEAN countries. Food and Agricultural Organization of the United Nations, New York, NY. [Google Scholar]
- 36.Van Boeckel TP, Browner C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R. 2015. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anonymous. 2013. Sales of veterinary antimicrobial agents in 25 EU/EEA countries in 2011: third ESVAC report. European Medicines Agency, London, United Kingdom. [Google Scholar]
- 38.Anonymous. 2013. The life of broiler chickens. Compassion in World Farming, Decatur, GA: https://www.ciwf.org.uk/media/5235306/The-life-of-Broiler-chickens.pdf. [Google Scholar]
- 39.Lawlor P. 2010. What is the optimum slaughter weight for pigs? Pig Development Unit, Animal and Grassland Research Centre, Teagasc, Moorepark, Fermoy, Ireland. [Google Scholar]
- 40.Anonymous. 2009. Modern beef production. Cattlemen's Beef Board and National Cattlemen's Beef Association, Washington, DC. [Google Scholar]
- 41.Lestari ES, Severin JA, Verbrugh HA. 2012. Antimicrobial resistance among pathogenic bacteria in Southeast Asia. Southeast Asian J Trop Med Public Health 43:385–422. [PubMed] [Google Scholar]
- 42.Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP). 2013. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Rosendahls-Schultz Grafisk, Albertslund, Denmark. [Google Scholar]
- 43.World Organization for Animal Health. 2010. FAO-OIE-WHO: a tripartite alliance for ‘one health’. World Organization for Animal Health, Paris, France. [Google Scholar]
- 44.Teale CJ, Moulin G. 2012. Prudent use guidelines: a review of existing veterinary guidelines. Rev Sci Tech 31:343–354. [DOI] [PubMed] [Google Scholar]
- 45.Duong VN, Paulsen P, Suriyasathaporn W, Smulders FJ, Kyule MN, Baumann MP, Zessin KH, Pham HN. 2006. Preliminary analysis of tetracycline residues in marketed pork in Hanoi, Vietnam. Ann N Y Acad Sci 1081:534–542. doi: 10.1196/annals.1373.081. [DOI] [PubMed] [Google Scholar]
- 46.European Medicines Agency. 2011. Trends in the sales of veterinary antimicrobial agents in nine European countries (2005–2009). European Medicines Agency, London, United, Kingdom. [Google Scholar]
- 47.Smith P. 2008. Antimicrobial resistance in aquaculture. Rev Sci Tech 27:243–264. [PubMed] [Google Scholar]
- 48.World Health Organization. 2011. Critically important antimicrobials for human medicine, 3rd revision 2011 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 49.Managaki S, Murata A, Takada H, Tuyen BC, Chiem NH. 2007. Distribution of macrolides, sulfonamides and trimethoprim in tropical waters: ubiquitous occurrence of veterinary antibiotics in the Mekong delta. Environ Sci Technol 41:8004–801010. doi: 10.1021/es0709021. [DOI] [PubMed] [Google Scholar]
- 50.Suandy I, Suparno X. 2012. Antimicrobial use and resistance in livestock in the Asia-Pacific region: Indonesia country report. 36th session of the APHCA-Animal Production and Health Commission for Asia and the Pacific. Food and Agricultural Organization of the United Nations, Geneva, Switzerland. [Google Scholar]
- 51.Stoesser N, Crook DW, Moore CE, Phetsouvanh R, Chansamouth V, Newton PN, Jones N. 2012. Characteristics of CTX-M ESBL-producing Escherichia coli isolates from the Lao People's Democratic Republic, 2004–09. J Antimicrob Chemother 67:240–242. doi: 10.1093/jac/dkr434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sone P, Aung YH. 2012. Antimicrobial use and resistance in livestock in the Asia-Pacific region: Myanmar country report. 36th session of the APHCA-Animal Production and Health Commission for Asia and the Pacific. Food and Agricultural Organization of the United Nations, Geneva, Switzerland. [Google Scholar]
- 53.Casewell M, Friis C, Marco E, McMullin P, Phillips I. 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother 52:159–161. doi: 10.1093/jac/dkg313. [DOI] [PubMed] [Google Scholar]
- 54.Paul JH, Rose JB, Jiang S, Kellogg C, Shinn EA. 1995. Occurrence of fecal indicator bacteria in surface waters and the subsurface aquifer in Key Largo, Florida. Appl Environ Microbiol 61:2235–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tansuphasiri U, Khaminthakul D, Pandii W. 2006. Antibiotic resistance of enterococci isolated from frozen foods and environmental water. Southeast Asian J Trop Med Public Health 37:162–170. [PubMed] [Google Scholar]
- 56.McIntosh AC. 2014. Urban water supply and sanitation in Southeast Asia: a guide to good practice. Asian Development Bank, Mandaluyong, Philippines. [Google Scholar]
- 57.Anh N, Ha TD, Nhue TH, Heinss U, Morel A, Moura M, Schertenleib R. 2002. Decentralized wastewater treatment-new concept and technologies for Vietnamese conditions. Proceedings of the 5th Specialized Conference on Small Water and Wastewater Treatment Systems, Istanbul, Turkey. [Google Scholar]
- 58.Anonymous. 2010. A rapid assessment of septage management in Asia: policies and practices in India, Indonesia, Malaysia, the Philippines, Sri Lanka, Thailand, and Vietnam. U. S. Agency for International Development, Washington, DC: http://pdf.usaid.gov/pdf_docs/PNADS118.pdf. [Google Scholar]
- 59.Al-Gheethi AA, Ismail N, Lalung J, Talib A, Efaq AN, Ab Kadir MO. 2013. Susceptibility for antibiotics among fecal indicators and pathogenic bacteria in sewage treated effluents. Water Pract Technol 8:1–6. doi: 10.2166/wpt.2013.001. [DOI] [Google Scholar]
- 60.Dada AC, Ahmad A, Usup G, Heng LY. 2013. Speciation and antimicrobial resistant of enterococci isolated from recreational beaches in Malaysia. Environ Monit Assess 185:1583–1599. doi: 10.1007/s10661-012-2653-6. [DOI] [PubMed] [Google Scholar]
- 61.Hammerum AM, Lester CH, Heuer OE. 2010. Antimicrobial-resistant enterococci in animals and meat: a human health hazard? Foodborne Pathog Dis 7:1137–1146. doi: 10.1089/fpd.2010.0552. [DOI] [PubMed] [Google Scholar]
- 62.Getachew Y, Hassan L, Zakaria Z, Zaid CZM, Yardi A, Shukor RA, Marawin LT. 2012. Characterization and risk factors of vancomycin-resistant enterococci (VRE) among animal-affiliated workers in Malaysia. J Appl Microbiol 113:1184–1195. doi: 10.1111/j.1365-2672.2012.05406.x. [DOI] [PubMed] [Google Scholar]
- 63.Hayes RJ, English LL, Carter PJ, Proescholdt T, Lee KY, Wagner DD, White DG. 2003. Prevalence and antimicrobial resistance of Enterococcus species isolated from retail Meats. Appl Environ Microbiol 69:7153–7160. doi: 10.1128/AEM.69.12.7153-7160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aerestrup FM, Butaye P, Witte W. 2002. Nonhuman reservoirs of enterococci, p 79 In Gilmore MS. (ed), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC. [Google Scholar]
- 65.Poulsen LL, Bisgaard M, Son NT, Trung NV, An HM, Dlasgaard A. 2012. Enterococcus faecium clones in poultry and in humans with urinary tract infections, Vietnam. Emerg Infect Dis 18:1096–1100. doi: 10.3201/eid1807.111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weng PL, Ramli R, Shamsudin MN, Cheah YK, Hamat RA. 2013. High genetic diversity of Enterococcus faecium and Enterococcus faecalis clinical isolates by pulsed-field gel electrophoresis and multilocus sequence typing from a hospital in Malaysia. Biomed Res Int 2013:938937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anonymous. 1995. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Morbid Mortal Wkly Rep 23:87–94. [DOI] [PubMed] [Google Scholar]
- 68.Diaz L, Kiratisin P, Mendes RE, Panesso D, Singh KV, Arias CA. 2012. Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother 56:3917–3922. doi: 10.1128/AAC.00419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hadi U, van den Broek P, Kolopaking EP, Zairina N, Gardjito W, Gyssens IC, Study Group Antimicrobial Resistance in Indonesia: Prevalence and Prevention (AMRIN). 2010. Cross-sectional study of availability and pharmaceutical quality of antibiotics requested with or without prescription (over the counter) in Surabaya, Indonesia. BMC Infect Dis 10:203. doi: 10.1186/1471-2334-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anonymous. 2013. National surveillance of antibiotic resistance report. Ministry of Health Malaysia, Putrajaya, Malaysia. [Google Scholar]
- 71.Poh LW, Rukman AW, Cheah YK, Norital Z, Nazri AM, Mariana NS. 2012. Vancomycin-resistant Enterococcus faecium of multi locus sequence type 18 in Malaysia. Med J Malaysia 67:639–640. [PubMed] [Google Scholar]
- 72.Chlebicki MP, Kurup A. 2008. Vancomycin-resistant Enterococcus: a review from a Singapore perspective. Ann Acad Med Singapore 37:861–869. [PubMed] [Google Scholar]
- 73.Yang KS, Fong YT, Lee HY, Kurup A, Koh TH, Koh D, Lim MK. 2007. Predictors of vancomycin-resistant enterococcus (VRE) carriage in the first major VRE outbreak in Singapore. Ann Acad Med Singapore 36:379–383. [PubMed] [Google Scholar]
- 74.Hsu LY, Tan TY, Jureen R, Koh TH, Krishnan P, Lin RTP, Tee NWS, Tambyah PA. 2007. Antimicrobial drug resistance in Singapore hospitals. Emerg Infect Dis 13:1944–1947. doi: 10.3201/eid1312.070299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Myat TO, Prasad N, Thinn KK, Win KK, Htike WW, Zin KN, Murdoch DR, Crump JA. 2014. Bloodstream infections at a tertiary referral hospital in Yangon, Myanmar. Trans R Soc Trop Med Hyg 108:692–698. doi: 10.1093/trstmh/tru151. [DOI] [PubMed] [Google Scholar]
- 76.Seol CA, Park JS, Sung H, Kim MN. 2014. Co-colonization of vanA and vanB Enterococcus faecium of clonal complex 17 in a patient with bacteremia due to vanA E. faecium. Diagn Microbiol Infect Dis 79:141–143. doi: 10.1016/j.diagmicrobio.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 77.Ostrowsky BE, Trick WE, Sohn AH, Quick SB, Holt S, Carson LA, Hill BC, Arduino MJ, Kuehnert MJ, Jarvis WR. 2001. Control of vancomycin resistant Enterococcus in health care facilities in a region. N Engl J Med 344:1427–1433. doi: 10.1056/NEJM200105103441903. [DOI] [PubMed] [Google Scholar]
- 78.Ang SW, Seah CS, Lee ST. 1996. Vancomycin-resistant Enterococcus in the Singapore National Burns Centre: a case report. Ann Acad Med Singapore 25:270–272. [PubMed] [Google Scholar]
- 79.Fisher K, Phillips C. 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155:1749. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 80.Getachew YM, Hassan L, Zakaria Z, Saleha AA, Kamaruddin MI, Che Zalina MZ. 2009. Characterization of vancomycin-resistant Enterococcus isolates from broilers in Selangor, Malaysia. Trop Biomed 26:280–288. [PubMed] [Google Scholar]


